Soil Nitrogen Sorption Using Charcoal and Wood Ash
Abstract
1. Introduction
2. Acidic Soils of Malaysia
3. Soil Acidification
4. Forms of Nitrogen in Soil-Plant Systems
5. Nitrogen Fractions and Pools in Soils
6. Soil Organic Nitrogen
7. Soil Inorganic Nitrogen
8. Soil Inorganic Nitrogen (Nitrate-Nitrogen)
9. Soil Inorganic Nitrogen (Ammonium-Nitrogen)
10. Nitrogen as a Fertiliser
11. Urea
12. Role of Nitrogen in Crops Productivity
13. Nitrogen Availability in Soils
14. Factors Affecting Nitrogen Availability to Crops
15. Nitrogen Transformation in Soil-Crops Systems
16. Mineralisation and Immobilisation
17. Nitrification
18. Denitrification
19. Volatilisation
20. Leaching
21. Crop Removal, Soil Erosion and Runoff
22. Nitrogen Use Efficiency
23. Factors Affecting Nitrogen Use Efficiency
24. Ammonium Adsorption and Desorption
25. Kinetics of Sorption Model
26. Adsorption Isotherm
27. Types of Spectroscopy
28. Ashes and Their Chemical Composition
29. Properties of Ashes
30. Use of Ashes in Agriculture
31. Mechanism of Bark Ash on the pH of Tropical Acid Soils
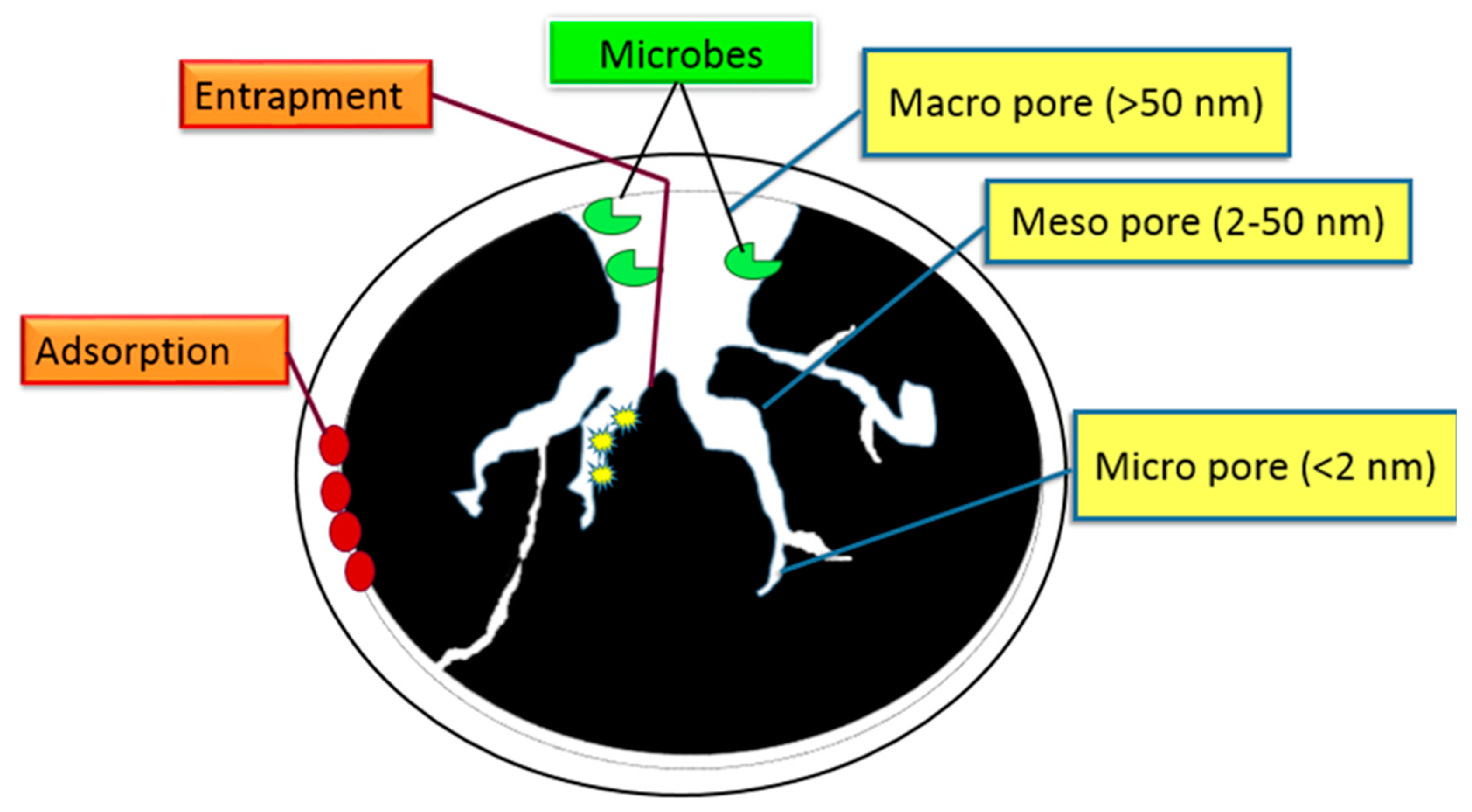
32. Charcoal
33. Charcoal Porosity
34. Charcoal as an Amendment That Retains Nutrients in Agriculture
35. Nutrient Sorption Mechanism of Charcoal
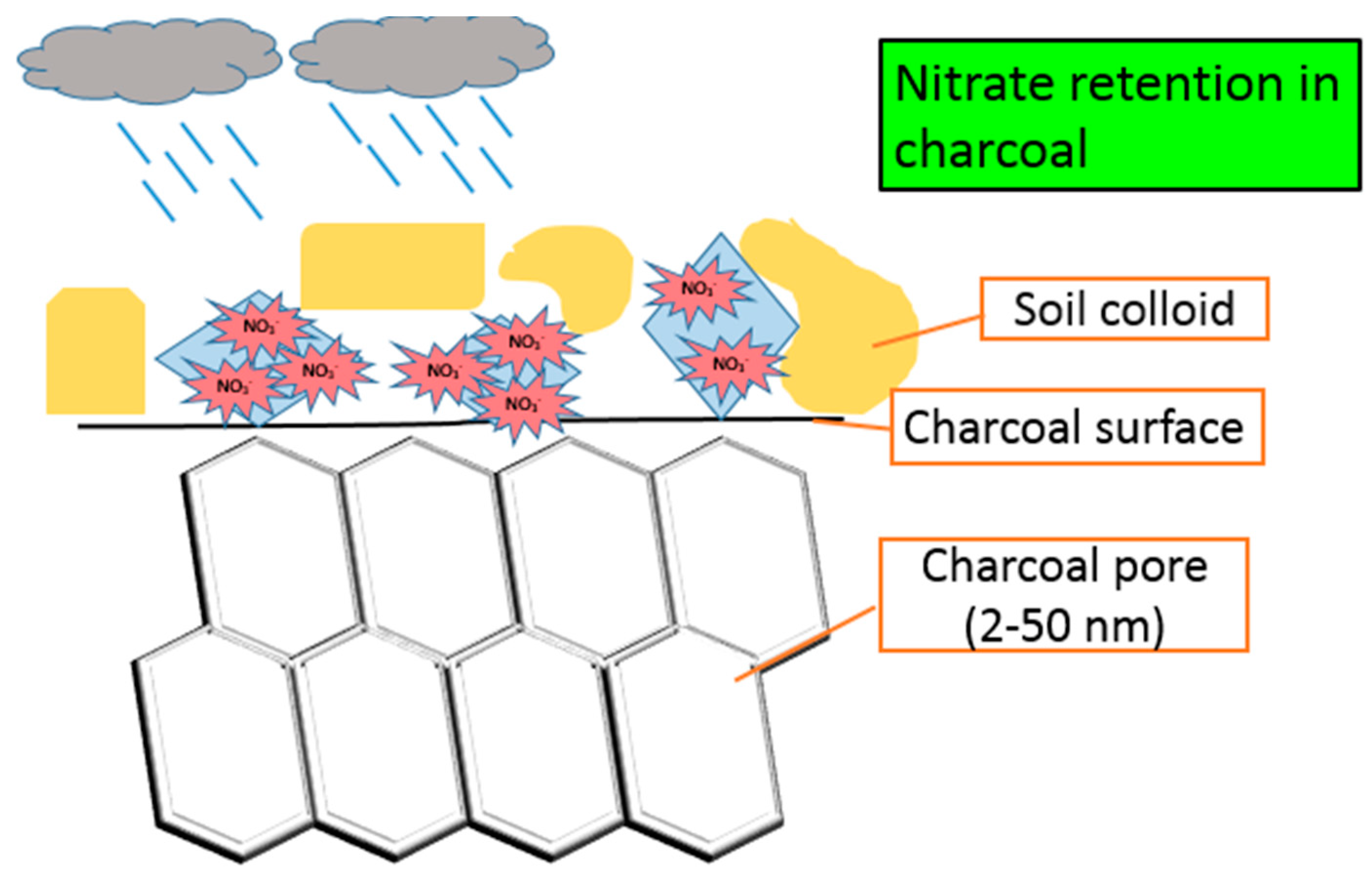
36. Water Retention Mechanism of Charcoal
37. Nitrate Retention Mechanism by Charcoal
38. Ammonium Retention Mechanism by Charcoal
39. Adverse Effect of Charcoal as Soil Amendment
40. Humic Substances
41. Humic Acids
42. Fulvic Acids
43. Humin
44. Changes of Carbon to Nitrogen
45. Current Challenges in Using Charcoal and Wood Ash as Adsorbents
46. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Godfray, H.C.J.; Beddington, J.R.; Crute, I.R.; Haddad, L.; Lawrence, D.; Muir, J.F.; Pretty, J.; Robinson, S.; Thomas, S.M.; Toulmin, C. Food Security: The Challenge of Feeding 9 Billion People. Science 2010, 327, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Lim, L.Y.; Lee, C.T.; Bong, C.P.C.; Lim, J.S.; Sarmidi, M.R.; Klemes, J.J. A Review on the Impacts of Compost on Soil Nitrogen Dynamics. Chem. Eng. Trans. 2018, 63, 349–354. [Google Scholar]
- Liu, Q.; Liu, B.; Zhang, Y.; Hu, T.; Lin, Z.; Liu, G.; Wang, X.; Ma, J.; Wang, H.; Jin, H.; et al. Biochar Application as a Tool to Decrease Soil Nitrogen Losses [NH3 Volatilization, N2O Emissions, and N Leaching] from Croplands: Options and Mitigation Strength in a Global Perpective. Glob. Chang. Biol. 2019, 25, 2077–2093. [Google Scholar] [CrossRef]
- Naz, M.Y.; Sulaiman, S.A. Slow Release Coating Remedy for Nitrogen Loss from Conventional Urea: A Review. J. Control. Release 2016, 225, 109–120. [Google Scholar] [CrossRef] [PubMed]
- Nweke, I.A. Effect of Land Use on Organic Matter Concentration of Aggregate Fractions of Fallow and Cultivated Soils. Indian J. Appl. Res. 2015, 5, 507–511. [Google Scholar]
- Brady, N.C.; Weil, R.R. The Nature and Properties of Soils, 14th ed.; Pearson Education: Upper Saddle River, NJ, USA, 2008. [Google Scholar]
- Liu, D.; Huang, Y.; Yan, H.; Jiang, Y.; Zhao, T.; An, S. Dynamics of Soil Nitrogen Fractions and Their Relationship with Soil Microbial Communities in Two Forest Species of Northern China. PLoS ONE 2018, 13, e0196567. [Google Scholar] [CrossRef]
- Li, S.; Barreto, V.; Li, R.; Chen, G.; Hsieh, Y.P. Nitrogen Retention of Biochar Derived from Different Feedstocks at Variable Pyrolysis Temperatures. J. Anal. Appl. Pyrolysis 2018, 133, 136–146. [Google Scholar] [CrossRef]
- Fan, M.X.; Mackenzie, A.F. Urea and Phosphate Interactions in Fertilizer Microsites: Ammonia Volatilization and pH Changes. Soil Sci. Soc. Am. J. 1993, 57, 839–845. [Google Scholar] [CrossRef]
- Siva, K.B.; Aminuddin, H.; Husni, M.H.A.; Manas, A.R. Ammonia Volatilization from Urea as Affected by Tropical-Based Palm Oil Mill Effluent (Pome) and Peat. Commun. Soil Sci. Plant Anal. 1999, 30, 785–804. [Google Scholar] [CrossRef]
- Theeba, M.; Husni, M.A.; Samsuri, A.W.; Robert, T.B.; Illani, Z.H. Nutrient Retention Capacity of Rice Husk Biocharcoal in Co-Composted Poultry Manure. J. Trop. Agric. Food. Sci. 2016, 44, 197–209. [Google Scholar]
- Ariyaratne, R.M. Integrated Plant Nutrition Systems (IPNS) Training Manual (Sri Lanka); The Fertilizer Advisory, Development Information Network for Asia and the Pacific (FADINAP): Bangkok, Thailand, 2000; p. 140. [Google Scholar]
- Tiessen, H.; Cuevas, E.; Chacon, P. The Role of Soil Organic Matter in Sustaining Soil Fertility. Nature 1994, 371, 783–785. [Google Scholar] [CrossRef]
- Fearnside, P.M. Global Warming and Tropical Land-Use Change: Greenhouse Gas Emissions from Biomass Burning, Decomposition and Soils in Forest Conversion, Shifting Cultivation and Secondary Vegetation. Clim. Chang. 2000, 46, 115–158. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K. Biochemical Cycling of Nitrogen and Phosphorus in Biochar-Amended Soils. Soil Biol. Biochem. 2016, 103, 1–15. [Google Scholar] [CrossRef]
- Brewer, C.E.; Brown, R.C. Biochar. In Comprehensive Renewable Energy; Sayigh, A., Ed.; Elsevier: Amsterdam, The Netherlands; Oxford, UK, 2012; pp. 357–384. [Google Scholar]
- Lehmann, J. Biochar for Environmental Management: Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK; Washington, DC, USA, 2009. [Google Scholar]
- Kocsis, T.; Biró, B.; Ulmer, Á.; Szántó, M.; Kotroczó, Z. Time-Lapse Effect of Ancient Plant Coal Biochar on Some Soil Agrochemical Parameters and Soil Characteristics. Environ. Sci. Pollut. Res. 2018, 25, 990–999. [Google Scholar] [CrossRef] [PubMed]
- Yu, X.-Y.; Ying, G.-G.; Kookana, R.S. Sorption and Desorption Behaviors of Diuron in Soils Amended with Charcoal. J. Agric. Food Chem. 2006, 54, 8545–8550. [Google Scholar] [CrossRef] [PubMed]
- Patel, H. Charcoal as an Adsorbent for Textile Wastewater Treatment. Sep. Sci. Technol. 2018, 53, 2797–2812. [Google Scholar] [CrossRef]
- Zackrisson, O.; Nilsson, M.-C.; Wardle, D.A. Key Ecological Function of Charcoal from Wildfire in the Boreal Forest Key Ecological Function of Charcoal from Wildfire in the Boreal Forest. Oikos 1996, 77, 10–19. [Google Scholar] [CrossRef]
- Pluchon, N.; Gundale, M.J.; Nilsson, M.-C.; Kardol, P.; Wardle, D.A. Stimulation of Boreal Tree Seedling Growth by Wood-Derived Charcoal: Effects of Charcoal Properties, Seedling Species and Soil Fertility. Funct. Ecol. 2014, 28, 766–775. [Google Scholar] [CrossRef]
- Mohammad-khah, A.; Ansari, R. Activated Charcoal: Preparation, Characterization and Applications: A Review Article. Int. J. ChemTech Res. 2009, 1, 859–864. [Google Scholar]
- Masek, O. Biochar Production Technologies. In Biochar for Environmental Management: Science, Technology and Implementation, 2nd Ed.; Chapter: Biochar Production Technology; Johannes, L., Stephen, J., Eds.; Routledge: Oxfordshire, UK, 2014. [Google Scholar]
- Demirbas, A.; Ahmad, W.; Alamoudi, R.; Sheikh, M. Sustainable Charcoal Production from Biomass. Energy Sources Part A Recover. Util. Environ. Eff. 2016, 38, 1882–1889. [Google Scholar] [CrossRef]
- Zhang, Z.H.; Wang, W.X.; Yang, X.Y.; Hu, Q.X.; Li, Y.F. Preparation of 4A Zeolite from Coal Gangue through a Alkali Fusion Method. China Surfactant Deterg Cosmet 2008, 5, 294–297. [Google Scholar]
- Kookana, R.S.; Sarmah, A.K.; Zwieten, L.V.; Krull, E.; Singh, B. Biochar Application to Soil: Agronomic and Environmental Benefits and Unintendedsystem in California. Agric. Ecosyst. Environ. 2011, 191, 17–26. [Google Scholar]
- Jindo, K.; Mizumoto, H.; Sawada, Y.; Sanchez-Monedero, M.A.; Sonoki, T. Physical and Chemical Characterization of Biochars Derived from Different Agricultural Residues. Biogeosciences 2014, 11, 6613. [Google Scholar] [CrossRef]
- DeLuca, T.H.; MacKenzie, M.D.; Gundale, M.J. Biochar Effects on Soil Nutrient Transformations. In Biochar for Environmental Management Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 251–270. [Google Scholar]
- Glaser, B.; Lehmann, J.; Zech, W. Ameliorating Physical and Chemical Properties of Highly Weathered Soils in the Tropics with Charcoal-A Review. Biol. Fertil. Soils 2002, 35, 219–230. [Google Scholar] [CrossRef]
- Kalyani, P.; Ariharaputhiran, A.; Darchen, A. Activated Carbon from Grass-A Green Alternative Catalyst Support for Water Electrolysis. Int. J. Hydrog. Energy 2013, 38, 10364–10372. [Google Scholar] [CrossRef]
- Van Laer, T.; De Smedt, P.; Ronsse, F.; Ruysschaert, G.; Boeckx, P.; Verstraete, W.; Lavrysen, L.J. Legal Constraints and Opportunities for Biochar: A Case Analysis of EU Law. Gcb Bioenergy 2015, 7, 14–24. [Google Scholar] [CrossRef]
- Demeyer, A.; Nkana, J.V.; Verloo, M.G. Characteristics of Wood Ash and Influence on Soil Properties and Nutrient Uptake: An Overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Nweke, I.A.; Mbah, C.N.; Oweremadu, E.O.; Dambaba, N.; Orji, E.C.; Ekesiobi, A.I.; Nnabuife, E.L.C. Soil pH, Available P of an Ultisol and Castor Performance as Influenced by Contrasting Tillage Methods and Wood Ash. Afr. J. Agric. Res. 2017, 12, 606–616. [Google Scholar] [CrossRef][Green Version]
- Quilliam, R.S.; Glanville, H.C.; Wade, S.C.; Jones, D.L. Life in the ’Charosphere’ Does Biochar in Agricultural Soil Provide a Significant Habitat for Microorganisms? Soil Biol. Biochem. 2013, 65, 287–293. [Google Scholar] [CrossRef]
- Quilliam, R.S.; DeLuca, T.H.; Jones, D.L. Biochar Application Rate Reduces Root Nodulation in Clover but Increases Nitrogenase Activity in Nodules. Plant Soil 2013, 366, 83–92. [Google Scholar] [CrossRef]
- Kilpimaa, S.; Kuokannen, T.; Lassi, U. Characterization and Utilization Potential of Wood Ash Form Combustion Process and Carbon Residue from Gasification Process. BioResources 2013, 8, 1011–1027. [Google Scholar] [CrossRef]
- Scheepers, G.P.; Du Toit, B. Potential Use of Wood Ash in South African Forestry: A Review. South. For. A J. For. Sci. 2016, 78, 255–266. [Google Scholar] [CrossRef]
- Anda, M.; Shamshuddin, J.; Fauziah, C.I.; Omar, S.S. Mineralogy and Factors Controlling Charge Development of Three Oxisols Developed from Different Parent Materials. Geoderma 2008, 143, 153–167. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Daud, N.W. Classification and Management of Highly Weathered Soils in Malaysia for Production of Plantation Crops. Princ. Appl. Assess. Soil Sci. 2011, 2011, 75–86. [Google Scholar]
- Kidd, P.S.; Proctor, J. Why Plants Grow Poorly on Very Acid Soils: Are Ecologists Missing the Obvious? J. Exp. Bot. 2001, 52, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Zech, W.; Senesi, N.; Guggenberger, G.; Kaiser, K.; Lehmann, J.; Miano, T.M.; Miltner, A.; Schroth, G. Factors Controlling Humification and Mineralization of Soil Organic Matter in the Tropics. Geoderma 1997, 79, 117–161. [Google Scholar] [CrossRef]
- Brown, T.T.; Koenig, R.T.; Huggins, D.R.; Harsh, J.B.; Rossi, R.E. Lime Effects on Soil Acidity, Crop Yield, and Aluminum Chemistry in Direct-Seeded Cropping Systems. Soil Sci. Soc. Am. J. 2008, 72, 634–640. [Google Scholar] [CrossRef]
- Shamshuddin, J.; Fauziah, C.I.; Bell, L.C. Soil Solution Properties and Yield of Corn and Groundnut Grown on Ultisols as Affected by Dolomitic Limestone and Gypsum Applications. Malays. J. Soil Sci. 2009, 13, 1–12. [Google Scholar]
- Ohno, T.; Amirbahman, A. Phosphorus Availability in Boreal Forest Soils: A Geochemical and Nutrient Uptake Modeling Approach. Geoderma 2010, 155, 46–54. [Google Scholar] [CrossRef]
- Ohno, T.; Fernandez, I.J.; Hiradate, S.; Sherman, J.F. Effects of Soil Acidification and Forest Type on Water Soluble Soil Organic Matter Properties. Geoderma 2007, 140, 176–187. [Google Scholar] [CrossRef]
- Harter, R.D. Acid Soils of the Tropics. ECHO Technical Note, ECHO. 2007. Volume 11. Available online: http://courses.umass.edu/psoil370/Syllabus-files/Acid_Soils_of_the_Tropics.pdf (accessed on 1 May 2021).
- Masclaux-Daubresse, C.; Daniel-Vedele, F.; Dechorgnat, J.; Chardon, F.; Gaufichon, L.; Suzuki, A. Nitrogen Uptake, Assimilation and Remobilization in Plants: Challenges for Sustainable and Productive Agriculture. Ann. Bot. 2010, 105, 1141–1157. [Google Scholar] [CrossRef] [PubMed]
- Follett, R.F. Transformation and Transport Processes of Nitrogen in Agricultural Systems. Nitrogen Environ. 2008, 2008, 19–50. [Google Scholar] [CrossRef]
- Grain Research and Development Corporation (GRDC). Maximising Nitrogen Availability to Crops. Plant Available Nitrogen [Fact Sheet]. Australian Government. 2013. Available online: https://grdc.com.au/resources-and-publications/all-publications/factsheets/2013/08/grdc-fs-plantavailablenitrogen (accessed on 1 May 2021).
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Enhancing Nitrogen Availability from Urea Using Clinoptilolite Zeolite. Geoderma 2017, 306, 152–159. [Google Scholar] [CrossRef]
- Brady, N.C.; Weil, R.R.; Brady, N.C. Elements of the Nature and Properties of Soils; Pearson Educational International: Upper Saddle River, NJ, USA, 2010. [Google Scholar]
- Korhonen, J.F.J.; Pihlatie, M.; Pumpanen, J.; Aaltonen, H.; Hari, P.; Levula, J.; Ilvesniemi, H. Nitrogen Balance of a Boreal Scots Pine Forest. Biogeosciences 2013, 10, 1083–1095. [Google Scholar] [CrossRef]
- Sainju, U.M.; Caesar-Tonthat, T.; Lenssen, A.W.; Evans, R.G.; Kolberg, R. Tillage and Cropping Sequence Impacts on Nitrogen Cycling in Dryland Farming in Eastern Montana. Soil Tillage Res. 2009, 103, 332–341. [Google Scholar] [CrossRef]
- Zhang, J.; Song, C.; Wenyan, Y. Tillage Effects on Soil Carbon Fractions in the Sanjiang Plain, Northeast China. Soil Tillage Res. 2007, 93, 102–108. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.W.; Caesar-TonThat, T.; Jabro, J.D.; Lartey, R.T.; Evans, R.G.; Allen, B.L. Dryland Soil Nitrogen Cycling Influenced by Tillage, Crop Rotation, and Cultural Practice. Nutr. Cycl. Agroecosystems 2012, 93, 309–322. [Google Scholar] [CrossRef]
- Sainju, U.M.; Lenssen, A.W.; Allen, B.L.; Stevens, W.B.; Jabro, J.D. Nitrogen Balance in Response to Dryland Crop Rotations and Cultural Practices. Agric. Ecosyst. Environ. 2016, 233, 25–32. [Google Scholar] [CrossRef]
- Kelley, K.R.; Stevenson, F.J. Forms and Nature of Organic N in Soil. In Nitrogen Economy in Tropical Soils; Springer: Dordrecht, The Netherlands, 1995; pp. 1–11. [Google Scholar]
- Amelung, W.; Zhang, X. Determination of Amino Acid Enantiomers in Soils. Soil Biol. Biochem. 2001, 33, 553–562. [Google Scholar] [CrossRef]
- Nannipieri, P.; Eldor, P. The Chemical and Functional Characterization of Soil N and Its Biotic Components. Soil Biol. Biochem. 2009, 41, 2357–2369. [Google Scholar] [CrossRef]
- Schulten, H.R.; Schnitzer, M. The Chemistry of Soil Organic Nitrogen: A Review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Schulten, H.R.; Leinweber, P.; Schnitzer, M. Analytical Pyrolysis and Computer Modelling of Humic and Soil Particles. In Structure and Surface Reactions of Soil Particles; John Wiley and Sons Ltd.: Hoboken, NJ, USA, 1998; pp. 281–324. [Google Scholar]
- Giese, A.C. Cell Physiology; Saunders Co.: Philadelphia, PA, USA, 1979. [Google Scholar]
- Stevenson, F.J. Humus Chemistry: Genesis, Composition, Reactions; John Wiley Sons: Hoboken, NJ, USA, 1994; pp. 59–95. [Google Scholar]
- Parsons, J.W. Chemistry and Distribution of Amino Sugars in Soils and Soil Organisms. Soil Biochem. 1981, 5, 197–227. [Google Scholar]
- Schmidt, B.H.; Kalbitz, K.; Braun, S.; Fuß, R.; McDowell, W.H.; Matzner, E. Microbial Immobilization and Mineralization of Dissolved Organic Nitrogen from Forest Floors. Soil Biol. Biochem. 2011, 43, 1742–1745. [Google Scholar] [CrossRef]
- Miller, A.J.; Cramer, M.D. Root Nitrogen Acquisition and Assimilation. Plant Soil 2005, 274, 1–36. [Google Scholar] [CrossRef]
- Ashton, I.W.; Miller, A.E.; Bowman, W.D.; Suding, K.N. Niche Complementarity Due to Plasticity in Resource Use: Plant Partitioning of Chemical N Forms. Ecology 2010, 91, 3252–3260. [Google Scholar] [CrossRef] [PubMed]
- Walworth, J. Nitrogen in the Soil and the Environment. 2013. Available online: https://repository.arizona.edu/bitstream/handle/10150/267773/az1591-2013.pdf?sequence=1 (accessed on 1 May 2021).
- Vigil, M.F.; Kissel, D.E. Equations for Estimating the Amount of Nitrogen Mineralized from Crop Residues. Soil Sci. Soc. Am. J. 1991, 55, 757–761. [Google Scholar] [CrossRef]
- Keuper, F.; Dorrepaal, E.; Van Bodegom, P.M.; Van Logtestijn, R.; Venhuizen, G.; Van Hal, J.; Aerts, R. Experimentally Increased Nutrient Availability at The Permafrost Thaw Front Selectively Enhances Biomass Production of Deep-Rooting Subarctic Peatland Species. Glob. Chang. Biol. 2017, 23, 4257–4266. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, B.J.; Robbins, S. PH Affects Ammonium, Nitrate and Proton Fluxes in the Apical Region of Conifer and Soybean Roots. Physiol. Plant. 2010, 138, 238–247. [Google Scholar] [CrossRef] [PubMed]
- Xu, G.; Fan, X.; Miller, A.J. Plant Nitrogen Assimilation and Use Efficiency. Annu. Rev. Plant Biol. 2012, 63, 153–182. [Google Scholar] [CrossRef] [PubMed]
- Strahm, B.D.; Harrison, R.B. Nitrate Sorption in a Variable-Charge Forest Soil of the Pacific Northwest. Soil Sci. 2006, 171, 313–321. [Google Scholar] [CrossRef]
- Marschner, P. Marschner’s Mineral Nutrition of Higher Plants, 3th ed.; Academic Press: London, UK; Waltham, MA, USA, 2012; pp. 135–151. [Google Scholar]
- Britto, D.T.; Kronzucker, H.J. NH4+ Toxicity in Higher Plants: A Critical Review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Dodds, W.K.; Whiles, M.R. Nitrogen, Sulfur, Phosphorus, and Other Nutrients. Freshw. Ecol. 2010, 2010, 345–373. [Google Scholar] [CrossRef]
- Gupta, P.K. A Handbook of Soil, Fertilizer and Manure; Agrobios: Chopasni Road, Jodhpur, India, 2003. [Google Scholar]
- Hirel, B.; Tétu, T.; Lea, P.J.; Dubois, F. Improving Nitrogen Use Efficiency in Crops for Sustainable Agriculture. Sustainability 2011, 3, 1452–1485. [Google Scholar] [CrossRef]
- Omar, L. Development, Beneficial Effects, and Economic Viability of Rice Straw and Paddy Husk Composts in Combination with Clinoptilolite Zeolite. Doctoral Thesis, Universiti Putra Malaysia, Seri Kembangan, Malaysia, 2016. [Google Scholar]
- Havlin, J.L.; Beaton, J.D.; Tisdale, S.L.; Nelson, W.L. Soil Fertility and Fertilizers: An Introduction to Nutrient Management, 17th ed.; Prientice Hall: Hoboken, NJ, USA, 2005. [Google Scholar]
- Guo, J.H.; Liu, X.J.; Zhang, Y.; Shen, J.L.; Han, W.X.; Zhang, W.F.; Zhang, F.S. Significant Acidification in Major Chinese Croplands. Science 2010, 327, 1008–1010. [Google Scholar] [CrossRef] [PubMed]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic Approaches toward Understanding Nitrogen Metabolism in Plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Wang, M.; Shen, Q.; Xu, G.; Guo, S. New Insight into the Strategy for Nitrogen Metabolism in Plant Cells. Int. Rev. Cell Mol. Boil. 2014, 310, 1–37. [Google Scholar]
- Coruzzi, G.; Bush, D.R. Nitrogen and Carbon Nutrient and Metabolite Signaling in Plants. Plant Physiol. 2001, 125, 61–64. [Google Scholar] [CrossRef] [PubMed]
- Miller, A.J.; Fan, X.; Orsel, M.; Smith, S.J.; Wells, D.M. Nitrate Transport and Signalling. J. Exp. Bot. 2007, 58, 2297–2306. [Google Scholar] [CrossRef] [PubMed]
- Schachtman, D.P.; Shin, R. Nutrient Sensing and Signaling: NPKS. Annu. Rev. Plant Biol. 2007, 58, 47–69. [Google Scholar] [CrossRef] [PubMed]
- Krouk, G.; Crawford, N.M.; Coruzzi, G.M.; Tsay, Y.F. Nitrate Signaling: Adaptation to Fluctuating Environments. Curr. Opin. Plant Biol. 2010, 13, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.; Forde, B.G. An Arabidopsis MADS Box Gene that Controls Nutrient-Induced Changes in Root Architecture. Science 1998, 279, 407–409. [Google Scholar] [CrossRef] [PubMed]
- Walch-Liu, P.; Neumann, G.; Bangerth, F.; Engels, C. Rapid Effects of Nitrogen Form on Leaf Morphogenesis in Tobacco. J. Exp. Bot 2000, 51, 227–237. [Google Scholar] [CrossRef] [PubMed]
- Stitt, M.; Müller, C.; Matt, P.; Gibon, Y.; Carillo, P.; Morcuende, R.; Scheible, W.R.; Krapp, A. Steps towards an Integrated View of Nitrogen Metabolism. J. Exp. Bot 2002, 53, 959–970. [Google Scholar] [CrossRef]
- Alboresi, A.; Gestin, C.; Leydecker, M.T.; Bedu, M.; Meyer, C.; Truong, H.N. Nitrate, a Signal Relieving Seed Dormancy in Arabidopsis. Plant Cell Environ. 2005, 28, 500–512. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, O.H.; Ab Majid, N.M. Towards Efficient Utilization of Nitrogen Based Fertilizers; Universiti Putra Malaysia Press: Serdang, Selangor, Malaysia, 2014. [Google Scholar]
- Scarsbrook, C.E. Nitrogen Availability. Soil Nitrogen 1965, 10, 481–502. [Google Scholar]
- Gouveia, G.A.; Eudoxie, G.D. Distribution of Fertilizer N among fixed Ammonium Fractions as Affected by Moisture and Fertilizer Source and Rate. Biol. Fertil. Soils 2007, 44, 9–18. [Google Scholar] [CrossRef]
- Gonçalves, J.D.M.; Carlyle, J.C. Modelling the Influence of Moisture and Temperature on Net Nitrogen Mineralization in a Forested Sandy Soil. Soil Biol. Biochem. 1994, 26, 1557–1564. [Google Scholar] [CrossRef]
- Sugihara, S.; Funakawa, S.; Kilasara, M.; Kosaki, T. Dynamics of Microbial Biomass Nitrogen in Relation to Plant Nitrogen Uptake during the Crop Growth Period in a Dry Tropical Cropland in Tanzania. Soil Sci. Plant Nutr. 2010, 56, 105–114. [Google Scholar] [CrossRef]
- Abera, G.; Wolde-meskel, E.; Beyene, S.; Bakken, L.R. Nitrogen Mineralization Dynamics under Different Moisture Regimes in Tropical Soils. Int. J. Soil Sci. 2012, 7, 132. [Google Scholar] [CrossRef]
- Baulch, H.M. Asking the Right Questions about Nutrient Control in Aquatic Ecosystems. Environ. Sci. Technol. 2013, 47, 1188–1189. [Google Scholar] [CrossRef] [PubMed]
- Robertson, G.P.; Groffman, P.M. Nitrogen Transformations. In Soil Microbiology, Ecology and Biochemistry; Academic Press: Cambridge, MA, USA, 2007; pp. 341–364. [Google Scholar]
- Myrold, D.D.; Bottomley, P.J. Nitrogen Mineralization and Immobilization. Nitrogen Agric. Syst. 2008, 49, 157–172. [Google Scholar]
- Liu, D.; Keiblinger, K.M.; Leitner, S.; Mentler, A.; Zechmeister-Boltenstern, S. Is There a Convergence of Deciduous Leaf Litter Stoichiometry, Biochemistry and Microbial Population during Decay? Geoderma 2016, 272, 93–100. [Google Scholar] [CrossRef]
- Ameloot, N.; Sleutel, S.; Das, K.C.; Kanagaratnam, J.; De Neve, S. Biochar Amendment to Soils with Contrasting Organic Matter Level: Effects on N Mineralization and Biological Soil Properties. Gcb Bioenergy 2015, 7, 135–144. [Google Scholar] [CrossRef]
- Rousk, K.; Michelsen, A.; Rousk, J. Microbial Control of Soil Organic Matter Mineralization Responses to Labile Carbon in Subarctic Climate Change Treatments. Glob. Chang. Biol. 2016, 22, 4150–4161. [Google Scholar] [CrossRef] [PubMed]
- Clough, T.J.; Condron, L.M.; Kammann, C.; Müller, C. A Review of Biochar and Soil Nitrogen Dynamics. Agronomy 2013, 3, 275–293. [Google Scholar] [CrossRef]
- Prosser, J.I.; Embley, T.M.; Webster, G. The Influence of Selection Pressures on Species Diversity, Functional Gene Diversity and Activity of Ammonia-Oxidizing Bacteria. In ‘Genes in the Environment’. The Fifteenth Special Symposium of the British Ecological Society, Oxford, UK, 17–19 September 2001; Blackwell Publishing: Hoboken, NJ, USA, 2003; pp. 187–202. [Google Scholar]
- Dobrovol’skaya, T.G.; Zvyagintsev, D.G.; Chernov, I.Y.; Golovchenko, A.V.; Zenova, G.M.; Lysak, L.V.; Manucharova, N.A.; Marfenina, O.E.; Polysnskaya, L.M.; Stepanov, A.L.; et al. The Role of Microorganisms in the Ecological Functions of Soils. Eurasian Soil Sci. 2015, 48, 959–967. [Google Scholar] [CrossRef]
- Berg, P.; Rosswall, T. Abiotic Factors Regulating Nitrification in a Swedish Arable Soil. Biol. Fertil. Soils 1989, 8, 247–254. [Google Scholar] [CrossRef]
- Macura, J.; Stotzky, G. Effect of Montmorillonite and Kaolinite on Nitrification in Soil. Folia Microbiol. 1980, 25, 90–105. [Google Scholar] [CrossRef] [PubMed]
- Castaldi, S.; Riondino, M.; Baronti, S.; Esposito, F.R.; Marzaioli, R.; Rutigliano, F.A. Impact of Biochar Application to a Mediterranean Wheat Crop on Soil Microbial Activity and Greenhouse Gas Fluxes. Chemosphere 2012, 85, 1464–1471. [Google Scholar] [CrossRef]
- Ball, P.N.; MacKenzie, M.D.; DeLuca, T.H.; Holben, W.E. Wildfire and Charcoal Enhance Nitrification and Ammonium-Oxidizing Bacterial Abundance in Dry Montane Forest Soils. J. Environ.Qual. 2010, 39, 1243–1253. [Google Scholar] [CrossRef]
- Cheng, Y.; Cai, Z.C.; Chang, S.X.; Wang, J.; Zhang, J.B. Wheat Straw and Its Biochar Have Contrasting Effects on Inorganic N Retention and N2O Production in a Cultivated Black Chernozem. Biol. Fertil. Soils 2012, 48, 941–946. [Google Scholar] [CrossRef]
- Klemedtsson, L.; Svensson, B.H.; Rosswall, T. Relationships between Soil Moisture Content and Nitrous Oxide Production during Nitrification and Denitrification. Biol. Fertil. Soils 1988, 6, 106–111. [Google Scholar] [CrossRef]
- Firestone, M.K.; Davidson, E.A. Microbiological Basis of NO and N2O Production and Consumption in Soil. Exch. Trace Gases Terr. Ecosyst. Atmos. 1988, 47, 7–21. [Google Scholar]
- Mosier, A.R. Exchange of Gaseous Nitrogen Compounds between Agricultural SYSTEMS and the atmosphere. Plant Soil 2001, 228, 17–27. [Google Scholar] [CrossRef]
- Bornø, M.L.; Rønn, R.; Ekelund, F. Is Wood Ash Amendment a Suitable Mitigation Strategy for N2O Emissions from Soil? Sci. Total Environ. 2020, 713, 136581. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Zhang, X.; Xu, W.; Liu, X.; Li, Y.; Wei, J.; Wang, Z.; Lu, X. Ammonia Volatilization as the Major Nitrogen Loss Pathway in Dryland Agro-Ecosystems. Environ. Pollut. 2020, 265, 114–862. [Google Scholar] [CrossRef] [PubMed]
- Galloway, J.N.; Townsend, A.R.; Erisman, J.W.; Bekunda, M.; Cai, Z.; Freney, J.R.; Martinelli, L.A.; Seitzinger, S.P.; Sutton, M.A. Transformation of the Nitrogen Cycle: Recent Trends, Questions, and Potential Solutions. Science 2008, 320, 889–892. [Google Scholar] [CrossRef] [PubMed]
- Gu, B.; Ge, Y.; Ren, Y.; Xu, B.; Luo, W.; Jiang, H.; Chang, J. Atmospheric Reactive Nitrogen in China: Sources, Recent Trends, and Damage Costs. Environ. Sci. Technol. 2012, 46, 9420–9427. [Google Scholar] [CrossRef] [PubMed]
- Harrison, R.; Webb, J. A Review of the Effect of N Fertilizer Type on Gaseous Emissions. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2001. [Google Scholar]
- Rochette, P.; Angers, D.A.; Chantigny, M.H.; Gasser, M.O.; MacDonald, J.D.; Pelster, D.E.; Bertrand, N. NH3 Volatilization, Soil Concentration and Soil pH Following Subsurface Banding of Urea at Increasing Rates. Can. J. Soil Sci. 2013, 93, 261–268. [Google Scholar] [CrossRef]
- Peng, X.; Maharjan, B.; Yu, C.; Su, A.; Jin, V.; Ferguson, R.B. A Laboratory Evaluation of Ammonia Volatilization and Nitrate Leaching Following Nitrogen Fertilizer Application on a Coarse-Textured Soil. Agron. J. 2015, 107, 871–879. [Google Scholar] [CrossRef]
- Stevenson, F.J.; Cole, M.A. Cycles of Soil: Carbon, Nitrogen, Phosphorus, Sulfur, Micronutrients; John Wiley Sons: New York, NY, USA, 1999. [Google Scholar]
- Liyanage, L.R.M.C.; Jayakody, A.N.; Gunaratne, G.P. Ammonia Volatilization from Frequently Applied Fertilizers for the Low-Country Tea Growing Soils of Sri Lanka. Trop. Agric. Res. 2014, 26, 48–61. [Google Scholar] [CrossRef]
- Guimarães, G.G.; Mulvaney, R.L.; Cantarutti, R.B.; Teixeira, B.C.; Vergütz, L. Value of Copper, Zinc, and Oxidized Charcoal for Increasing Forage Efficiency of Urea N Uptake. Agric. Ecosyst. Environ. 2016, 224, 157–165. [Google Scholar] [CrossRef]
- Paiva, D.M.D.; Cantarutti, R.B.; Guimarães, G.G.F.; Silva, I.R.D. Urea Coated with Oxidized Charcoal Reduces Ammonia Volatilization. Rev. Bras. De Ciênc. Do Solo 2012, 36, 1221–1230. [Google Scholar] [CrossRef]
- Puga, A.P.; Melo, L.C.A.; De Abreu, C.A.; Coscione, A.R.; Paz-Ferreiro, J. Leaching and Fractionation of Heavy Metals in Mining Soils Amended with Biochar. Soil Tillage Res. 2016, 164, 25–33. [Google Scholar] [CrossRef]
- Jury, W.A.; Nielsen, D.R. Nitrate Transport and Leaching Mechanisms. In Developments in Agricultural and Managed Forest Ecology; Elsevier: Amsterdam, The Netherlands, 1989; Volume 21, pp. 139–157. [Google Scholar]
- Di, H.J.; Cameron, K.C. Nitrate Leaching in Temperate Agroecosystems: Sources, Factors and Mitigating Strategies. Nutr. Cycl. Agroecosyst. 2002, 64, 237–256. [Google Scholar] [CrossRef]
- Addiscott, T.M. Measuring and Modelling Nitrogen Leaching: Parallel Problems. In Progress in Nitrogen Cycling Studies; Springer: Dordrecht, The Netherlands, 1996; pp. 665–670. [Google Scholar]
- Meisinger, J.J.; Delgado, J.A. Principles for Managing Nitrogen Leaching. J. Soil Water Conserve. 2002, 57, 485–498. [Google Scholar]
- O’Leary, M.; Rehm, G.; Schmitt, M. Understanding Nitrogen in Soils; Project no. 89-EWQI-1–9180; U.S. Department of Agriculture, Extension Service: Minneapolis, MN, USA, 2002.
- Foster, G.R.; Young, R.A.; Römkens, M.J.M.; Onstad, C.A. Processes of Soil Erosion by Water. Soil Eros. Crop Product. 1985, 1985, 137–162. [Google Scholar] [CrossRef]
- Lehman, O.R.; Ahuja, L.R. Interflow of Water and Tracer Chemical on Sloping Field Plots with Exposed Seepage Faces. J. Hydrol. 1985, 76, 307–317. [Google Scholar] [CrossRef]
- Lassaletta, L.; Billen, G.; Grizzetti, B.; Anglade, J.; Garnier, J. 50 Year Trends in Nitrogen Use Efficiency of World Cropping Systems: The Relationship between Yield and Nitrogen Input to Cropland. Environ. Res. Lett. 2014, 9, 105011. [Google Scholar] [CrossRef]
- Good, A.G.; Shrawat, A.K.; Muench, D.G. Can Less Yield More? Is Reducing Nutrient Input into the Environment Compatible with Maintaining Crop Production? Trends Plant Sci. 2004, 9, 597–605. [Google Scholar] [CrossRef]
- Nielsen, R.L. N Loss Mechanisms and Nitrogen Use Efficiency. In Purdue Nitrogen Management Workshops; Purdue University: West Lafayette, IN, USA, 2006; pp. 1–5. [Google Scholar]
- Kant, S.; Bi, Y.M.; Rothstein, S.J. Understanding Plant Response to Nitrogen Limitation for the Improvement of Crop Nitrogen Use Efficiency. J. Exp. Bot. 2011, 62, 1499–1509. [Google Scholar] [CrossRef]
- Lin, H.C.; Huber, J.A.; Gerl, G.; Hülsbergen, K.J. Nitrogen Balances and Nitrogen-Use Efficiency of Different Organic and Conventional Farming Systems. Nutr. Cycl. Agroecosystems 2016, 105, 1–23. [Google Scholar] [CrossRef]
- Andrews, M.; Lea, P.J.; Raven, J.A.; Lindsey, K. Can Genetic Manipulation of Plant Nitrogen Assimilation Enzymes Result in Increased Crop Yield and Greater N-Use Efficiency? An assessment. Ann. Appl. Biol. 2004, 145, 25–40. [Google Scholar] [CrossRef]
- Andrews, M.; Raven, J.A.; Lea, P.J.; Sprent, J.I. A Role for Shoot Protein in Shoot–Root Dry Matter Allocation in Higher Plants. Ann. Bot. 2006, 97, 3–10. [Google Scholar] [CrossRef] [PubMed]
- Keating, B.A.; Carberry, P.S.; Bindraban, P.S.; Asseng, S.; Meinke, H.; Dixon, J. Eco-Efficient Agriculture: Concepts, Challenges, and Opportunities. Crop Sci. 2010, 50, S-109. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, Sustainable Agriculture and Food Security: A review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; Volume 2009, pp. 635–651. [Google Scholar] [CrossRef]
- Zhang, F.; Cui, Z.; Fan, M.; Zhang, W.; Chen, X.; Jiang, R. Integrated Soil–Crop System Management: Reducing Environmental Risk While Increasing Crop Productivity and Improving Nutrient Use Efficiency in China. J. Environ. Qual. 2011, 40, 1051–1057. [Google Scholar] [CrossRef] [PubMed]
- Kuosmanen, N. Estimating Stocks and Flows of Nitrogen: Application of Dynamic Nutrient Balance to European Agriculture. Ecol. Econ. 2014, 108, 68–78. [Google Scholar] [CrossRef]
- Al-Naggar, A.M.M.; Shabana, R.; Abd El-Aleem, M.M.; El-Rashidy, Z. Selection Criteria for High Nitrogen Use Efficiency in Wheat [Triticum aestivum L.] Parents and their F1 and F2 Progenies. J. Agric. Ecol. Res. Int. 2016, 5, 1–15. [Google Scholar] [CrossRef]
- Zhang, X.; Davidson, E.A.; Mauzerall, D.L.; Searchinger, T.D.; Dumas, P.; Shen, Y. Managing Nitrogen for Sustainable Development. Nature 2015, 528, 51–59. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Beusen, A.H.W.; Lassaletta, L.; Van Apeldoorn, D.F.; Van Grinsven, H.J.M.; Zhang, J. Lessons from Temporal and Spatial Patterns in Global Use of N and P Fertilizer on Cropland. Sci. Rep. 2017, 7, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Schenk, M.K. Nutrient Efficiency of Vegetable Crops. Acta Horticulturae 2006, 700, 21–34. [Google Scholar] [CrossRef]
- Burns, L.G. Assessing N Fertilizer Requirements and the Reliability of Different Recommendation Systems. Acta Hortic. 2006, 35–48. [Google Scholar] [CrossRef]
- Han, M.; Okamoto, M.; Beatty, P.H.; Rothstein, S.J.; Good, A.G. The Genetics of Nitrogen Use Efficiency in Crop Plants. Annu. Rev. Genet. 2015, 49, 269–289. [Google Scholar] [CrossRef] [PubMed]
- Agostini, F.; Tei, F.; Silgram, M.; Farneselli, M.; Benincasa, P.; Aller, M.F. Decreasing Nitrate Leaching in Vegetable Crops with Better N Management. In Genetic Engineering, Biofertilisation, Soil Quality and Organic Farming; Springer Science&Business Media: Berlin/Heidelberg, Germany, 2010; pp. 147–200. [Google Scholar]
- Darnhofer, I.; Bellon, S.; Dedieu, B.; Milestad, R. Adaptiveness to Enhance the Sustainability of Farming Systems. A Review. Agron. Sustain. Dev. 2010, 30, 545–555. [Google Scholar] [CrossRef]
- Schaap, B.F.; Blom-Zandstra, M.; Hermans, C.M.; Meerburg, B.G.; Verhagen, J. Impact Changes of Climatic Extremes on Arable Farming in the North of the Netherlands. Reg. Environ. Chang. 2011, 11, 731–741. [Google Scholar] [CrossRef]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, Nitrogen-Use Efficiency, and Nitrogen Management. AMBIO A J. Hum. Environ. 2002, 31, 132–140. [Google Scholar] [CrossRef] [PubMed]
- Lützow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of Organic Matter in Temperate Soils: Mechanisms and Their Relevance under Different Soil Conditions–A Review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Kleber, M. What is Recalcitrant Soil Organic Matter? Environ. Chem. 2010, 7, 320–332. [Google Scholar] [CrossRef]
- Raworth, K. A Safe and Just Space for Humanity: Can We Live within the Doughnut? Oxfam: Nairobi, Kenya, 2012. [Google Scholar]
- Buss, S.R.; Herbert, A.W.; Morgan, P.; Thornton, S.F.; Smith, J.W.N. A Review of Ammonium Attenuation in Soil and Groundwater. Q. J. Eng. Geol. Hydrogeol. 2004, 37, 347–359. [Google Scholar] [CrossRef]
- Sharifnia, S.; Khadivi, M.A.; Shojaeimehr, T.; Shavisi, Y. Characterization, Isotherm and Kinetic Studies for Ammonium Ion Adsorption by Light Expanded Clay Aggregate (LECA). J. Saudi Chem. Soc. 2016, 20, S342–S351. [Google Scholar] [CrossRef]
- Yusof, A.M.; Keat, L.K.; Ibrahim, Z.; Majid, Z.A.; Nizam, N.A. Kinetic and Equilibrium Studies of the Removal of Ammonium Ions from Aqueous Solution by Rice Husk Ash-Synthesized Zeolite Y and Powdered and Granulated Forms of Mordenite. J. Hazard. Mater. 2010, 174, 380–385. [Google Scholar] [CrossRef]
- Liu, H.; Dong, Y.; Liu, Y.; Wang, H. Screening of Novel Low-Cost Adsorbents from Agricultural Residues to Remove Ammonia Nitrogen From Aqueous Solution. J. Hazard. Mater. 2010, 178, 1132–1136. [Google Scholar] [CrossRef] [PubMed]
- Ji, Z.Y.; Yuan, J.S.; Li, X.G. Removal of Ammonium from Wastewater Using Calcium Form Clinoptilolite. J. Hazard. Mater. 2007, 141, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Fiol, N.; Villaescusa, I. Determination of Sorbent Point Zero Charge: Usefulness in Sorption Studies. Environ. Chem. Lett. 2009, 7, 79–84. [Google Scholar] [CrossRef]
- Makoto, K.; Koike, T. Charcoal Ecology: Its Function as a Hub for Plant Succession and Soil Nutrient Cycling in Boreal Forests. Ecol. Res. 2021, 36, 4–12. [Google Scholar] [CrossRef]
- Andersson, K.I.; Eriksson, M.; Norgren, M. Removal of Lignin from Wastewater Generated by Mechanical Pulping Using Activated Charcoal and Fly Ash: Adsorption Isotherms and Thermodynamics. Ind. Eng. Chem. Res. 2011, 50, 7722–7732. [Google Scholar] [CrossRef]
- Gómez-Rey, M.X.; Madeira, M.; Coutinho, J. Wood Ash Effects on Nutrient Dynamics and Soil Properties under Mediterranean Climate. Ann. For. Sci. 2012, 69, 569–579. [Google Scholar] [CrossRef]
- Özcan, A.S.; Erdem, B.; Özcan, A. Adsorption of Acid Blue 193 from Aqueous Solutions onto BTMA-Bentonite. Colloids Surfaces A Physicochem. Eng. Asp. 2005, 266, 73–81. [Google Scholar] [CrossRef]
- Ho, Y. Review of Second-Order Models for Adsorption Systems. J. Hazard. Mater. 2006, 136, 681–689. [Google Scholar] [CrossRef] [PubMed]
- Ruíz-Baltazar, A.; Pérez, R. Kinetic Adsorption Study of Silver Nanoparticles on Natural Zeolite: Experimental and Theoretical Models. Appl. Sci. 2015, 5, 1869–1881. [Google Scholar] [CrossRef]
- Ravichandran, J.; Sivasankar, B. Properties and Catalytic Activity of Acid-Modified Montmorillonite and Vermiculite. Clays Clay Miner. 1997, 45, 854–858. [Google Scholar] [CrossRef]
- Weber, W.J., Jr.; Morris, J.C. Kinetics of Adsorption on Carbon from Solution. J. Sanit. Eng. Div. 1963, 89, 31–59. [Google Scholar] [CrossRef]
- Gürses, A.; Karaca, S.; Doğar, Ç.; Bayrak, R.; Açıkyıldız, M.; Yalçın, M. Determination of Adsorptive Properties of Clay/Water System: Methylene Blue Sorption. J. Colloid Interface Sci. 2004, 269, 310–314. [Google Scholar] [CrossRef] [PubMed]
- Özcan, A.; Öncü, E.M.; Özcan, A.S. Adsorption of Acid Blue 193 from Aqueous Solutions onto DEDMA-Sepiolite. J. Hazard. Mater. 2006, 129, 244–252. [Google Scholar] [CrossRef]
- Lagergren, S. Zur Theorie der Sogenannten Adsorption Geloster Stoffe. K. Sven. Vetenskapsakademiens. Handl. 1898, 24, 1–39. [Google Scholar]
- Sieczka, A.; Koda, E. Kinetic and Equilibrium Studies of Sorption of Ammonium in the Soil-Water Environment in Agricultural Areas of Central Poland. Appl. Sci. 2016, 6, 269. [Google Scholar] [CrossRef]
- Yuan, Q.; Li, N.; Chi, Y.; Geng, W.; Yan, W.; Zhao, Y.; Li, X.; Dong, B. Effect of Large Pore Size of Multifunctional Mesoporous Microsphere on Removal of Heavy Metal Ions. J. Hazard. Mater. 2013, 254, 157–165. [Google Scholar] [CrossRef] [PubMed]
- Srividya, K.; Mohanty, K. Biosorption of Hexavalent Chromium from Aqueous Solutions by Catla Catla Scales: Equilibrium and Kinetics Studies. Chem. Eng. J. 2009, 155, 666–673. [Google Scholar] [CrossRef]
- Abdelnaeim, M.Y.; El Sherif, I.Y.; Attia, A.A.; Fathy, N.A.; El-Shahat, M.F. Impact of Chemical Activation on the Adsorption Performance of Common Reed Towards Cu [II] and Cd [II]. Int. J. Min. Process. 2016, 157, 80–88. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, B.; Zhang, X.; Wang, J.; Liu, J.; Chen, R. Preparation of Highly Ordered Cubic NaA Zeolite from Halloysite Mineral for Adsorption of Ammonium Ions. J. Hazard. Mater. 2010, 178, 658–664. [Google Scholar] [CrossRef] [PubMed]
- Ravichandran, T.; Arivoli, S. Adsorption of Rhodamine-B from Aqueous Solution by Activated Calcite Powder Studies on Equilibrium Isotherm, Kinetics and Thermodynamics. Int. J. Pollut. Abate. Technol. 2013, 2, 6–12. [Google Scholar]
- Ünlü, N.; Ersoz, M. Adsorption Characteristics of Heavy Metal Ions onto a Low Cost Biopolymeric Sorbent from Aqueous Solutions. J. Hazard. Mater. 2006, 136, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Hui, K.S.; Chao, C.Y.H.; Kot, S.C. Removal of Mixed Heavy Metal Ions in Wastewater by Zeolite 4A and Residual Products from Recycled Coal Fly Ash. J. Hazard. Mater. 2005, 127, 89–101. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Alling, V.; Martinsen, V.; Mulder, J.; Breedveld, G.D.; Cornelissen, G. The Sorption and Desorption of Phosphate-P, Ammonium-N and Nitrate-N in Cacao Shell and Corn Cob Biochars. Chemosphere 2013, 91, 1612–1619. [Google Scholar] [CrossRef] [PubMed]
- Van Erp, T.S.; Martens, J.A. A Standardization for Bet Fitting of Adsorption Isotherms. Microporous Mesoporous Mater. 2011, 145, 188–193. [Google Scholar] [CrossRef]
- Mel’gunov, M.S.; Ayupov, A.B. Direct Method for Evaluation of BET Adsorbed Monolayer Capacity. Microporous Mesoporous Mater. 2017, 243, 147–153. [Google Scholar] [CrossRef]
- Ambroz, F.; Macdonald, T.J.; Martis, V.; Parkin, I.P. Evaluation of the BET Theory for the Characterization of Meso and Microporous MOFs. Small Methods 2018, 2, 1800173. [Google Scholar] [CrossRef]
- Yin, Q.; Zhang, B.; Wang, R.; Zhao, Z. Phosphate and Ammonium Adsorption of Sesame Straw Biochars Produced at Different Pyrolysis Temperatures. Environ. Sci. Pollut. Res. 2018, 25, 4320–4329. [Google Scholar] [CrossRef] [PubMed]
- Thommes, M.; Kaneko, K.; Neimark, A.V.; Olivier, J.P.; Rodriguez-Reinoso, F.; Rouquerol, J.; Sing, K.S. Physisorption of Gases, with Special Reference to the Evaluation of Surface Area and Pore Size Distribution (IUPAC Technical Report). Pure Appl. Chem. 2015, 87, 1051–1069. [Google Scholar] [CrossRef]
- Karthik, R.M.; Philip, L. Sorption of Pharmaceutical Compounds and Nutrients by Various Porous Low Cost Adsorbents. J. Environ. Chem. Eng. 2021, 9, 104916. [Google Scholar] [CrossRef]
- Walton, K.S.; Snurr, R.Q. Applicability of the BET Method for Determining Surface Areas of Microporous Metal−Organic Frameworks. J. Am. Chem. Soc. 2007, 129, 8552–8556. [Google Scholar] [CrossRef] [PubMed]
- Machado, A.I.; Dordio, A.; Fragoso, R.; Leitão, A.E.; Duarte, E. Furosemide Removal in Constructed Wetlands: Comparative Efficiency of LECA and Cork granulates as support matrix. J. Environ. Manag. 2017, 203, 422–428. [Google Scholar] [CrossRef]
- Lombi, E.; Susini, J. Synchrotron-Based Techniques for Plant and Soil Science: Opportunities, Challenges and Future Perspectives. Plant Soil 2009, 320, 1–35. [Google Scholar] [CrossRef]
- Khandaker, S.; Kuba, T.; Toyohara, Y.; Kamida, S.; Uchikawa, Y. Development of Ion-Exchange Properties of Bamboo Charcoal Modified With Concentrated Nitric Acid. In IOP Conference Series: Earth and Environmental Science; IOP Publishing: Bristol, UK, 2017; Volume 82, p. 012002. [Google Scholar]
- Mourdikoudis, S.; Pallares, R.M.; Thanh, N.T. Characterization Techniques for Nanoparticles: Comparison and Complementarity upon Studying Nanoparticle Properties. Nanoscale 2018, 10, 12871–12934. [Google Scholar] [CrossRef] [PubMed]
- Cai, W.; Li, Z.; Wei, J.; Liu, Y. Synthesis of Peanut Shell Based Magnetic Activated Carbon With Excellent Adsorption Performance Towards Electroplating Wastewater. Chem. Eng. Res. Des. 2018, 140, 23–32. [Google Scholar] [CrossRef]
- Cai, W.; Wei, J.; Li, Z.; Liu, Y.; Zhou, J.; Han, B. Preparation of Amino-Functionalized Magnetic Biochar with Excellent Adsorption Performance for Cr (VI) by a Mild One-Step Hydrothermal Method from Peanut Hull. Colloids Surf. A Physicochem. Eng. Asp. 2019, 563, 102–111. [Google Scholar] [CrossRef]
- Nabeela, F.; Murad, W.; Khan, I.; Mian, I.A.; Rehman, H.; Adnan, M.; Azizullah, A. Effect of Wood Ash Application on the Morphological, Physiological and Biochemical Parameters of Brassica Napus L. Plant Physiol. Biochem. 2015, 95, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Ozolinčius, R.; Varnagiryte, I.; Armolaitis, K.; Karltun, E. Initial Effects of Wood Ash Fertilization on Soil, Needle and Litterfall Chemistry in a Scots Pine [Pinus Sylvestris L.] Stand. Balt. For. 2005, 11, 59–67. [Google Scholar]
- Eriksson, H.M.; Nilsson, T.; Nordin, A. Early Effects of Lime and Hardened and Non-Hardened Ashes on pH and Electrical Conductivity of the Forest Floor, an Relations to Some Ash and Lime Qualities. Scand. J. For. Res. Suppl. 1998, 2, 56–66. [Google Scholar]
- Mohamed, S.A.; Ewees, S.A.; Sawsan, A.; Seaf, E.Y.; Dalia, M.S. Improving Maize Grain Yield and Its Quality Grown on a Newly Reclaimed Sandy Soil by Applying Micronutrients, Organic Manure and Biological Inoculation. Res. J. Agric. Biol. Sci. 2008, 4, 537–544. [Google Scholar]
- Pitman, R.M. Wood Ash Use in Forestry–A Review of the Environmental Impacts. For. An Int. J. For. Res. 2006, 79, 563–588. [Google Scholar] [CrossRef]
- Park, B.B.; Yanai, R.D.; Sahm, J.M.; Lee, D.K.; Abrahamson, L.P. Wood Ash Effects on Plant and Soil in a Willow Bioenergy Plantation. Biomass Bioenergy 2005, 28, 355–365. [Google Scholar] [CrossRef]
- Etiegni, L.; Campbell, A.G. Physical and Chemical Characteristics of Wood Ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Fritze, H.; Pennanen, T.; Pietikäinen, J. Recovery of Soil Microbial Biomass and Activity from Prescribed Burning. Can. J. For. Res. 1993, 23, 1286–1290. [Google Scholar] [CrossRef]
- Martikainen, P.J.; Ohtonen, R.; Silvola, J.; Vuorinen, A. The Effects of Fertilization on Forest Soil Biology. Eff. Fertil. For. Ecosyst. 1994, 1994, 40–79. [Google Scholar]
- Nottfige, D.O.; Ojeniyi, S.O.; Asawalam, D.O. Comparative Effect of Plant Residues and NPK Fertilizer on Nutrient Status and Yield of Maize [Zea Mays L.] in a Humid Ultisol. Niger. J. Soil Sci. 2005, 15, 1–8. [Google Scholar]
- Mbah, C.N.; Nwite, J.N.; Njoku, C.; Nweke, I.A. Response of Maize (Zea Mays L.) to Different Rates of Wood-Ash Application in Acid Ultisol in Southeast Niger. Afr. J. Agric. Res. 2010, 5, 580–583. [Google Scholar]
- Owolabi, O.; Ojeniyi, S.O.; Amodu, A.O.; Hazzan, K. Response of Cowpea, Okra and Tomato Sawdust Ash Manure. Moor J. Agric. Res. 2003, 4, 178–182. [Google Scholar] [CrossRef]
- Perkiömäki, J. Wood Ash Use in Coniferous Forests: A Soil Microbiological Study into the Potential Risk of Cadmium Release. 2004. Available online: https://helda.helsinki.fi/bitstream/handle/10138/20875/woodashu.pdf?sequence=1 (accessed on 1 May 2021).
- Lundborg, A. Ecological and Economical Evaluation of Biomass Ash Utilization–The Swedish Approach. Ashes and particulate emissions from biomass combustion, Series Thermal Biomass Utilization. Inst. Chem. Engin. Techn. Univ. Graz. 1998, 3, 29–41. [Google Scholar]
- Augusto, L.; Bakker, M.R.; Meredieu, C. Wood Ash Applications to Temperate Forest Ecosystems—Potential Benefits and Drawbacks. Plant Soil 2008, 306, 181–198. [Google Scholar] [CrossRef]
- Insam, H.; Franke-Whittle, I.H.; Knapp, B.A.; Plank, R. Use of Wood Ash and Anaerobic Sludge for Grassland Fertilization: Effects on Plants and Microbes. Die Bodenkult. 2009, 60, 39–50. [Google Scholar]
- Füzesi, I.; Heil, B.; Kovács, G. Effects of Wood Ash on the Chemical Properties of Soil and Crop Vitality in Small Plot Experiments. Acta Silv. Lignaria Hung. 2015, 11, 55–64. [Google Scholar] [CrossRef]
- Arvidsson, H.; Lundkvist, H. Effects of Crushed Wood Ash on Soil Chemistry in Young Norway Spruce Stands. For. Ecol. Manag. 2003, 176, 121–132. [Google Scholar] [CrossRef]
- Krevelen, D.W. Coal: Typology, Chemistry, Physics, Constitution; Elsevier Publishing Company: Amsterdam, The Netherlands, 1961. [Google Scholar]
- Major, J.; Rondon, M.; Molina, D.; Riha, S.J.; Lehmann, J. Maize Yield and Nutrition during 4 Years after Biochar Application to a Colombian Savanna Oxisol. Plant Soil 2010, 333, 117–128. [Google Scholar] [CrossRef]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the Effects of Biochar on Manure Composting: Evidence Supporting the Relationship between N2O Emission and Denitrifying Community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef] [PubMed]
- Cheng, C.H.; Lehmann, J.; Engelhard, M.H. Natural Oxidation of Black Carbon in Soils: Changes in Molecular Form and Surface Charge along a Climosequence. Geochim. Cosmochim. Acta 2008, 72, 1598–1610. [Google Scholar] [CrossRef]
- Spokas, K.A.; Novak, J.M.; Masiello, C.A.; Johnson, M.G.; Colosky, E.C.; Ippolito, J.A.; Trigo, C. Physical Disintegration of Biochar: An Overlooked Process. Environ. Sci. Technol. Lett. 2014, 1, 326–332. [Google Scholar] [CrossRef]
- Bird, M.I.; Ascough, P.L.; Young, I.M.; Wood, C.V.; Scott, A.C. X-ray Microtomographic Imaging of Charcoal. J. Archaeol. Sci. 2008, 35, 2698–2706. [Google Scholar] [CrossRef]
- Hockaday, W.C.; Grannas, A.M.; Kim, S.; Hatcher, P.G. Direct Molecular Evidence for the Degradation and Mobility of BLACK Carbon in Soils from Ultrahigh-Resolution Mass Spectral Analysis of Dissolved Organic Matter from a Fire-Impacted Forest Soil. Org. Geochem. 2006, 37, 501–510. [Google Scholar] [CrossRef]
- Kasin, I.; Ohlson, M. An Experimental Study of Charcoal Degradation in a Boreal Forest. Soil Biol. Biochem. 2013, 65, 39–49. [Google Scholar] [CrossRef]
- Jaafar, N.M.; Clode, P.L.; Abbott, L.K. Microscopy Observations of Habitable Space in Biochar for Colonization by Fungal Hyphae from Soil. J. Integr. Agric. 2014, 13, 483–490. [Google Scholar] [CrossRef]
- Brewer, C.E.; Chuang, V.J.; Masiello, C.A.; Gonnermann, H.; Gao, X.; Dugan, B.; Davies, C.A. New Approaches to Measuring Biochar Density and Porosity. Biomass Bioenergy 2014, 66, 176–185. [Google Scholar] [CrossRef]
- Hardie, M.; Clothier, B.; Bound, S.; Oliver, G.; Close, D. Does Biochar Influence Soil Physical Properties and Soil Water Availability? Plant Soil 2014, 376, 347–361. [Google Scholar] [CrossRef]
- Gao, X.; Driver, L.E.; Kasin, I.; Masiello, C.A.; Pyle, L.A.; Dugan, B.; Ohlson, M. Effect of Environmental Exposure on Charcoal Density and Porosity in a Boreal Forest. Sci. Total Environ. 2017, 592, 316–325. [Google Scholar] [CrossRef] [PubMed]
- Gray, M.; Johnson, M.G.; Dragila, M.I.; Kleber, M. Water Uptake in Biochars: The Roles of Porosity and Hydrophobicity. Biomass Bioenergy 2014, 61, 196–205. [Google Scholar] [CrossRef]
- Trubetskaya, A.; Jensen, P.A.; Jensen, A.D.; Glarborg, P.; Larsen, F.H.; Andersen, M.L. Characterization of Free Radicals by Electron Spin Resonance Spectroscopy in Biochars from Pyrolysis at High Heating Rates and at High Temperatures. Biomass Bioenergy 2016, 94, 117–129. [Google Scholar] [CrossRef]
- Barnes, R.T.; Gallagher, M.E.; Masiello, C.A.; Liu, Z.; Dugan, B. Biochar-Induced Changes in Soil Hydraulic Conductivity and Dissolved Nutrient Fluxes Constrained by Laboratory Experiments. PLoS ONE 2014, 9, 108–340. [Google Scholar]
- Jeffery, S.; Meinders, M.B.; Stoof, C.R.; Bezemer, T.M.; Van De Voorde, T.F.; Mommer, L.; Van Groenigen, J.W. Biochar application Does not Improve the Soil Hydrological Function of a Sandy Soil. Geoderma 2015, 251, 47–54. [Google Scholar] [CrossRef]
- Liu, Z.; Dugan, B.; Masiello, C.A.; Barnes, R.T.; Gallagher, M.E.; Gonnermann, H. Impacts of Biochar Concentration and Particle Size on Hydraulic Conductivity and DOC Leaching of Biochar–Sand Mixtures. J. Hydrol. 2016, 533, 461–472. [Google Scholar] [CrossRef]
- Warnock, D.D.; Lehmann, J.; Kuyper, T.W.; Rillig, M.C. Mycorrhizal Responses to Biochar in Soil–Concepts and Mechanisms. Plant Soil 2007, 300, 9–20. [Google Scholar] [CrossRef]
- Hermann, B.G.; Debeer, L.; De Wilde, B.; Blok, K.; Patel, M.K. To Compost or not to Compost: Carbon and Energy Footprints of Biodegradable Materials’ Waste Treatment. Polym. Degrad. Stab. 2011, 96, 1159–1171. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M.S. Rice Husk Biochar for rice Based Cropping System in Acid Soil 1. The Characteristics of Rice Husk Biochar and Its Influence on the Properties of Acid Sulfate Soils and Rice Growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39. [Google Scholar] [CrossRef]
- Lehmann, J.; Rillig, M.C.; Thies, J.; Masiello, C.A.; Hockaday, W.C.; Crowley, D. Biochar Effects on Soil Biota–A Review. Soil Boil. Biochem. 2011, 43, 1812–1836. [Google Scholar] [CrossRef]
- Martina, S.M.; Kookana, R.S.; Van Zwieten, L.; Krull, E. Marked Changes in Herbicide Sorption–Desorption Upon Ageing of Biochars in Soil. J. Hazard. Mater. 2012, 231, 70–78. [Google Scholar] [CrossRef] [PubMed]
- Tryon, E.H. Effect of Charcoal on Certain Physical, Chemical, and Biological Properties of Forest Soils. Ecol. Monogr. 1948, 18, 81–115. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Temperature and Source Material Influence Ecological Attributes of Ponderosa Pine and Douglas-Fir Charcoal. For. Ecol. Manag. 2006, 231, 86–93. [Google Scholar] [CrossRef]
- Gundale, M.J.; DeLuca, T.H. Charcoal Effects on Soil Solution Chemistry and Growth of Koeleria Macrantha in the Ponderosa Pine/Douglas-Fir Ecosystem. Biol. Fertil. Soils 2007, 43, 303–311. [Google Scholar] [CrossRef]
- Rajkovich, S.; Enders, A.; Hanley, K.; Hyland, C.; Zimmerman, A.R.; Lehmann, J. Corn Growth and Nitrogen Nutrition after Additions of Biochars with Varying Properties to a Temperate Soil. Biol. Fertil. Soils 2012, 48, 271–284. [Google Scholar] [CrossRef]
- Ulery, A.L.; Graham, R.C.; Amrhein, C. Wood-Ash Composition and Soil PH Following Intense Burning. Soil Sci. 1993, 156, 358–364. [Google Scholar] [CrossRef]
- Brockhoff, S.R.; Christians, N.E.; Killorn, R.J.; Horton, R.; Davis, D.D. Physical and Mineral-Nutrition Properties of Sand-Based Turfgrass Root Zones Amended with Biochar. Agron. J. 2010, 102, 1627–1631. [Google Scholar] [CrossRef]
- Biederman, L.A.; Harpole, W.S. Biochar and Its Effects on Plant Productivity and Nutrient Cycling: A meta-Analysis. GCB Bioenergy 2013, 5, 202–214. [Google Scholar] [CrossRef]
- Wardle, D.A.; Nilsson, M.-C.; Zackrisson, O. Fire-Derived Charcoal Causes Loss of Forest Humus. Science 2008, 320, 629. [Google Scholar] [CrossRef] [PubMed]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar induced soil microbial community change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Khodadad, C.L.; Zimmerman, A.R.; Green, S.J.; Uthandi, S.; Foster, J.S. Taxa-Specific Changes in Soil Microbial Community Composition Induced by Pyrogenic Carbon Amendments. Soil Biol. Biochem. 2011, 43, 385–392. [Google Scholar] [CrossRef]
- Chen, J.; Sun, X.; Zheng, J.; Zhang, X.; Liu, X.; Bian, R.J.; Li, L.; Cheng, K.; Zheng, J.W.; Pan, G. Biochar Amendment Changes Temperature Sensitivity of Soil Respiration and Composition of Microbial Communities 3 Years after Incorporation in an Organic Carbon-Poor Dry Cropland Soil. Biol. Fertil. Soils 2018, 54, 175–188. [Google Scholar] [CrossRef]
- Liang, B.; Lehmann, J.; Sohi, S.P.; Thies, J.E.; O’Neill, B.; Trujillo, L.; Gaunt, J.; Solomon, D.; Grossman, J.M.; Neves, E.G.; et al. Black Carbon Affects the Cycling of Non-Black Carbon in Soil. Org. Geochem. 2010, 41, 206–213. [Google Scholar] [CrossRef]
- Qiu, Y.; Cheng, H.; Xu, C.; Sheng, G.D. Surface Characteristics of Crop-Residue-Derived Black Carbon and Lead [II] Adsorp-Tion. Water Res. 2008, 42, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, L.; Yang, H.; Yan, G.; Xu, Z.; Chen, C.; Zhang, D. Biochar Nutrient Availability Rather than Its Water Holding Capacity Governs the Growth of Both C3 and C4 Plants. J. Soils Sediments 2016, 16, 801–810. [Google Scholar] [CrossRef]
- Prendergast-Miller, M.T.; Duvall, M.; Sohi, S.P. Localisation of Nitrate in the Rhizosphere of Biochar-amended Soils. Soil Biol. Biochem. 2011, 43, 2243–2246. [Google Scholar] [CrossRef]
- Gul, S.; Whalen, J.K.; Thomas, B.W.; Sachdeva, V.; Deng, H. Physico-Chemical Properties and Microbial Responses in Bio-Char-Amended Soils: Mechanisms and Future Directions. Agric. Ecosyst. Environ. 2015, 206, 46–59. [Google Scholar] [CrossRef]
- Pietikäinen, J.; Kiikkilä, O.; Fritze, H. Charcoal as a Habitat for Microbes and Its Effect on the Microbial Community of the Underlying Humus. Oikos 2000, 89, 231–242. [Google Scholar] [CrossRef]
- Makoto, K.; Tamai, Y.; Kim, Y.S.; Koike, T. Buried Charcoal Layer and Ectomycorrhizae Cooperatively Promote the Growth of Larix Gmelinii Seedlings. Plant Soil 2010, 327, 143–152. [Google Scholar] [CrossRef]
- Haider, G.; Steffens, D.; Müller, C.; Kammann, C.I. Standard Extraction Methods May Underestimate Nitrate Stocks Captured by Field-Aged Biochar. J. Environ. Qual. 2016, 45, 1196–1204. [Google Scholar] [CrossRef]
- Noel, J.D.; Biswas, P.; Giammar, D.E. Evaluation of a Sequential Extraction Process Used for Determining Mercury Binding Mechanisms to Coal Combustion Byproducts. J. Air Waste Manag. Assoc. 2007, 57, 856–867. [Google Scholar] [CrossRef] [PubMed]
- Gierak, A.; Łazarska, I. Adsorption of Nitrate, Nitrite, and Ammonium Ions on Carbon Adsorbents. Adsorpt. Sci. Technol. 2017, 35, 721–727. [Google Scholar] [CrossRef]
- Figueiredo, J.L.; Pereira, M.F.R.; Freitas, M.M.A.; Orfao, J.J.M. Modification of the Surface Chemistry of Activated Carbons. Carbon 1999, 37, 1379–1389. [Google Scholar] [CrossRef]
- Karhu, K.; Mattila, T.; Bergström, I.; Regina, K. Biochar Addition to Agricultural Soil Increased CH4 Uptake and Water Holding Capacity–Results from a Short-Term Pilot Field Study. Agric. Ecosyst. Environ. 2011, 140, 309–313. [Google Scholar] [CrossRef]
- Jankowska, H. (Ed.) Adsorpcja jonów na węglu aktywnym; Naukowe PWN: Warsaw, Poland, 1991. [Google Scholar]
- Freddo, A.; Cai, C.; Reid, B.J. Environmental Contextualisation of Potential Toxic Elements and Polycyclic Aromatic Hydrocarbons in Biochar. Environ. Pollut. 2012, 171, 18–24. [Google Scholar] [CrossRef] [PubMed]
- Méndez, A.; Gómez, A.; Paz-Ferreiro, J.; Gascó, G. Effects of Sewage Sludge Biochar on Plant Metal Availability after Application to a Mediterranean Soil. Chemosphere 2012, 89, 1354–1359. [Google Scholar] [CrossRef] [PubMed]
- Farrell, M.; Rangott, G.; Krull, E. Difficulties in Using Soil-Based Methods to Assess Plant Availability of Potentially Toxic Elements in Biochars and Their Feedstocks. J. Hazard. Mater. 2013, 250, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Chen, T.; Zhang, Y.; Wang, H.; Lu, W.; Zhou, Z.; Zhang, Y.; Ren, L. Influence of Pyrolysis Temperature on Characteristics and Heavy Metal Adsorptive Performance of Biochar Derived from Municipal Sewage Sludge. Bioresour. Technol. 2014, 164, 47–54. [Google Scholar] [CrossRef]
- Keiluweit, M.; Kleber, M.; Sparrow, M.A.; Simoneit, B.R.; Prahl, F.G. Solvent-Extractable Polycyclic Aromatic Hydrocarbons in Biochar: Influence of Pyrolysis Temperature and Feedstock. Environ. Sci. Technol. 2012, 46, 9333–9341. [Google Scholar] [CrossRef]
- Lyu, H.; He, Y.; Tang, J.; Hecker, M.; Liu, Q.; Jones, P.D.; Codling, G.; Giesy, J.P. Effect of Pyrolysis Temperature on Potential TOXICITY of biochar If Applied to the Environment. Environ. Pollut. 2016, 218, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Hale, S.E.; Lehmann, J.; Rutherford, D.; Zimmerman, A.R.; Bachmann, R.T.; Shitumbanuma, V.; O’Toole, A.; Sundqvist, K.L.; Arp, H.P.H.; Cornelissen, G. Quantifying the Total and Bioavailable Polycyclic Aromatic Hydrocarbons and Dioxins in Biochars. Environ. Sci. Technol. 2012, 46, 2830–2838. [Google Scholar] [CrossRef] [PubMed]
- Shackley, S.; Carter, S.; Knowles, T.; Middelink, E.; Haefele, S.; Sohi, S.; Cross, A.; Haszeldine, S. Sustainable Gasification-Biochar Systems? A Case-Study of Rice-Husk Gasification in Cambodia, Part I: Context, Chemical Properties, Environmental and Health and Safety Issues. Energy Policy 2012, 42, 49–58. [Google Scholar] [CrossRef]
- Mohammadi, A.; Cowie, A.L.; Mai, T.L.A.; Brandao, M.; De La Rosa, R.A.; Kristiansen, P.; Joseph, S. Climate-Change and Health Effects of Using Rice Husk for Biochar-Compost: Comparing Three Pyrolysis Systems. J. Clean. Prod. 2017, 162, 260–272. [Google Scholar] [CrossRef]
- Anyanwu, I.N.; Alo, M.N.; Onyekwere, A.M.; Crosse, J.D.; Nworie, O.; Chamba, E.B. Influence of Biochar Aged in Acidic soil on Ecosystem Engineers and Two Tropical Agricultural Plants. Ecotoxicol. Environ. Saf. 2018, 153, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Chen, J.; Pan, G.; Liu, X.; Zhang, X.; Li, L.; Jinwei, Z. Biochar Decreased Microbial Metabolic Quotient and Shifted Community Composition Four Years after a Single Incorporation in a Slightly Acid Rice Paddy from Southwest China. Sci. Total. Environ. 2016, 571, 206–217. [Google Scholar] [CrossRef]
- Safaei Khorram, M.; Fatemi, A.; Khan, M.A.; Kiefer, R.; Jafarnia, S. Potential Risk of Weed Outbreak by Increasing Biochar’s Application Rates in Slow-Growth Legume, Lentil (Lens Culinaris Medik.). J. Sci. Food Agric. 2018, 98, 2080–2088. [Google Scholar] [CrossRef] [PubMed]
- Ukalska-Jaruga, A.; Debaene, G.; Smreczak, B. Particle and Structure Characterization of Fulvic Acids from Agricultural soils. J. Soils Sediments 2018, 18, 2833–2843. [Google Scholar] [CrossRef]
- Chang, R.R.; Mylotte, R.; Hayes, M.H.B.; Mclnerney, R.; Tzou, Y.M. A Comparison of the Compositional Differences between Humic Fractions Isolated by the IHSS and Exhaustive Extraction Procedures. Naturwissenschaften 2014, 101, 197–209. [Google Scholar] [CrossRef] [PubMed]
- Sposito, G. The Chemistry of Soils; Oxford University Press: Oxford, UK, 2008. [Google Scholar]
- Liu, M.; Wang, C.; Wang, F.; Xie, Y. Maize (Zea Mays) Growth and Nutrient Uptake Following Integrated Improvement of Vermicompost and Humic Acid Fertilizer on Coastal Saline Soil. Appl. Soil Ecol. 2019, 142, 147–154. [Google Scholar] [CrossRef]
- Ali, J.; Li, Y.; Wang, X.; Zhao, J.; Xi, N.; Zhang, Z.; Xia, X. Climate-Zone-Dependent Effect Mechanism of Humic Acid and Fulvic Acid Extracted from River Sediments on Aggregation Behavior of Graphene Oxide. Sci. Total Environ. 2020, 721, 137–682. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, I.; Ali, S.; Khan, K.S.; Hassan, F.U.; Ijaz, S.S.; Bashir, K. Use of Coal Derived Humic Acid as Soil Conditioner for Soil Physical Properties and Its Impact on Wheat Crop Yield. Int. J. Biosci. 2015, 6, 81–89. [Google Scholar]
- Gholami, H.; Fard, F.R.; Saharkhiz, M.J.; Ghani, A. Yield and Physicochemical Properties of Inulin Obtained from Iranian Chicory Roots under Vermicompost and Humic Acid Treatments. Ind. Crop. Prod. 2018, 123, 610–616. [Google Scholar] [CrossRef]
- Turgay, O.C.; Karaca, A.; Unver, S.; Tamer, N. Effects of Coal-Derived Humic Substance on Some Soil Properties and Bread Wheat Yield. Commun. Soil Sci. Plant Anal. 2011, 42, 1050–1070. [Google Scholar] [CrossRef]
- Zhang, S.Q.; Liang, Y.; Wei, L.; Lin, Z.A.; Li, Y.T.; Hu, S.W.; Zhao, B.Q. Effects of Urea Enhanced with Different Weathered Coal-derived Humic Acid Components on Maize Yield and Fate of Fertilizer Nitrogen. J. Integr. Agric. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Jin, J.; Sun, K.; Yang, Y.; Wang, Z.; Han, L.; Wang, X.; Wu, F.; Xing, B. Comparison between Soil-And Biochar-Derived Humic acids: Composition, Conformation, and Phenanthrene Sorption. Environ. Sci. Technol. 2018, 52, 1880–1888. [Google Scholar] [CrossRef]
- Schnitzer, M.; Skinner, S.I.M. Alkali versus Acid Extraction of Soil Organic Matter. Soil Sci. 1968, 105, 392–396. [Google Scholar] [CrossRef]
- Schnitzer, M. Humic Substances: Chemistry and Reactions. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 1978; Volume 8, pp. 1–64. [Google Scholar]
- Schaumann, G.E. Soil Organic Matter beyond Molecular Structure Part I: Macromolecular and Supramolecular Characteristics. J. Plant Nutr. Soil Sci. 2006, 169, 145–156. [Google Scholar] [CrossRef]
- Schaumann, G.E. Soil Organic Matter beyond Molecular Structure Part II: Amorphous Nature and Physical Aging. J. Plant Nutr. Soil Sci. 2006, 169, 157–167. [Google Scholar] [CrossRef]
- Tan, K.H. Humic Matter in Soil and the Environment: Principles and Controversies; CRC Press: Boca Raton, FL, USA, 2014. [Google Scholar]
- Hayes, M.H.; Mylotte, R.; Swift, R.S. Humin: Its Composition and Importance in Soil Organic Matter. Adv. Agrono. 2017, 143, 47–138. [Google Scholar]
- Lehmann, J.; Kleber, M. The Contentious Nature of Soil Organic Matter. Nature 2015, 528, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Contreras, C.; De La Rosa, G.; Peralta-Videa, J.R.; Gardea-Torresdey, J.L. Lead Adsorption by Silica-Immobilized Humin under Flow and Batch Conditions: Assessment of Flow Rate and Calcium and Magnesium Interference. J. Hazard. Mater. 2006, 133, 79–84. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Li, D.; Li, J.; Li, G.; Zhang, B. Evaluation of Humic Substances during Co-Composting of Sewage Sludge and Corn Stalk under Different Aeration Rates. Bioresour. Technol. 2017, 245, 1299–1302. [Google Scholar] [CrossRef] [PubMed]
- Pan, W.L.; Port, L.E.; Xiao, Y.; Bary, A.I.; Cogger, C.G. Soil Carbon and Nitrogen Fraction Accumulation with Long-Term Biosolids Applications. Soil Sci. Soc. Am. J. 2017, 81, 1381. [Google Scholar] [CrossRef]
- Wang, Y.; Zheng, J.; Liu, X.; Yan, Q.; Hu, Y. Short-Term Impact of Fire-Deposited Charcoal on Soil Microbial Community Abundance and Composition in a Subtropical Plantation in China. Geoderma 2020, 359, 113992. [Google Scholar] [CrossRef]
- Bremner, J.M. Studies on Soil Organic Matter: Part I. The Chemical Nature of Soil Organic Nitrogen. J. Agric. Sci. 1949, 39, 183–193. [Google Scholar] [CrossRef]
- Plante, A.F.; Conant, R.T.; Paul, E.A.; Paustian, K.; Six, J. Acid Hydrolysis of Easily Dispersed and Microaggregate-Derived Silt-and Clay-Sized Fractions to Isolate Resistant Soil Organic Matter. Eur. J. Soil Sci. 2006, 57, 456–467. [Google Scholar] [CrossRef]
- Martel, Y.A.; Paul, E.A. The Use of Radiocarbon Dating of Organic Matter in the Study of Soil Genesis. Soil Sci. Soc. Am. J. 1974, 38, 501–506. [Google Scholar] [CrossRef]
- Schnitzer, M.; Preston, C.M. Effects of Acid Hydrolysis on the 13 C NMR Spectra of Humic Substances. Plant Soil 1983, 75, 201–211. [Google Scholar] [CrossRef]
- Helfrich, M.; Flessa, H.; Mikutta, R.; Dreves, A.; Ludwig, B. Comparison of Chemical Fractionation Methods for Isolating Stable Soil Organic Carbon Pools. Eur. J. Soil Sci. 2007, 58, 1316–1329. [Google Scholar] [CrossRef]
- Pessenda, L.C.; Gouveia, S.E.; Aravena, R. Radiocarbon Dating of Total Soil Organic Matter and Humin Fraction and Its Comparison with 14C Ages of Fossil Charcoal. Radiocarbon 2001, 43, 595–601. [Google Scholar] [CrossRef]
- Sun, X.; Zhong, T.; Zhang, L.; Zhang, K.; Wu, W. Reducing Ammonia Volatilization from Paddy Field with Rice Straw Derived Biochar. Sci. Total. Environ. 2019, 660, 512–518. [Google Scholar] [CrossRef] [PubMed]

| Fertiliser | % Nitrogen | Composition |
|---|---|---|
| Ammonium sulfate | 20.5 | (NH4)2SO4 |
| Calcium nitrate | 16 | Ca(NO3)2 |
| Urea | 45 | (NH2)2CO |
| Anhydrous ammonia | 82 | NH3 |
| Diammonium phosphate | 20 | (NH4)2HPO4 |
| Ammonium polyphosphate | 10–15 | (NH4PO3)n |
| Urea ammonium nitrate | 28–32 | (NH2)2CO, NH4NO3 |
| Name | Chemical Formula | Oxidation State |
|---|---|---|
| Nitrate | NO3− | +5 |
| Nitrogen dioxide [g] | NO2 | +4 |
| Nitrite | NO2− | +3 |
| Nitric oxide [g] | NO | +2 |
| Nitrous oxides [g] | N2O | +1 |
| Dinitrogen [g] | N2 | 0 |
| Ammonia [g] | NH3 | −3 |
| Ammonium | NH4+ | −3 |
| Organic N | RNH3 | −3 |
| Factor | Will Increase N2O or N2 |
|---|---|
| NO3− or NO2− | Increasing oxidant |
| Oxygen | Increasing oxygen |
| Carbon | Decreasing C availability |
| pH | Decreasing pH |
| H2S | Increasing Sulfide |
| Temperature | Decreasing temperature |
| Enzyme status | Low N2O reductase activity |
| Kinetic Models | Equation | Plot | Reference |
|---|---|---|---|
| Pseudo-first order | log(qe − qt) vs. t | [175] | |
| Pseudo-second order | vs. t | [172,175] | |
| Intra-particle diffusion | qt vs. t0.5 | [172] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hamidi, N.H.; Ahmed, O.H.; Omar, L.; Ch’ng, H.Y. Soil Nitrogen Sorption Using Charcoal and Wood Ash. Agronomy 2021, 11, 1801. https://doi.org/10.3390/agronomy11091801
Hamidi NH, Ahmed OH, Omar L, Ch’ng HY. Soil Nitrogen Sorption Using Charcoal and Wood Ash. Agronomy. 2021; 11(9):1801. https://doi.org/10.3390/agronomy11091801
Chicago/Turabian StyleHamidi, Nur Hidayah, Osumanu Haruna Ahmed, Latifah Omar, and Huck Ywih Ch’ng. 2021. "Soil Nitrogen Sorption Using Charcoal and Wood Ash" Agronomy 11, no. 9: 1801. https://doi.org/10.3390/agronomy11091801
APA StyleHamidi, N. H., Ahmed, O. H., Omar, L., & Ch’ng, H. Y. (2021). Soil Nitrogen Sorption Using Charcoal and Wood Ash. Agronomy, 11(9), 1801. https://doi.org/10.3390/agronomy11091801







