3.1. Phytoaccumulation of Total Nitrogen and Total Phosphorus in Common Reed (P. australis)
The ranges of concentration of total nitrogen and total phosphorus in individual parts of reeds, accounting for the place where they were collected, have been presented in
Figure 2. The results of the studies show that young plants contained the most total nitrogen and total phosphorus during the phase of their intense growth. The amount of accumulated nitrogen and phosphorus dropped in the following months of the vegetative period.
Leaves and stems of reeds were characterized by the highest variability in nitrogen and phosphorus concentration in the subsequent months of growth. The accumulation of nitrogen and phosphorus in the subterranean parts of macrophytes and the inflorescence changed to a lesser degree.
The highest amounts of total nitrogen were accumulated in the leaves of reeds 17.50 ÷ 49.29 g/kg d.m. (average: 32.21 ± 9.7 g/kg d.m.). The total nitrogen content in the subterranean parts of reeds was from 6.67 g/kg d.m. to 27.71 g/kg d.m. (which corresponds to the average value of: 18.56 ± 4.3 g/kg d.m.). The stems contained from 3.89 to 33.60 g/kg d.m. of total nitrogen (average value: 17.25 ± 8.3 g/kg d.m.) The reed panicle accumulated from 19.53 to 29.53 g total nitrogen/kg d.m. (average: 24.79 ± 3.5 g/kg d.m.).
The content of total phosphorus in the roots of reeds ranged from 0.55 to 3.10 g/kg d.m. (average value: 1.63 ± 0.6 g/kg d.m.). The stems of plants contained from 0.13 to 2.60 g/kg d.m. of total phosphorus (average: 1.13 ± 0.8 g/kg d.m.). The phosphorus concentration in the leaves of the plants was in the range from 0.39 to 3.08 g/kg s.m. (with an average value of: 1.54 ± 0.8 g/kg d.m.). The reed panicle contained from 0.58 g/kg d.m. to 2.71 g/kg d.m. total phosphorus (average: 1.77 ± 0.6 g/kg d.m.).
According to Vymazal et al. [
26], the total nitrogen content in reeds inhabiting single-phase HF-CW beds with subsurface sewage flow in Czech constructed wetlands varied from: 9.0 to 20.8 g/kg d.m. in the stems, from 15.0 to 43.0 g/kg d.m. in the leaves, and from 16.0 to 46.0 g/kg d.m. in the subterranean parts. According to the authors, the accumulation of nitrogen in the stems of reeds growing in natural sites can reach as much as 51.0 g/kg d.m.
The constructed wetlands analyzed by Gajewska and Obarska-Pempkowiak [
27] were characterized by an average accumulation of nitrogen in reeds amounting to: object I—24.2 g/kg d.m., and object II—25.8 g/kg d.m. The authors believe that the higher the accumulation of nitrogen in the reed in the constructed wetland in Sarbsk, the more likely it was caused by a higher concentration of contaminants in the sewage flowing through it.
The average values of total nitrogen and phosphorus in individual parts of the reeds were higher than values given by Ozimek and Renman [
23]. The authors report that the concentration of nitrogen in the leaves of reeds was 3.5%, 0.9% in the stems, and 0.6% in the rhizomes; total phosphorus, on the other hand, was characterized by values of 0.15% in the leaves and 0.08% in the stems and rhizomes.
The studies of Vymazal [
28] showed the direct role of plants in nutrient removal in HF CWs. The common values of standing stock vary in the range of 30–80 g N m
−2 and 2–6 g P m
−2. Under the conditions of low loadings, the portion of removed nitrogen or phosphorus could be substantially higher (>100 g N m
−2 and 10 g P m
−2).
The study confirmed that during the vegetative period of plants, the amount of accumulated total nitrogen and phosphorus in individual parts of reeds was higher than post-vegetation (
Figure 3). The highest differences in total nitrogen and phosphorus contents in the vegetative and post-vegetative periods were observed in the stems of the plant. In the vegetative period, reed stems accumulated on average 2.77 g/kg d.m. of nitrogen and 1.51 g/kg d.m. of phosphorus, whereas in the post-vegetative period, the respective values of these elements were 6.69 and 0.22 g/kg d.m.
High seasonal changes in phytoaccumulation were also noted in the leaves of P. australis. The average value of nitrogen in the vegetative period was 37.56 g/kg d.m. In the off-season, the leaves contained 21.54 g/kg d.m. nitrogen. Both during the vegetative period as well as the period of dormancy, the nitrogen contents in the leaves of reeds were higher in plants collected near the end of the constructed wetland than in reeds from the first part of the bed. The average value of nitrogen in leaf samples collected from the beginning of the subplot was 35.30 g/kg and 39.78 g/kg/d.m. in the end part. In the off-season, the plant leaves contained significantly less total nitrogen, which amounted to anywhere from 19.12 in the initial part of the bed to 23.61 gN/kg d.m. in the end section.
During the vegetative period, the accumulation of total phosphorus in the leaves of P. australis was, on average, 1.95 gP/kg d.m., with a lower value of 0.73 gP/kg d.m. noted in the off-season. During vegetation, the leaves of reeds collected near the inflow contained, on average, 1.84 gP/kg d.m., as compared to 2.09 gP/kg d.m. determined at the end of the bed. In the post-vegetative season, these respective values were 0.63 gP/kg d.m. and 0.75 gP/kg d.m.
Total nitrogen content in the rhizomes and inflorescence of reed did not undergo major seasonal changes. The average value of nitrogen in the subterranean parts of the plant varied from 19.23 g/kg d.m. in the vegetative period to 16.99 g/kg d.m. in the post-vegetative period. The reed panicle contained 27.46 g/kg d.m. of nitrogen during vegetation, whereas in the winter period, the amount of nitrogen was lower and averaged 22.12 gN/kg d.m.
During the vegetative period, the average amount of total phosphorus contained in the rhizomes of reeds was approximately 1.74 g/kg d.m. After the vegetation period, the average total phosphorus content in subterranean parts of the plant was somewhat lower: 1.27 g/kg d.m. of phosphorus. The reed panicle collected during the plant’s vegetative period accumulated 2.06 g/kg d.m. phosphorus. In the off-season, the dry panicles contained lesser amounts of total phosphorus, i.e., 1.49 g/kg d.m.
In the initial part of the bed, intensive growth of reeds was observed, the plants in the period of full development were high (approx. 3 m), with the number approximately 384 pcs/m2.. In the middle of the wetland bed, the reeds were lower (approx. 224 pieces/m2). At the outflow of sewage from the treatment plant, the reed height was about 1.5 m, the amount was about 66 pcs/m2.
3.2. Accumulation of Total Nitrogen and Phosphorus in the Constructed Wetland Bed
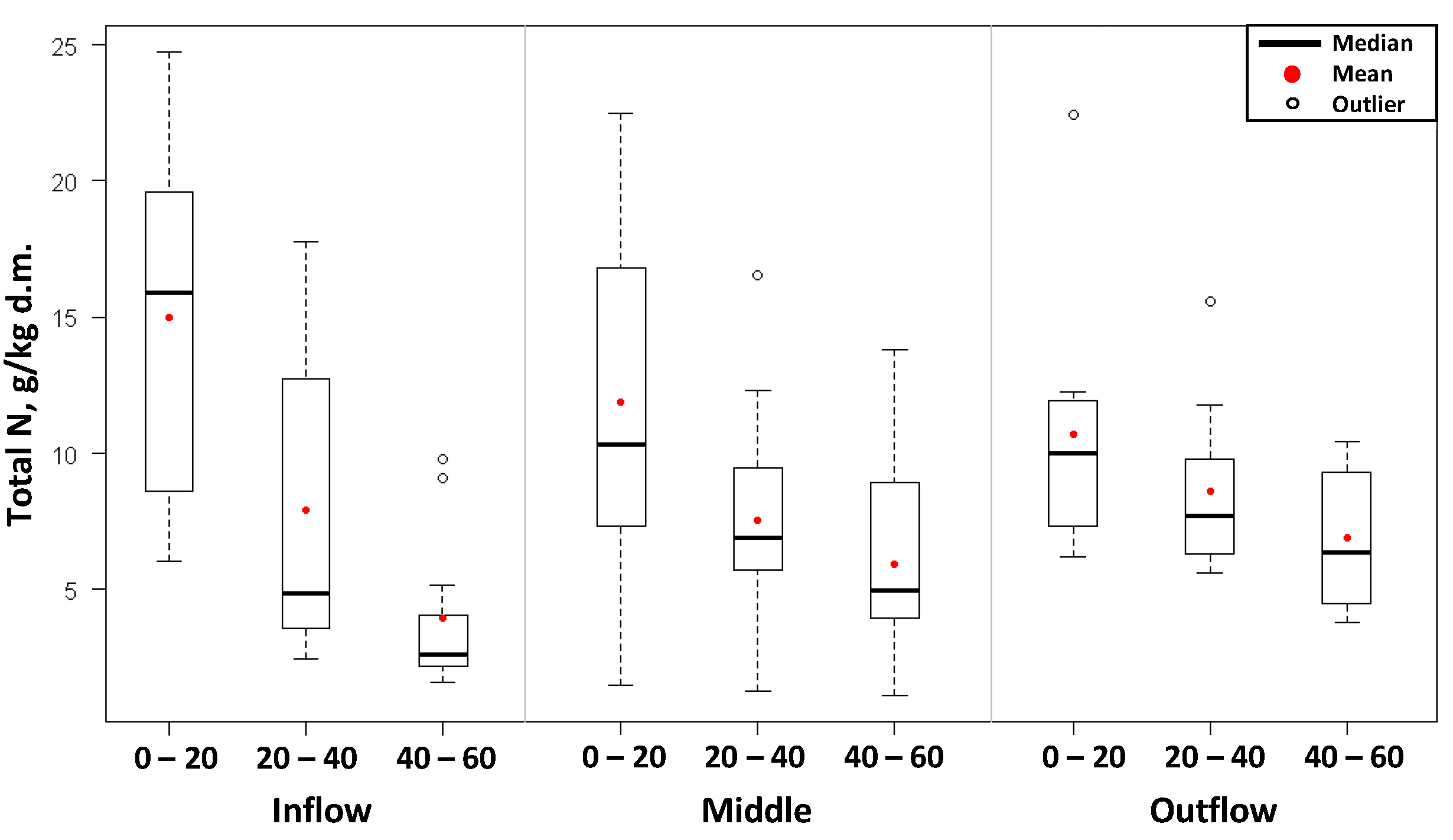
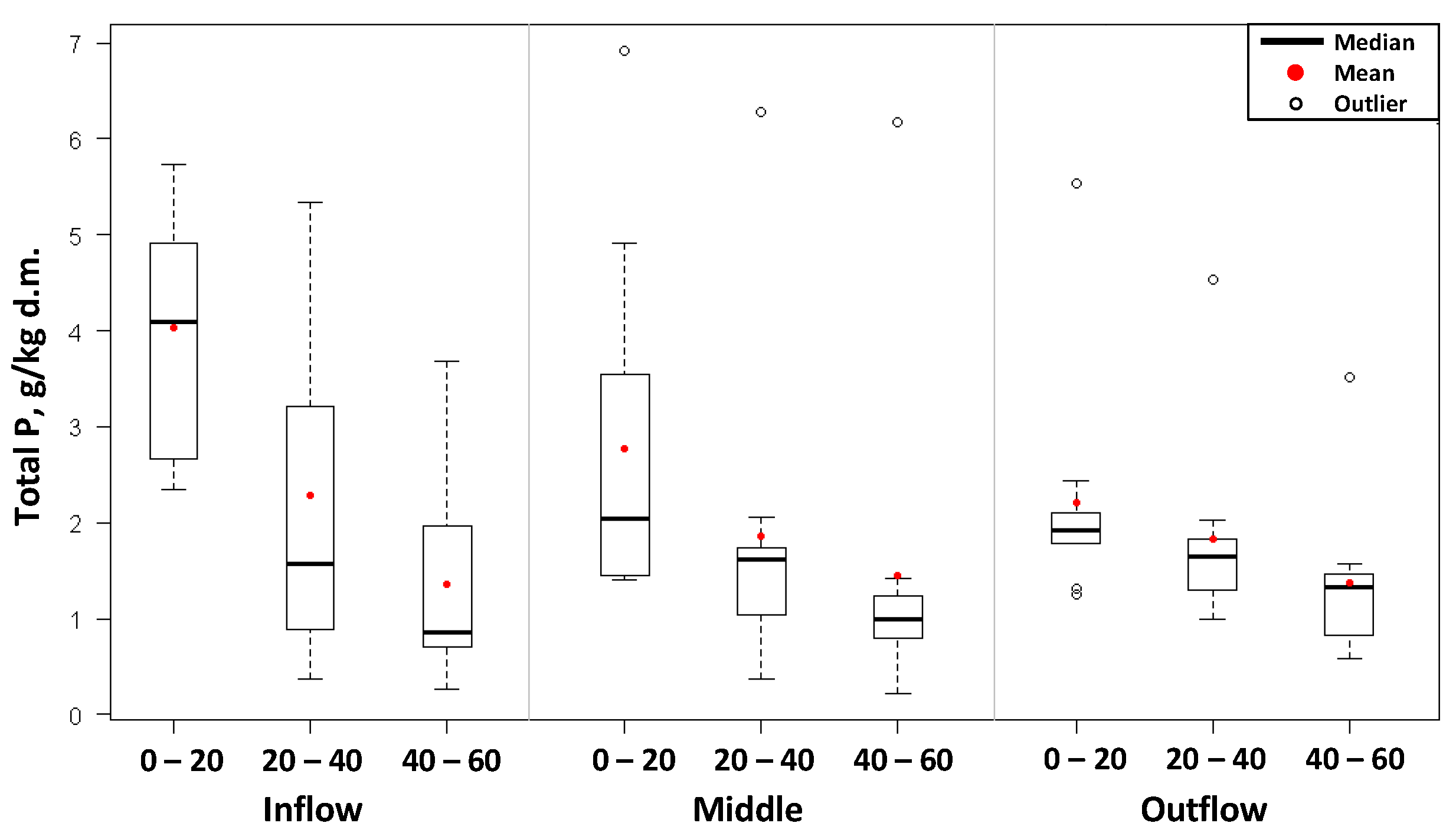
The accumulation of total nitrogen and total phosphorus was also studied in the constructed wetland bed. The distribution of nitrogen and phosphorus contents in the bed, as well as the basic values of descriptive statistics, depending on the location and depth of their collection, have been presented in
Figure 4. The greatest changes in the concentration of total nitrogen and phosphorus during the course of the study period occurred in the surface layer 0–20 cm, especially in bed samples collected at the inflow of sewage into the wetlands. A comparison of the values of arithmetic means of nitrogen and phosphorus contents indicates that irrespective of the location where the samples were collected, these differ at each of the depths, and the average nitrogen and phosphorus consents in the bed is lower for subsequent depths.
The surface layer of the wetland bed, up to 20 cm in depth, was characterized by the highest content of total nitrogen and total phosphorus. The amount of total nitrogen was on average: 12.53 ± 6.0 gN/kg d.m. (range: 1.51 ÷ 24.74 g/kg d.m.). In deeper layers of the bed, the nitrogen content was lower. At the level of 20–40 cm, total nitrogen accumulated in the leaves in amounts from 1.30 to 17.79 g/kg d.m., with an average content of 8.01 ± 4.4 gN/kg d.m. At the depth of 40–60 cm, the amount of nitrogen fell into the range from 1.12 to 13.82, averaging: 5.60 ± 3.3 gN/kg d.m.
Similar relationships were observed in regards to total phosphorus. The average concentration of phosphorus at a depth of up to 20 cm was 3.01 ± 1.6 gP/kg d.m. (range of values: 1.41 ÷ 6.92 g/kg d.m.). In deeper layers of the bed, phosphorus content was lower. At the level of 20–40 cm, the accumulation of phosphorus was in the range from 0.37 to 6.28 gP/kg d.m., on average, 1.99 ± 1.5 gP/kg d.m. On the other hand, at the depth of 40–60 cm, the accumulation of phosphorus averaged 1.39 ± 1.2 gN/kg d.m. (range of values: 0.22 ÷ 6.18 g/kg d.m.).
The accumulation of nitrogen and phosphorus changed not only along with the depth but also depending on the location of collecting samples. In the initial part of the hydrophyte subplot, higher amounts of total nitrogen and total phosphorus were confirmed in the 0–20 cm layer than in samples taken near the sewage outflow. The average value of total nitrogen up to 20 cm in depth in the initial part of the bed was 14.99 ± 6.2 gN/kg d.m., whereas the average accumulation of nitrogen at the end of the bed was lower, and amounted to 10.73 ± 4.7 gN/kg d.m. The total phosphorus content at this depth was found to be 4.03 ± 1.2 gP/kg d.m. and 2.22 ± 1.2 gP/kg d.m. respectively at the initial part of the bed and near the outflow of sewage.
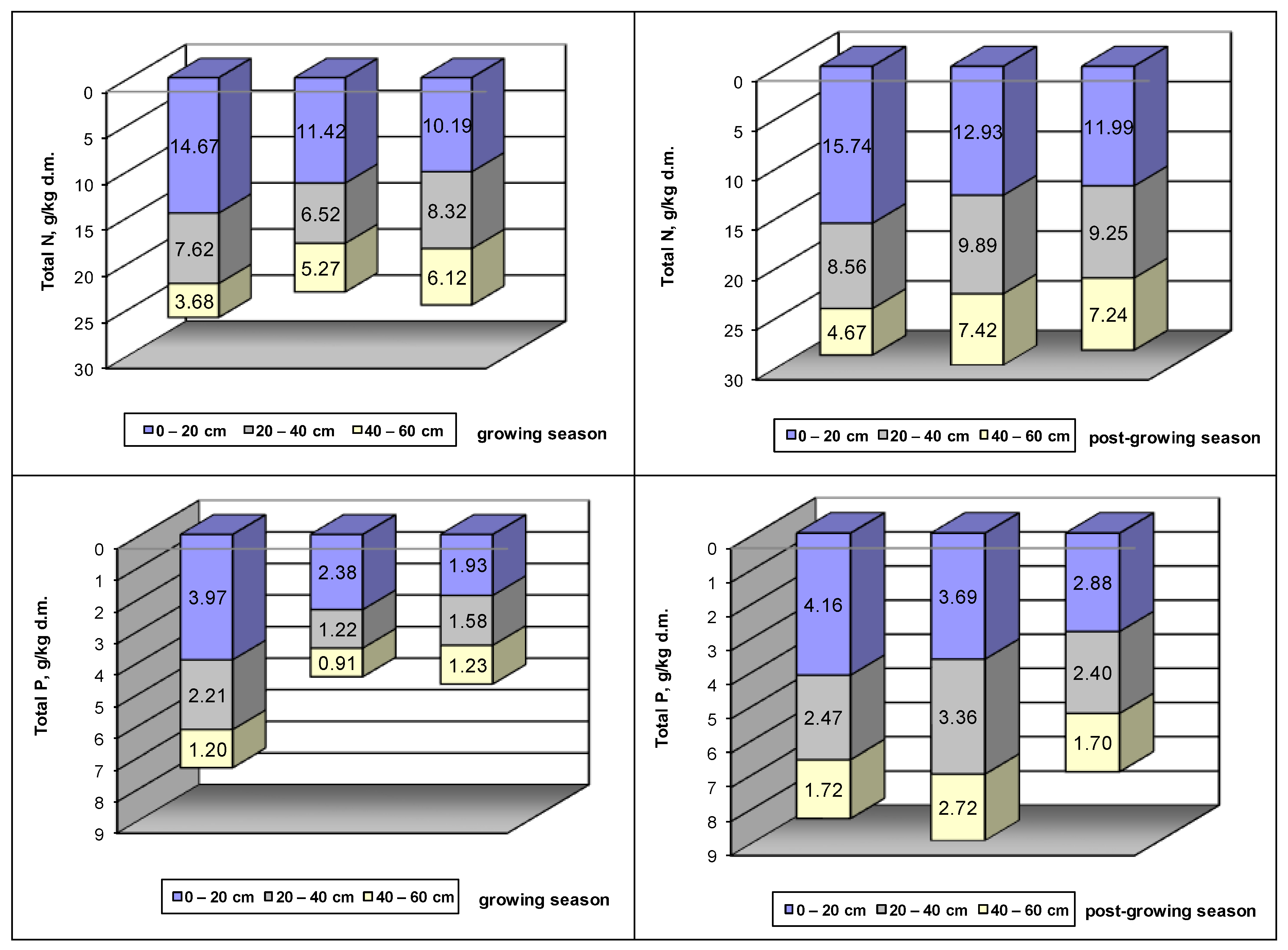
The penetration of nutrients deeper into the hydrophytic subplot increased with decreasing distance to the outflow of sewage from the wetlands. The amount of nitrogen and phosphorus at individual depths of the bed undergoes seasonal changes. The results of the studies carried out showed that, during the post-vegetative period, the content of total nitrogen and total phosphorus in the soil at individual depths was higher than in the vegetative season (
Figure 5). These differences were the highest in the layer of up to 20 cm depth. During the period of full plant development, when the contents of total nitrogen in individual parts of the reed were high, a lower concentration of nitrogen in the wetland bed was noted. In January, the concentration of nitrogen in the plant was low, whereas the nitrogen contents in the wetland bed were the highest for the entire period of the studies. In the off-season, increased penetration of nutrients to deeper layers of the bed was also observed.
The increase in the biomass of reed in the initial part of the bed was higher than in the final part, thus making it possible to conclude that the amount of biomass is directly proportional to the nitrogen content in the ground of the wetlands. The panicle and leaves of reeds, which accumulate the highest amounts of total nitrogen, do not take it up proportionately to the amounts found in the bed. The results of the studies revealed that the nitrogen content in these parts of reeds is higher than in plants taken from the end of the wetlands, hence from the area where it is less abundant in the bed.
The efficiency of the accumulation of phosphorus depends on the filtration properties of the bed as well as the pH of the soil-water environment. The removal effect decreases along with the passing of time and the sorption capacities of the soil being used up. In the new objects, where the removal of phosphorus occurs in the sorption process of phosphorus on the surface of the bed, the efficiency of removing phosphorus compounds may be very high. After this period, the removal of phosphorus takes place only as a result of accumulation on the surfaces in the entire cross-section of the soil.
3.3. Analysis of Statistical Results of the Research
Two-way analysis of variance (ANOVA) in the randomized block design was applied for the statistic assessment of the results of bed and reed samples. The analysis concerned the effect of the influence of factor A, which was assumed as the sample collection location (inflow, middle, outflow), factor B, and the effects of the interaction between these factors in affecting the analyzed property. When preparing the reed sample results, plant organs (rhizomes, stem, leaves) were assumed as factor B. In the analysis of variance for the bed results, factor B indicated the depth of sample collection (0–20, 20–40, 40–60).
The analyses showed a rather strong positive correlation between the accumulation of nitrogen in the rhizome of reed collected at the initial part of the bed and the nitrogen content at individual depths (r = 0.65; r = 0.66; r = 0.71). On the other hand, the nitrogen content in the rhizomes of reeds growing at the end of the hydrophytic subplot was negatively correlated with its amount in the bed, and, compared to the correlation in the initial part of the bed, was rather low (
Table S1).
Analysis of variance showed that from a statistical point of view, the location of collecting reed samples (factor A) did not influence the total nitrogen and total phosphorus contents in individual plant organs. Differences between the nitrogen and phosphorus contents in individual plant parts, however, turned out to be highly significant. The analysis did not also indicate the interactions between the analyzed factors A and B.
The applied multiple comparison test revealed highly significant differences between the average nitrogen content in the leaves and rhizomes as well as in the leaves and stems. Absolute differences for the average contents of nitrogen were 14.25 g/kg d.m. and 16.52 g/kg d.m. respectively, whereas LSD (Least Significant Difference) LSDB = 3.17 gN/kg d.m.
The Tukey test shows that significant differences between the average total phosphorus contents occurred between the following pairs of plant organs: rhizomes and stem, and stem and leaves. Absolute differences in measurements between average values of phosphorus amounted to 0.65 g/kg d.m. and 0.56 g/kg d.m. respectively (LSDB = 0.34 gP/kg d.m.).
The analysis of variance confirmed that the location of sample collection did not significantly diversify the total nitrogen content in the wetland bed (
Table S2). On the other hand, the depth at which the samples were collected had a very significant influence on the average nitrogen content in the collected soil samples. The influence of interaction between the depth and location of sample collection on the nitrogen content was also determined to be highly significant. The applied test of multiple comparisons revealed that significant differences occurred between the average nitrogen content in the bed samples for each pair of depths: 0–20 and 20–40; 0–20 and 40–60; 20–40 and 40–60 cm (LSD
B = 1.89 gN/kg d.m.). At the same time, the highest absolute difference in measurements between averages was noted for nitrogen contained in samples taken from a depth of 0–20 cm and 40–60 cm. The most significant differences in the nitrogen content for these depths occurred in the first part of the bed (the absolute difference was 11.02 g/kg d.m. whereas LSD
A–B = 3.27 gN/kg d.m.). Statistically, large differences in the average measurement of nitrogen content were also observed at the inflow in the 0–20 cm layer, and its accumulation in the middle and at the end of the subplot in the 40–60 cm layer.
The concentration of total phosphorus in the bed samples depended on both the depth of collecting the samples and the location from which they were taken (beginning, middle, or end of the constructed wetland plot). These factors also influence the total phosphorus content in the bed through interaction. The test of multiple comparisons showed significant differences between the average phosphorus content in the bed samples for each pair of depths: 0–20 and 20–40; 0–20 and 40–60; and 20–40 and 40–60 cm. At the same time, the highest absolute difference in the measurement between averages occurred for phosphorus contained in samples collected at depths of 0–20 cm and 40–60 cm (LSDB = 0.49 gP/kg d.m.). The test confirms that significant differences also occurred between the average contents of total phosphorus in the bed samples collected at the inflow and outflow of the bed, as well as between samples collected from the inflow and the center of the subplot (LSDA = 0.49 gP/kg d.m.). Differences between the average concentration of phosphorus in samples collected from the middle and end of the bed, on the other hand, were not statically significant. The most significant differences in the average measurements were observed between the phosphorus content in the initial part of the subplot in the 0–20 cm layer and the accumulation of this element in the 40–60 cm layer, at the beginning, middle, and end of the subplot (LSDAB = 0.84 gP/kg d.m.).