Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L.
Abstract
:1. Introduction
2. Materials and Methods
2.1. Microgreens Experiment
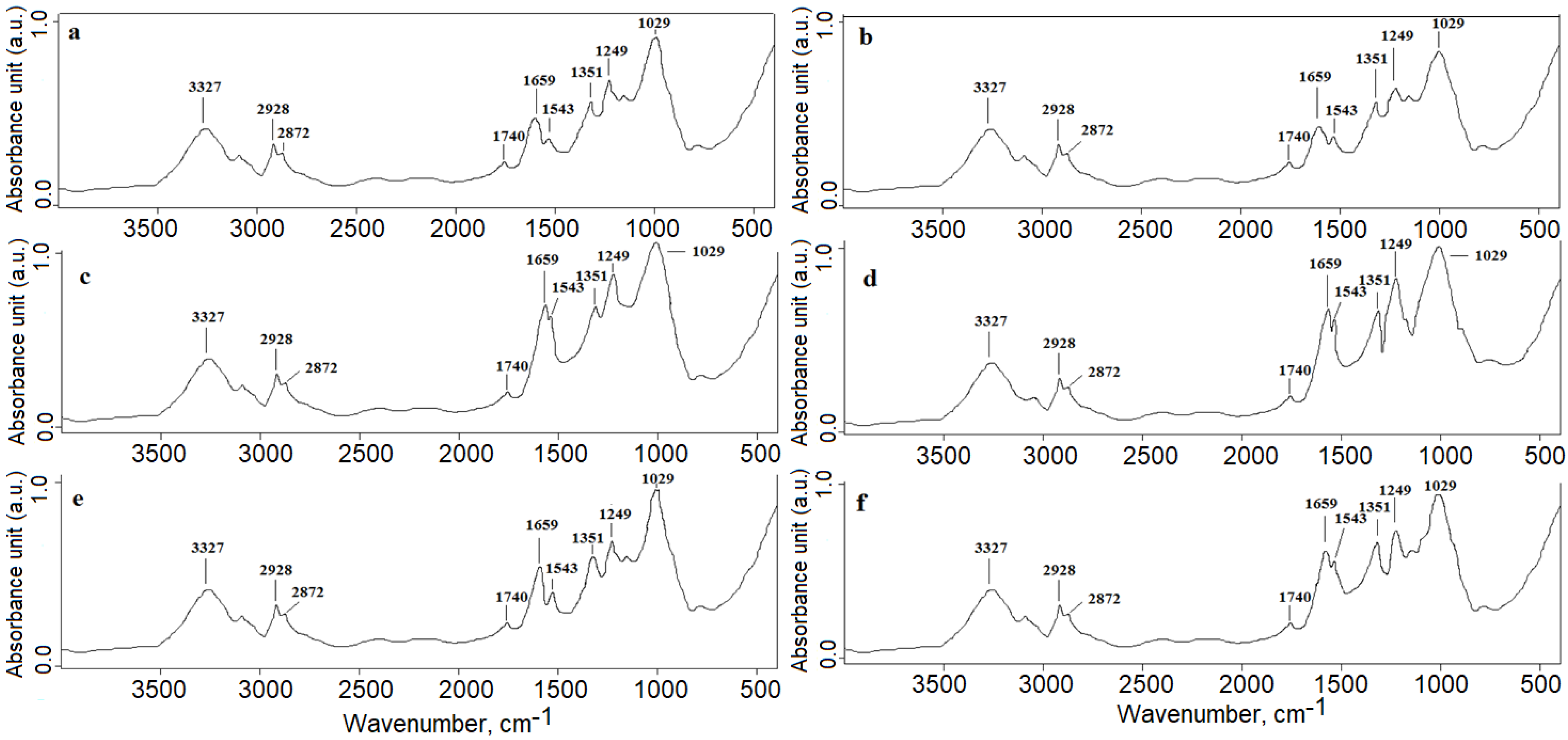
- (a)
- control (dose 0) seeds soaked in water without the addition of algae;
- (b)
- dose 0 without soaking;
- (c)
- 0.5% algae;
- (d)
- 1% of algae;
- (e)
- 5% of algae;
- (f)
- 10% of algae.
- Bright period imitating day: 16 h (350 µmol·m−2·s−1) at 23 °C; dark period imitating night: 8 h at 16 °C.
- Air humidity 75–80%.
- Universal soil Sterlux (HolLas) was used; pH: 5.5–6.5 NPK (nitrogen–phosphorus–potassium) fertilizer + Mg (magnesium) 14–16–18 + (5)–0.6 kg·m−3, maximum salinity 1.9 g NaCl dm−3.
- Each object (=pot containing seeds treated with the same dose) in four repetitions.
- Material for analysis: 10-day-old plants (above-ground part—young leaves).
2.1.1. FTIR Spectroscopy
2.1.2. Statistical Analysis
2.2. Seed Experiment
- (1)
- Control (dose 0) seeds soaked in water without the addition of algae;
- (2)
- 0.025% of algae
- (3)
- 0.01% of algae;
- (4)
- 0.05% of algae;
- (5)
- 0.1% of algae;
- (6)
- 0.25% of algae;
- (7)
- 0.5% of algae;
- (8)
- 1% of algae;
- (9)
- 2.5% of algae;
- (10)
- 5% of algae;
- (11)
- 10% of algae;
- (12)
- 15% of algae.
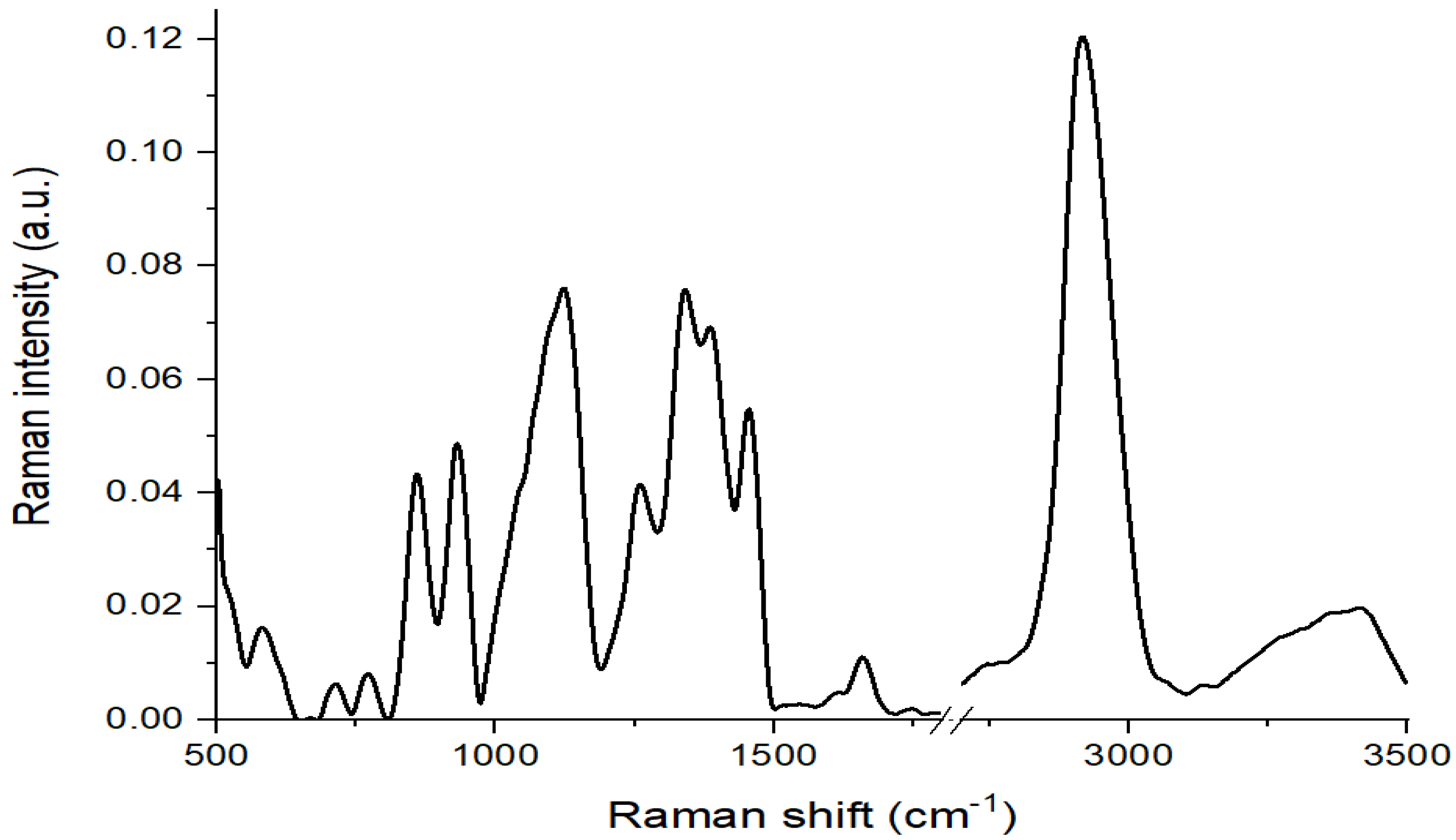
2.2.1. FT–Raman Measurements
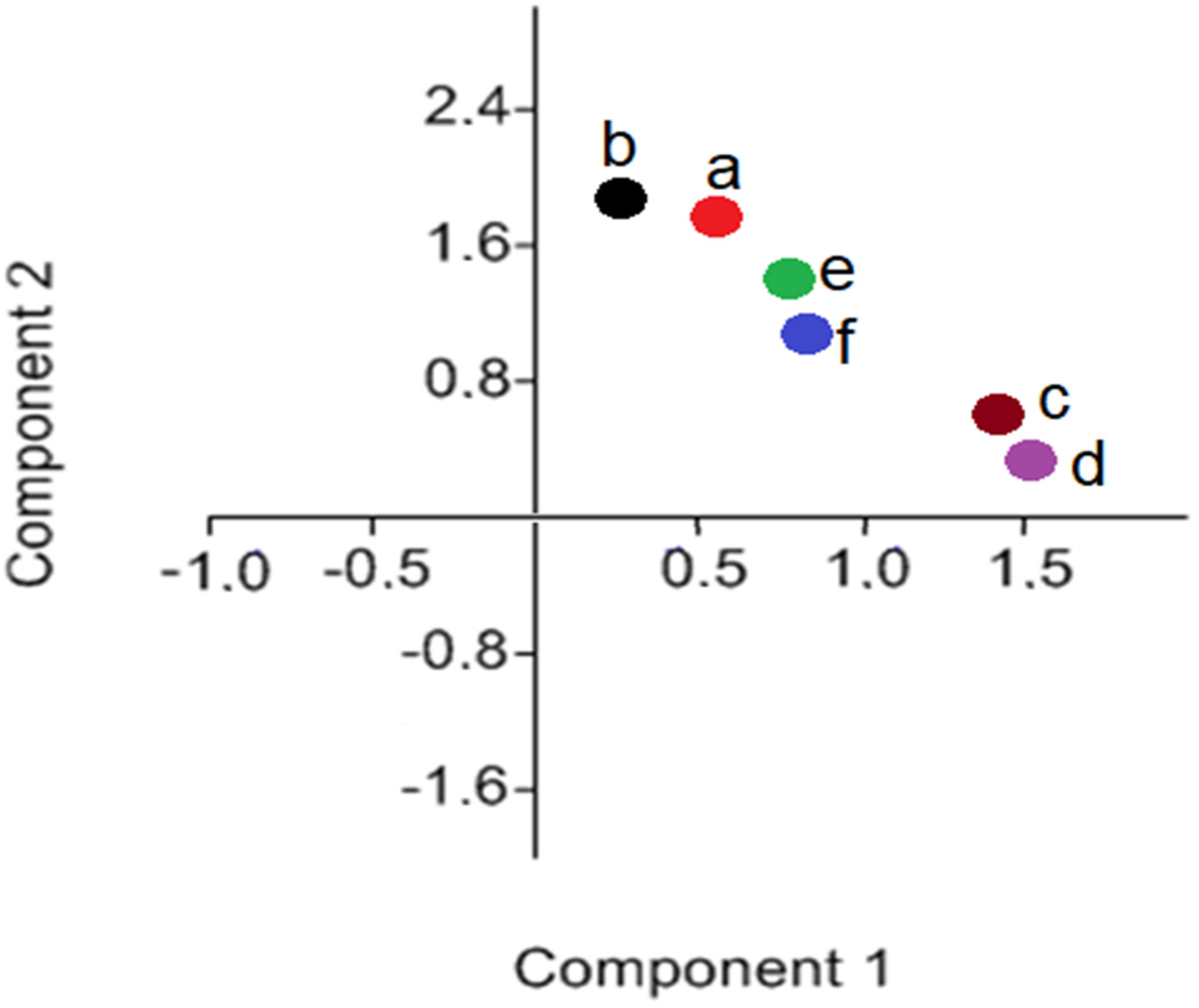
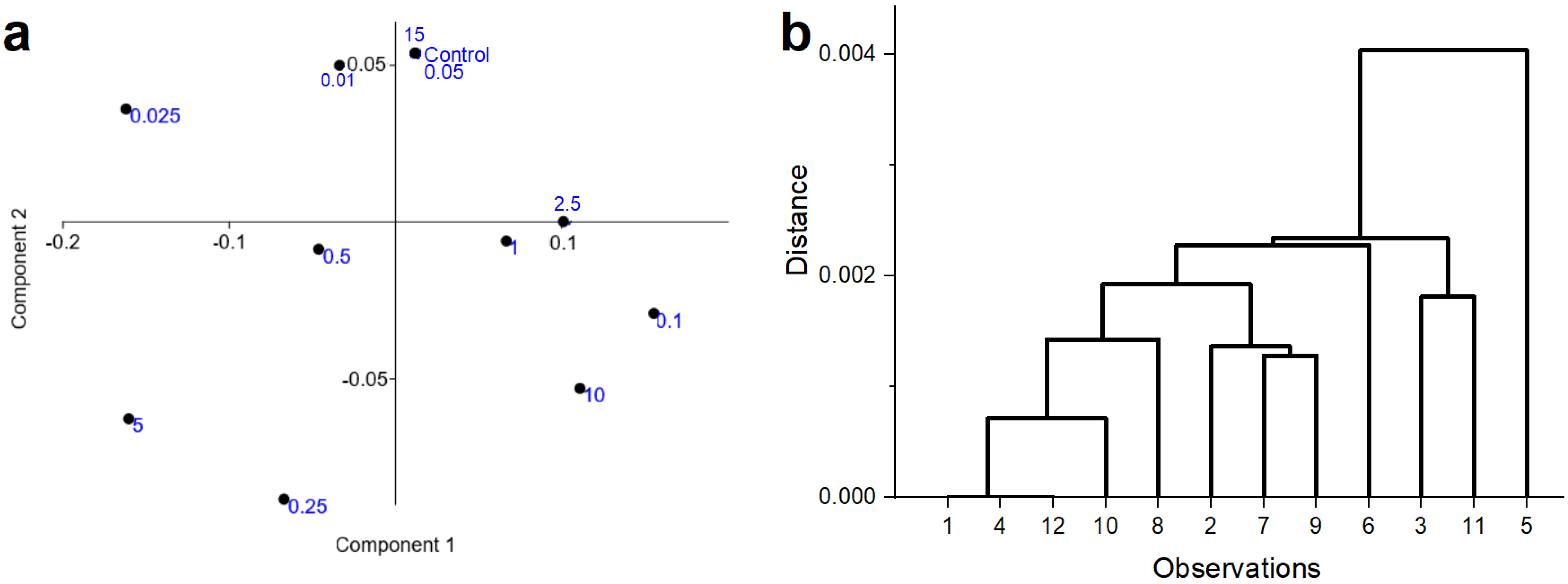
2.2.2. Statistics—Multivariate Analysis, Pearson Correlation Test
2.3. Field Experiment
3. Results
3.1. FTIR Analysis (Microgreens Experiment)
3.2. Seed Experiment (Raman Spectroscopic Measurements)
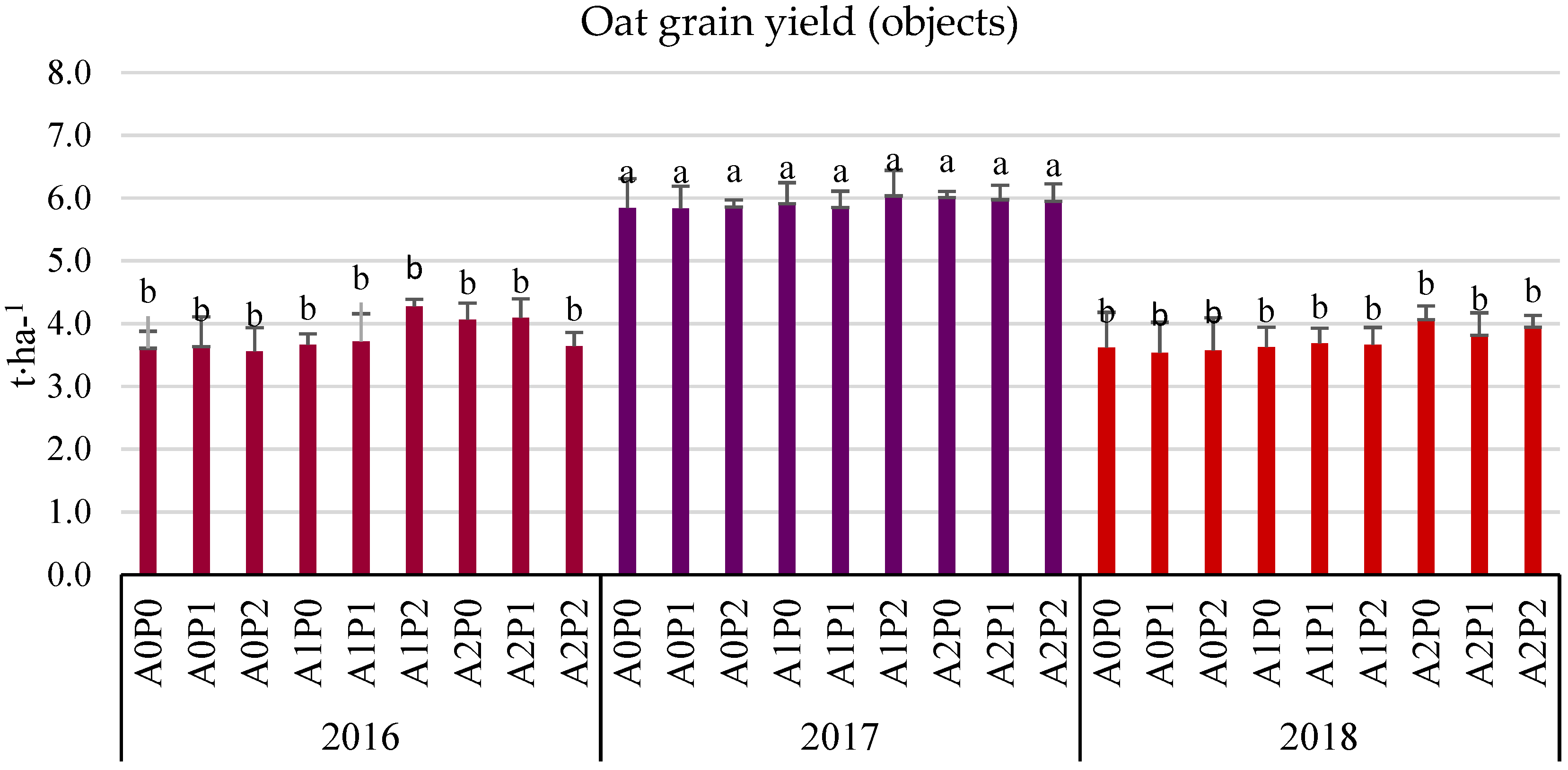
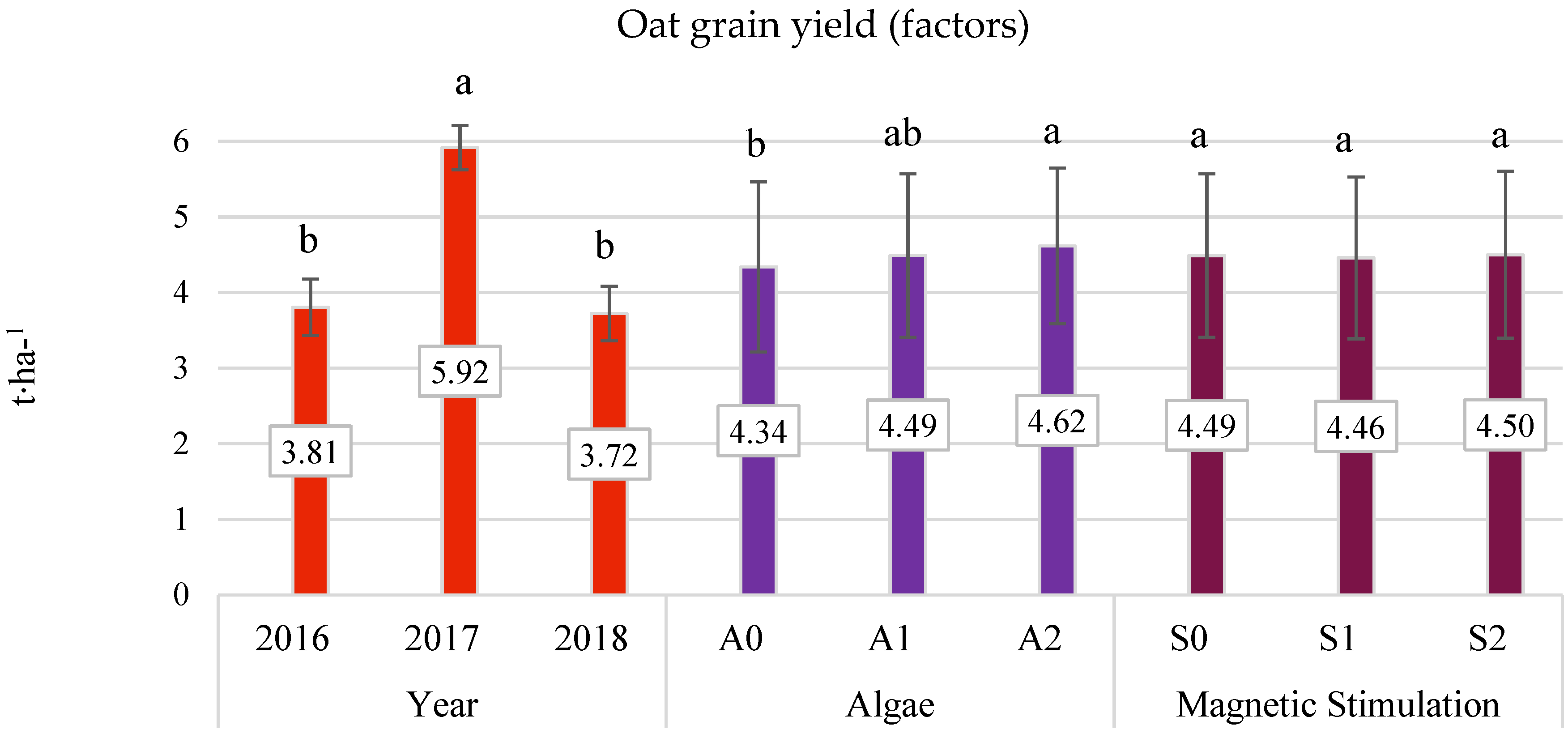
3.3. Grain Yield (Field Experiment)
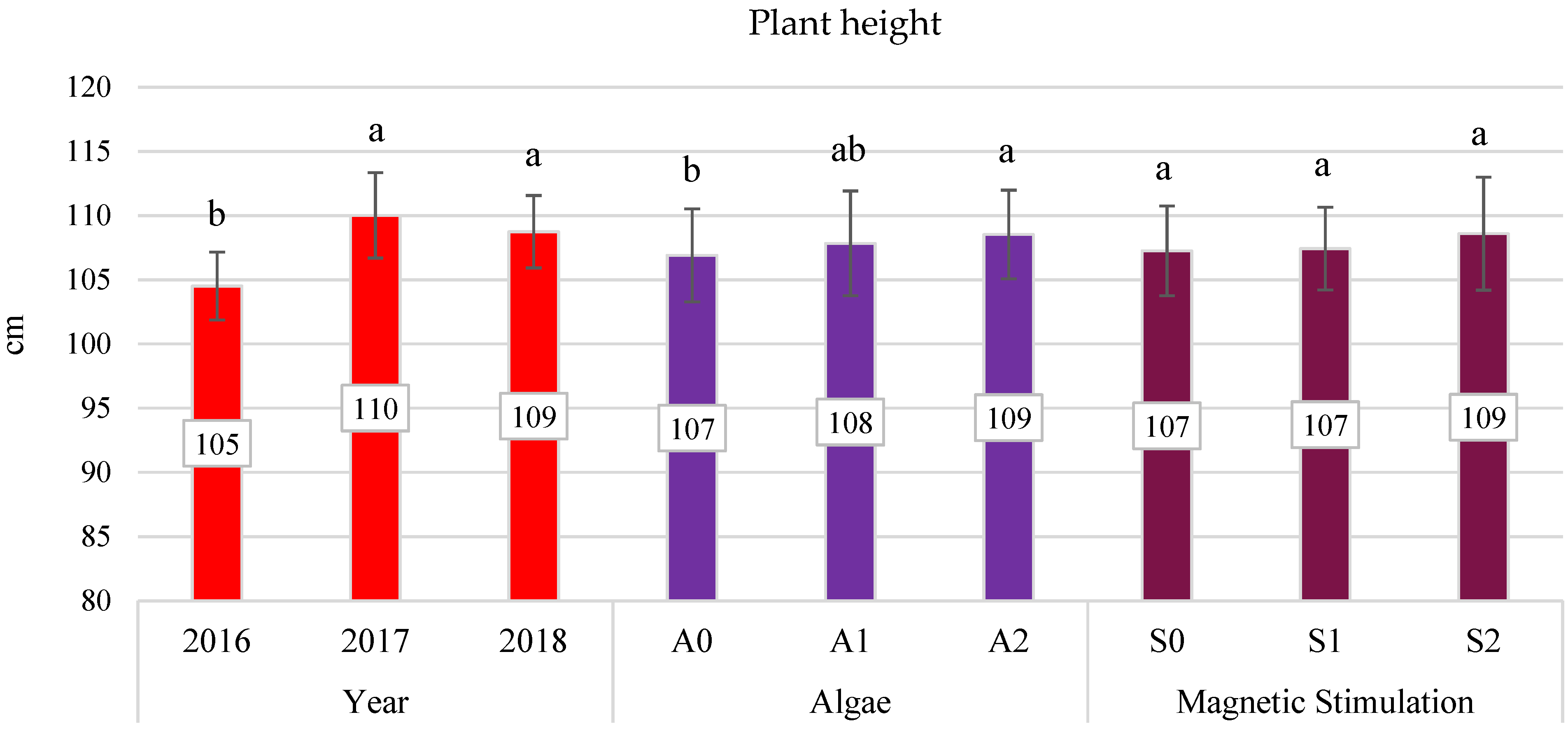
3.4. Yield Components
4. Discussion
5. Conclusions
- Soaking in the algae suspension affected the germination, metabolism, and chemical composition of oats. The plants from grains that were soaked in suspensions showed higher absorbance values for the sugar-building and protein-building functional groups than the control oat microgreens. No differences in the lipid content were found.
- The average three-year oat grain yield increased under the influence of algae application, especially after the highest dose of A2 (by 6.38% compared to the control). This effect was particularly significant in the years with less favorable hydrometeorological conditions. In 2016, the increase was 9.28%, and in 2018, it was 10.21%.
- No beneficial effect of pre-sowing stimulation with a low-frequency magnetic field on the yield of oats was found.
- The application of algae in dose A2 improved the characteristics of oats, such as panicle grain weight and the number of grains in the panicle by 14.01% and 4.3%, respectively.
- The interaction of the experimental factors (algae application × pre-sowing magnetic field stimulation) in shaping yield, structure, and oat grain composition was not relevant from 2016 to 2018.
- A. nodosum can stimulate the growth and yield of oats.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Edmondson, J.L.; Davies, Z.G.; Gaston, K.J.; Leake, J.R. Urban cultivation in allotments maintains soil qualities adversely affected by conventional agriculture. J. Appl. Ecol. 2014, 51, 880–889. [Google Scholar] [CrossRef]
- Pretty, J.N.; Noble, A.D.; Bossio, D.; Dixon, J.; Hine, R.E.; de Vries, F.W.T.P.; Morison, J.I.L. Resource-Conserving Agriculture Increases Yields in Developing Countries. Environ. Sci. Technol. 2005, 40, 1114–1119. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pretty, J.; Bharucha, Z.P. Sustainable intensification in agricultural systems. Ann. Bot. 2014, 114, 1571–1596. [Google Scholar] [CrossRef] [PubMed]
- Pretty, J.; Benton, T.G.; Bharucha, Z.P.; Dicks, L.V.; Flora, C.B.; Godfray, H.C.J.; Goulson, D.; Hartley, S.; Lampkin, N.; Morris, C.; et al. Global assessment of agricultural system redesign for sustainable intensification. Nat. Sustain. 2018, 1, 441–446. [Google Scholar] [CrossRef]
- Araújo, S.D.S.; Epaparella, S.; Edondi, D.; Ebentivoglio, A.; Ecarbonera, D.; Balestrazzi, A.; Araújo, S.D.S.; Epaparella, S.; Edondi, D.; Ebentivoglio, A.; et al. Physical Methods for Seed Invigoration: Advantages and Challenges in Seed Technology. Front. Plant Sci. 2016, 7, 646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aladjadjiyan, A. Physical factors for plant growth stimulation improve food quality. In Food Production—Approaches, Challenges and Tasks; IntechOpen: London, UK, 2012; Volume 270, pp. 145–168. [Google Scholar]
- Shukla, P.S.; Mantin, E.; Adil, M.; Bajpai, S.; Critchley, A.T.; Prithiviraj, B. Ascophyllum nodosum-Based Biostimulants: Sustainable Applications in Agriculture for the Stimulation of Plant Growth, Stress Tolerance, and Disease Management. Front. Plant Sci. 2019, 10, 655. [Google Scholar] [CrossRef] [Green Version]
- Kahane, R.; Hodgkin, T.; Jaenicke, H.; Hoogendoorn, C.; Hermann, M.; Keatinge, J.D.H.; Hughes, J.D.; Padulosi, S.; Looney, N. Agrobiodiversity for food security, health and income. Agron. Sustain. Dev. 2013, 33, 671–693. [Google Scholar] [CrossRef] [Green Version]
- Dmytryk, A.; Michalak, I.; Wilk, R.; Chojnacka, K.; Górecka, H.; Górecki, H. Innovative Seed Treatment with Algae Homog-enate. Waste Biomass Valorization 2015, 6, 441–448. [Google Scholar] [CrossRef]
- Halpern, M.; Bar-Tal, A.; Ofek, M.; Minz, D.; Muller, T.; Yermiyahu, U. The Use of Biostimulants for Enhancing Nutrient Uptake. Adv. Agron. 2015, 130, 141–174. [Google Scholar] [CrossRef]
- Du Jardin, P. Plant biostimulants: Definition, concept, main categories and regulation. Sci. Hortic. 2015, 196, 3–14. [Google Scholar] [CrossRef] [Green Version]
- EIBC. European Biostimulant Industry Council. Available online: https://biostimulants.eu/ (accessed on 29 December 2020).
- Du Jardin, P. The Science of Plant Biostimulants—A Bibliographic Analysis, Ad Hoc Study Report; European Commission: Brussels, Belgium, 2012; Available online: http://hdl.handle.net/2268/169257 (accessed on 20 December 2020).
- Dz.U.2020.796 t.j. Available online: https://sip.lex.pl/akty-prawne/dzu-dziennik-ustaw/nawozy-i-nawozenie-17370471 (accessed on 20 May 2021).
- Craigie, J.S. Seaweed extract stimuli in plant science and agriculture. J. Appl. Phycol. 2011, 23, 371–393. [Google Scholar] [CrossRef]
- Khan, W.; Rayirath, U.P.; Subramanian, S.; Jithesh, M.N.; Rayorath, P.; Hodges, D.M.; Critchley, A.; Craigie, J.S.; Norrie, J.; Prithiviraj, B. Seaweed Extracts as Biostimulants of Plant Growth and Development. J. Plant Growth Regul. 2009, 28, 386–399. [Google Scholar] [CrossRef]
- Eris, A.; Sivritepe, H.; Sivritepe, N. The effect of seaweed (Ascophyllum nodosum) extract on yield and quality criteria in peppers. ISHS Acta Hortic. 412 1995, 185–192. [Google Scholar] [CrossRef]
- Norrie, J.; Branson, T.; Keathley, P. Marine plant extracts impact on grape yield and quality. Acta Hortic. 2002, 594, 315–319. [Google Scholar] [CrossRef]
- Sabir, A.; Yazar, K.; Sabir, F.; Kara, Z.; Yazici, M.A.; Goksu, N. Vine growth, yield, berry quality attributes and leaf nutrient content of grapevines as influenced by seaweed extract (Ascophyllum nodosum) and nanosize fertilizer pulverizations. Sci. Hortic. 2014, 175, 1–8. [Google Scholar] [CrossRef]
- Fornes, F.; Sánchez-Perales, M.; Guardiola, J.L. Effect of a Seaweed Extract on the Productivity of ‘de Nules’ Clementine Mandarin and Navelina Orange. Bot. Mar. 2002, 45, 486–489. [Google Scholar] [CrossRef]
- Abdel-Mawgoud, A.M.R.; Tantaway, A.S.; Hafez, M.M.; Habib, H.A. Seaweed extract improves growth, yield and quality of different watermelon hybrids. Res. J. Agric. Biol. Sci. 2010, 6, 161–168. [Google Scholar]
- Spinelli, F.; Fiori, G.; Noferini, M.; Sprocatti, M.; Costa, G. A novel type of seaweed extract as a natural alternative to the use of iron chelates in strawberry production. Sci. Hortic. 2010, 125, 263–269. [Google Scholar] [CrossRef]
- Fan, D.; Hodges, D.M.; Critchley, A.; Prithiviraj, B. A Commercial Extract of Brown Macroalga (Ascophyllum nodosum) Affects Yield and the Nutritional Quality of SpinachIn Vitro. Commun. Soil Sci. Plant Anal. 2013, 44, 1873–1884. [Google Scholar] [CrossRef]
- Hidangmayum, A.; Sharma, R. Effect of different concentrations of commercial seaweed liquid extract of Ascophyllum nodosum as a plant bio stimulant on growth, yield and biochemical constituents of onion (Allium cepa L.). J. Pharmacogn. Phytochem. 2017, 6, 658–663. [Google Scholar]
- Keleszade, E.; Patterson, M.; Trangmar, S.; Guinan, K.; Costabile, A. Clinical Efficacy of Brown Seaweeds Ascophyllum nodosum and Fucus vesiculosus in the Prevention or Delay Progression of the Metabolic Syndrome: A Review of Clinical Trials. Molecules 2021, 26, 714. [Google Scholar] [CrossRef] [PubMed]
- Wally, O.S.D.; Critchley, A.T.; Hiltz, D.; Han, X.; Zaharia, L.I.; Abrams, S.R.; Prithiviraj, B. Regulation of pytohormone bo-synthesis and accumulation in Arabidopsis following treatment with commercial contract from the Marine Macroalga Ascophyllum nodosum. J. Plant Growth Regul. 2013, 32, 324–339. [Google Scholar] [CrossRef]
- Sarraf, M.; Kataria, S.; Taimourya, H.; Santos, L.; Menegatti, R.; Jain, M.; Ihtisham, M.; Liu, S.; Sarraf, M.; Kataria, S.; et al. Magnetic Field (MF) Applications in Plants: An Overview. Plants 2020, 9, 1139. [Google Scholar] [CrossRef] [PubMed]
- Gusta, L.V.; Kirkland, K.J.; Austenson, H.M. Effects of a brief magnetic exposure on cereal germination and seedling growth. Can. J. Plant Sci. 1978, 58, 79–86. [Google Scholar] [CrossRef]
- Radhakrishnan, R. Magnetic field regulates plant functions, growth and enhances tolerance against environmental stresses. Physiol. Mol. Biol. Plants 2019, 25, 1107–1119. [Google Scholar] [CrossRef] [PubMed]
- Index Mundi. Available online: https://www.indexmundi.com/ (accessed on 30 August 2020).
- Ebert, A.W. Sprouts, Microgreens, and Edible Flowers: The Potential for High Value Specialty Produce in Asia. In Proceedings of the SEAVEG Regional Symposium. High Value Vegetables in Southeast Asia: Production, Supply and Demand, Chiang Mai, Thailand, 24–26 January 2012. [Google Scholar]
- Benincasa, P.; Falcinelli, B.; Lutts, S.; Stagnari, F.; Galieni, A. Sprouted Grains: A Comprehensive Review. Nutrients 2019, 11, 421. [Google Scholar] [CrossRef] [Green Version]
- Di Gioia, F.; Renna, M.; Santamaria, P. Sprouts, Microgreens and “Baby Leaf” Vegetables; Springer: Boston, MA, USA, 2017; pp. 403–432. [Google Scholar] [CrossRef]
- Singh, N.; Verma, P.; Pandey, B.R. Therapeutic potential of organic Triticum aestivum linn. (wheat grass) in prevention and treatment of chronic diseases: An overview. Int. J. Pharm. Sci. Drug Res. 2012, 4, 10–14. [Google Scholar]
- Mitchell, A.L.; Gajjar, K.B.; Theophilou, G.; Martin, F.L.; Martin-Hirsch, P.L. Vibrational spectroscopy of biofluids for disease screening or diagnosis: Translation from the laboratory to a clinical setting. J. Biophotonics 2014, 7, 153–165. [Google Scholar] [CrossRef]
- Calabrò, E.; Magazù, S. The helix alignment of proteins in water solution toward a high-frequency electromagnetic field: A FTIR spectroscopy study. Electromagn. Biol. Med. 2017, 36, 279–288. [Google Scholar] [CrossRef]
- Baker, M.; Trevisan, J.; Bassan, P.; Bhargava, R.; Butler, H.; Dorling, K.M.; Fielden, P.R.; Fogarty, S.W.; Fullwood, N.J.; A Heys, K.; et al. Using Fourier transform IR spectroscopy to analyze biological materials. Nat. Protoc. 2014, 9, 1771–1791. [Google Scholar] [CrossRef] [Green Version]
- Grdadolnik, J. ATR-FTIR spectroscopy: Its advantages and limitations. Acta Chim. Slov. 2002, 49, 631–642. [Google Scholar]
- Lasch, P. Spectral pre-processing for biomedical vibrational spectroscopy and micro spectroscopic imaging. Chemom. Intell. Lab. Syst. 2012, 117, 100–114. [Google Scholar] [CrossRef] [Green Version]
- Gracia, M.-B.; Armstrong, P.R.; Rongkui, H.; Mark, S. Quantification of betaglucans, lipid and protein contents in whole oat groats (Avena sativa L.) using near infrared reflectance spectroscopy. J. Near Infrared Spectrosc. 2017, 25, 172–179. [Google Scholar] [CrossRef]
- Grube, M.; Marauska, M.; Bekers, M. Quantitative analysis of oat by Infrared spectroscopy. In Spectroscopy of Biological Molecules: New Directions; Springer: Berlin/Heidelberg, Germany, 1999; pp. 617–618. [Google Scholar] [CrossRef]
- Witkowicz, R.; Biel, W.; Chłopicka, J.; Galanty, A.; Gleń-Karolczyk, K.; Skrzypek, E.; Krupa, M.; Witkowicz, R.; Biel, W.; Chłopicka, J.; et al. Biostimulants and Microorganisms Boost the Nutritional Composition of Buckwheat (Fagopyrum esculentum Moench) Sprouts. Agronomy 2019, 9, 469. [Google Scholar] [CrossRef] [Green Version]
- Tobiasz-Salach, R.; Bobrecka-Jamro, D. The effect of nitrogen fertilization on the grain yield, content and elements of the oat yield structure. Bull. Plant Breed. Acclim. Inst. 2006, 239, 41–47. (In Polish) [Google Scholar]
- Pereira, L.; Morrison, L.; Shukla, P.S.; Critchley, A.T. A concise review of the brown macroalga Ascophyllum nodosum (Linnaeus) Le Jolis. J. Appl. Phycol. 2020, 32, 3561–3584. [Google Scholar] [CrossRef]
- De Seager, J.; Van Praet, S.; Vareecke, D.; Park, J.; Jacques, S.; Han, T.; Depuydt, D. Toward the molecular understanding of the action mechanism of Ascophyllum nodosum extracts on plants. J. Appl. Phycol. 2020, 32, 573–597. [Google Scholar] [CrossRef] [Green Version]
- Yakhin, O.; Lubyanov, A.; Yakhin, I.; Brown, P. Biostimulants in Plant Science: A Global Perspective. Front. Plant Sci. 2017, 7, 2049. [Google Scholar] [CrossRef] [Green Version]
- Caradonia, F.; Battaglia, V.; Righi, L.; Pascali, G.; La Torre, A. Plant Biostimulant Regulatory Framework: Prospects in Europe and Current Situation at International Level. J. Plant Growth Regul. 2018, 38, 438–448. [Google Scholar] [CrossRef]
- Artyszak, A. The Best-Yielding Oat Varieties in 2018 Agropolska.pl (In Polish). Available online: https://www.agropolska.pl/uprawa/zboza/najlepiej-plonujace-odmiany-owsa-w-2018-roku,315.html (accessed on 12 October 2019).
- Tobiasz-Salach, R.; Bobrecka-Jamro, D.; Pyrek-Bajcar, E.; Buczek, J. Response of hulled and naked oat to foliar fertilization. Acta Sci. Pol. Agric. 2016, 15, 77–78. [Google Scholar]




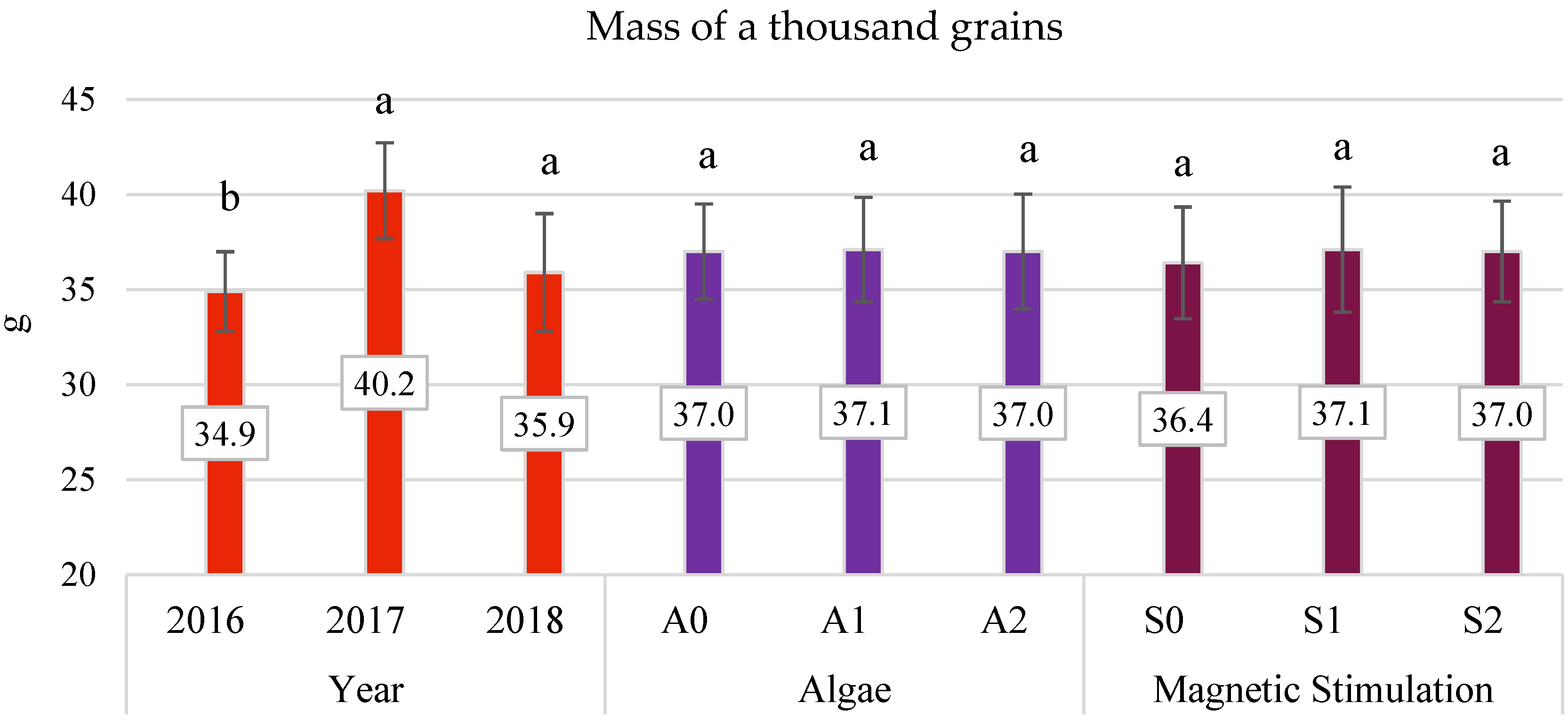

| g⋅ha−1 | kg⋅ha−1 | ||||
|---|---|---|---|---|---|
| B | Mn | Cu | Zn | Fe | |
| A0 | - | - | - | - | - |
| A1 | 3.2 | 2.5 | 2.1 | 6.2 | 0.43 |
| A2 | 6.4 | 5 | 4.2 | 12.4 | 0.86 |
| Location | Krasne (50°30′ N and 22°06′ E), Poland |
|---|---|
| Tested species, cultivar | Oat (Avena sativa) cv. Bingo |
| System of experiment | Split-block |
| 16 m2 | |
| Seeding density | 550 grains⋅m2 |
| Soil | Heavy, brown, in class IIIa |
| Forecrop | Spring barley (Hordeum vulgare) in 2015 and spring wheat (Triticum L.) in 2016 and 2017 |
| Sowing dates | 5 May 2016, 4 April 2017, 11 April 2018 |
| Harvest dated | 18 August 2016, 10 August 2017, and 8 August 2018 |
| NPK constant fertilization (ha−1) | 80 kg N⋅ha−1 80 kg P2O5⋅ha−1 100 kg K2O⋅ha−1 |
| Nutrient previous content | Moderate manganese and iron contents and low boron, zinc, and copper contents |
| Year | k Value for Month | |||||
|---|---|---|---|---|---|---|
| III | IV | V | VI | VII | VIII | |
| 2016 | 1.6 | 1.7 | 0.46 | 0.73 | 0.53 | 0.63 |
| 2017 | 1 | 2 | 1 | 0.36 | 0.83 | 0.13 |
| 2018 | 8.61 | 0.16 | 0.31 | 0.86 | 1.92 | 0.56 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Drygaś, B.; Depciuch, J.; Puchalski, C. Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L. Agronomy 2021, 11, 1446. https://doi.org/10.3390/agronomy11071446
Drygaś B, Depciuch J, Puchalski C. Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L. Agronomy. 2021; 11(7):1446. https://doi.org/10.3390/agronomy11071446
Chicago/Turabian StyleDrygaś, Barbara, Joanna Depciuch, and Czesław Puchalski. 2021. "Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L." Agronomy 11, no. 7: 1446. https://doi.org/10.3390/agronomy11071446
APA StyleDrygaś, B., Depciuch, J., & Puchalski, C. (2021). Effect of Ascophyllum nodosum Alga Application on Microgreens, Yield, and Yield Components in Oats Avena sativa L. Agronomy, 11(7), 1446. https://doi.org/10.3390/agronomy11071446






