Spatial Distribution and Development of Sequential Sampling Plans for Diaphorina citri Kuwayama (Hemiptera: Liviidae)
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Collection
2.2. Spatial Distribution of the ACP
2.2.1. Variance–Mean Relationship (DI)
2.2.2. Taylor’s Power Law
2.2.3. The Negative Binomial Distribution and Its Parameter k
2.3. Sequential Sampling Plan (SeqSP) Based on Sequential Probability Ratio Test (SPRT)
2.4. Field Evaluation of the SPRT Sequential Sampling Plan for D. Citri
2.5. Statistical Analysis
3. Results
3.1. Exploratory Analysis and Dispersion Indices
3.2. Taylor’s Power Law
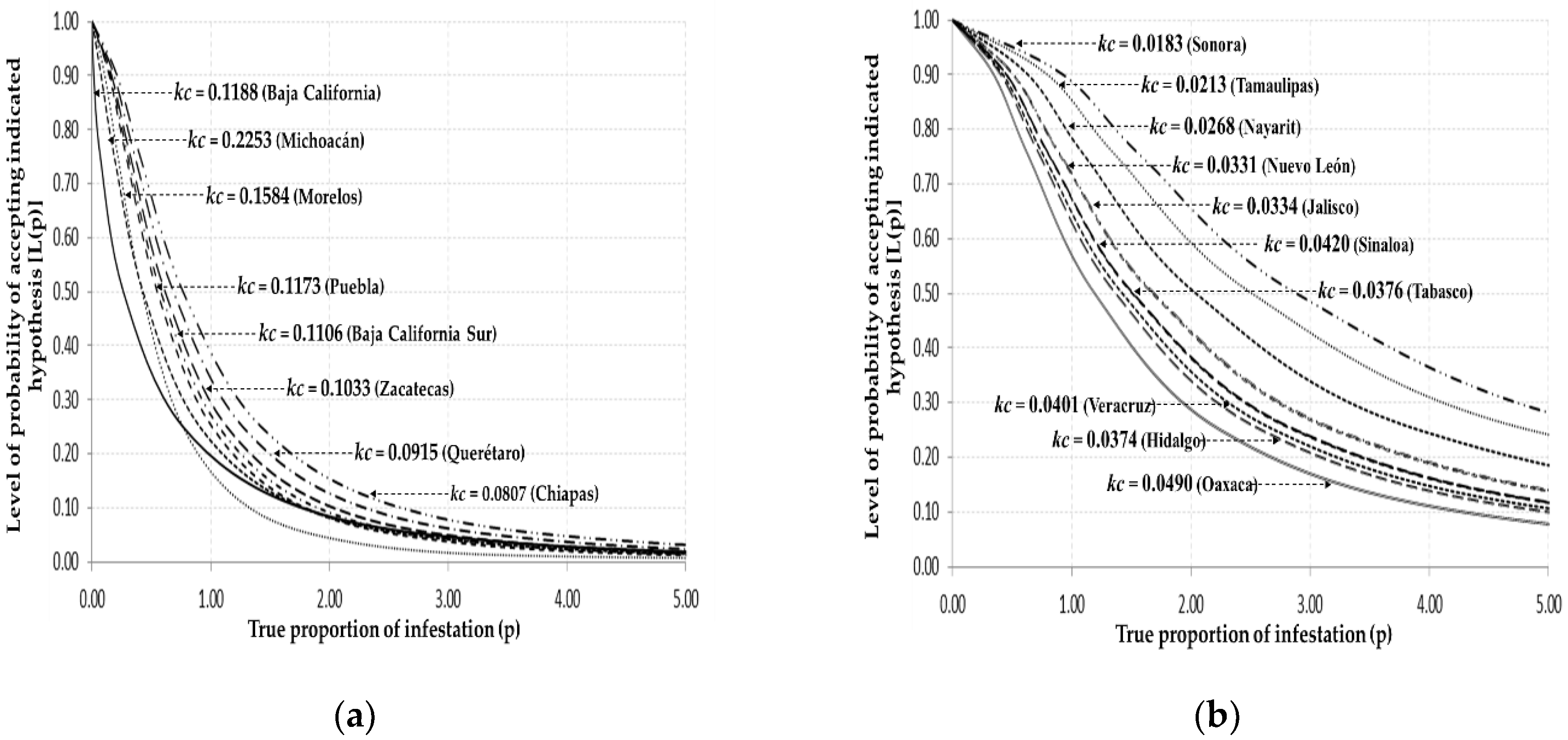
3.3. Common k
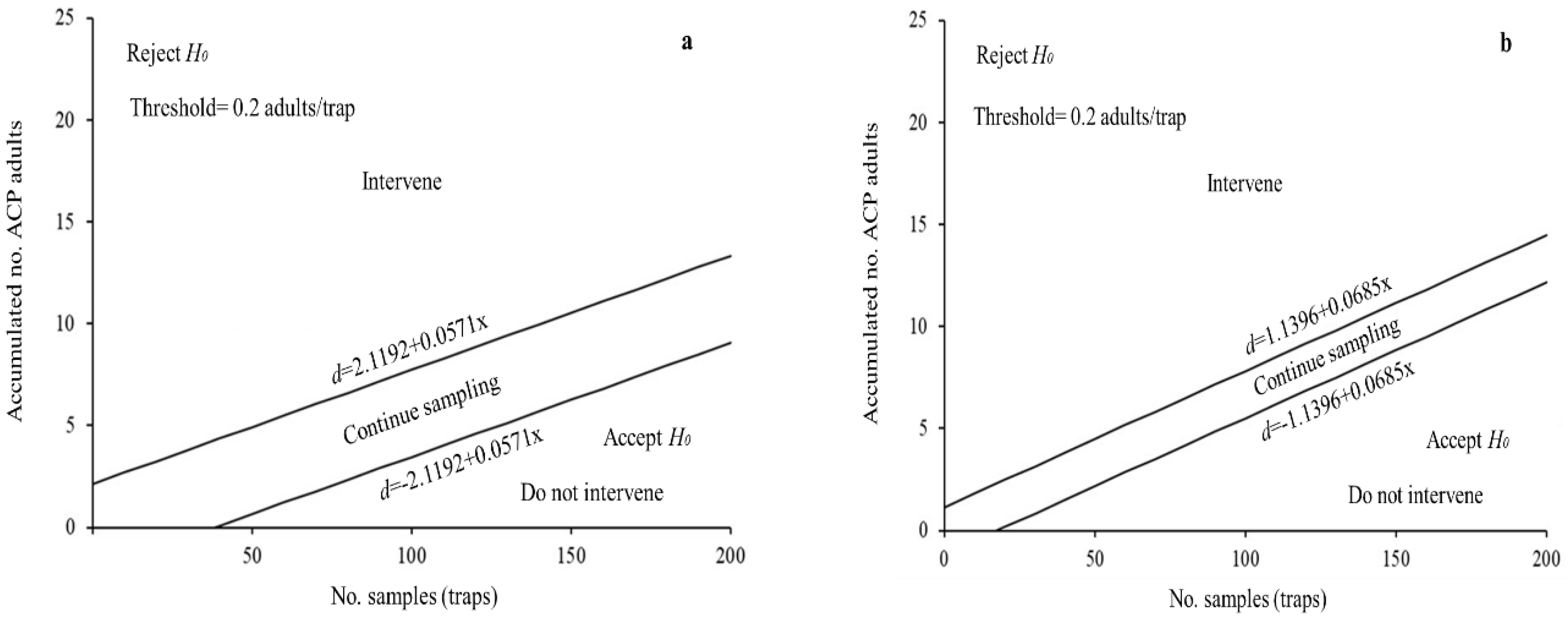
3.4. SPRT Sequential Sampling Plans for D. Citri
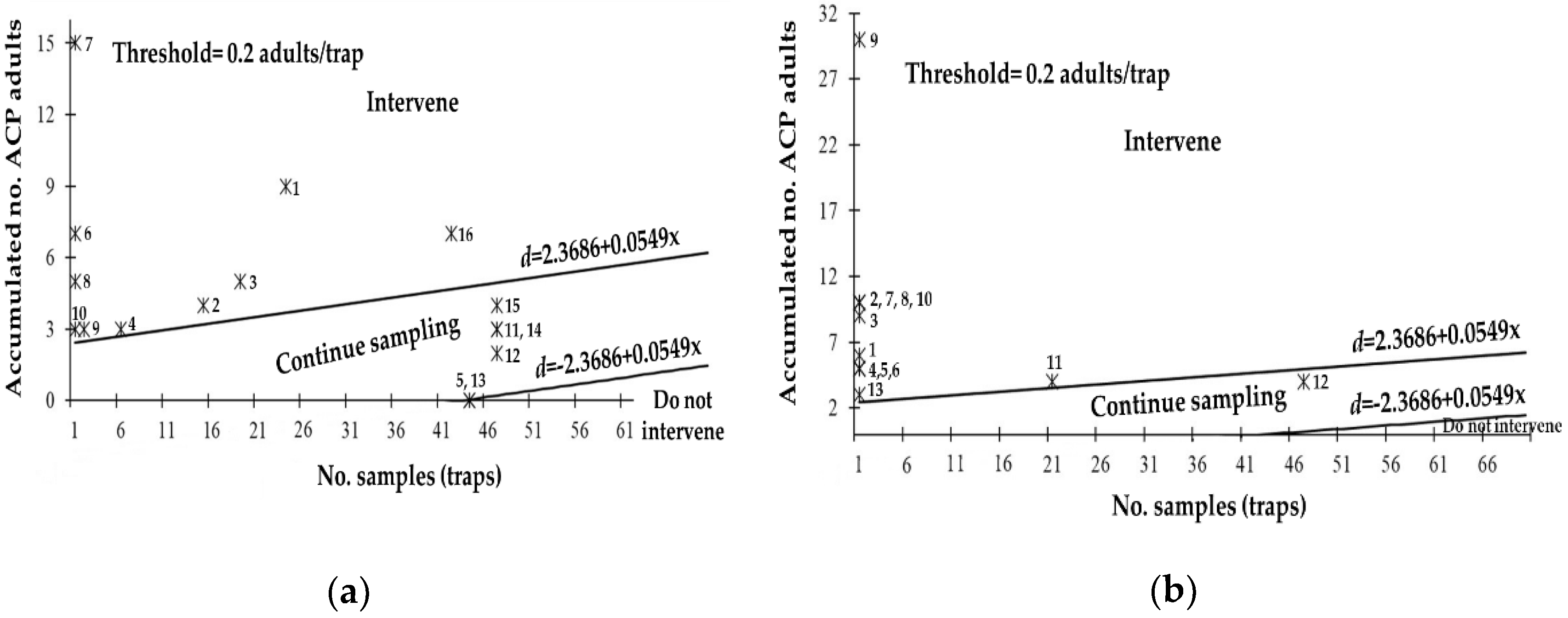
3.5. Field Evaluation of the SPRT Sequential Sampling Plan for D. citri
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Da Graca, J.V. Citrus greening disease. Annu. Rev. Phytopathol. 1991, 29, 109–136. [Google Scholar] [CrossRef]
- Da Graca, J.V.; Korsten, L. Citrus Huanglongbing: Review, Present Status and Future Strategies. In Diseases of Fruits and Vegetables, 1st ed.; Naqvi, S.A.M.H., Ed.; Kluwer Academic Publishers: Dordrecht, The Netherlands, 2004; Volume 1, pp. 229–245. [Google Scholar]
- Gottwald, T.R. Current epidemiological understanding of citrus Huanglongbing. Annu. Rev. Phytopathol. 2010, 48, 119–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- CABI. Invasive Species Compendium. Citrus Huanglongbing (Greening) Disease (Citrus Greening). 2021. Available online: https://www.cabi.org/isc/datasheet/16567 (accessed on 15 March 2021).
- Bové, J.M. Huanglongbing: A destructive, newly emerging, century-old disease of citrus. J. Plant Pathol. 2006, 88, 7–37. [Google Scholar]
- National Research Council. Strategic Planning for the Florida Citrus Industry: Addressing Citrus Greening Disease; The National Academies Press: Washington, DC, USA, 2010. [Google Scholar]
- Santivañez, T.; Vernal, P.; Mora, G.; Díaz, G.; López, J.I. Citrus: Marco Estratégico Para la Gestión Regional del Huanglongbing en América Latina y el Caribe; FAO: Roma, Italia, 2014; 72p, ISBN 978-92-5-107711-5. [Google Scholar]
- Halbert, S.E.; Manjunath, K.L. Asian Citrus Psyllids (Sternorrhyncha: Psyllidae) and greening disease of citrus: A literature review and assessment of risk in Florida. Fla. Entomol. 2004, 87, 401–402. [Google Scholar] [CrossRef]
- Ammar, E.-D.; George, J.; Sturgeon, K.; Stelinski, L.L.; Shatters, R.G. Asian citrus psyllid adults inoculate huanglongbing bacterium more efficiently than nymphs when this bacterium is acquired by early instar nymphs. Sci. Rep. 2020, 10, 18244. [Google Scholar] [CrossRef] [PubMed]
- Inoue, H.; Ohnishi, J.; Ito, T.; Tomimura, K.; Miyata, S.; Iwanami, T.; Ashihara, W. Enhanced proliferation and efficient transmission of Candidatus Liberibacter asiaticus by adult Diaphorina citri after acquisition feeding in the nymphal stage. Ann. Appl. Biol. 2009, 155, 29–36. [Google Scholar] [CrossRef]
- Pelz-Stelinski, K.S.; Brlansky, R.H.; Ebert, T.A.; Rogers, M.E. Transmission parameters for Candidatus Liberibacter asiaticus by Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 1531–1541. [Google Scholar] [CrossRef] [PubMed]
- George, J.; Ammar, E.-D.; Hall, D.G.; Shatters, R.G., Jr.; Lapointe, S.L. Prolonged phloem ingestion by Diaphorina citri nymphs compared to adults is correlated with increased acquisition of citrus greening pathogen. Sci. Rep. 2018, 8, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.A.; Halbert, S.E.; Dawson, W.O.; Robertson, C.J.; Keesling, J.E.; Singerd, B.H. Asymptomatic spread of huanglongbing and implications for disease control. Proc. Natl. Acad. Sci. USA 2015, 112, 7605–7610. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.; Gottwald, T.; Setamou, M. Status of Huanglongbing (HLB) outbreaks in Florida, California and Texas. Trop. Plant Pathol. 2020, 45, 265–278. [Google Scholar] [CrossRef]
- Li, S.; Wu, F.; Duan, Y.; Singerman, A.; Guan, Z. Citrus greening: Management strategies and their economic impact. HortScience 2020, 55, 604–612. [Google Scholar] [CrossRef] [Green Version]
- Hall, D.G.; Richardson, M.L.; Ammar, E.-D.; Halbert, S.E. Asian citrus psyllid, Diaphorina citri, vector of citrus huanglongbing disease. Entomol. Exp. Appl. 2013, 146, 207–223. [Google Scholar] [CrossRef]
- Qureshi, J.A.; Kostyk, B.C.; Stansly, P.A. Insecticidal Suppression of Asian Citrus Psyllid Diaphorina citri (Hemiptera: Liviidae) Vector of Huanglongbing Pathogens. PLoS ONE 2014, 9, e112331. [Google Scholar] [CrossRef] [Green Version]
- Stansly, P.A.; Arevalo, H.A.; Qureshi, J.A.; Jones, M.M.; Hendricks, K.; Roberts, P.D.; Roka, F.M. Vector control and foliar nutrition to maintain economic sustainability of bearing citrus in Florida groves affected by Huanglongbing. Pest Manag. Sci. 2014, 70, 415–426. [Google Scholar] [CrossRef]
- Boina, D.R.; Bloomquist, J.R. Chemical control of the Asian citrus psyllid and of huanglongbing disease in citrus. Pest Manag. Sci. 2015, 71, 808–823. [Google Scholar] [CrossRef] [PubMed]
- Singerman, A.; Rogers, M.E. The economic challenges of dealing with citrus greening: The case of Florida. J. Integ. Pest Manag. 2020, 11, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Yuan, X.; Chen, C.; Bassanezi, R.; Wu, F.; Feng, Z.; Shi, D.; Du, Y.; Zhong, L.; Zhong, B.; Lu, Z.; et al. Region-wide comprehensive implementation of roguing infected trees, tree replacement, and insecticide applications successfully controls citrus HLB. Phytopathology 2020, 23, 1–29. [Google Scholar]
- Bassanezi, R.B.; Lopes, S.A.; de Miranda, M.P.; Wulff, N.A.; Volpe, H.X.L.; Ayres, A.J. Overview of citrus huanglongbing spread and management strategies in Brazil. Trop. Plant Pathol. 2020, 45, 251–264. [Google Scholar] [CrossRef]
- Stelinski, L.L. Ecological aspects of the vector-borne bacterial disease, citrus greening (Huanglongbing): Dispersal and host use by Asian citrus psyllid, Diaphorina citri Kuwayama. Insects 2019, 10, 208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andrade, M.; Li, J.; Wang, N. Candidatus Liberibacter asiaticus: Virulence traits and control strategies. Trop. Plant Pathol. 2020, 45, 285–297. [Google Scholar] [CrossRef]
- Monzo, C.; Stansly, P.A. Economic injury levels for Asian citrus psyllid control in process oranges from mature trees with high incidence of Huanglongbing. PLoS ONE 2017, 12, e0175333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wald, A. Sequential Analysis; J. Wiley & Sons: New York, NY, USA, 1947; 212p. [Google Scholar]
- Onsager, J.A. The rationale of sequential sampling, with emphasis on its use in pest management. United States Department of Agriculture, Economic Research Service. Tech. Bull. 1976, 158539, 1–19. [Google Scholar]
- Fowler, G.W. Accuracy of sequential sampling plans based on Wald’s sequential probability ratio test. Can. J. For. Res. 1983, 13, 1197–1203. [Google Scholar] [CrossRef]
- Binns, M.R.; Nyrop, J.P.; van der Werf, W. Sampling and Monitoring in Crop Protection: The Theoretical Basis for Developing Practical Decision Guides, 1st ed.; CABI Publishing: Oxon, UK, 2000; 284p. [Google Scholar]
- Oakland, G.B. An application of sequential analysis to whitefish sampling. Biometrics 1950, 6, 59–67. [Google Scholar] [CrossRef]
- Kipchirchir, I. An analysis of sequential sampling strategy in pest control based on negative binomial distribution. ICASTOR J. Math. Sci. 2011, 5, 217–228. [Google Scholar]
- Pereira, P.S.; Sarmento, R.A.; Galdino, T.V.S.; Lima, C.H.O.; dos Santos, F.A.; Silva, J.; dos Santos, F.R.; Picanço, M.C. Economic injury levels and sequential sampling plans for Frankliniella schultzei in watermelon crops. Pest Manag. Sci. 2016, 73, 1438–1445. [Google Scholar] [CrossRef] [PubMed]
- Binns, M.R.; Nyrop, J.P. Sampling insect populations for the purpose of IPM decision making. Annu. Rev. Entomol. 1992, 37, 427–453. [Google Scholar] [CrossRef]
- Flores-Sánchez, J.L.; Mora-Aguilera, G.; Loeza-Kuk, E.; López-Arroyo, J.I.; Gutiérrez-Espinosa, M.A.; Velázquez-Monreal, J.J.; Domínguez-Monge, S.; Bassanezi, R.B.; Acevedo-Sánchez, G.; Robles-García, P. Diffusion model for describing the regional spread Huanglongbing from first-reported outbreaks and basing an area-wide disease management strategy. Plant Dis. 2017, 101, 1119–1127. [Google Scholar] [CrossRef] [PubMed]
- García-Ávila, C.J.; Trujillo-Arriaga, F.J.; Quezada-Salinas, A.; Ruiz-Galván, I.; Bravo-Pérez, D.; Pineda-Ríos, J.M.; Florencio-Anastasio, J.G.; Robles-García, P.L. Holistic Area-Wide Approach for Successfully Managing Citrus Greening (Huanglongbing) in Mexico. In Area-Wide Integrated Pest Management: Development and Field Application, 1st ed.; Hendrichs, J., Pereira, R., Vreysen, M.J.B., Eds.; CRC Press: Boca Raton, FL, USA, 2021; pp. 33–49. [Google Scholar]
- Pedigo, L.; Zeiss, M.R. Analyses in insect ecology and management. Bul. Entomol. Res. 1996, 87, 219–220. [Google Scholar]
- Southwood, T.R.E.; Henderson, A.P. Ecological Methods, 3rd ed.; Blackwell Science Ltd.: Oxford, UK, 2000; 592p. [Google Scholar]
- Tsai, J.H.; Wang, J.J.; Liu, Y.-H. Sampling of Diaphorina citri (Homoptera: Psyllidae) on orange jessamine in southern Florida. Fl. Entomol. 2000, 83, 446–459. [Google Scholar] [CrossRef]
- Sétamou, M.; Flores, D.; French, J.V.; Hall, D.G. Dispersion patterns and sampling plans for Diaphorina citri (Hemiptera: Psyllidae) in citrus. J. Econ. Entomol. 2008, 101, 1478–1487. [Google Scholar] [CrossRef] [PubMed]
- Soemargono, A.; Ibrahim, Y.; Ibrahim, R.; Osman, M.S. Spatial distribution of the Asian citrus psyllid, Diaphorina citri Kuwayama (Homoptera: Psyllidae) on citrus and orange jasmine. J. Biosci. 2008, 19, 9–19. [Google Scholar]
- Krebs, C.J. Ecological Methodology, 2nd ed.; Addison-Wesley Educational: Menlo Park, CA, USA, 1999; 620p. [Google Scholar]
- Prager, S.M.; Butler, C.D.; Trumble, J.T. A sequential binomial sampling plan for potato psyllid (Hemiptera: Triozidae) on bell pepper (Capsicum annum). Pest Manag. Sci. 2013, 69, 1131–1135. [Google Scholar] [CrossRef] [PubMed]
- Taylor, L.R. Aggregation, variance and the mean. Nature 1961, 189, 732. [Google Scholar] [CrossRef]
- Patil, G.P.; Stiteler, W.M. Concepts of aggregation and their quantification: A critical review with some new results and applications. Res. Popul. Ecol. 1974, 15, 238–254. [Google Scholar] [CrossRef]
- Banerjee, B. Variance to mean ratio and the spatial distribution of animals. Experientia 1976, 32, 993–994. [Google Scholar] [CrossRef]
- Taylor, L.R. Assessing and interpreting the spatial distributions of insect populations. Ann. Rev. Entomol. 1984, 29, 321–357. [Google Scholar] [CrossRef]
- Greig-Smith, P. Quantitative Plant Ecology; Academic Press Inc.: New York, NY, USA, 1957; 198p. [Google Scholar]
- Downing, J.A. Spatial heterogeneity: Evolved behavior or mathematical artifact? Nature 1986, 323, 255–257. [Google Scholar] [CrossRef]
- Bliss, C.I.; Fisher, R.A. Fitting the negative binomial distribution to biological data. Biometrics 1953, 9, 176–200. [Google Scholar] [CrossRef]
- Anscombe, F.J. The statistical analysis of insect counts based on the negative binomial distribution. Biometrics 1949, 5, 165–173. [Google Scholar] [CrossRef]
- Bliss, C.I.; Owen, A.R.G. Negative binomial distributions with a common k. Biometrika 1958, 45, 37–58. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2018. [Google Scholar]
- Lima, C.H.O.; Sarmento, R.A.; Pereira, P.S.; Galdino, T.V.S.; Santos, F.A.; Silva, J.; Picanço, M.C. Feasible sampling plan for Bemisia tabaci control decision-making in watermelon fields. Pest Manag. Sci. 2017, 73, 2345–2352. [Google Scholar] [CrossRef]
- Monzo, C.; Arevalo, H.A.; Jones, M.M.; Vanaclocha, P.; Croxton, S.D.; Qureshi, J.A.; Stansly, P.A. Sampling methods for detection and monitoring of the Asian Citrus Psyllid (Hemiptera: Psyllidae). Environ. Entomol. 2015, 44, 780–788. [Google Scholar] [CrossRef] [PubMed]
- Costa, M.G. Distribuição Espacial e Amostragem Seqüencial de Ninfas e Adultos de Diaphorina Citri Kuwayama (Hemiptera: Psyllidae) na Cultura de Citros. Ph.D. Thesis, Universidade Estadual Paulista, Jaboticabal, São Paulo, Brazil, 2009. [Google Scholar]
- Hall, H.G.; Hentz, M.G. Sticky trap and stem–tap sampling protocols for the Asian citrus psyllid (Hemiptera: Psyllidae). J. Econ. Entomol. 2010, 103, 541–549. [Google Scholar] [CrossRef]
- Hall, D.G.; Hentz, M.G.; Ciomperlink, M.A. A comparison of traps and stem tap sampling for monitoring adult Asian citrus psyllid (Hemiptera: Psyllidae) in citrus. Fl. Entomol. 2007, 90, 327–333. [Google Scholar] [CrossRef]
- Auguie, B.; Antonov, A. gridExtra: Miscellaneous Functions for “Grid” Graphics. 2016. Available online: https://cran.r-project.org/package=gridExtra (accessed on 14 July 2018).
- Wickham, H. ggplot2: Elegant Graphics for Data Analysis, 1st ed.; Springer: New York, NY, USA, 2009; 213p. [Google Scholar]
- Taylor, L.R.; Taylor, R.A.J.; Woiwod, I.P.; Perry, J.N. Behavioural dynamycs. Nature 1983, 303, 801–804. [Google Scholar] [CrossRef]
- Rakhshani, E.; Saeedifar, A. Seasonal fluctuation, spatial distribution and natural enemies of Asian citrus psyllid Diaphorina citri Kuwayama (Hemiptera: Psyllidae) in Iran. Entomol. Sci. 2013, 16, 17–25. [Google Scholar] [CrossRef]
- Costa, M.G.; Barbosa, J.C.; Yamamoto, P.T.; Leal, R.M. Spatial distribution of Diaphorina citri Kuwayama (Hemiptera: Psyllidae) in citrus orchards. Scientia Agric. 2010, 67, 546–554. [Google Scholar] [CrossRef] [Green Version]
- Nyrop, J.P.; Binns, M.R. Algorithms for computing operating characteristic and average sample number functions for sequential sampling plans based on binomial count models and revised plans for European red mite (Acari: Tetranychidae) on apple. J. Econ. Entomol. 1992, 85, 1253–1273. [Google Scholar] [CrossRef] [Green Version]
- Warren, W.G.; Chen, P.W. The impact of misspecification of the negative binomial shape parameter in sequential sampling plans. Biometrics 1986, 47, 419–427. [Google Scholar] [CrossRef]
- Hubbard, D.J.; Allen, O.B. Robustness of the SPRT for a negative binomial to misspecification of the dispersion parameter. Biometrics 1991, 47, 419–427. [Google Scholar] [CrossRef]
- Fuentes, A.; Braswell, W.E.; Ruiz-Arce, R.; Racelis, A. Genetic variation and population structure of Diaphoria citri using cytochrome oxidase I sequencing. PLoS ONE 2018, 13, e0198399. [Google Scholar] [CrossRef] [Green Version]
- Ammar, E.-D.; Ramos, J.E.; Hall, D.G.; Dawson, W.O.; Shatters, R.G., Jr. Acquisition, replication and inoculation of Candidatus Liberibacter asiaticus following various acquisition periods on Huanglongbing-infected citrus by nymphs and adults of the Asian citrus psyllid. PLoS ONE 2016, 11, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Monzo, C.; Qureshi, J.A.; Stansly, P.A. Insecticide sprays, natural enemy assemblages and predation on Asian citrus psyllid, Diaphorina citri (Hemiptera: Psyllidae). Bull. Entomol. Res. 2014, 104, 576–585. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Casique-Valdés, R.; Sánchez-Lara, B.M.; Ek-Maas, J.; Hernández-Guerra, C.; Bidochka, M.; Guízar-Guzmán, L.; López-Arroyo, J.I.; Sánchez-Peña, S.R. Field Trial of Aqueous and Emulsion Preparations of Entomopathogenic Fungi Against the Asian Citrus Psyllid (Hemiptera: Liviidae) in a Lime Orchard in Mexico. J. Entomol. Sci. 2015, 50, 79–87. [Google Scholar] [CrossRef] [Green Version]
- Cortez-Mondaca, E.; López-Arroyo, J.I.; Rodríguez-Ruíz, L.; Partida-Valenzuela, M.P.; Pérez-Márquez, J. Especies de Chrysopidae asociadas a Diaphorina citri Kuwayama en cítricos y capacidad de depredación en Sinaloa, México. Rev. Mex. Ciencias Agríc. 2016, 7, 363–374. [Google Scholar]
- Peña-Carrillo, K.; González-Hernández, A.; López-Arroyo, J.I.; Mercado-Hernández, R.; Favela-Lara, S. Mophological and genetic variation in Mexican wild populations of Tamarixia radiata (Hymenoptera: Eulophidae). Fl. Ent. 2015, 98, 1093–1100. [Google Scholar] [CrossRef]
- Singerman, A.; Burani-Arouca, M. Evolution of Citrus Disease Management Programs and Their Economic Implications: The Case of Florida’s Citrus Industry; Department of Food and Resource Economics, UF/IFAS Extension: Gainesville, FL, USA, 2017; 5p. [Google Scholar]
- Fowler, G.W.; Lynch, A.M. Sampling plans in insect pest management based on Wald’s sequential probability ratio test. Environ. Entomol. 1987, 16, 345–354. [Google Scholar] [CrossRef]
- Monzó, C.; Stansly, P.A. Monitoring Asian citrus psyllid populations. Citrus Ind. 2015, 96, 10–12. [Google Scholar]
- Miranda, M.P.; dos Santos, F.L.; Bassanezi, R.B.; Montesino, L.H.; Barbosa, J.C.; Sétamou, M. Monitoring methods for Diaphorina citri Kuwayama (Hemiptera: Liviidae) on citrus groves with different insecticide application programmes. J. Appl. Ento. 2017, 141, 1–8. [Google Scholar] [CrossRef]
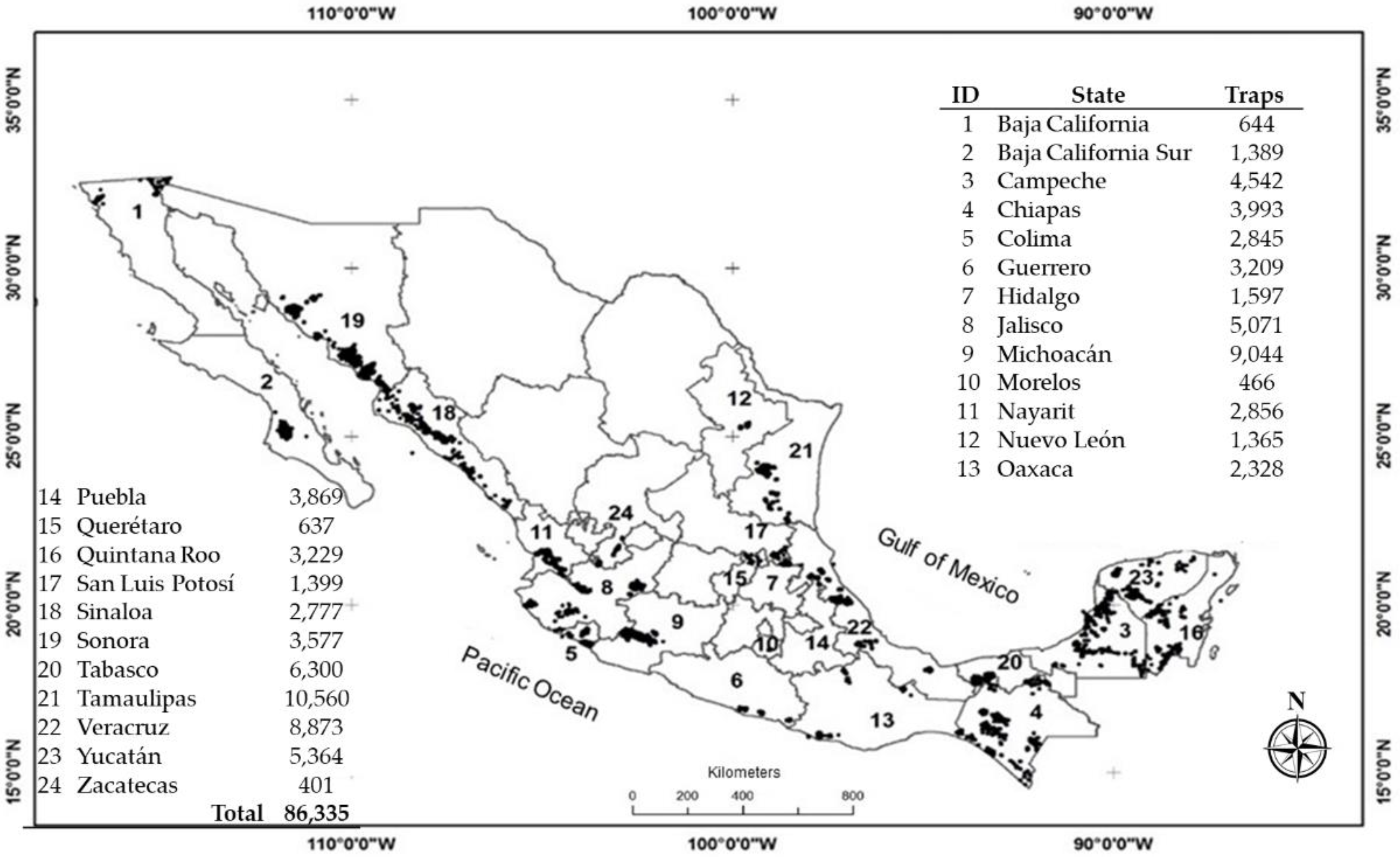

| State | No. Traps | No. Weeks | No. Records | No. Total Adults 1 | DI | AT_GS | ||
|---|---|---|---|---|---|---|---|---|
| Baja California | 644 | 52 | 32,775 | 1027 | 0.031 ± 0.008 | 0.042 | 1.331 | ** |
| Baja California Sur | 1389 | 30 | 41,005 | 28,272 | 0.689 ± 0.107 | 15.052 | 21.832 | ** |
| Campeche | 4542 | 52 | 180,274 | 2677 | 0.015 ± 0.009 | 0.043 | 2.870 | ** |
| Chiapas | 3993 | 52 | 194,974 | 19,081 | 0.098 ± 0.018 | 0.279 | 2.855 | ** |
| Colima | 2845 | 50 | 106,085 | 128,421 | 1.211 ± 0.177 | 19.967 | 16.494 | ** |
| Guerrero | 3209 | 53 | 152,561 | 5757 | 0.038 ± 0.014 | 0.176 | 4.676 | ** |
| Hidalgo | 1597 | 51 | 81,212 | 836 | 0.010 ± 0.004 | 0.014 | 1.389 | ** |
| Jalisco | 5071 | 53 | 204,574 | 49,652 | 0.243 ± 0.049 | 4.780 | 19.694 | ** |
| Michoacán | 9044 | 52 | 216,546 | 233,852 | 1.080 ± 0.113 | 8.438 | 7.813 | ** |
| Morelos | 466 | 48 | 22,436 | 4227 | 0.188 ± 0.030 | 0.526 | 2.790 | ** |
| Nayarit | 2856 | 52 | 118,467 | 11,757 | 0.099 ± 0.031 | 0.607 | 6.112 | ** |
| Nuevo León | 1365 | 48 | 60,043 | 3141 | 0.052 ± 0.016 | 0.161 | 3.075 | ** |
| Oaxaca | 2328 | 52 | 90,101 | 95,541 | 1.060 ± 0.211 | 28.087 | 26.488 | ** |
| Puebla | 3869 | 50 | 85,432 | 23,595 | 0.276 ± 0.043 | 1.054 | 3.818 | ** |
| Querétaro | 637 | 53 | 32,853 | 8123 | 0.247 ± 0.043 | 1.540 | 6.227 | ** |
| Quintana Roo | 3229 | 53 | 162,353 | 1504 | 0.009 ± 0.010 | 0.079 | 8.579 | ** |
| San Luis Potosí | 1399 | 38 | 52,080 | 113 | 0.002 ± 0.003 | 0.007 | 3.210 | ** |
| Sinaloa | 2777 | 52 | 97,207 | 41,230 | 0.424 ± 0.079 | 7.788 | 18.362 | ** |
| Sonora | 3577 | 31 | 24,812 | 10,085 | 0.406 ± 0.089 | 8.707 | 21.421 | ** |
| Tabasco | 6300 | 47 | 269,904 | 32,582 | 0.121 ± 0.034 | 0.681 | 5.642 | ** |
| Tamaulipas | 10,560 | 53 | 421,360 | 37,007 | 0.088 ± 0.026 | 0.482 | 5.490 | ** |
| Veracruz | 8873 | 53 | 450,382 | 127,304 | 0.283 ± 0.070 | 3.427 | 12.124 | ** |
| Yucatán | 5364 | 46 | 149,693 | 3941 | 0.026 ± 0.006 | 0.475 | 18.055 | ** |
| Zacatecas | 401 | 52 | 17,531 | 545 | 0.031 ± 0.009 | 0.044 | 1.406 | ** |
| National | 86,335 | 49 | 3264,660 | 870,270 | 0.280 ± 0.050 | 4.269 | 9.240 | ** |
| State | aa | bb | r2 | SEbc | SEb/b | ||
|---|---|---|---|---|---|---|---|
| Baja California | 0.5440 | 1.0847 | 0.9648 | 0.0296 | 0.0273 | 2.8624 | 0.0031 ** |
| Baja California Sur | 2.2095 | 1.7778 | 0.9068 | 0.1115 | 0.0627 | 6.9733 | 0.0000 ** |
| Campeche | 2.0424 | 1.2839 | 0.8763 | 0.0681 | 0.0530 | 4.1667 | 0.0001 ** |
| Chiapas | 1.6144 | 1.3785 | 0.8024 | 0.0965 | 0.0700 | 3.9225 | 0.0001 ** |
| Colima | 2.5770 | 1.3324 | 0.6721 | 0.1365 | 0.1024 | 2.4352 | 0.0095 ** |
| Guerrero | 2.1404 | 1.3844 | 0.6601 | 0.1398 | 0.1010 | 2.7502 | 0.0042 ** |
| Hidalgo | 1.1907 | 1.2124 | 0.9231 | 0.0500 | 0.0412 | 4.2524 | 0.0000 ** |
| Jalisco | 3.5527 | 2.2087 | 0.7931 | 0.1560 | 0.0706 | 7.7461 | 0.0000 ** |
| Michoacán | 1.3019 | 2.7259 | 0.5847 | 0.3226 | 0.1183 | 5.3493 | 0.0000 ** |
| Morelos | 1.7263 | 1.6068 | 0.8873 | 0.0843 | 0.0525 | 7.1959 | 0.0000 ** |
| Nayarit | 2.8138 | 1.6340 | 0.7615 | 0.1289 | 0.0789 | 4.9175 | 0.0000 ** |
| Nuevo León | 1.6360 | 1.2524 | 0.8683 | 0.0770 | 0.0615 | 3.2793 | 0.0011 ** |
| Oaxaca | 2.7448 | 1.6605 | 0.7968 | 0.1183 | 0.0712 | 5.5840 | 0.0000 ** |
| Puebla | 1.7102 | 1.4875 | 0.8255 | 0.0975 | 0.0655 | 5.0010 | 0.0000 ** |
| Querétaro | 2.2730 | 1.7590 | 0.8859 | 0.0875 | 0.0497 | 8.6788 | 0.0000 ** |
| Quintana Roo | 3.8170 | 1.5144 | 0.9116 | 0.0680 | 0.0449 | 7.5637 | 0.0000 ** |
| San Luís Potosí | 3.2868 | 1.4777 | 0.8302 | 0.1451 | 0.0982 | 3.2917 | 0.0018 ** |
| Sinaloa | 3.6446 | 3.0126 | 0.6704 | 0.2944 | 0.0977 | 6.8362 | 0.0000 ** |
| Sonora | 2.0218 | 1.7323 | 0.3692 | 0.4021 | 0.2321 | 1.8211 | 0.0395 ** |
| Tabasco | 3.1510 | 1.8911 | 0.7734 | 0.1521 | 0.0804 | 5.8592 | 0.0000 ** |
| Tamaulipas | 2.2533 | 1.3330 | 0.8610 | 0.0749 | 0.0562 | 4.4464 | 0.0000 ** |
| Veracruz | 2.8035 | 1.7274 | 0.8537 | 0.1000 | 0.0579 | 7.2752 | 0.0000 ** |
| Yucatán | 2.9285 | 1.6237 | 0.7646 | 0.1354 | 0.0834 | 4.6079 | 0.0000 ** |
| Zacatecas | 0.9577 | 1.2024 | 0.9681 | 0.0309 | 0.0257 | 6.5560 | 0.0000 ** |
| State | ||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Baja California | 0.0740 | 0.1188 | 53.94 | 8.41 | ± | 1.36 | 5.74 | 11.09 | 0.09 | 0.17 | 34.039 | ** | 1.277 | NS |
| B.C. Sur | 0.1100 | 0.1106 | 309.43 | 9.03 | ± | 0.25 | 8.53 | 9.54 | 0.10 | 0.11 | 96.694 | ** | 0.304 | NS |
| Campeche | NaN | NaN | NaN | NaN | ± | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN | |
| Chiapas | 0.0793 | 0.0807 | 1647.55 | 12.38 | ± | 0.28 | 11.86 | 12.93 | 0.07 | 0.08 | 57.531 | ** | 0.367 | NS |
| Colima | 0.0812 | 0.0797 | 818.64 | 12.54 | ± | 0.21 | 12.12 | 12.96 | 0.07 | 0.08 | 247.388 | ** | 14.668 | ** |
| Guerrero | 0.0000 | NaN | NaN | NaN | ± | Inf | NaN | NaN | NaN | NaN | NaN | NaN | NaN | |
| Hidalgo | 0.0407 | 0.0374 | 133.24 | 26.72 | ± | 3.03 | 20.79 | 32.66 | 0.03 | 0.04 | 27.467 | ** | 0.044 | NS |
| Jalisco | 0.0331 | 0.0334 | 8792.10 | 29.90 | ± | 0.62 | 28.61 | 31.12 | 0.03 | 0.03 | 13.471 | ** | 0.983 | NS |
| Michoacán | 0.2397 | 0.2253 | 22939.04 | 4.43 | ± | 0.03 | 4.36 | 4.50 | 0.22 | 0.22 | 34.668 | ** | 3.528 | NS |
| Morelos | 0.1878 | 0.1584 | 376.78 | 6.31 | ± | 0.26 | 5.79 | 6.83 | 0.14 | 0.17 | 69.659 | ** | 2.530 | NS |
| Nayarit | 0.0269 | 0.0268 | 728.81 | 37.29 | ± | 1.29 | 34.75 | 39.83 | 0.02 | 0.02 | 54.612 | ** | 0.125 | NS |
| Nuevo León | 0.0252 | 0.0331 | 100.25 | 30.16 | ± | 2.46 | 25.34 | 34.99 | 0.02 | 0.03 | 60.623 | ** | 2.487 | NS |
| Oaxaca | 0.0463 | 0.0490 | 1782.34 | 20.39 | ± | 0.53 | 19.35 | 21.43 | 0.04 | 0.05 | 41.759 | ** | 2.272 | NS |
| Puebla | 0.1131 | 0.1173 | 2119.12 | 8.51 | ± | 0.20 | 8.12 | 8.91 | 0.11 | 0.12 | 39.503 | ** | 0.013 | NS |
| Querétaro | 0.0995 | 0.0915 | 560.69 | 10.92 | ± | 0.40 | 10.12 | 11.72 | 0.08 | 0.09 | 65.974 | ** | 1.202 | NS |
| Quintana Roo | NaN | NaN | NaN | NaN | ± | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN | |
| San Luis Potosí | NaN | NaN | NaN | NaN | ± | NaN | NaN | NaN | NaN | NaN | NaN | NaN | NaN | |
| Sinaloa | 0.0385 | 0.0420 | 12,765.02 | 23.78 | ± | 0.67 | 22.44 | 25.11 | 0.04 | 0.04 | 4.899 | ** | 2.012 | NS |
| Sonora | 0.0505 | 0.0183 | 4768.86 | 54.59 | ± | 0.94 | 52.75 | 56.44 | 0.01 | 0.01 | 19.796 | ** | 0.025 | NS |
| Tabasco | 0.0405 | 0.0376 | 3317.34 | 26.53 | ± | 0.47 | 25.60 | 27.46 | 0.03 | 0.03 | 41.196 | ** | 1.080 | NS |
| Tamaulipas | 0.0184 | 0.0213 | 1376.50 | 46.87 | ± | 1.16 | 44.59 | 49.15 | 0.02 | 0.02 | 61.775 | ** | 3.410 | NS |
| Veracruz | 0.0374 | 0.0401 | 6239.36 | 24.88 | ± | 0.36 | 24.17 | 25.59 | 0.03 | 0.04 | 37.915 | ** | 1.590 | NS |
| Yucatán | 0.0110 | 0.0100 | 8503.92 | 99.40 | ± | 5.02 | 89.55 | 109.24 | 0.01 | 0.01 | 1.953 | NS | 0.392 | NS |
| Zacatecas | 0.0894 | 0.1033 | 22.72 | 9.68 | ± | 1.67 | 6.39 | 12.96 | 0.07 | 0.15 | 70.929 | ** | 0.427 | NS |
| State | Stop Lines | MSS | N = 1 | OC | ASN |
|---|---|---|---|---|---|
| Baja California | d = ±1.3512 + 0.0644x | 21 | 1.4156 | 0.83 | 23 |
| Baja California Sur | d = ±1.3796 + 0.0640x | 22 | 1.4436 | 0.85 | 24 |
| Chiapas | d = ±1.5435 + 0.0618x | 25 | 1.6053 | 0.90 | 28 |
| Hidalgo | d = ±2.1989 + 0.0567x | 39 | 2.2556 | 0.98 | 43 |
| Jalisco | d = ±2.3395 + 0.0560x | 42 | 2.3955 | 0.98 | 47 |
| Michoacán | d = ±1.1396 + 0.0685x | 17 | 1.2081 | 0.65 | 17 |
| Morelos | d = ±1.2401 + 0.0663x | 19 | 1.3064 | 0.76 | 20 |
| Nayarit | d = ±2.6591 + 0.0547x | 49 | 2.7138 | 0.99 | 54 |
| Nuevo León | d = ±2.3686 + 0.0549x | 44 | 2.4235 | 0.90 | 47 |
| Oaxaca | d = ±1.9161 + 0.0584x | 33 | 1.9745 | 0.96 | 37 |
| Puebla | d = ±1.3537 + 0.0644x | 22 | 1.4181 | 0.83 | 23 |
| Querétaro | d = ±1.4728 + 0.0627x | 24 | 1.5355 | 0.88 | 26 |
| Sinaloa | d = ±2.0689 + 0.0574x | 37 | 2.1263 | 0.97 | 40 |
| Sonora | d = ±3.3954 + 0.0528x | 65 | 3.4482 | 1.0 | 69 |
| Tabasco | d = ±2.1926 + 0.0567x | 39 | 2.2493 | 0.98 | 43 |
| Tamaulipas | d = ±3.0703 + 0.0535x | 58 | 3.1238 | 1.0 | 62 |
| Veracruz | d = ±2.1192 + 0.0571x | 38 | 2.1763 | 0.97 | 42 |
| Zacatecas | d = ±1.4114 + 0.0635x | 23 | 1.4749 | 0.86 | 25 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Díaz-Padilla, G.; López-Arroyo, J.I.; Guajardo-Panes, R.A.; Sánchez-Cohen, I. Spatial Distribution and Development of Sequential Sampling Plans for Diaphorina citri Kuwayama (Hemiptera: Liviidae). Agronomy 2021, 11, 1434. https://doi.org/10.3390/agronomy11071434
Díaz-Padilla G, López-Arroyo JI, Guajardo-Panes RA, Sánchez-Cohen I. Spatial Distribution and Development of Sequential Sampling Plans for Diaphorina citri Kuwayama (Hemiptera: Liviidae). Agronomy. 2021; 11(7):1434. https://doi.org/10.3390/agronomy11071434
Chicago/Turabian StyleDíaz-Padilla, Gabriel, J. Isabel López-Arroyo, Rafael A. Guajardo-Panes, and Ignacio Sánchez-Cohen. 2021. "Spatial Distribution and Development of Sequential Sampling Plans for Diaphorina citri Kuwayama (Hemiptera: Liviidae)" Agronomy 11, no. 7: 1434. https://doi.org/10.3390/agronomy11071434
APA StyleDíaz-Padilla, G., López-Arroyo, J. I., Guajardo-Panes, R. A., & Sánchez-Cohen, I. (2021). Spatial Distribution and Development of Sequential Sampling Plans for Diaphorina citri Kuwayama (Hemiptera: Liviidae). Agronomy, 11(7), 1434. https://doi.org/10.3390/agronomy11071434





