Evaluation of Algae-Based Fertilizers Produced from Revolving Algal Biofilms on Kentucky Bluegrass
Abstract
:1. Introduction
2. Materials and Methods
2.1. Turfgrass Greenhouse Establishment and Maintenance
2.2. Treatment Structure
2.3. Data Collection
2.4. Statistical Analysis
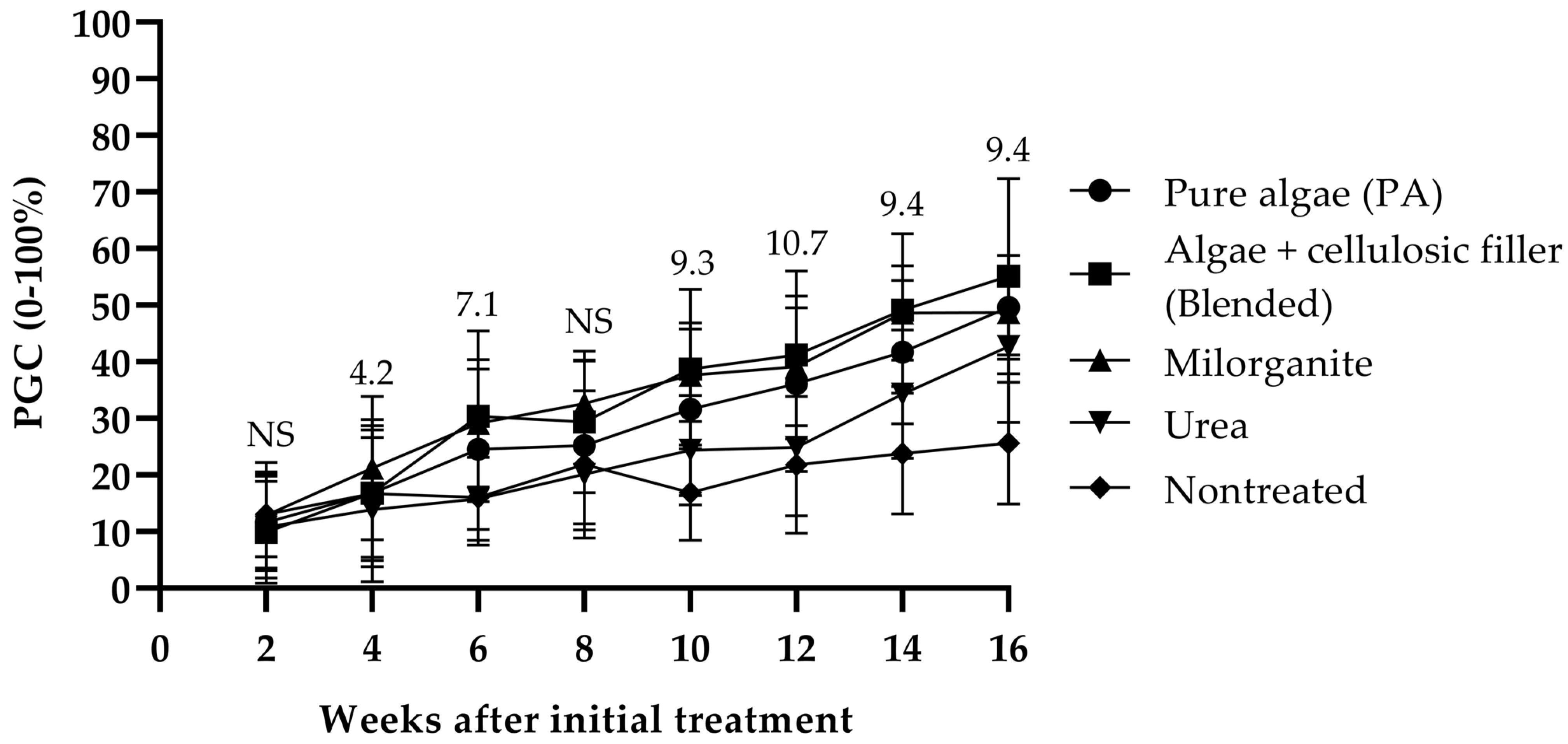
3. Results
4. Discussion
4.1. Effect of Fertilizer on Shoot Growth, Cover, and Color
4.2. Effect of Fertilizer on Root Growth
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- West, T.; Marland, G. A synthesis of carbon sequestration, carbon emissions, and net carbon flux in agriculture: Comparing tillage practices in the United States. Agric. Ecosyst. Environ. 2002, 91, 217–232. [Google Scholar] [CrossRef]
- Carey, R.; Hochmuth, G.; Martinez, C.; Boyer, T.; Nair, V.; Dukes, M.; Toor, G.; Shober, A.; Cisar, J.; Trenholm, L.; et al. A review of turfgrass fertilizer management practices: Implications for urban water quality. HortTechnology 2012, 22, 280–291. [Google Scholar] [CrossRef] [Green Version]
- Gómez-Armayones, C.; Kvalbein, A.; Aamlid, T.; Knox, J. Assessing evidence on the agronomic and environmental impacts of turfgrass irrigation management. J. Agron. Crop Sci. 2018, 204, 333–346. [Google Scholar] [CrossRef] [Green Version]
- Motavalli, P.; Goyne, K.; Udawatta, R. Environmental impacts of enhanced-efficiency nitrogen fertilizers. Crop Manag. 2008, 7, 1–5. [Google Scholar] [CrossRef]
- Soldat, D.; Petrovic, A. The fate and transport of phosphorus in turfgrass ecosystems. Crop Sci. 2008, 48, 2051–2065. [Google Scholar] [CrossRef]
- Marshall, S.; Orr, D.; Bradley, L.; Moorman, C. A review of organic lawn care practices and policies in North America and the implications of lawn plant diversity and insect pest management. HortTechnology 2015, 25, 437–446. [Google Scholar] [CrossRef] [Green Version]
- Khachatryan, H.; Suh, D.; Zhou, G.; Dukes, M. Sustainable urban landscaping: Consumer preferences and willingness to pay for turfgrass fertilizers. Can. J. Agric. Econ. 2017, 65, 385–407. [Google Scholar] [CrossRef]
- Association of American Plant Food Control Officials Official Publication, 71; AAPFCO: Little Rock, AR, USA, 2018.
- Pelletier, N.; Arsenault, N.; Tyedmers, P. Scenario modeling potential eco-efficiency gains from a transition to organic agriculture: Life cycle perspectives on Canadian canola, corn, soy, and wheat production. Environ. Manag. 2008, 42, 989–1001. [Google Scholar] [CrossRef] [PubMed]
- Braun, R.; Bremer, D. Nitrous oxide emissions from turfgrass receiving different irrigation amounts and nitrogen fertilizer forms. Crop Sci. 2018, 58, 1762–1775. [Google Scholar] [CrossRef]
- Guillard, K.; Kopp, K. Nitrogen fertilizer form and associated nitrate leaching from cool-season lawn turf. J. Environ. Qual. 2004, 33, 1822–1827. [Google Scholar] [CrossRef] [Green Version]
- LeMonte, J.; Jolley, V.; Summerhays, J.; Terry, R.; Hopkins, B. Polymer coated urea in turfgrass maintains vigor and mitigates nitrogen’s environmental impacts. PLoS ONE 2016, 11, e0146761. [Google Scholar] [CrossRef] [Green Version]
- Gross, M.; Henry, W.; Michael, C.; Wen, Z. Development of a rotating algal biofilm growth system for attached microalgae growth with in situ biomass harvest. Bioresour. Technol. 2013, 150, 195–201. [Google Scholar] [CrossRef]
- Zhao, X.; Kumar, K.; Gross, M.; Kunetz, T.; Wen, Z. Evaluation of revolving algae biofilm reactors for nutrients and metals removal from sludge thickening supernatant in a municipal wastewater treatment facility. Water Res. 2018, 143, 467–478. [Google Scholar] [CrossRef]
- Peacock, C.; Bowman, D.; Cooper, R. Bermudagrass (Cynodon dactylon x C. transvaalensis ‘Tifway’) response to slow release fertilizers. Int. Turfgrass Soc. Res. J. 2013, 12, 551–559. [Google Scholar]
- Griffith, S.; Bero, N.; Stier, J.; Obear, G.; Ruis, S.; Soldat, D. Biosolids as an alternative fertilizer for Kentucky bluegrass sod production in Wisconsin. Crop Sci. 2017, 57, 227–237. [Google Scholar] [CrossRef]
- Lee, S.; Minner, D.; Christians, N. Evaluation of various slow-release nitrogen sources for growth and establishment of Poa pratensis on sand-based systems. Asian J. Turfgrass Sci. 2010, 24, 145–148. [Google Scholar]
- Badzmierowski, M.; Evanylo, G.; Ervin, E.; Boyd, A.; Brewster, C. Biosolids-based amendments improve tall fescue establishment and urban soils. Crop Sci. 2019, 59, 1273–1284. [Google Scholar] [CrossRef]
- Tian, G.; Granato, T.; Dinelli, F.; Cox, A. Effectiveness of biosolids in enhancing soil microbial populations and N mineralization in golf course putting greens. Appl. Soil Ecol. 2008, 40, 381–386. [Google Scholar] [CrossRef]
- Ham, S.; Kim, Y. Growth effect and nutrient uptake by application interval of developed slurry composting and biofiltration (DSCB) liquid fertilizer on Kentucky bluegrass. Weed Turfgrass Sci. 2014, 3, 362–369. [Google Scholar] [CrossRef]
- Ham, S.; Kim, Y.; Kim, T.; Kim, K.; Park, C. The effect of SCB (slurry compost and biofilter) liquid fertilizer on growth of creeping bentgrasss. Asian J. Turfgrass Sci. 2009, 23, 91–100. [Google Scholar]
- Ham, S.; Kim, Y.; Park, C. The growth effects of creeping bentgrass by SCB (slurry composting and biofilteration) liquid fertilizer application. Asian J. Turfgrass Sci. 2010, 24, 56–61. [Google Scholar]
- Kim, Y.; Ham, S.; Lim, H. Monitoring of soil chemical properties and pond water quality in golf courses after application of SCB liquid fertilizer. Asian J. Turfgrass Sci. 2012, 26, 44–53. [Google Scholar]
- Kim, S.; Kim, D.; Lee, S. Effects of liquid fertilizer produced from fermented clippings for creeping bentgrass growth. Asian J. Turfgrass Sci. 2011, 25, 202–207. [Google Scholar]
- Lee, S. Effects of liquid fertilizer produced from fermented clippings for Kentucky bluegrass. Asian J. Turfgrass Sci. 2012, 26, 67–71. [Google Scholar]
- Fetter, J.; Brown, R.; Amador, J. Effectiveness of squid hydrolysate as a home lawn fertilizer. HortScience 2013, 48, 380–385. [Google Scholar] [CrossRef]
- Carillo, P.; Ciarmiello, L.; Woodrow, P.; Corrado, G.; Chiaiese, P.; Rouphael, Y. Enhancing sustainability by improving plant salt tolerance through macro- and micro-algal biostimulants. Biology 2020, 9, 253. [Google Scholar] [CrossRef]
- Gimondo, J.; Currey, C.; Jarboe, D.; Gross, M.; Graves, W. Wastewater-grown algae pellets and paste as fertilizers for containerized crops. HortScience 2019, 54, 528–536. [Google Scholar] [CrossRef] [Green Version]
- Lindsey, A.J.; Thoms, A.; Christians, N. Kentucky bluegrass and bermudagrass rooting response to humic fertilizers during greenhouse establishment. Agron. J. 2020, 112, 3396–3401. [Google Scholar] [CrossRef]
- Hummel, N. Rationale for the revisions of the USGA green construction specifications. USGA Green Sec. Rec. 1993, 31, 7–21. [Google Scholar]
- USGA. USGA Recommendations for a Method of Putting Green Construction; USGA Green Section: Far Hills, NJ, USA, 2018. [Google Scholar]
- Brosnan, J.; Horvath, B.; Elmore, M.; Breeden, G.; Sorochan, J. Greenhouse investigation of strobilurin fungicide applications on creeping bentgrass root characteristics under two irrigation regimes. Crop Sci. 2010, 50, 2605–2612. [Google Scholar] [CrossRef]
- Archer, M. Marketing biosolids: The experience of Milorganite with special reference to Canada. In Proceedings of the Wastewater Biosolids Sustainability: Technical, Managerial, and Public Synergy, Moncton, NB, Canada, 24–27 June 2007; pp. 1017–1019. [Google Scholar]
- What Is Milorganite? Available online: https://www.milorganite.com/using-milorganite/what-is-milorganite (accessed on 5 May 2021).
- Thoms, A.; Sorochan, J.; Brosnan, J.; Samples, T. Perennial ryegrass (Lolium perenne L.) and grooming affect bermudagrass traffic tolerance. Crop Sci. 2011, 51, 2204–2211. [Google Scholar] [CrossRef]
- Richardson, M.; Karcher, D.; Purcell, L. Quantifying turfgrass cover using digital image analysis. Crop Sci. 2001, 41, 1884–1888. [Google Scholar] [CrossRef]
- Karcher, D.; Richardson, M. Quantifying turfgrass color using digital image analysis. Crop Sci. 2003, 43, 943–951. [Google Scholar] [CrossRef]
- Aamlid, T.; Hanslin, H. Evaluation of organic fertilizers and biostimulants on sand-based golf greens and football pitches under Scandinavian climate conditions. Int. Turfgrass Soc. Res. J. 2009, 11, 919–931. [Google Scholar]
- Green, B. Nitrogen Release Characteristics of Commercial Organic Fertilizers in Turfgrass. Master’s Thesis, Auburn University, Auburn, AL, USA, 2011. [Google Scholar]
- Acikgoz, E.; Bilgili, U.; Sahin, F.; Guillard, K. Effect of plant growth-promoting Bacillus sp. on color and clipping yield of three turfgrass species. J. Plant Nutr. 2016, 39, 1404–1411. [Google Scholar] [CrossRef]
| Treatment | Nitrogen % | Phosphorus % | Potassium % | Calcium % | Iron % |
|---|---|---|---|---|---|
| Pure algae (PA) | 5.6 | 1.9 | 0.3 | 3.8 | 1.6 |
| Algae + cellulosic filler (Blended) | 2.9 | 1.1 | 0.2 | 2.3 | 1.8 |
| Milorganite | 6.0 | 1.7 | 0 | 1.2 | 2.5 |
| Urea | 46.0 | 0 | 0 | 0 | 0 |
| Nontreated | 0 | 0 | 0 | 0 | 0 |
| Treatment 1 | DGCI (0–1) 2 | |||||||
|---|---|---|---|---|---|---|---|---|
| Weeks after Initial Treatment | ||||||||
| 2 | 4 | 6 | 8 | 10 | 12 | 14 | 16 | |
| Pure algae (PA) | 0.56 (+/−0.02) 3 | 0.56 (+/−0.03) | 0.55 (+/−0.02) | 0.54 (+/−0.04) | 0.54 (+/−0.03) | 0.53 (+/−0.03) | 0.53 (+/−0.02) | 0.54 (+/−0.03) |
| Algae + cellulosic filler (Blended) | 0.56 (+/−0.02) | 0.54 (+/−0.03) | 0.56 (+/−0.02) | 0.54 (+/−0.03) | 0.55 (+/−0.02) | 0.53 (+/−0.02) | 0.54 (+/−0.02) | 0.55 (+/−0.03) |
| Milorganite | 0.57 (+/−0.02) | 0.54 (+/−0.02) | 0.54 (+/−0.02) | 0.52 (+/−0.03) | 0.52 (+/−0.02) | 0.51 (+/−0.02) | 0.52 (+/−0.02) | 0.53 (+/−0.02) |
| Urea | 0.56 (+/−0.02) | 0.54 (+/−0.03) | 0.53 (+/−0.03) | 0.53 (+/−0.04) | 0.53 (+/−0.03) | 0.52 (+/−0.02) | 0.55 (+/−0.02) | 0.56 (+/−0.03) |
| Nontreated | 0.56 (+/−0.03) | 0.53 (+/−0.03) | 0.51 (+/−0.03) | 0.51 (+/−0.04) | 0.50 (+/−0.04) | 0.50 (+/−0.03) | 0.51 (+/−0.03) | 0.53 (+/−0.03) |
| LSD0.05 4 | NS 5 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 | 0.02 |
| Treatment 1 | Total Root Length 2 cm | Root Surface Area 2 cm2 | Root Volume 2 cm3 | Longest Root 3 cm | Root Biomass 4 g | Longest Shoot 3 cm | Shoot Biomass 4 g |
|---|---|---|---|---|---|---|---|
| Pure algae (PA) | 2403.3 (+/−633.4) 5 | 176.4 (+/−50.0) | 1.1 (+/−0.5) | 46.8 (+/−8.5) | 0.12 (+/−0.06) | 4.8 (+/−1.1) | 0.12 (+/−0.07) |
| Algae + cellulosic filler (Blended) | 2868.3 (+/−452.1) | 231.9 (+/−76.2) | 1.6 (+/−0.9) | 48.5 (+/−4.8) | 0.16 (+/−0.04) | 4.7 (+/−1.6) | 0.14 (+/−0.07) |
| Milorganite | 2655.9 (+/−265.5) | 230.7 (+/−67.4) | 1.7 (+/−1.1) | 48.3 (+/−4.3) | 0.14 (+/−0.06) | 4.7 (+/−0.9) | 0.14 (+/−0.10) |
| Urea | 1758.5 (+/−366.0) | 130.1 (+/−36.4) | 0.8 (+/−0.4) | 39.6 (+/−11.3) | 0.11 (+/−0.03) | 6.4 (+/−2.5) | 0.10 (+/−0.03) |
| Nontreated | 1252.7 (+/−496.5) | 93.6 (+/−62.5) | 0.6 (+/−0.6) | 31.5 (+/−6.3) | 0.07 (+/−0.04) | 2.6 (+/−0.9) | 0.07 (+/−0.08) |
| LSD0.05 6 | 473.0 | 44.9 | 0.4 | 8.0 | 0.05 | 1.7 | 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lindsey, A.J.; Thoms, A.W.; Dancer, J.; Gross, M. Evaluation of Algae-Based Fertilizers Produced from Revolving Algal Biofilms on Kentucky Bluegrass. Agronomy 2021, 11, 1288. https://doi.org/10.3390/agronomy11071288
Lindsey AJ, Thoms AW, Dancer J, Gross M. Evaluation of Algae-Based Fertilizers Produced from Revolving Algal Biofilms on Kentucky Bluegrass. Agronomy. 2021; 11(7):1288. https://doi.org/10.3390/agronomy11071288
Chicago/Turabian StyleLindsey, Alex J., Adam W. Thoms, Jens Dancer, and Martin Gross. 2021. "Evaluation of Algae-Based Fertilizers Produced from Revolving Algal Biofilms on Kentucky Bluegrass" Agronomy 11, no. 7: 1288. https://doi.org/10.3390/agronomy11071288
APA StyleLindsey, A. J., Thoms, A. W., Dancer, J., & Gross, M. (2021). Evaluation of Algae-Based Fertilizers Produced from Revolving Algal Biofilms on Kentucky Bluegrass. Agronomy, 11(7), 1288. https://doi.org/10.3390/agronomy11071288






