Exogenous Application of Thiourea for Improving the Productivity and Nutritional Quality of Bread Wheat (Triticum aestivum L.)
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Weather Condition
2.2. Experimental Design and Treatments
2.3. Crop Husbandry
2.4. Observations Recorded
2.4.1. Morphological and Yield Related
2.4.2. Nutritional Quality
2.5. Statistical Analysis
3. Results
3.1. Morphological Traits
3.2. Yield Related Traits
3.3. Nutritional Quality Traits
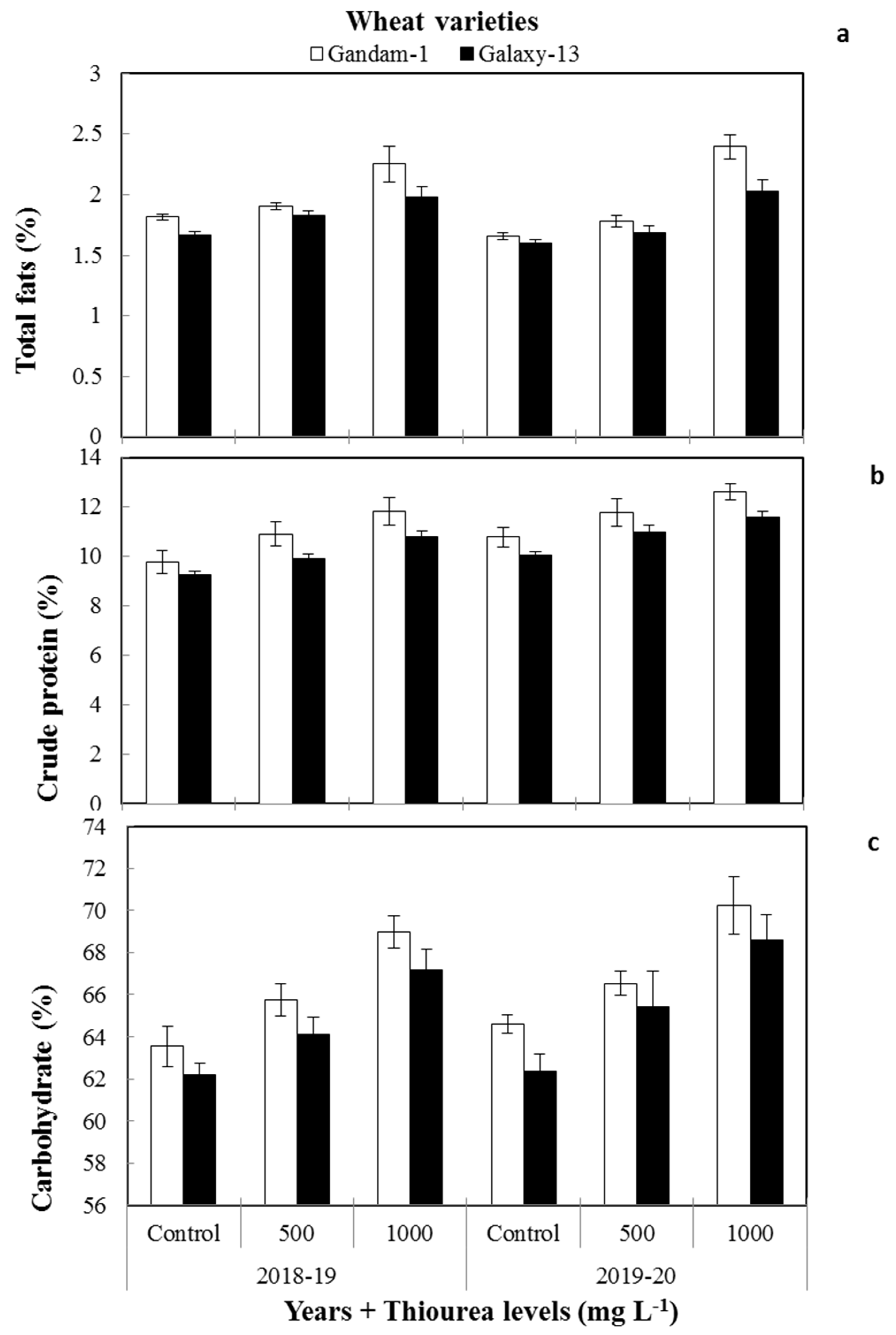
3.3.1. Total Fats
3.3.2. Crude Protein
3.3.3. Carbohydrate
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tahir, S.; Ahmad, A.; Khaliq, T.; Cheema, M.J. Evaluating the impact of seed rate and sowing dates on wheat productivity in semi-arid environment. Int. J. Agric. Biol. 2019, 22, 57–64. [Google Scholar]
- Gbegbelegbe, S.; Cammarano, D.; Asseng, S.; Robertson, R.; Chung, U.; Adam, M.; Shiferaw, B. Baseline simulation for global wheat production with CIMMYT mega-environment specific cultivars. Field Crops Res. 2017, 202, 122–135. [Google Scholar] [CrossRef]
- Zampieri, M.; Ceglar, A.; Dentener, F.; Toreti, A. Wheat yield loss attributable to heat waves, drought and water excess at the global, national and subnational scales. Environ. Res. Lett. 2017, 12, 064008. [Google Scholar] [CrossRef]
- Ahmad, M.J.; Iqbal, M.A.; Choi, K.S. Climate-driven constraints in sustaining future wheat yield and water productivity. Agric. Water Manag. 2020, 231, 105991. [Google Scholar] [CrossRef]
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2020. Available online: http://www.fao.org/faostat/en (accessed on 22 May 2021).
- FAOSTAT. Food and Agriculture Organization of the United Nations. 2018. Available online: http://www.fao.org/faostat/en (accessed on 22 May 2021).
- Yu, S.; Tian, L. Breeding major cereal grains through the lens of nutrition sensitivity. Mol. Plant 2018, 11, 23–30. [Google Scholar] [CrossRef]
- Abid, M. Physiological and biochemical changes during drought and recovery periods at tillering and jointing stages in wheat (Triticum aestivum L.). Sci. Rep. 2019, 8, 4615. [Google Scholar] [CrossRef] [PubMed]
- Shah, F.; Wu, W. Soil and crop management strategies to ensure higher crop productivity within sustainable environments. Sustainability 2019, 11, 1485. [Google Scholar] [CrossRef]
- Giraldo, P.; Benavente, E.; Manzano-Agugliaro, F.; Gimenez, E. Worldwide research trends on wheat and barley: A bibliometric comparative analysis. Agronomy 2019, 9, 352. [Google Scholar] [CrossRef]
- Bhatt, R.; Kukal, S.S.; Busari, M.A.; Arora, S.; Yadav, M. Sustainability issues on rice–wheat cropping system. Int. Soil Water Conserv. Res. 2016, 4, 64–74. [Google Scholar] [CrossRef]
- Krupnik, T.J.; Ahmed, Z.U.; Timsina, J.; Shahjahan, M.; Kurishi, A.A.; Miah, A.A.; McDonald, A.J. Forgoing the fallow in Bangladesh’s stress-prone coastal deltaic environments: Effect of sowing date, nitrogen, and genotype on wheat yield in farmers’ fields. Field Crops Res. 2015, 170, 7–20. [Google Scholar] [CrossRef]
- Anwar, S.; Ali, W.; Imanullah, K.; Islam, M.; Bashir, S.; Shafi, M.; Bakht, J. Effect of sowing dates and seed rates on the agro-physiological traits of wheat. Int. J. Environ. Sci. 2015, 5, 135–140. [Google Scholar]
- Khattak, S.I.; Nadim, M.A.; Baloch, M.S.; Waseem, K.; Sohail, M. Impact of Variable Seeding Dates on Cereals ‘performance under Agro-Ecological Conditions Of Dera Ismail Khan. Pak. J. Agric. Res. 2016, 29. [Google Scholar]
- Humphreys, E.; Gaydon, D.S.; Eberbach, P.L. Evaluation of the effects of mulch on optimum sowing date and irrigation management of zero till wheat in central Punjab, India using APSIM. Field Crops Res. 2016, 197, 83–96. [Google Scholar]
- Humphreys, E.; Gaydon, D.S. Options for increasing the productivity of the rice–wheat system of North West India while reducing groundwater depletion. Part 2. Is conservation agriculture the answer? Field Crops Res. 2015, 173, 81–94. [Google Scholar]
- Mottaleb, K.A.; Singh, P.K.; Sonder, K.; Kruseman, G.; Tiwari, T.P.; Barma, N.C.; Erenstein, O. Threat of wheat blast to South Asia’s food security: An ex-ante analysis. PLoS ONE 2018, 13, e0197555. [Google Scholar] [CrossRef] [PubMed]
- Kajla, M.; Yadav, V.K.; Chhokar, R.S.; Sharma, R.K. Management practices to mitigate the impact of high temperature on wheat. J. Wheat Res. 2015, 7, 1–12. [Google Scholar]
- Zain, M.; Khan, I.; Chattha, M.U.; Qadri RW, K.; Anjum, S.A.; Hassan, M.U.; Ilyas, M. Foliar applied thiourea at different growth stages modulated late sown wheat. Pak. J. Sci. 2017, 69, 39. [Google Scholar]
- Hassanein, R.A.; Amin AA, E.; Rashad ES, M.; Ali, H. Effect of thiourea and salicylic acid on antioxidant defense of wheat plants under drought stress. Int. J. ChemTech Res. 2015, 7, 346–354. [Google Scholar]
- Seleiman, M.F.; Kheir, A.M. Saline soil properties, quality and productivity of wheat grown with bagasse ash and thiourea in different climatic zones. Chemosphere 2018, 193, 538–546. [Google Scholar] [CrossRef]
- Amin, A.A.; El-Kader, A.A.A.; Abouziena, H.F.; El-Awadi, M.; Gharib, F.A. Effects of benzoic acid and thiourea on growth and productivity of wheat (Triticum aestivum L.) plants. Int. J. Sci. Res. 2016, 72, 1032–1037. [Google Scholar]
- Kaya, C.; Sönmez, O.; Ashraf, M.; Polat, T.; Tuna, L.; Aydemir, S. Exogenous application of nitric oxide and thiourea regulates on growth and some key physiological processes in maize (Zea mays L.) plants under saline stress. Toprak Su Dergisi 2015, 61–66. [Google Scholar] [CrossRef][Green Version]
- Kaya, C.; Ashraf, M.; Sönmez, O. Promotive effect of exogenously applied thiourea on key physiological parametersand oxidative defense mechanism in salt-stressed Zea mays L. plants. Turk. J. Bot. 2015, 39, 786–795. [Google Scholar] [CrossRef]
- Hafez, E.; Farig, M. Efficacy of salicylic acid as a cofactor for ameliorating effects of water stress and enhancing wheat yield and water use efficiency in saline soil. Int. J. Plant Prod. 2019, 13, 163–176. [Google Scholar] [CrossRef]
- Hafez, E.M. Influence of salicylic acid onion distribution, enzymatic activity and some agro morphological characteristics of wheat under salt-affected soil. Egypt. J. Agron. 2016, 38, 455–469. [Google Scholar] [CrossRef]
- Arif, Y.; Sami, F.; Siddiqui, H.; Bajguz, A.; Hayat, S. Salicylic acid in relation to other phytohormones in plant: A study towards physiology and signal transduction under challenging environment. Environ. Exp. Bot. 2020, 175, 104040. [Google Scholar] [CrossRef]
- Boukari, N.; Jelali, N.; Renaud, J.B.; Youssef, R.B.; Abdelly, C.; Hannoufa, A. Salicylic acid seed priming improves tolerance to salinity, iron deficiency and their combined effect in two ecotypes of alfalfa. Environ. Exp. Bot. 2019, 167, 103820. [Google Scholar] [CrossRef]
- Askari, E.; Ehsanzadeh, P. Drought stress mitigation by foliar application of salicylic acid and their interactive effects on physiological characteristics of fennel (Foeniculum vulgare Mill.) genotypes. Acta Physiol. Plant. 2015, 37, 4. [Google Scholar] [CrossRef]
- Jackson, M.L. Soil Chemical Analysis; Prentice Hall of India Pvt. Ltd.: New Delhi, India, 1973; pp. 10–144. [Google Scholar]
- Anon. Approved Methods of American Association of Cereal Chemists; American Association Cereal Chemistry Inc.: St. Paul, MN, USA, 2000. [Google Scholar]
- Official Method of Analysis, 17th ed.; Association of Official Analytical Chemist (AOAC): Arlington, VA, USA, 2002.
- Steel, R.G.D.; Torrie, J.H.; Dickey, D.A. Principles and Procedures of Statistics: A Biometrical Approach, 3rd ed.; McGraw Hill Book Co. Inc.: New York, NY, USA, 1997; pp. 400–428. [Google Scholar]
- Premaradhya, N.; Shashidhar, K.S.; Samuel Jeberson, R.K. Effect and profitability of foliar application of thiourea on growth and yield attributes of lentil (Lens culinaris L.) under Manipur Conditions of North-East, India. Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1040–1050. [Google Scholar] [CrossRef]
- Khanna, P.; Kaur, K.; Gupta, A.K. Root biomass partitioning, differential antioxidant system and thiourea spray are responsible for heat tolerance in spring maize. Proc. Natl. Acad. Sci. USA India Sect. B Biol. Sci. 2017, 87, 351–359. [Google Scholar] [CrossRef]
- Sharma, K.M.; Asarey, R.; Verma, H. Response of wheat (Triticum aestivum L.) to the foliar applied brassinosteroid and thiourea with recommended fertilization practice on farmer’s fields. Plant Arch. 2015, 15, 729–732. [Google Scholar]
- Dadhich, R.K.; Meena, R.S. Performance of Indian mustard (Brassica juncea L.) in response to foliar spray of thiourea and thioglycollic acid under different irrigation levels. Indian J. Ecol. 2014, 41, 376–378. [Google Scholar]
- Asthir, B.; Thapar, R.; Bains, N.S.; Farooq, M. Biochemical responses of thiourea in ameliorating high temperature stress by enhancing antioxidant defense system in wheat. Russ. J. Plant Physiol. 2015, 62, 875–882. [Google Scholar] [CrossRef]
- Perveen, S.; Farooq, R.; Shahbaz, M. Thiourea-induced metabolic changes in two mung bean [Vigna radiata (L.) Wilczek] (Fabaceae) varieties under salt stress. Braz. J. Bot. 2016, 39, 41–54. [Google Scholar] [CrossRef]
- Akladious, S.A. Influence of thiourea application on some physiological and molecular criteria of sunflower (Helianthus annuus L.) plants under conditions of heat stress. Protoplasma 2014, 251, 625–638. [Google Scholar] [CrossRef]
- Asthir, B.; Kaur, R.; Bains, N.S. Variation of invertase activities in four wheat cultivars as influenced by thiourea and high temperature. Acta Physiol. Plant. 2015, 37, 1712. [Google Scholar] [CrossRef]
- Srivastava, A.K.; Sablok, G.; Hackenberg, M.; Deshpande, U.; Suprasanna, P. Thiourea priming enhances salt tolerance through co-ordinated regulation of microRNAs and hormones in Brassica juncea. Sci. Rep. 2017, 7, 45490. [Google Scholar] [CrossRef]
- Belwal, T.; Bisht, A.; Bhatt, I.D.; Rawal, R.S. Influence of seed priming and storage time on germination and enzymatic activity of selected Berberis species. Plant Growth Regul. 2015, 77, 189–199. [Google Scholar] [CrossRef]
- Chattha, M.B.; Khan, I.; Mahmood, A.; Chattha, M.U.; Anjum, S.A.; Ashraf, U.; Bilal, U. Seed priming with thiourea improves the performance of late sown wheat. J. Agric. Res 2017, 55, 29–39. [Google Scholar]
- Kumar, A.; Hemantaranjan, A.; Kumari, V.; Chaubey, R.K. Assessment of the impact of thiourea on biochemical and physiological characteristics of wheat under heat stress. J. Pharmacogn. Phytochem. 2020, 9, 434–441. [Google Scholar]
- Ahmad, M.; Waraich, E.A.; Tanveer, A.; Anwar-ul-Haq, M. Foliar Applied Thiourea Improved Physiological Traits and Yield of Camelina and Canola Under Normal and Heat Stress Conditions. J. Soil Sci. Plant Nutr. 2021, 21, 1666–1678. [Google Scholar] [CrossRef]
- Dreccer, M.F.; Fainges, J.; Whish, J.; Ogbonnaya, F.C.; Sadras, V.O. Comparison of sensitive stages of wheat, barley, canola, chickpea and field pea to temperature and water stress across Australia. Agric. For. Meteorol. 2018, 248, 275–294. [Google Scholar] [CrossRef]
- Sanaullah, T.; Wahid, A.; Javed, F.; Sadia, B. Optimization of thiourea level at cellular and whole plant level for maize hybrids (Zea mays L.). Appl. Ecol. Environ. Res. 2009, 14, 1–18. [Google Scholar] [CrossRef]
- Parveen, A.; Hussain, I.; Rasheed, R.; Mahmood, S.; Wahid, A. Potential of Thiourea in Modifying Membrane Stability, Osmoprotectants, Vitamins and Antioxidants Levels under Cadmium Stress in Maize. Int. J. Agric. Biol. 2018, 20, 2862–2870. [Google Scholar]
- Xalxo, R.; Keshavkant, S. Melatonin, glutathione and thiourea attenuates lead and acid rain-induced deleterious responses by regulating gene expression of antioxidants in Trigonella foenum graecum L. Chemosphere 2019, 221, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kasajima, S.; Araki, H. Improvement of yield performance by examining the morphological aspects of a leading winter wheat variety, ‘Kitahonami’, in Hokkaido, the northernmost region of Japan. Plant Prod. Sci. 2020, 23, 226–233. [Google Scholar] [CrossRef]
- Thapa, S.; Ghimire, A.; Adhikari, J.; Thapa, A.; Thapa, B. Impacts of sowing and climatic conditions on wheat yield in Nepal. Malays. J. Halal Res. 2020, 3, 38–40. [Google Scholar] [CrossRef]
- Marasini, D.; Marahatta, S.; Dhungana, S.M.; Acharya, R. Effect of Date of Sowing on Yield and Yield Attributes of Different Wheat Varieties under Conventional Tillage in Sub-Humid Condition of Chitwan District of Nepal. Int. J. Appl. Sci. Biotechnol. 2016, 4, 27–31. [Google Scholar] [CrossRef]
- Acharya, R.; Marahatta, S.; Amgain, L.P. Response of Wheat Cultivars in Different Agricultural Practices Differed by Sowing Date. Int. J. Appl. Sci. Biotechnol. 2017, 5, 250–255. [Google Scholar] [CrossRef]
- Oakes, J.; Heiniger, R.; Crozier, C.; Murphy, J.; Wilkerson, G. Phyllochron interval and yield response to planting date and fertility in wheat. Crop Forage Turfgrass Manag. 2016, 2, 1–9. [Google Scholar] [CrossRef]
- Atayde, R.J. The Effect of Low Temperature during Seed Development on Flowering in Wheat (Triticum aestivum L.). Honours Dissertation, Charles Sturt University, Bathurst, NSW, Australia, 2019. [Google Scholar]
- Flohr, B.M.; Hunt, J.R.; Kirkegaard, J.A.; Evans, J.R. Water and temperature stress define the optimal flowering period for wheat in south-eastern Australia. Field Crops Res. 2017, 209, 108–119. [Google Scholar] [CrossRef]
- Feng, L.; Wang, G.; Han, Y.; Li, Y.; Zhu, Y.; Zhou, Z.; Cao, W. Effects of planting pattern on growth and yield and economic benefits of cotton in a wheat-cotton double cropping system versus monoculture cotton. Field Crops Res. 2017, 213, 100–108. [Google Scholar] [CrossRef]

| Determinant | Determination Method | Values | Units |
|---|---|---|---|
| Texture of soil | - | Sandy loam | |
| Sand | - | 60 | % |
| Clay | - | 10 | % |
| Silt | - | 30 | % |
| Electrical conductivity | saturation extract method | 1.49 | dSm−1 |
| pH | glass electrode making a 1:2.5 soil-water suspension | 8.2 | - |
| Nitrogen | Bremner and Mulvaney, 1982 | 0.4 | % |
| Available phosphorus | Olsen and Watanabe, 1957 | 3 | ppm |
| Available potassium | flame photometry | 96 | ppm |
| Wheat Varieties | Plant Height (cm) | Number of Leaves/Plant | Number of Tillers/Plant | Leaf Area (cm2) | ||||
|---|---|---|---|---|---|---|---|---|
| 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | |
| Gandam-1 | 75.9 | 74.8 | 12.1 | 11.6 | 3.3 B | 8.2 | 18.1 | 17.8 |
| Galaxy-13 | 76.9 | 77.3 | 11.3 | 11.8 | 4.2 A | 8.3 | 18.3 | 18.8 |
| LSD (p ≤ 0.05) | ns | ns | ns | ns | 0.08 | ns | ns | ns |
| Thiourea level (mg L−1) | ||||||||
| Control | 71.3 C | 72.3 C | 11.1 | 10.4 C | 4.0 C | 6.3 B | 17.1 C | 16.3 C |
| 500 | 77.2 B | 76.3 B | 11.1 | 11.6 B | 5.8 B | 4.1 C | 18.0 B | 18.0 B |
| 1000 | 80.8 A | 79.6 A | 13.1 | 13.2 A | 6.1 A | 8.1 A | 19.5 A | 20.5 A |
| LSD (p ≤ 0.05) | 3.35 | 2.93 | ns | 0.55 | 0.10 | 0.06 | 0.55 | 0.93 |
| Growth stages | ||||||||
| Tillering | 73.7 B | 72.4 C | 11.3 | 11.3 | 6.7 A | 8.3 A | 17.5 | 18.6 |
| Booting | 78.8 A | 79.2 A | 12.4 | 12.0 | 4.7 B | 5.0 C | 18.1 | 18.4 |
| Heading | 76.5 A | 75.9 B | 11.4 | 11.8 | 4.6 B | 6.1 B | 19.2 | 17.2 |
| LSD (p ≤ 0.05) | 2.83 | 3.23 | ns | ns | 0.12 | 0.04 | ns | ns |
| Wheat Varieties | Number of Spikelets/Spike | Number of Grains/Spike | 1000-Grain Weight (g) | Biological Yield (t ha−1) | Seed Yield (t ha−1) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | 2018–2019 | 2019–2020 | |
| Gandam-1 | 20.5 | 31.4 | 29.3 B | 30.2 | 33.5 B | 35.0 | 3.42 | 3.02 | 1.95 | 1.99 |
| Galaxy-13 | 20.6 | 27.8 | 31.5 A | 30.6 | 34.7 A | 35.4 | 3.61 | 3.37 | 2.14 | 2.10 |
| LSD (p ≤ 0.05) | ns | ns | 1.86 | ns | 0.68 | ns | ns | ns | ns | ns |
| Thiourea level (mg L−1) | ||||||||||
| Control | 19.9 | 19.0 | 27.9 B | 29.9 | 33.8 B | 33.7 B | 3.70 C | 3.36 C | 1.69 C | 2.07 C |
| 500 | 20.2 | 32.3 | 31.0 A | 30.4 | 33.7 B | 34.8 B | 3.86 B | 3.93 B | 2.07 B | 2.18 B |
| 1000 | 21.5 | 37.5 | 33.2 A | 31.0 | 34.7 A | 37.2 A | 3.98 A | 4.10 A | 2.37 A | 2.32 A |
| LSD (p ≤ 0.05) | ns | ns | 1.46 | ns | 0.83 | 1.47 | 0.09 | 0.15 | 0.20 | 0.10 |
| Growth stages | ||||||||||
| Tillering | 21 | 28.5 | 29.6 B | 27.2 C | 34.0 | 35.5 | 3.34 B | 3.39 C | 1.91 B | 1.97 C |
| Booting | 20.5 | 21.8 | 30.4 AB | 31.9 B | 34.2 | 34.8 | 3.67 B | 3.59 B | 2.02 B | 2.11 B |
| Heading | 20.1 | 38.0 | 31.2 A | 33.2 A | 34.1 | 35.3 | 3.95 A | 3.72 A | 2.17 A | 2.23 A |
| LSD (p ≤ 0.05) | ns | ns | 0.96 | 1.20 | ns | ns | 0.25 | 0.12 | 0.10 | 0.11 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sher, A.; Wang, X.; Sattar, A.; Ijaz, M.; Ul-Allah, S.; Nasrullah, M.; Bibi, Y.; Manaf, A.; Fiaz, S.; Qayyum, A. Exogenous Application of Thiourea for Improving the Productivity and Nutritional Quality of Bread Wheat (Triticum aestivum L.). Agronomy 2021, 11, 1432. https://doi.org/10.3390/agronomy11071432
Sher A, Wang X, Sattar A, Ijaz M, Ul-Allah S, Nasrullah M, Bibi Y, Manaf A, Fiaz S, Qayyum A. Exogenous Application of Thiourea for Improving the Productivity and Nutritional Quality of Bread Wheat (Triticum aestivum L.). Agronomy. 2021; 11(7):1432. https://doi.org/10.3390/agronomy11071432
Chicago/Turabian StyleSher, Ahmad, Xiukang Wang, Abdul Sattar, Muhammad Ijaz, Sami Ul-Allah, Muhammad Nasrullah, Yamin Bibi, Abdul Manaf, Sajid Fiaz, and Abdul Qayyum. 2021. "Exogenous Application of Thiourea for Improving the Productivity and Nutritional Quality of Bread Wheat (Triticum aestivum L.)" Agronomy 11, no. 7: 1432. https://doi.org/10.3390/agronomy11071432
APA StyleSher, A., Wang, X., Sattar, A., Ijaz, M., Ul-Allah, S., Nasrullah, M., Bibi, Y., Manaf, A., Fiaz, S., & Qayyum, A. (2021). Exogenous Application of Thiourea for Improving the Productivity and Nutritional Quality of Bread Wheat (Triticum aestivum L.). Agronomy, 11(7), 1432. https://doi.org/10.3390/agronomy11071432







