Nitrogen Immobilisation and Microbial Biomass Build-Up Induced by Miscanthus x giganteus L. Based Fertilisers
Abstract
:1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant and Soil Analyses
2.3. Statistical Analyses
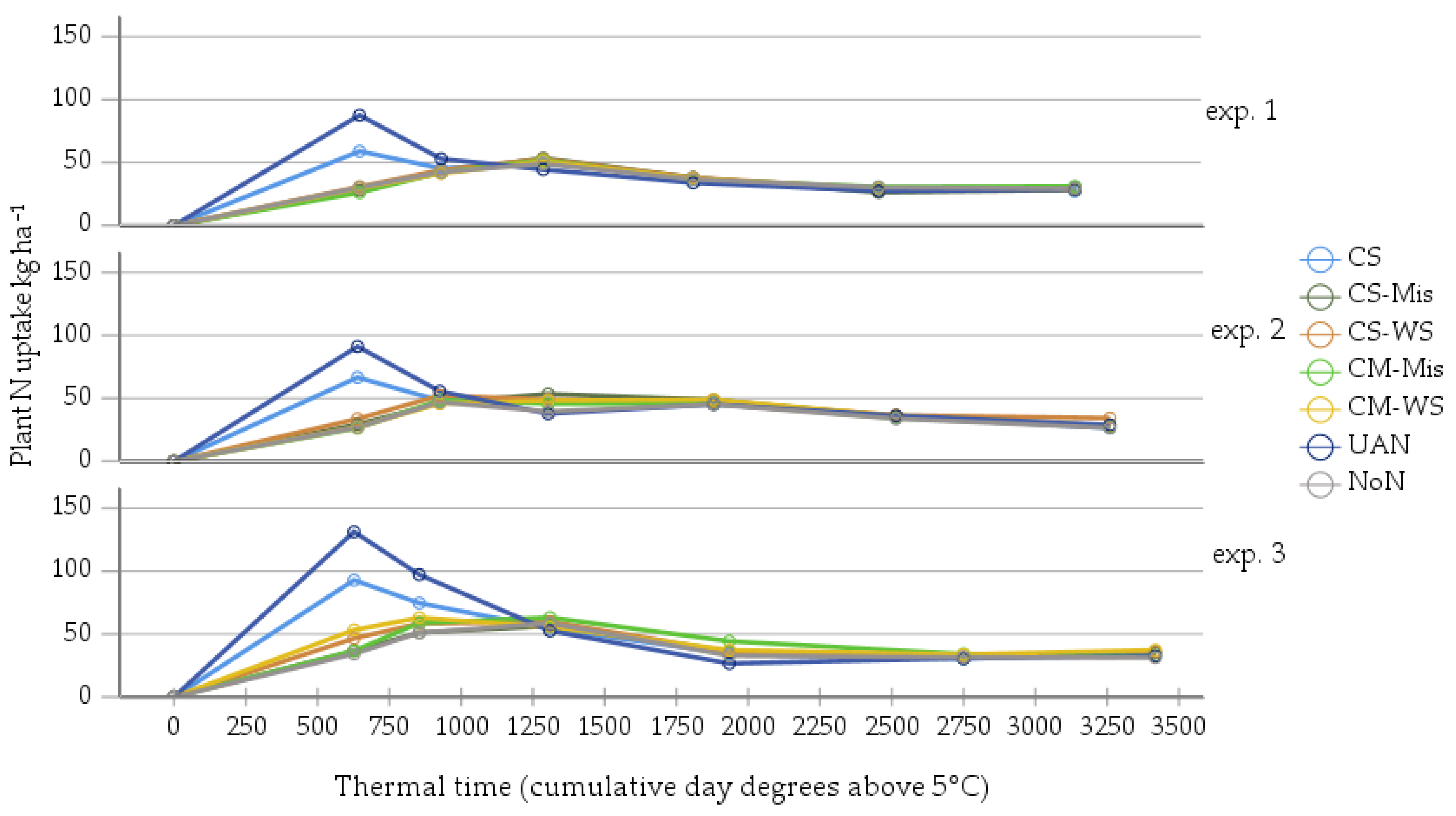
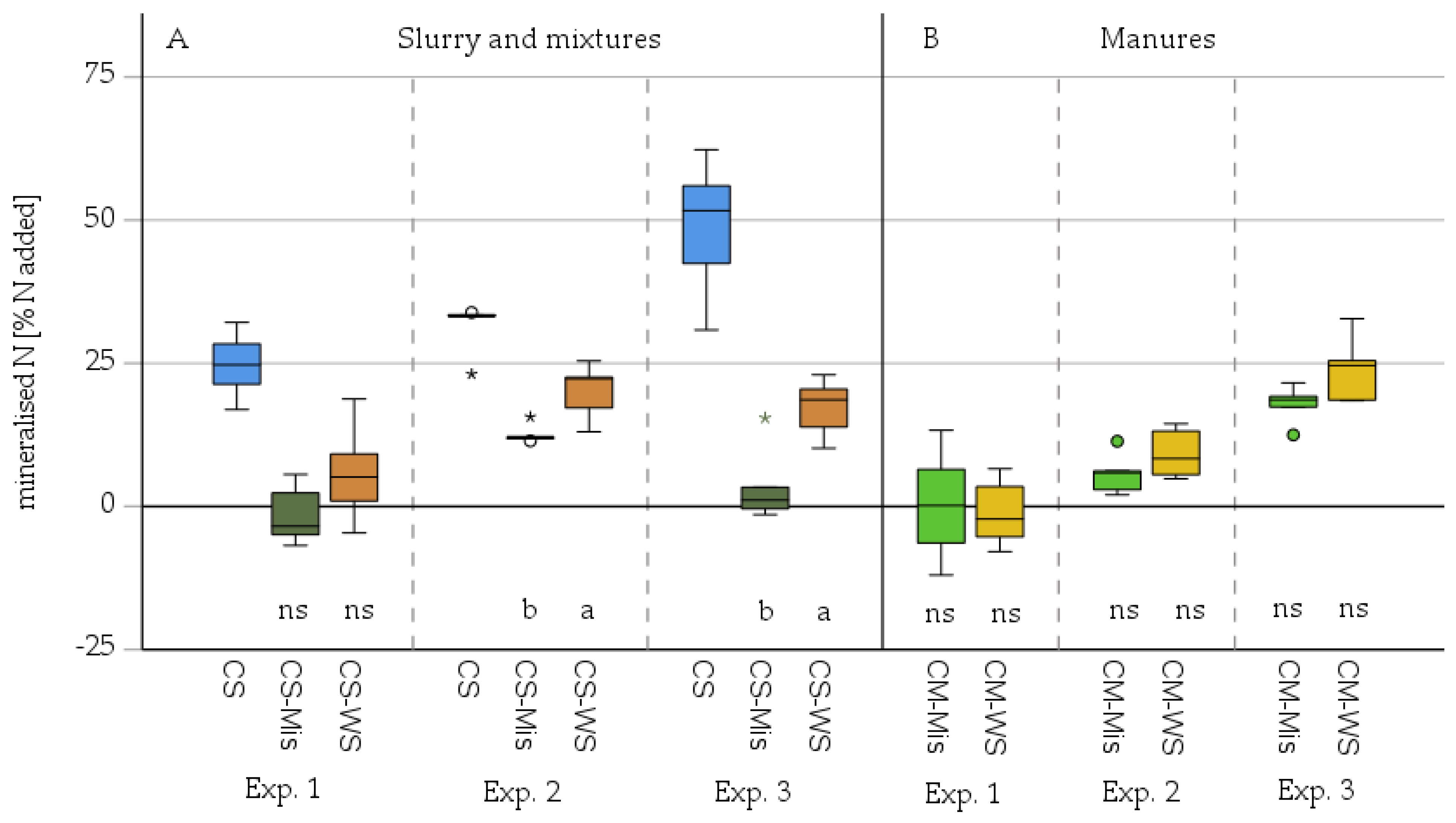
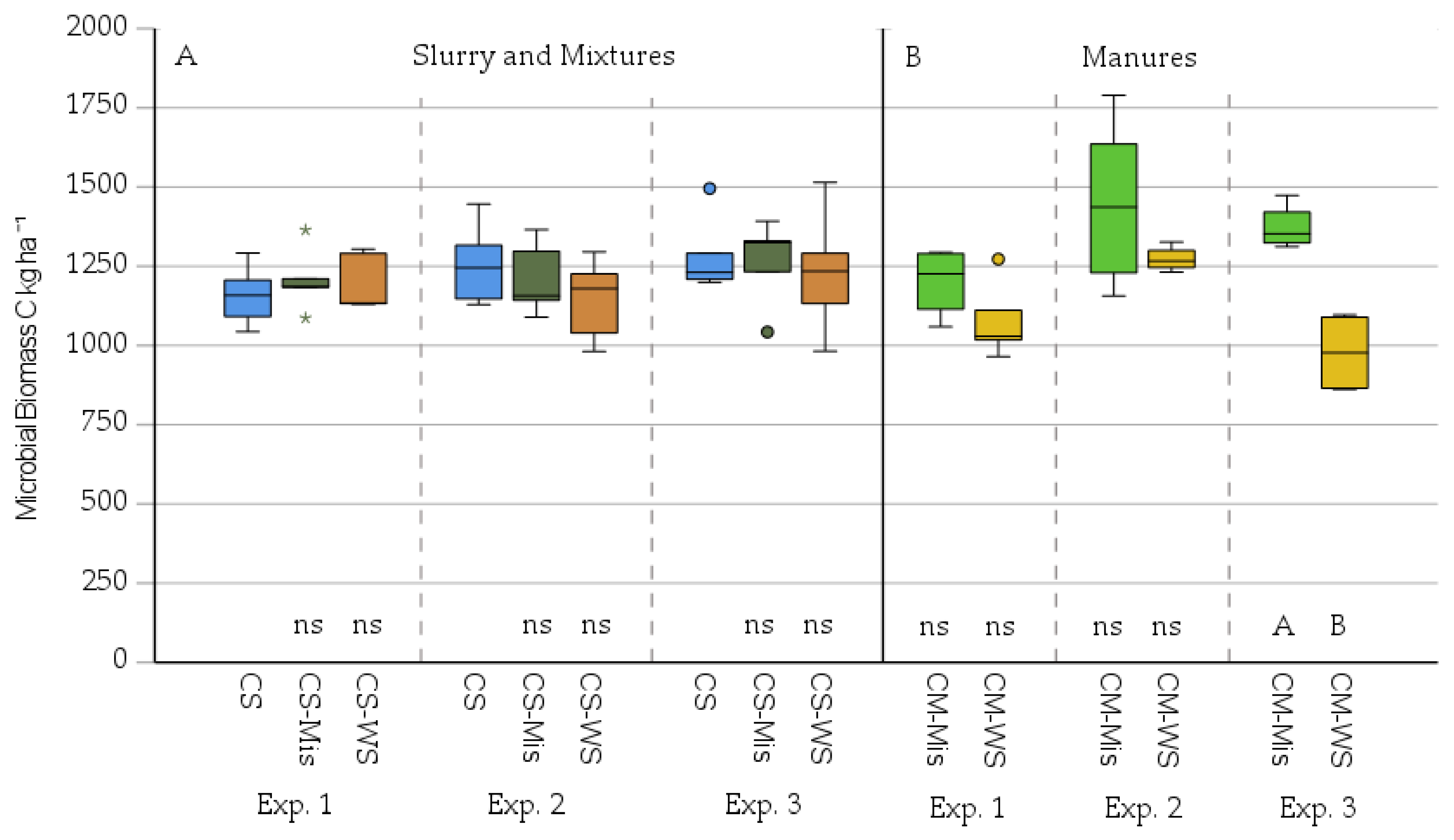
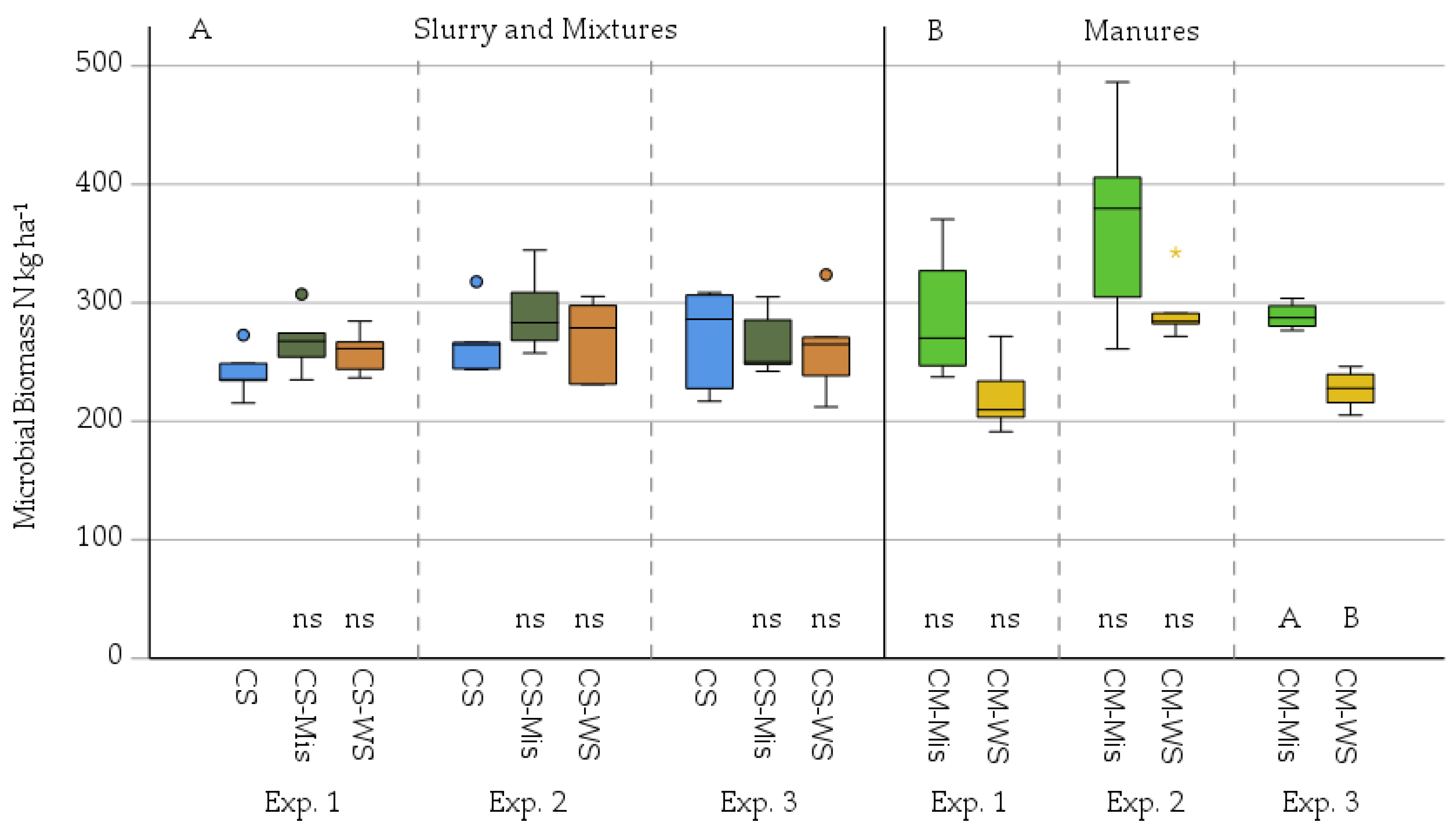
3. Results
3.1. Plant N-Uptake
3.2. Microbial Mineralisation-Immobilisation as Affected by Added Miscanthus Straw
4. Discussion
4.1. Miscanthus-Induced N Immobilisation
4.2. Miscanthus as C Source for Microbial-Derived C Sequestration and SOM Build-Up
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Tilman, D.; Cassman, K.G.; Matson, P.A.; Naylor, R.; Polasky, S. Agricultural sustainability and intensive production practices. Nature 2002, 418, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Bouwman, L.; Goldewijk, K.K.; Van Der Hoek, K.W.; Beusen, A.; van Vuuren, D.; Willems, J.; Rufino, M.; Stehfest, E. Exploring global changes in nitrogen and phosphorus cycles in agriculture induced by livestock production over the 1900–2050 period. Proc. Natl. Acad. Sci. USA 2013, 110, 20882–20887. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Naylor, R.L. Energy and Resource Constraints on Intensive Agricultural Production. Annu. Rev. Energy Environ. 1996, 21, 99–123. [Google Scholar] [CrossRef]
- Cassman, K.G. Ecological intensification of cereal production systems: Yield potential, soil quality, and precision agriculture. Proc. Natl. Acad. Sci. USA 1999, 96, 5952–5959. [Google Scholar] [CrossRef] [Green Version]
- Smil, V. Nitrogen in crop production: An account of global flows. Glob. Biogeochem. Cycles 1999, 13, 647–662. [Google Scholar] [CrossRef] [Green Version]
- Cassman, K.G.; Dobermann, A.; Walters, D.T. Agroecosystems, Nitrogen-use Efficiency, and Nitrogen Management. AMBIO 2002, 31, 132–140. [Google Scholar] [CrossRef]
- Galloway, J.N.; Dentener, F.J.; Capone, D.G.; Boyer, E.W.; Howarth, R.W.; Seitzinger, S.P.; Asner, G.P.; Cleveland, C.C.; Green, P.A.; Holland, E.A.; et al. Nitrogen Cycles: Past, Present, and Future. Biogeochemistry 2004, 70, 153–226. [Google Scholar] [CrossRef]
- Sandel, B.; Arge, L.; Dalsgaard, B.; Davies, R.G.; Gaston, K.J.; Sutherland, W.; Svenning, J.-C. The Influence of Late Quaternary Climate-Change Velocity on Species Endemism. Science 2011, 334, 660–664. [Google Scholar] [CrossRef] [Green Version]
- Goidts, E.; van Wesemael, B. Regional assessment of soil organic carbon changes under agriculture in Southern Belgium (1955–2005). Geoderma 2007, 141, 341–354. [Google Scholar] [CrossRef]
- Sleutel, S.; De Neve, S.; Hofman, G. Assessing causes of recent organic carbon losses from cropland soils by means of regional-scaled input balances for the case of Flanders (Belgium). Nutr. Cycl. Agroecosyst. 2007, 78, 265–278. [Google Scholar] [CrossRef]
- Meersmans, J.; van Wesemael, B.; Goidts, E.; Van Molle, M.; De Baets, S.; De Ridder, F. Spatial analysis of soil organic carbon evolution in Belgian croplands and grasslands, 1960–2006. Glob. Chang. Biol. 2010, 17, 466–479. [Google Scholar] [CrossRef]
- Steinmann, T.; Welp, G.; Holbeck, B.; Amelung, W. Long-term development of organic carbon contents in arable soil of North Rhine-Westphalia, Germany, 1979–2015. Eur. J. Soil Sci. 2016, 67, 616–623. [Google Scholar] [CrossRef]
- Steinmann, T.; Welp, G.; Wolf, A.; Holbeck, B.; Amelung, W.; Große-Rüschkamp, T. Repeated monitoring of organic carbon stocks after eight years reveals carbon losses from intensively managed agricultural soils in Western Germany. J. Plant Nutr. Soil Sci. 2016, 179, 355–366. [Google Scholar] [CrossRef]
- Bellamy, P.; Loveland, P.J.; Bradley, R.I.; Lark, R.; Kirk, G. Carbon losses from all soils across England and Wales 1978–2003. Nat. Cell Biol. 2005, 437, 245–248. [Google Scholar] [CrossRef] [Green Version]
- Geisseler, D.; Horwath, W.R.; Joergensen, R.G.; Ludwig, B. Pathways of nitrogen utilization by soil microorganisms-A review. Soil Biol. Biochem. 2010, 42, 2058–2067. [Google Scholar] [CrossRef]
- Geisseler, D.; Horwath, W.R. Investigating amino acid utilization by soil microorganisms using compound specific stable isotope analysis. Soil Biol. Biochem. 2014, 74, 100–105. [Google Scholar] [CrossRef]
- Joergensen, R.; Wichern, F. Quantitative assessment of the fungal contribution to microbial tissue in soil. Soil Biol. Biochem. 2008, 40, 2977–2991. [Google Scholar] [CrossRef]
- Dilly, O.; Blume, H.-P.; Munch, J.C. Soil microbial activities in Luvisols and Anthrosols during 9 years of region-typical tillage and fertilisation practices in northern Germany. Biogeochemistry 2003, 65, 319–339. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Emmerling, C. Methods for evaluating human impact on soil microorganisms based on their activity, biomass, and diversity in agricultural soils. J. Plant Nutr. Soil Sci. 2006, 169, 295–309. [Google Scholar] [CrossRef]
- Sørensen, P. Immobilisation, remineralisation and residual effects in subsequent crops of dairy cattle slurry nitrogen compared to mineral fertiliser nitrogen. Plant Soil 2004, 267, 285–296. [Google Scholar] [CrossRef]
- Daudén, A.; Quilez, D.; Martínez, C. Residual effects of pig slurry applied to a Mediterranean soil on yield and N uptake of a subsequent wheat crop. Soil Use Manag. 2004, 20, 156–162. [Google Scholar] [CrossRef]
- Sørensen, P.; Thomsen, I.K. Separation of Pig Slurry and Plant Utilization and Loss of Nitrogen-15-labeled Slurry Nitrogen. Soil Sci. Soc. Am. J. 2005, 69, 1644–1651. [Google Scholar] [CrossRef]
- Spiertz, J.H.J. Nitrogen, Sustainable Agriculture and Food Security: A Review. In Sustainable Agriculture; Lichtfouse, E., Navarrete, M., Debaeke, P., Véronique, S., Alberola, C., Eds.; Springer: Dordrecht, The Netherlands, 2009; pp. 635–651. ISBN 9789048126651. [Google Scholar]
- Richards, J.E.; Webster, C.P. Denitrification in the subsoil of the Broadbalk Continuous Wheat Experiment. Soil Biol. Biochem. 1999, 31, 747–755. [Google Scholar] [CrossRef]
- Lal, R. World cropland soils as a source or sink for atmospheric carbon. Adv. Agron. 2001, 71, 145–191. [Google Scholar] [CrossRef]
- Poissant, L.; Beauvais, C.; Lafrance, P.; Deblois, C. Pesticides in fluvial wetlands catchments under intensive agricultural activities. Sci. Total. Environ. 2008, 404, 182–195. [Google Scholar] [CrossRef] [PubMed]
- Ilumäe, E.; Akk, E.; Hansson, A.; Kastanje, V. Changes the content of organic matter in soil during the whole cycle of crop rotation. Agronomy 2009, 7, 263–268. [Google Scholar]
- Smith, P. How long before a change in soil organic carbon can be detected? Glob. Chang. Biol. 2004, 10, 1878–1883. [Google Scholar] [CrossRef]
- Powlson, D.; Prookes, P.; Christensen, B. Measurement of soil microbial biomass provides an early indication of changes in total soil organic matter due to straw incorporation. Soil Biol. Biochem. 1987, 19, 159–164. [Google Scholar] [CrossRef]
- Kallenbach, C.M.; Frey, S.D.; Grandy, A.S. Direct evidence for microbial-derived soil organic matter formation and its ecophysiological controls. Nat. Commun. 2016, 7, 13630. [Google Scholar] [CrossRef]
- Khan, K.S.; Mack, R.; Castillo, X.; Kaiser, M.; Joergensen, R.G. Microbial biomass, fungal and bacterial residues, and their relationships to the soil organic matter C/N/P/S ratios. Geoderma 2016, 271, 115–123. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Mueller, T. The fumigation-extraction method to estimate soil microbial biomass: Calibration of the kEN value. Soil Biol. Biochem. 1996, 28, 33–37. [Google Scholar] [CrossRef]
- Chen, B.; Liu, E.; Tian, Q.; Yan, C.; Zhang, Y. Soil nitrogen dynamics and crop residues. A review. Agron. Sustain. Dev. 2014, 34, 429–442. [Google Scholar] [CrossRef] [Green Version]
- Abbasi, M.K.; Tahir, M.M.; Sabir, N.; Khurshid, M. Impact of the addition of different plant residues on nitrogen mineralization–immobilization turnover and carbon content of a soil incubated under laboratory conditions. Solid Earth 2015, 6, 197–205. [Google Scholar] [CrossRef] [Green Version]
- Reichel, R.; Wei, J.; Islam, M.S.; Schmid, C.; Wissel, H.; Schröder, P.; Schloter, M.; Bruggemann, N. Potential of Wheat Straw, Spruce Sawdust, and Lignin as High Organic Carbon Soil Amendments to Improve Agricultural Nitrogen Retention Capacity: An Incubation Study. Front. Plant Sci. 2018, 9, 900. [Google Scholar] [CrossRef] [PubMed]
- Wei, J.; Reichel, R.; Islam, M.S.; Wissel, H.; Amelung, W.; Brüggemann, N. Chemical Composition of High Organic Carbon Soil Amendments Affects Fertilizer-Derived N2O Emission and Nitrogen Immobilization in an Oxic Sandy Loam. Front. Environ. Sci. 2020, 8, 115. [Google Scholar] [CrossRef]
- Nishio, T.; Oka, N. Effect of Organic matter application on the fate of15N-labeled ammonium fertilizer in an upland soil. Soil Sci. Plant Nutr. 2003, 49, 397–403. [Google Scholar] [CrossRef] [Green Version]
- Shindo, H.; Nishio, T. Immobilization and remineralization of N following addition of wheat straw into soil: Determination of gross N transformation rates by 15N-ammonium isotope dilution technique. Soil Biol. Biochem. 2005, 37, 425–432. [Google Scholar] [CrossRef]
- Simon, T.; Kunzová, E.; Friedlová, M. The effect of digestate, cattle slurry and mineral fertilization on the winter wheat yield and soil quality parameters. Plant Soil Environ. 2016, 61, 522–527. [Google Scholar] [CrossRef] [Green Version]
- García-Ruiz, R.; Carranza-Gallego, G.; Aguilera, E.; De Molina, M.G.; Guzmán, G.I. C and N mineralisation of straw of traditional and modern wheat varieties in soils of contrasting fertility. Nutr. Cycl. Agroecosyst. 2019, 113, 167–179. [Google Scholar] [CrossRef]
- Joergensen, R.G.; Wichern, F. Alive and kicking: Why dormant soil microorganisms matter. Soil Biol. Biochem. 2018, 116, 419–430. [Google Scholar] [CrossRef]
- Schröder, J.J. The Position of Mineral Nitrogen Fertilizer in Efficient Use of Nitrogen and Land: A Review. Nat. Resour. 2014, 05, 936–948. [Google Scholar] [CrossRef] [Green Version]
- Blagodatsky, S.; Richter, O. Microbial growth in soil and nitrogen turnover: A theoretical model considering the activity state of microorganisms. Soil Biol. Biochem. 1998, 30, 1743–1755. [Google Scholar] [CrossRef]
- Schulten, H.-R.; Schnitzer, M. The chemistry of soil organic nitrogen: A review. Biol. Fertil. Soils 1997, 26, 1–15. [Google Scholar] [CrossRef]
- Lutzow, M.V.; Kögel-Knabner, I.; Ekschmitt, K.; Matzner, E.; Guggenberger, G.; Marschner, B.; Flessa, H. Stabilization of organic matter in temperate soils: Mechanisms and their relevance under different soil conditions-a review. Eur. J. Soil Sci. 2006, 57, 426–445. [Google Scholar] [CrossRef]
- Olk, D.; Cassman, K.; Schmidt-Rohr, K.; Anders, M.; Mao, J.-D.; Deenik, J. Chemical stabilization of soil organic nitrogen by phenolic lignin residues in anaerobic agroecosystems. Soil Biol. Biochem. 2006, 38, 3303–3312. [Google Scholar] [CrossRef] [Green Version]
- Emmerling, C.; Pude, R. Introducing Miscanthus to the greening measures of the EU Common Agricultural Policy. GCB Bioenergy 2016, 9, 274–279. [Google Scholar] [CrossRef]
- European Union. Regulation (EU) No. 2017/2393 of the european parliament and of the council-of 13 December 2017-amending Regulations (EU) No 1305/2013 on support for rural development by the European Agricultural Fund for Rural Development (EAFRD), (EU) No 1306/2013 on the financing, management and monitoring of the common agricultural policy, (EU) No 1307/2013 establishing rules for direct payments to farmers under support schemes within the framework of the common agricultural policy, (EU) No 1308/2013 establishing a common organisation of the markets in agricultural products and (EU) No 652/2014 laying down provisions for the management of expenditure relating to the food chain, animal health and animal welfare, and relating to plant health and plant reproductive material. Off. J. Eur. Union 2017, 15–49. [Google Scholar]
- Ruf, T.; Emmerling, C. Impact of premature harvest of Miscanthus x giganteus for biogas production on organic residues, microbial parameters and earthworm community in soil. Appl. Soil Ecol. 2017, 114, 74–81. [Google Scholar] [CrossRef]
- Schmidt, A.; Lemaigre, S.; Ruf, T.; Delfosse, P.; Emmerling, C. Miscanthus as biogas feedstock: Influence of harvest time and stand age on the biochemical methane potential (BMP) of two different growing seasons. Biomass-Convers. Biorefinery 2017, 8, 245–254. [Google Scholar] [CrossRef]
- Nguyen, V.; Elfers, J.; Kühn, H.; Kraska, T.; Pude, R. Different Miscanthus genotypes as growing media in soilless tomato cultivation and its subsequent use for combustion. Acta Hortic. 2021, 301–308. [Google Scholar] [CrossRef]
- Pude, R. Nachwachsende Rohstoffe aus der Region und für die Region. Berichte über Landwirtschaft-Zeitschrift für Agrarpolitik und Landwirtschaft Aktuelle Beiträge 2021, 99, 1–12. [Google Scholar] [CrossRef]
- Van Weyenberg, S.; Ulens, T.; De Reu, K.; Zwertvaegher, I.; Demeyer, P.; Pluym, L. Feasibility of Miscanthus as alternative bedding for dairy cows. Vet. Med. 2016, 60, 121–132. [Google Scholar] [CrossRef]
- Wilke, B.-M. Determination of Chemical and Physical Soil Properties. In Manual for Soil Analysis: Monitoring and Assessing Soil Bioremediation; Margesin, R., Schinner, F., Eds.; Springer: Berlin/Heidelberg, Germany, 2005; pp. 47–95. ISBN 3540253467. [Google Scholar]
- ISO; DIN. Bodenbeschaffenheit–Bestimmung der Partikelgrößenverteilung in Mineralböden–Verfahren Mittels Siebung und Sedimentation; Deutsches Institut für Normung e. V.: Berlin, Germany, 2002; p. 11277. [Google Scholar]
- VDLUFA. Methode A 5.1.1. Bestimmung des pH-Wertes. In Methodenbuch I Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 2016. [Google Scholar]
- VDLUFA. Methode A 6.2.1.1. Bestimmung von Phosphor und Kalium im Calcium-Acetat-Lactat-Auszug. In Methodenbuch I Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 2012. [Google Scholar]
- VDLUFA. Methode A 6.2.4.1 Bestimmung des pflanzenverfügbaren Magnesiums im Calciumchlorid-Auszug. In Methodenbuch I Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 1997. [Google Scholar]
- VDLUFA. Methode A 6.4.1. Bestimmung von Magnesium, Natrium, und den Spurennährstoffen Kupfer, Mangan, Zink und Bor im Calciumchlorid/DTPA-Auszug. In Methodenbuch I Die Untersuchung von Böden; VDLUFA-Verlag: Darmstadt, Germany, 2002. [Google Scholar]
- ISO; DIN. Bodenbeschaffenheit-Bestimmung von Organischem Kohlenstoff und Gesamtkohlenstoff Nach Trockener Verbrennung (Elementaranalyse); Deutsches Institut für Normung e. V.: Berlin, Germany, 1996; p. 10694. [Google Scholar]
- ISO; DIN. Bodenbeschaffenheit-Bestimmung des Gesamt-Stickstoffs Durch Trockene Verbrennung (Elementaranalyse); Deutsches Institut für Normung e. V.: Berlin, Germany, 1998; p. 13878. [Google Scholar]
- Bundesministerium der Justiz und für Verbraucherschutz. Düngeverordnung 2017; BGBl: Berlin, Germany, 2017; pp. 1305–1348. [Google Scholar]
- Brookes, P.; Landman, A.; Pruden, G.; Jenkinson, D. Chloroform fumigation and the release of soil nitrogen: A rapid direct extraction method to measure microbial biomass nitrogen in soil. Soil Biol. Biochem. 1985, 17, 837–842. [Google Scholar] [CrossRef]
- Vance, E.; Brookes, P.; Jenkinson, D. Microbial biomass measurements in forest soils: Determination of kC values and tests of hypotheses to explain the failure of the chloroform fumigation-incubation method in acid soils. Soil Biol. Biochem. 1987, 19, 689–696. [Google Scholar] [CrossRef]
- Wu, J.; Joergensen, R.; Pommerening, B.; Chaussod, R.; Brookes, P. Measurement of soil microbial biomass C by fumigation-extraction—an automated procedure. Soil Biol. Biochem. 1990, 22, 1167–1169. [Google Scholar] [CrossRef]
- Cabrera, M.L.; Kissel, D.E.; Vigil, M.F. Nitrogen Mineralization from Organic Residues. J. Environ. Qual. 2005, 34, 75–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dittmar, T.; Stubbins, A. Dissolved organic matter in aquatic systems. In Treatise on Geochemistry; Elsevier: Amsterdam, The Netherlands, 2014; pp. 125–156. ISBN 9780080983004. [Google Scholar]
- Bhogal, A.; Williams, J.R.; Nicholson, F.A.; Chadwick, D.R.; Chambers, K.H.; Chambers, B.J. Mineralization of organic nitrogen from farm manure applications. Soil Use Manag. 2016, 32, 32–43. [Google Scholar] [CrossRef] [Green Version]
- Rahn, C.R.; Bending, G.; Lillywhite, R.D.; Turner, M.K. Chemical characterisation of vegetable and arable crop residue materials: A comparison of methods. J. Sci. Food Agric. 1999, 79, 1715–1721. [Google Scholar] [CrossRef]
- Corbeels, M.; Hofman, G.; Van Cleemput, O. Nitrogen cycling associated with the decomposition of sunflower stalks and wheat straw in a Vertisol. Plant Soil 2000, 218/2, 71–82. [Google Scholar] [CrossRef]
- Eiland, F.; Leth, M.; Klamer, M.; Lind, A.-M.; Jensen, H.; Iversen, J. C and N Turnover and Lignocellulose Degradation During Composting of Miscanthus Straw And Liquid Pig Manure. Compos. Sci. Util. 2001, 9, 186–196. [Google Scholar] [CrossRef]
- Van Kuijk, S.J.; Sonnenberg, A.S.; Baars, J.J.; Hendriks, W.H.; del Río, J.C.; Rencoret, J.; Gutiérrez, A.; De Ruijter, N.; Cone, J.W. Chemical changes and increased degradability of wheat straw and oak wood chips treated with the white rot fungi Ceriporiopsis subvermispora and Lentinula edodes. Biomass-Bioenergy 2017, 105, 381–391. [Google Scholar] [CrossRef]
- Pude, R.; Treseler, C.-H.; Trettin, R.; Noga, G. Suitability of Miscanthus Genotypes for Lightweight Concrete. Die Bodenkultur 2005, 56, 61–69. [Google Scholar]
- Congreves, K.A.; Vyn, R.J.; Van Eerd, L.L. Evaluation of Post-Harvest Organic Carbon Amendments as a Strategy to Minimize Nitrogen Losses in Cole Crop Production. Agronomy 2013, 3, 181–199. [Google Scholar] [CrossRef]
- Eiland, F.; Klamer, M.; Lind, A.-M.; Leth, M.; Bååth, E. Influence of Initial C/N Ratio on Chemical and Microbial Composition during Long Term Composting of Straw. Microb. Ecol. 2001, 41, 272–280. [Google Scholar] [CrossRef] [PubMed]
- Nieder, R.; Benbi, D.K.; Scherer, H.W. Fixation and defixation of ammonium in soils: A review. Biol. Fertil. Soils 2011, 47, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Jensen, H.E.K.; Leth, M.; Iversen, J.J.L. Growth of Hedera helix L. Container Plants in Compost Substrates Made with Miscanthus ogiformis Honda Straw and Various N-Sources. Compos. Sci. Util. 2001, 9, 206–214. [Google Scholar] [CrossRef]
- Leth, M.; Jensen, H.; Iversen, J. Influence of Different Nitrogen Sources on Composting Of Miscanthus in Open and Closed Systems. Compos. Sci. Util. 2001, 9, 197–205. [Google Scholar] [CrossRef]
- Beuch, S.; Belau, L.; Boelcke, B. Modelluntersuchungen zur Mineralisierung der Biomasse von Miscanthus x giganteus (Mineralisierung von Miscanthusbiomasse). Arch. Agron. Soil Sci. 1998, 42, 347–357. [Google Scholar] [CrossRef]
- Mierzwa-Hersztek, M.; Gondek, K.; Klimkowicz-Pawlas, A.; Baran, A. Effect of wheat and Miscanthus straw biochars on soil enzymatic activity, ecotoxicity, and plant yield. Int. Agrophys. 2017, 31, 367–375. [Google Scholar] [CrossRef]
- O’Toole, A.; Moni, C.; Weldon, S.; Schols, A.; Carnol, M.; Bosman, B.; Rasse, D.P. Miscanthus Biochar had Limited Effects on Soil Physical Properties, Microbial Biomass, and Grain Yield in a Four-Year Field Experiment in Norway. Agriculture 2018, 8, 171. [Google Scholar] [CrossRef] [Green Version]
- Schimmelpfennig, S.; Kammann, C.; Moser, G.; Grünhage, L.; Muller, C. Changes in macro- and micronutrient contents of grasses and forbs following Miscanthus x giganteus feedstock, hydrochar and biochar application to temperate grassland. Grass Forage Sci. 2015, 70, 582–599. [Google Scholar] [CrossRef]
- Rex, D.; Schimmelpfennig, S.; Jansen-Willems, A.; Moser, G.; Kammann, C.; Muller, C. Microbial community shifts 2.6 years after top dressing of Miscanthus biochar, hydrochar and feedstock on a temperate grassland site. Plant Soil 2015, 397, 261–271. [Google Scholar] [CrossRef]
- Liangguang, C.; Cheng, G.; Wixon, D.L.; Balser, T.C. An Absorbing Markov Chain approach to understanding the microbial role in soil carbon stabilization. Biogeochemistry 2010, 106, 303–309. [Google Scholar] [CrossRef]
- Hobara, S.; Osono, T.; Hirose, D.; Noro, K.; Hirota, M.; Benner, R. The roles of microorganisms in litter decomposition and soil formation. Biogeochemistry 2013, 118, 471–486. [Google Scholar] [CrossRef]
- Yesufu, J.; McCalmont, J.P.; Clifton-Brown, J.C.; Williams, P.; Hyland, J.; Gibbons, J.; Styles, D. Consequential life cycle assessment of miscanthus livestock bedding, diverting straw to bioelectricity generation. GCB Bioenergy 2019, 12, 39–53. [Google Scholar] [CrossRef]
| pH (H2O) | P (mg kg−1) | K (mg kg−1) | Mg (mg kg−1) |
|---|---|---|---|
| 6.3 ± 0.06 | 11.4 ± 2.7 | 10.4 ± 1.6 | 14 ± 1.9 |
| B (mg kg−1) | Cu (mg kg−1) | Mn (mg kg−1) | Fe (mg kg−1) |
| 0.5 ± 0.04 | 6.3 ± 0.5 | 169.4 ± 47.4 | 196.3 ± 18.6 |
| SOM (%) | SOC (%) | Nt (%) | C/N (ratio) |
| 3.9 ± 0.7 | 2.3 ± 0.4 | 0.27 ± 0.02 | 8.5 ± 1.2 |
| Clay (g kg−1) | Silt (g kg−1) | Sand (g kg−1) | |
| 229 | 597 | 173 |
| Abbreviation | Fertiliser Description |
|---|---|
| CS | Cattle Slurry |
| CS-Mis | Cattle Slurry with Miscanthus addition (5 kg:1 kg) |
| CS-WS | Cattle Slurry with Wheat Straw addition (8.5 kg:1 kg) |
| CM-Mis | Cattle Manure from Miscanthus shredded bedding |
| CM-WS | Cattle Manure from Wheat Straw bedding |
| UAN | Urea Ammonium Nitrate solution |
| NoN | No Nitrogen applied |
| Mis | Miscanthus-shredding |
| WS | Wheat Straw-shredding |
| Test Parameter | Unit | CS 1 | CS-Mis 2 | CS-WS 2 | CM-Mis 2 | CM-WS 2 | UAN 1 | Mis 2 | WS 2 |
|---|---|---|---|---|---|---|---|---|---|
| Dry matter | % | 9.2 | 21.6 | 16.8 | 32.8 | 33.2 | - | 87.8 | 86.2 |
| Organic matter | % | 6.7 | 19.1 | 14.2 | 26.9 | 22 | - | 85.2 | 79.2 |
| Total N | kg m−3/kg t−1 | 4.0 | 3.8 | 4.2 | 7.4 | 12.4 | 358.4 | 1.7 | 6.3 |
| NH4+-N | kg m−3/kg t−1 | 1.8 | 1.2 | 1.3 | 0.2 | 0.1 | 89.6 | <0.1 | 0.2 |
| NH4-N in total N | % | 45 | 32 | 31 | 3 | 1 | 50 | 5 | 3 |
| C/N | ratio | 10 | 29 | 20 | 21 | 10 | - | 288 | 73 |
| Test Parameter | Unit | CS 1 | CS-Mis 2 | CS-WS 2 | CM-Mis 2 | CM-WS 2 | UAN 1 | Mis 2 | WS 2 |
|---|---|---|---|---|---|---|---|---|---|
| Dry matter | % | 8 | 20.7 | 16.5 | 25.4 | 15.5 | - | 90.1 | 90.9 |
| Organic matter | % | 5.3 | 18.0 | 13.5 | 22.9 | 12.4 | - | 86.9 | 86.3 |
| Total N | kg m−3/kg t−1 | 3.5 | 3.7 | 3.9 | 5.0 | 5.0 | 358.4 | 3.0 | 4.4 |
| NH4+ | kg m−3/kg t−1 | 2.1 | 1.5 | 1.3 | 1.4 | 1.8 | 89.6 | 0.2 | 0.2 |
| NH4-N in total N | % | 60 | 41 | 33 | 28 | 36 | 50 | 7 | 5 |
| C/N | ratio | 9 | 28 | 20 | 27 | 15 | - | 166 | 115 |
| Nutrient | Nutrient (mg pot−1) | Form of Supply |
|---|---|---|
| N | 188/266 a | Organic N, NH4+ b K2HPO4 |
| P | 220 | K2HPO4 |
| K | 1800 | K2SO4 |
| Mg | 400 | MgSO4•7H2O |
| B | 5 | H3BO3 |
| Zn | 20 | ZnSO4•7H2O |
| Harvest Number | ||||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | |
| Exp. | Thermal Time a/Days after Sowing | |||||
| 1 | 646/55 | 931/75 | 1286/102 | 1808/137 | 2455/173 | 3138/209 |
| 2 | 640/47 | 926/67 | 1304/96 | 1880/133 | 2516/168 | 3260/204 |
| 3 | 627/42 | 855/56 | 1310/84 | 1935/118 | 2751/155 | 3418/188 |
| Harvest Number | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | cum | ||
| Exp. | Treatment | N Uptake [% of CS] | ||||||
| 1 | CS | 100 a | 100 a | 100 ns | 100 ns | 100 ns | 100 ns | 100 a |
| CS-Mis | 47 c | 93 b | 107 ns | 101 ns | 95 ns | 101 ns | 87 b | |
| CS-WS | 52 b | 97 ab | 105 ns | 101 ns | 106 ns | 107 ns | 91 b | |
| 2 | CS | 100 a | 100 ab | 100 ns | 100 ns | 100 ns | 100 ns | 100 a |
| CS-Mis | 44 c | 96 b | 110 ns | 104 ns | 100 ns | 102 ns | 88 b | |
| CS-WS | 51 b | 108 a | 100 ns | 101 ns | 103 ns | 130 ns | 93 b | |
| 3 | CS | 100 a | 100 a | 100 ns | 100 ns | 100 ns | 100 ns | 100 a |
| CS-Mis | 39 b | 68 cc | 104 ns | 104 ns | 102 ns | 99 ns | 76 b | |
| CS-WS | 50 b | 78 b | 110 ns | 104 ns | 105 ns | 101 ns | 83 b | |
| Harvest Number | ||||||||
|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | cum | ||
| Exp. | Treatment | N Uptake [% of CM-WS] | ||||||
| 1 | CM-Mis | 88 B | 102 ns | 102 ns | 98 ns | 106 ns | 109 ns | 101 ns |
| CM-WS | 100 A | 100 ns | 100 ns | 100 ns | 100 ns | 100 ns | 100 ns | |
| 2 | CM-Mis | 97 ns | 106 ns | 95 ns | 94 ns | 95 ns | 98 ns | 97 ns |
| CM-WS | 100 ns | 100 ns | 100 ns | 100 ns | 100 ns | 100 ns | 100 ns | |
| 3 | CM-Mis | 69 B | 93 ns | 113 ns | 119 A | 101 ns | 88 B | 96 ns |
| CM-WS | 100 A | 100 ns | 100 ns | 100 B | 100 ns | 100 A | 100 ns | |
| Exp. | CS | CS-Mis | CS-WS | CM-Mis | CM-WS | UAN |
|---|---|---|---|---|---|---|
| N Immobilisation [kg ha−1] | ||||||
| 1 | 34 | 60 | 51 | 80 | 15 | 21 |
| 2 | 23 | 48 | 25 | 123 | 50 | 40 |
| 3 | 26 | 23 | 19 | 46 | −16 | 46 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stotter, M.; Wichern, F.; Pude, R.; Hamer, M. Nitrogen Immobilisation and Microbial Biomass Build-Up Induced by Miscanthus x giganteus L. Based Fertilisers. Agronomy 2021, 11, 1386. https://doi.org/10.3390/agronomy11071386
Stotter M, Wichern F, Pude R, Hamer M. Nitrogen Immobilisation and Microbial Biomass Build-Up Induced by Miscanthus x giganteus L. Based Fertilisers. Agronomy. 2021; 11(7):1386. https://doi.org/10.3390/agronomy11071386
Chicago/Turabian StyleStotter, Michael, Florian Wichern, Ralf Pude, and Martin Hamer. 2021. "Nitrogen Immobilisation and Microbial Biomass Build-Up Induced by Miscanthus x giganteus L. Based Fertilisers" Agronomy 11, no. 7: 1386. https://doi.org/10.3390/agronomy11071386
APA StyleStotter, M., Wichern, F., Pude, R., & Hamer, M. (2021). Nitrogen Immobilisation and Microbial Biomass Build-Up Induced by Miscanthus x giganteus L. Based Fertilisers. Agronomy, 11(7), 1386. https://doi.org/10.3390/agronomy11071386






