Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach
Abstract
:1. Introduction
2. Materials and Methods
2.1. Evaluation of the CSM–MANIHOT–Cassava Model
2.2. Model Application
3. Results and Discussion
3.1. Model Evaluation
3.2. Scenario Analysis
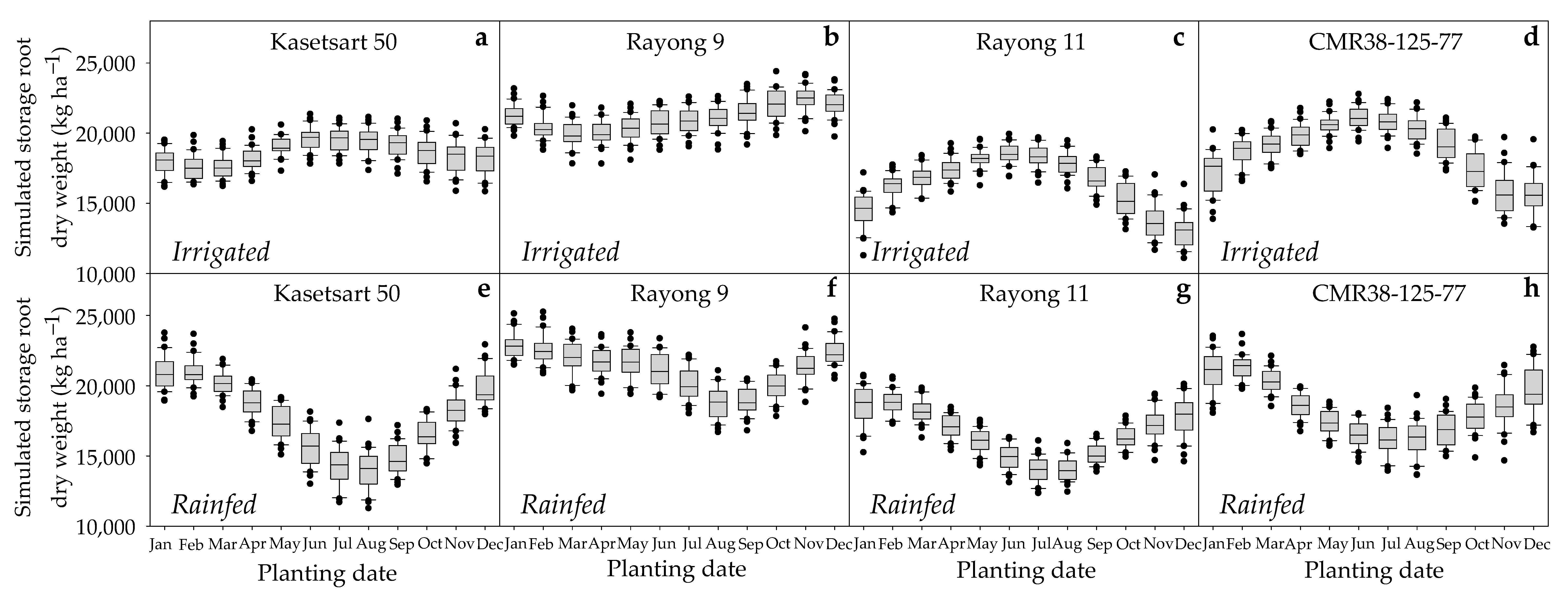
3.2.1. The Productivity among Locations
3.2.2. Yield Gap between Irrigated and Rainfed Conditions
3.2.3. Performances of the Four Cassava Genotypes
3.2.4. Variation of Cassava Productivity across Planting Dates
3.2.5. The Interaction between Planting Dates and Water Regimes
3.2.6. The Interaction between Genotypes, Plating Dates, and Water Regimes
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A


References
- Howeler, R.H. Sustainable Soil and Crop. Management of Cassava in Asia; CIAT Publication: Hanoi, Vietnam, 2014. [Google Scholar]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Office of Agricultural Economics. Agricultural Statistics of Thailand, 2018; Office of Agricultural Economics: Bangkok, Thailand, 2018. [Google Scholar]
- FAO. FAOSTAT—Crops. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 14 October 2020).
- Howeler, R.H. Save and Grow: Cassava, a Guide to Sustainable Production Intensification; Food and Agriculture Organization of the United Nations: Rome, Italy, 2013. [Google Scholar]
- Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Shelia, V.; Wilkens, P.W.; Singh, U.; White, J.W.; Asseng, S.; Lizaso, J.I.; Moreno, L.P.; et al. The DSSAT crop modeling ecosystem. In Advances in Crop Modeling for a Sustainable Agriculture; Boote, K.J., Ed.; Burleigh Dodds Science Publishing: Cambridge, UK, 2019; pp. 173–216. [Google Scholar]
- Hoogenboom, G.; Porter, C.H.; Shelia, V.; Boote, K.J.; Singh, U.; White, J.W.; Hunt, L.A.; Ogoshi, R.; Lizaso, J.I.; Koo, J.; et al. DSSAT—Decision Support System for Agrotechnology Transfer, version 4.7; DSSAT Foundation: Gainesville, FL, USA, 2019; Available online: www.DSSAT.net (accessed on 1 March 2021).
- Jones, J.W.; Hoogenboom, G.; Porter, C.H.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Wilkens, P.W.; Singh, U.; Gijsman, A.J.; Ritchie, J.T. The DSSAT cropping system model. Eur. J. Agron. 2003, 18, 235–265. [Google Scholar] [CrossRef]
- Tsuji, G.Y.; Hoogenboom, G.; Thornton, P.K. Understanding Options for Agricultural Production; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1998. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Wilkens, P.W.; Porter, C.H.; Tsuji, G.Y. Decision Support System for Agrotechnology Transfer, Version 4.5—DSSAT v.4.5: ICASA Tools; University of Hawaii: Honolulu, HI, USA, 2010; Volume 3. [Google Scholar]
- Jones, J.W.; Hoogenboom, G.; Wilkens, P.W.; Porter, C.H.; Tsuji, G.Y. Decision Support System for Agrotechnology Transfer, Version 4.0—DSSAT v.4.0: Crop. Model Documentation; University of Hawaii: Honolulu, HI, USA, 2010; Volume 4. [Google Scholar]
- Hoogenboom, G.; Jones, J.W.; Porter, C.H.; Wilkens, P.W.; Boote, K.J.; Batchelor, W.D.; Hunt, L.A.; Tsuji, G.Y. Decision Support System for Agrotechnology Transfer, Version 4—Overview; University of Hawaii: Honolulu, HI, USA, 2003; Volume 1. [Google Scholar]
- Banterng, P.; Patanothai, A.; Pannangpetch, K.; Jogloy, S.; Hoogenboom, G. Yield stability evaluation of peanut lines: A comparison of an experimental versus a simulation approach. Field Crops Res. 2006, 96, 168–175. [Google Scholar] [CrossRef]
- Putto, W.; Patanothai, A.; Jogloy, S.; Hoogenboom, G. Determination of mega-environments for peanut breeding using the CSM-CROPGRO-Peanut model. Crop Sci. 2008, 48, 973–982. [Google Scholar] [CrossRef]
- Phakamas, N.; Patanothai, A.; Pannangpetch, K.; Jogloy, S.; Hoogenboom, G. Determination of adaptive responses of peanut genotypes and patterns of genotype × location interaction using the CSM-CROPGRO-Peanut model. Int. J. Plant. Prod. 2010, 4, 223–234. [Google Scholar]
- Soler, C.M.T.; Sentelhas, P.C.; Hoogenboom, G. Application of the CSM-CERES-Maize model for planting date evaluation and yield forecasting for maize grown off-season in a subtropical environment. Eur. J. Agron. 2007, 27, 165–177. [Google Scholar] [CrossRef]
- Banterng, P.; Hoogenboom, G.; Patanothai, A.; Singh, P.; Wani, S.P.; Pathak, P.; Tongpoonpol, S.; Atichart, S.; Srihaban, P.; Buranaviriyakul, S.; et al. Application of the cropping system model (CSM)—CROPGRO-Soybean for determining optimum management strategies for soybean in tropical environments. J. Agron. Crop Sci. 2010, 196, 231–242. [Google Scholar] [CrossRef]
- Andarzian, B.; Hoogenboom, G.; Bannayan, M.; Shirali, M.; Andarzian, B. Determining optimum sowing date of wheat using CSM-CERES-Wheat model. J. Saudi Soc. Agric. Sci. 2015, 14, 189–199. [Google Scholar] [CrossRef] [Green Version]
- Vilayvong, S.; Banterng, P.; Patanothai, A.; Pannangpetch, K. CSM-CERES-Rice model to determine management strategies for lowland rice production. Sci. Agric. 2015, 72, 229–236. [Google Scholar] [CrossRef] [Green Version]
- Ahmed, M.; Akram, M.N.; Asim, M.; Aslam, M.; Hassan, F.; Higgins, S.; Stöckle, C.O.; Hoogenboom, G. Calibration and validation of APSIM-Wheat and CERES-Wheat for spring wheat under rainfed conditions: Models evaluation and application. Comput. Electron. Agric. 2016, 123, 384–401. [Google Scholar] [CrossRef]
- Paz, J.O.; Woli, P.; Garcia, A.G.; Hoogenboom, G. Cotton yields as influenced by ENSO at different planting dates and spatial aggregation levels. Agric. Syst. 2012, 111, 45–52. [Google Scholar] [CrossRef]
- Kaeomuangmoon, T.; Jintrawet, A.; Chotamonsak, C.; Singh, U.; Buddhaboon, C.; Naoujanon, P.; Kongton, S.; Kono, Y.; Hoogenboom, G. Estimating seasonal fragrant rice production in Thailand using a spatial crop modelling and weather forecasting approach. J. Agric. Sci. 2019, 157, 566–577. [Google Scholar] [CrossRef]
- Thornton, P.K.; Hoogenboom, G. A computer program to analyze single-season crop model outputs. Agron. J. 1994, 86, 860–868. [Google Scholar] [CrossRef]
- Moreno-Cadena, L.P.; Hoogenboom, G.; Fisher, M.J.; Ramirez-Villegas, J.; Prager, S.D.; Becerra Lopez-Lavalle, L.A.; Pypers, P.; Mejia de Tafur, M.S.; Wallach, D.; Muñoz-Carpena, R.; et al. Importance of genetic parameters and uncertainty of MANIHOT, a new mechanistic cassava simulation model. Eur. J. Agron. 2020, 115, 126031. [Google Scholar] [CrossRef] [PubMed]
- Kaweewong, J.; Tawornpruek, S.; Yampracha, S.; Yost, R.; Kongton, S.; Kongkeaw, T. Cassava nitrogen requirements in Thailand and crop simulation model predictions. Soil Sci. 2013, 178, 248–255. [Google Scholar] [CrossRef]
- Phoncharoen, P.; Banterng, P.; Moreno Cadena, L.P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Performance of the CSM-MANIHOT-Cassava model for simulating planting date response of cassava genotypes. Field Crop Res. 2021, 264, 108073. [Google Scholar] [CrossRef]
- Sawatraksa, N.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Moreno Cadena, L.P.; Hoogenboom, G. Performance of a model in simulating growth and stability for cassava grown after rice. Agron. J. 2021. [Google Scholar] [CrossRef]
- Hoogenboom, G.; Jones, J.W.; Traore, P.C.S.; Boote, K.J. Experiments and data for model evaluation and application. In Improving Soil Fertility Recommendations in Africa Using the Decision Support Systems for Agrotechnology Transfers (DSSAT); Kihara, J., Fatondji, D., Jones, J.W., Hoogenboom, G., Tabo, R., Bationo, A., Eds.; Springer: Dordrecht, The Netherlands, 2012; pp. 9–18. [Google Scholar]
- Hinthong, Y.; Banterng, P. Evaluation of the potential of the CSM-CSCRP-Cassava model. Khon Kaen Agr. J. 2013, 41, 469–482. [Google Scholar]
- Phuntupan, K.; Banterng, P. Physiological determinants of storage root yield in three cassava genotypes under different nitrogen supply. J. Agric. Sci. 2017, 155, 978–992. [Google Scholar] [CrossRef]
- Gomez, K.A.; Gomez, A.A. Statistical Procedures for Agricultural Research; John Wiley and Sons: New York, NY, USA, 1984. [Google Scholar]
- Willmott, C.T. Some comments on the evaluation of model performance. Bull. Am. Meteorol. Soc. 1982, 63, 1309–1313. [Google Scholar] [CrossRef] [Green Version]
- Wallach, D.; Goffinet, B. Mean squared error of prediction as a criterion for evaluating and comparing system models. Ecol. Modell. 1989, 44, 299–306. [Google Scholar] [CrossRef]
- Yang, J.M.; Yang, J.Y.; Liu, S.; Hoogenboom, G. An evaluation of the statistical methods for testing the performance of a crop simulation model with observed data. Agric. Syst. 2014, 127, 81–89. [Google Scholar] [CrossRef]
- Li, Z.T.; Yang, J.Y.; Drury, C.F.; Hoogenboom, G. Evaluation of the DSSAT-CSM for simulating yield and soil organic C and N of a long-term maize and wheat rotation experiment in the Loess Plateau of Northwestern China. Agric. Syst. 2015, 135, 90–104. [Google Scholar] [CrossRef]
- Phakamas, N.; Jintrawet, A.; Patanothai, A.; Sringam, P.; Hoogenboom, G. Estimation of solar radiation based on air temperature and application with the DSSAT v4.5 peanut and rice simulation models in Thailand. Agric. For. Meteorol. 2013, 180, 182–193. [Google Scholar] [CrossRef]
- Analytical Software. Statistix, version 10; Analytical Software: Tallahassee, FL, USA, 2013. [Google Scholar]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Growth rates and yields of cassava at different planting dates in a tropical savanna climate. Sci. Agric. 2019, 76, 376–388. [Google Scholar] [CrossRef] [Green Version]
- Keating, B.A.; Evenson, J.P.; Fukai, S. Environmental effects on growth and development of cassava (Manihot esculenta Crantz) II. Crop growth rate and biomass yield. Field Crop. Res. 1982, 5, 283–292. [Google Scholar] [CrossRef]
- Fukai, S.; Alcoy, A.B.; Llamelo, A.B.; Patterson, R.D. Effects of solar radiation on growth of cassava (Manihot esculenta crantz.). I. Canopy development and dry matter growth. Field Crop. Res. 1984, 9, 347–360. [Google Scholar] [CrossRef]
- Sawatraksa, N.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Hoogenboom, G. Chlorophyll fluorescence and biomass of four cassava genotypes grown under rain-fed upper paddy field conditions in the tropics. J. Agron. Crop Sci. 2018, 204, 554–565. [Google Scholar] [CrossRef]
- Sawatraksa, N.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Hoogenboom, G. Cassava growth analysis of production during the off-season of paddy rice. Crop Sci. 2019, 59, 760–771. [Google Scholar] [CrossRef]
- Wongnoi, S.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P. Physiology, growth and yield of different cassava genotypes planted in upland with dry environment during high storage root accumulation stage. Agronomy 2020, 10, 576. [Google Scholar] [CrossRef] [Green Version]
- Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. The impact of seasonal environments in a tropical savanna climate on forking, leaf area index, and biomass of cassava genotypes. Agronomy 2019, 9, 19. [Google Scholar] [CrossRef] [Green Version]
- Santanoo, S.; Vongcharoen, K.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Roytrakul, S.; Theerakulpisut, P. Canopy structure and photosynthetic performance of irrigated cassava genotypes growing in different seasons in a tropical savanna climate. Agronomy 2020, 10, 2018. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. Cassava biology and physiology. Plant. Mol. Biol. 2004, 53, 621–641. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A. International research on cassava photosynthesis, productivity, eco-physiology, and responses to environmental stresses in the tropics. Photosynthetica 2006, 45, 399. [Google Scholar] [CrossRef]
- El-Sharkawy, M.A.; Cock, J.H.; Lynam, J.K.; Hernàndez, A.D.P.; Cadavid, L.F.L. Relationships between biomass, root-yield and single-leaf photosynthesis in field-grown cassava. Field Crops Res. 1990, 25, 183–201. [Google Scholar] [CrossRef]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Seasonal variation in photosynthesis performance of cassava at two different growth stages under irrigated and rain-fed conditions in a tropical savanna climate. Photosynthetica 2018, 56, 1398–1413. [Google Scholar] [CrossRef]
- Vongcharoen, K.; Santanoo, S.; Banterng, P.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P. Diurnal and seasonal variations in the photosynthetic performance and chlorophyll fluorescence of cassava “Rayong 9” under irrigated and rainfed conditions. Photosynthetica 2019, 57, 268–285. [Google Scholar] [CrossRef] [Green Version]
- El-Sharkawy, M.A.; de Tafur, S.M.; Lopez, Y. Eco-physiological research for breeding improved cassava cultivars in favorable and stressful environments in tropical/subtropical bio-systems. Environ. Res. J. 2012, 6, 143–211. [Google Scholar]
- Mahakosee, S.; Jogloy, S.; Vorasoot, N.; Theerakulpisut, P.; Banterng, P.; Kesmala, T.; Holbrook, C.; Kvien, C. Seasonal variations in canopy size and yield of Rayong 9 cassava genotype under rainfed and irrigated conditions. Agronomy 2019, 9, 362. [Google Scholar] [CrossRef] [Green Version]
- Alves, A.A.C. Cassava botany and physiology. In Cassava: Biology, Production and Utilization; Hillocks, R.J., Thresh, J.M., Bellotti, A.C., Eds.; CABI Publishing: New York, NY, USA, 2002; pp. 67–89. [Google Scholar]
- Banterng, P.; Patanothai, A.; Pannangpetch, K.; Jogloy, S.; Hoogenboom, G. Determination and evaluation of genetic coefficients of peanut lines for breeding applications. Eur. J. Agron. 2004, 21, 297–310. [Google Scholar] [CrossRef]
- Rahman, M.H.; Ahmad, A.; Wajid, A.; Hussain, M.; Rasul, F.; Ishaque, W.; Islam, M.A.; Shelia, V.; Awais, M.; Ullah, A.; et al. Application of CSM-CROPGRO-Cotton model for cultivars and optimum planting dates: Evaluation in changing semi-arid climate. Field Crops Res. 2019, 238, 139–152. [Google Scholar] [CrossRef]
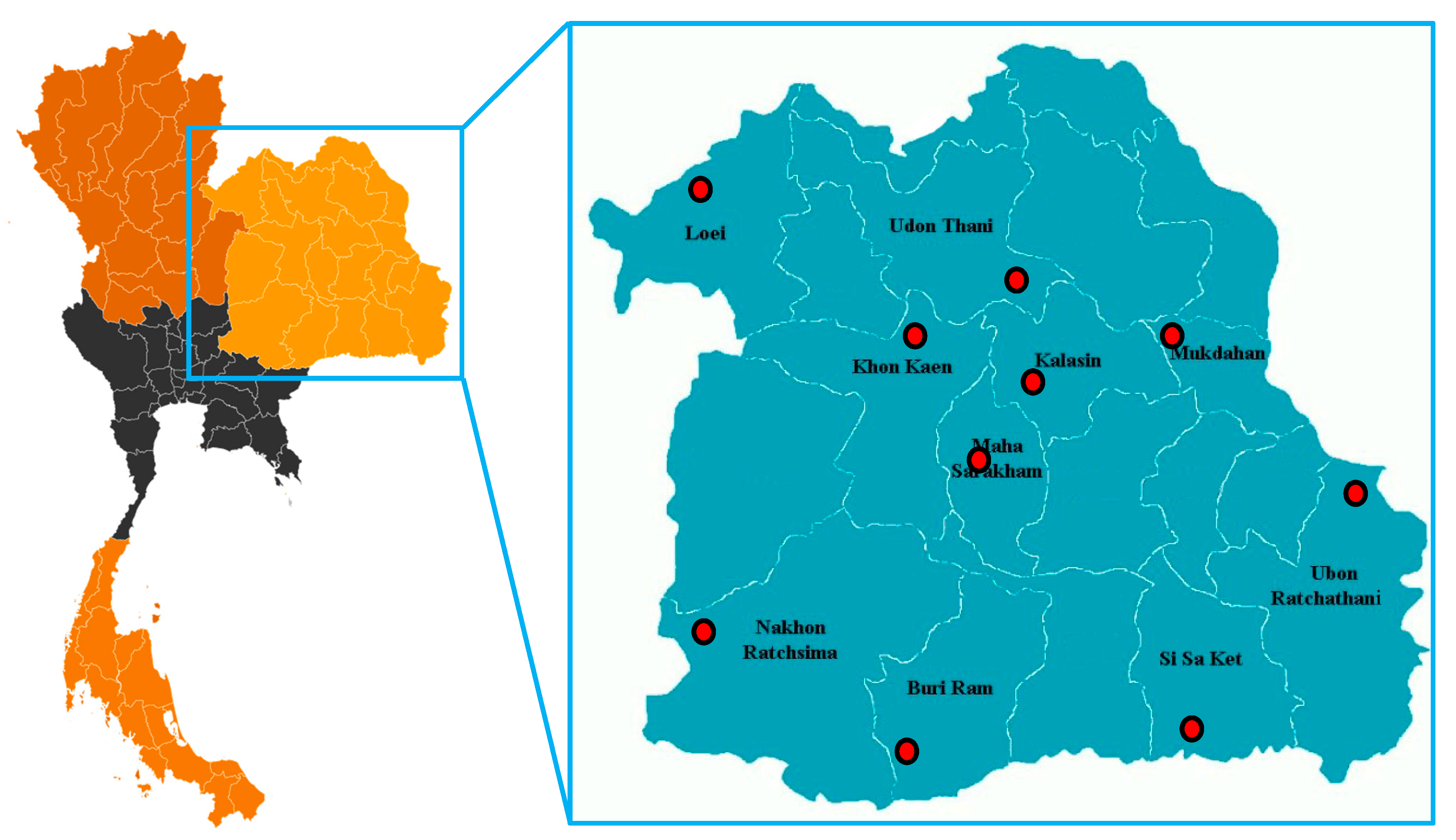
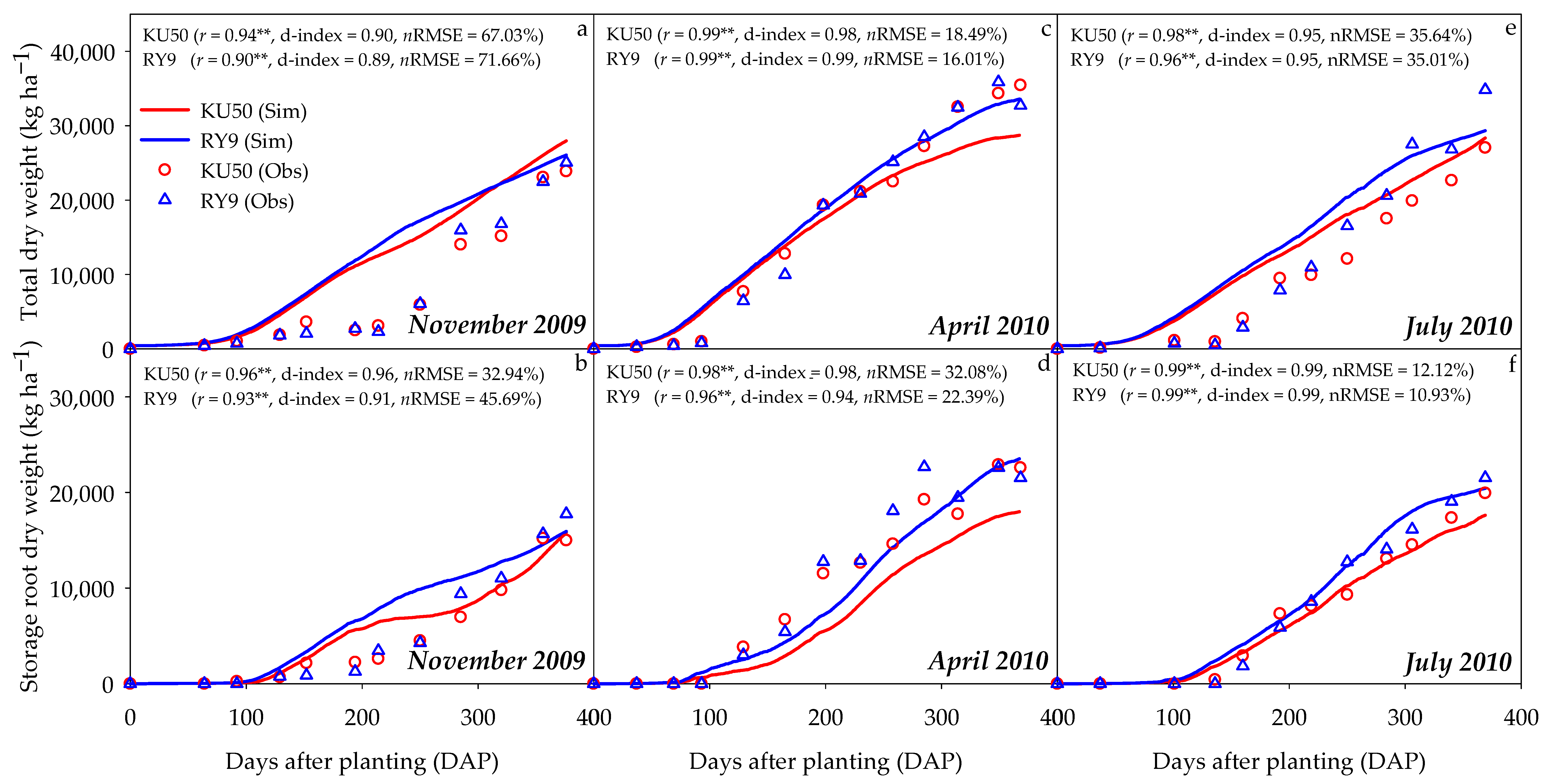

| Location | Latitude and Longitude | Soil Type | Soil Texture | Bulk Density (g cm−3) | pH | Season | Temperature (°C) | Solar Radiation (MJ m−2) | Rainfall (mm) | |
|---|---|---|---|---|---|---|---|---|---|---|
| 0–30 cm | Max | Min | ||||||||
| Buri Ram | 14°24′ N, 102°36′ E | Chok Chai (Rhodic Kandiustox) | Clay | 1.37 | 5.3 | Hot | 35.8 | 23.5 | 20.3 | 212.5 |
| Rainy | 31.7 | 23.7 | 16.2 | 1007.8 | ||||||
| Cool | 30.9 | 18.9 | 17.3 | 80.5 | ||||||
| Kalasin | 16°32′ N, 103°22′ E | Chom Phra (Paleustults) | Loamy Sand | 1.70 | 4.8 | Hot | 35.1 | 23.3 | 19.9 | 223.3 |
| Rainy | 31.7 | 24.4 | 15.1 | 1145.1 | ||||||
| Cool | 30.3 | 18.4 | 16.7 | 58.7 | ||||||
| Khon Kaen | 16°47′ N, 102°41′ E | Ban Phai (Grossarenic Kandiustalf) | Loamy Sand | 1.68 | 5.1 | Hot | 35.2 | 22.7 | 19.9 | 207.8 |
| Rainy | 32.7 | 23.8 | 17.3 | 938.6 | ||||||
| Cool | 31.0 | 18.3 | 16.9 | 64.1 | ||||||
| Loie | 17°40′ N, 101°26′ E | Tha Li (Ultic Haplust) | Loam | 1.50 | 5.8 | Hot | 35.0 | 21.3 | 20.6 | 245.5 |
| Rainy | 32.4 | 23.8 | 17.1 | 946.9 | ||||||
| Cool | 30.4 | 17.2 | 16.8 | 86.2 | ||||||
| Maha Sarakham | 16°05′ N, 103°06′ E | Ban Phai (Grossarenic Kandiustalf) | Loamy Sand | 1.68 | 5.1 | Hot | 35.8 | 23.5 | 20.4 | 231.0 |
| Rainy | 33.4 | 24.4 | 17.8 | 1034.3 | ||||||
| Cool | 31.7 | 18.8 | 17.3 | 57.2 | ||||||
| Mukdahan | 16°52′ N, 104°09′ E | Korat (Paleustults) | Sandy Clay Loam | 1.60 | 4.8 | Hot | 35.3 | 23.4 | 20.0 | 202.1 |
| Rainy | 32.7 | 24.6 | 16.3 | 1241.6 | ||||||
| Cool | 30.8 | 18.7 | 17.0 | 44.2 | ||||||
| Nakhon Ratchasima | 15°17′ N, 101°34′ E | Chum Phuang (Kandiustults) | Sandy Loam | 1.68 | 4.8 | Hot | 35.7 | 24.1 | 19.9 | 224.3 |
| Rainy | 33.4 | 24.8 | 17.4 | 810.3 | ||||||
| Cool | 30.8 | 20.3 | 16.4 | 77.7 | ||||||
| Si Sa Ket | 14°32′ N, 104°13′ E | Korat (Paleustults) | Sandy Clay Loam | 1.60 | 4.8 | Hot | 35.6 | 23.5 | 20.4 | 196.1 |
| Rainy | 32.5 | 24.7 | 16.0 | 1199.5 | ||||||
| Cool | 31.2 | 19.4 | 17.1 | 56.1 | ||||||
| Ubon Ratchathani | 16°03′ N, 105°09′ E | Chakkarat (Paleustults) | Sandy Loam | 1.68 | 4.9 | Hot | 35.8 | 23.4 | 20.6 | 209.6 |
| Rainy | 32.7 | 24.3 | 16.9 | 1333.2 | ||||||
| Cool | 32.0 | 19.5 | 17.6 | 64.6 | ||||||
| Udon Thani | 17°05′ N, 103°24′ E | Korat (Paleustults) | Sandy Clay Loam | 1.60 | 4.8 | Hot | 35.3 | 22.9 | 20.2 | 220.3 |
| Rainy | 32.7 | 24.6 | 16.4 | 1169.4 | ||||||
| Cool | 30.7 | 18.4 | 16.8 | 61.4 | ||||||
| Genotype | N Rate (kg ha−1) | December 2014 | June 2015 | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Total Dry Weight | Storage Root Dry Weight | Total Dry Weight | Storage Root Dry Weight | ||||||||||
| r | d-Index | nRMSE | r | d-Index | nRMSE | r | d-Index | nRMSE | r | d-Index | nRMSE | ||
| Kasetsart 50 | 46.9 | 1.00 ** | 0.99 | 13.26 | 0.99 ** | 0.98 | 15.78 | 0.96 ** | 0.97 | 16.91 | 0.86 ** | 0.79 | 39.44 |
| 90.0 | 0.99 ** | 0.86 | 48.36 | 0.99 ** | 0.70 | 56.05 | 0.97 ** | 0.97 | 17.13 | 0.90 ** | 0.83 | 35.93 | |
| 133.2 | 0.99 ** | 0.83 | 53.58 | 0.99 ** | 0.70 | 55.61 | 0.97 ** | 0.89 | 31.02 | 0.88 ** | 0.73 | 45.03 | |
| Rayong 9 | 46.9 | 1.00 ** | 0.99 | 19.76 | 0.99 ** | 0.99 | 9.91 | 0.99 ** | 0.87 | 56.01 | 0.97 ** | 0.85 | 55.34 |
| 90.0 | 0.99 ** | 0.99 | 16.19 | 0.99 ** | 0.97 | 18.74 | 0.96 ** | 0.92 | 36.61 | 0.88 ** | 0.84 | 44.64 | |
| 133.2 | 0.99 ** | 0.97 | 25.37 | 0.99 ** | 0.95 | 23.17 | 0.96 ** | 0.95 | 26.44 | 0.90 ** | 0.86 | 40.54 | |
| Rayong 11 | 46.9 | 1.00 ** | 0.98 | 17.57 | 0.99 ** | 0.98 | 15.87 | 0.98 ** | 0.98 | 17.57 | 0.93 ** | 0.92 | 29.12 |
| 90.0 | 1.00 ** | 0.99 | 15.18 | 0.99 ** | 0.90 | 34.80 | 0.97 ** | 0.98 | 15.18 | 0.90 ** | 0.89 | 33.88 | |
| 133.2 | 1.00 ** | 0.99 | 22.99 | 0.99 ** | 0.89 | 35.91 | 0.96 ** | 0.95 | 22.99 | 0.85 ** | 0.80 | 41.48 | |
| Treatment | Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buri Ram | Kalasin | Khon Kaen | Loei | Maha Sarakham | Mukdahan | Nakhon Ratchasima | Si Sa Ket | Ubon Ratchathani | Udon Thani | |
| Water | ||||||||||
| Irrigation | 35,395 A | 33,988 A | 36,436 A | 36,387 A | 37,748 A | 34,882 A | 35,921 A | 35,194 A | 36,558 A | 35,021 A |
| Rainfed | 29,007 B | 27,323 B | 29,082 B | 30,746 B | 29,592 B | 28,403 B | 27,863 B | 28,444 B | 29,800 B | 28,809 B |
| Genotype | ||||||||||
| Kasetsart 50 | 32,213 B | 30,505 B | 32,482 C | 33,290 C | 33,561 B | 31,610 B | 31,963 B | 31,874 B | 33,218 B | 31,877 B |
| Rayong 9 | 31,910 C | 30,357 C | 32,687 B | 33,494 B | 33,410 C | 31,384 C | 31,419 C | 31,432 C | 32,937 C | 31,625 C |
| Rayong 11 | 31,147 D | 29,748 D | 31,867 D | 32,647 D | 32,733 D | 30,580 D | 30,853 D | 30,790 D | 32,092 D | 30,856 D |
| CMR38–125–77 | 33,534 A | 32,013 A | 34,000 A | 34,835 A | 34,977 A | 32,997 A | 33,335 A | 33,178 A | 34,469 A | 33,300 A |
| Planting date | ||||||||||
| January | 34,416 A | 33,463 A | 35,526 A | 35,940 A | 36,560 B | 34,936 B | 34,174 A | 35,075 A | 36,741 A | 35,067 B |
| February | 34,093 B | 32,612 C | 34,559 B | 35,043 C | 35,273 D | 33,683 D | 33,277 C | 34,048 C | 35,713 B | 33,914 D |
| March | 33,473 C | 31,440 D | 33,339 C | 33,777 E | 34,018 E | 32,079 F | 32,376 D | 32,638 D | 34,216 C | 32,478 F |
| April | 32,331 D | 29,986 E | 32,238 D | 32,749 F | 32,862 F | 30,588 G | 31,485 E | 31,129 F | 32,590 E | 30,974 G |
| May | 31,386 E | 28,957 G | 31,464 E | 31,971 G | 32,096 H | 29,507 H | 30,886 F | 30,030 H | 31,281 G | 29,928 I |
| Jun | 30,393 G | 28,061 I | 30,583 F | 31,285 I | 31,343 I | 28,633 J | 300,87 H | 29,035 I | 30,240 H | 28,965 K |
| July | 29,620 H | 27,758 J | 30,222 G | 31,178 I | 31,031 J | 28,426 K | 29,564 I | 28,542 J | 29,792 I | 28,738 L |
| August | 29,533 H | 28,300 H | 30,312 FG | 31,707 H | 31,281 I | 28,939 I | 29,507 I | 28,994 I | 30,193 H | 29,254 J |
| September | 30,764 F | 29,627 F | 31,371 E | 32,869 F | 32,440 G | 30,461 G | 30,633 G | 30,394 G | 31,502 F | 30,638 H |
| October | 32,414 D | 31,320 D | 33,151 C | 34,470 D | 34,246 E | 32,598 E | 32,382 D | 32,369 E | 33,469 D | 32,802 E |
| November | 33,709 C | 32,847 B | 34,793 B | 35,714 B | 36,048 C | 34,561 C | 33,918 B | 34,318 B | 35,608 B | 34,752 C |
| December | 34,281 AB | 33,496 A | 35,552 A | 36,094 A | 36,844 A | 35,302 A | 34,420 A | 35,255 A | 36,805 A | 35,465 A |
| Treatment | Location | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Buri Ram | Kalasin | Khon Kaen | Loei | Maha Sarakham | Mukdahan | Nakhon Ratchasima | Si Sa Ket | Ubon Ratchathani | Udon Thani | |
| Water | ||||||||||
| Irrigation | 19,100 A | 16,742 B | 18,554 B | 18,141 B | 19,977 A | 18,488 A | 18,794 A | 18,678 A | 20,135 A | 18,477 A |
| Rainfed | 18,466 B | 16,850 A | 19,088 A | 19,797 A | 19,482 B | 17,801 B | 18,139 B | 17,707 B | 18,887 B | 17,924 B |
| Genotype | ||||||||||
| Kasetsart 50 | 18,480 C | 16,369 C | 18,218 C | 18,530 B | 19,103 C | 17,758 C | 17,926 C | 17,854 C | 19,156 C | 17,825 C |
| Rayong 9 | 21,217 A | 19,421 A | 21,759 A | 22,236 A | 22,248 A | 20,531 A | 20,504 A | 20,443 A | 21,866 A | 20,668 A |
| Rayong 11 | 16,567 D | 14,671 D | 16,678 D | 16,526 C | 17,765 D | 16,033 D | 16,595 D | 16,129 D | 17,407 D | 16,033 D |
| CMR38–125–77 | 18,867 B | 16,723 B | 18,629 B | 18,584 B | 19,802 B | 18,256 B | 18,840 B | 18,342 B | 19,615 B | 18,274 B |
| Planting date | ||||||||||
| January | 18,863 C | 17,249 C | 19,903 AB | 19,175 B | 21,103 A | 19,038 A | 19,282 A | 18,857 B | 20,632 A | 18,809 B |
| February | 19,439 B | 17,700 A | 20,122 A | 19,758 A | 20,995 A | 19,172 A | 19,338 A | 19,178 A | 20,820 A | 19,085 A |
| March | 19,653 AB | 17,681 AB | 19,729 B | 19,608 A | 20,388 B | 18,733 B | 18,988 B | 18,855 B | 20,361 B | 18,792 B |
| April | 19,637 AB | 17,485 AB | 19,203 C | 19,357 B | 19,734 C | 18,463 C | 18,560 CD | 18,585 C | 19,973 C | 18,481 C |
| May | 19,716 A | 17,474 B | 18,920 D | 19,212 B | 19,393 D | 18,401 C | 18,422 DE | 18,660 C | 19,763 D | 18,486 C |
| Jun | 19,410 B | 17,077 C | 18,486 E | 18,847 C | 19,111 EF | 18,069 D | 18,145 F | 18,356 D | 19,306 E | 18,195 D |
| July | 18,740 C | 16,443 D | 18,109 F | 18,594 DEF | 18,853 GH | 17,509 E | 17,864 G | 17,698 FG | 18,691 F | 17,719 F |
| August | 17,983 E | 16,119 E | 17,793 G | 18,601 DEF | 18,782 H | 17,211 F | 17,616 H | 17,305 H | 18,323 G | 17,475 G |
| September | 17,856 E | 16,027 E | 17,862 FG | 18,740 CD | 19,034 FG | 17,278 F | 17,928 G | 17,347 H | 18,407 G | 17,490 G |
| October | 17,914 E | 15,914 E | 18,131 F | 18,706 CDE | 19,321 DE | 17,579 E | 18,324 EF | 17,598 G | 18,767 F | 17,714 F |
| November | 17,918 E | 15,993 E | 18,537 E | 18,480 F | 19,746 C | 17,908 D | 18,461 DE | 17,777 F | 19,257 E | 17,951 E |
| December | 18,263 D | 16,391 D | 19,057 CD | 18,549 EF | 20,297 B | 18,373 C | 18,668 C | 18,087 E | 19,831 CD | 18,203 D |
| Planting Date | Solar Radiation (MJ−1 m−2) | Maximum Temperature (°C) | Minimum Temperature (°C) | Rainfall (mm) | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Growth Stage | Growth Stage | Growth Stage | Growth Stage | |||||||||||||
| 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | 1 | 2 | 3 | 4 | |
| January | 18.9 | 19.2 | 16.2 | 16.5 | 33.2 | 34.8 | 32.0 | 30.5 | 19.7 | 24.7 | 23.8 | 18.8 | 63.3 | 457.6 | 822.0 | 22.1 |
| February | 20.0 | 17.8 | 16.2 | 16.9 | 35.0 | 33.6 | 31.6 | 30.5 | 22.1 | 24.7 | 22.7 | 17.4 | 139.2 | 593.8 | 621.2 | 10.7 |
| March | 20.1 | 16.7 | 16.3 | 18.2 | 35.5 | 32.7 | 31.1 | 32.0 | 23.9 | 24.5 | 21.0 | 18.3 | 315.6 | 652.9 | 374.8 | 21.7 |
| April | 19.2 | 16.2 | 16.6 | 19.7 | 34.8 | 32.2 | 30.9 | 34.3 | 24.7 | 24.3 | 19.4 | 20.9 | 457.6 | 717.2 | 132.9 | 57.2 |
| May | 17.8 | 16.0 | 17.3 | 20.5 | 33.6 | 31.8 | 31.3 | 35.8 | 24.7 | 23.6 | 18.6 | 23.4 | 593.8 | 603.8 | 43.8 | 123.6 |
| June | 16.7 | 16.2 | 18.3 | 20.0 | 32.7 | 31.5 | 32.3 | 35.5 | 24.5 | 22.2 | 19.2 | 24.6 | 652.9 | 370.1 | 68.0 | 274.0 |
| July | 16.2 | 16.4 | 19.3 | 18.4 | 32.2 | 30.9 | 34.0 | 34.1 | 24.3 | 20.1 | 20.9 | 24.8 | 717.2 | 126.9 | 145.3 | 375.7 |
| August | 16.0 | 16.8 | 19.8 | 17.0 | 31.8 | 30.7 | 34.9 | 33.0 | 23.6 | 18.3 | 22.8 | 24.7 | 603.8 | 28.1 | 331.2 | 401.8 |
| September | 16.2 | 17.6 | 19.4 | 16.3 | 31.5 | 31.3 | 35.0 | 32.4 | 22.2 | 18.1 | 24.1 | 24.4 | 370.1 | 26.4 | 499.3 | 469.2 |
| October | 16.4 | 18.9 | 18.5 | 16.0 | 30.9 | 33.2 | 34.3 | 32.0 | 20.1 | 19.7 | 24.6 | 24.1 | 126.9 | 63.3 | 675.8 | 499.1 |
| November | 16.8 | 20.0 | 17.3 | 16.0 | 30.7 | 35.0 | 33.2 | 31.7 | 18.3 | 22.1 | 24.6 | 23.2 | 28.1 | 139.2 | 844.9 | 352.7 |
| December | 17.6 | 20.1 | 16.5 | 16.4 | 31.3 | 35.5 | 32.5 | 31.3 | 18.1 | 23.9 | 24.4 | 21.4 | 26.4 | 315.6 | 900.8 | 122.2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Phoncharoen, P.; Banterng, P.; Vorasoot, N.; Jogloy, S.; Theerakulpisut, P.; Hoogenboom, G. Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach. Agronomy 2021, 11, 1372. https://doi.org/10.3390/agronomy11071372
Phoncharoen P, Banterng P, Vorasoot N, Jogloy S, Theerakulpisut P, Hoogenboom G. Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach. Agronomy. 2021; 11(7):1372. https://doi.org/10.3390/agronomy11071372
Chicago/Turabian StylePhoncharoen, Phanupong, Poramate Banterng, Nimitr Vorasoot, Sanun Jogloy, Piyada Theerakulpisut, and Gerrit Hoogenboom. 2021. "Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach" Agronomy 11, no. 7: 1372. https://doi.org/10.3390/agronomy11071372
APA StylePhoncharoen, P., Banterng, P., Vorasoot, N., Jogloy, S., Theerakulpisut, P., & Hoogenboom, G. (2021). Identifying Suitable Genotypes for Different Cassava Production Environments—A Modeling Approach. Agronomy, 11(7), 1372. https://doi.org/10.3390/agronomy11071372







