Abstract
The increasing quantities of organic residues are becoming one of the most important problems for climate change mitigation. Sustainable utilization technologies are required to minimize the effect of recycling on the environment. Nevertheless, treated residues should be part of the circular bioeconomy. One of the most promising processes is the biogas system, with the final products biogas and digestate, which contain valuable nutrients and are therefore suitable as agricultural fertilizers. However, there is lack of research data on the effectiveness of digestate on environmental factors including soil quality as well as crop productivity and quality. In this study, we compare the roles of different digestates (chicken manure digestate, cow manure digestate, and pig manure digestate) on spring wheat productivity, soil microbial activities, and greenhouse gas emissions in loam and sandy loam soil under controlled climate conditions. The liquid digestate applied was equivalent to 170 kg N ha−1 of total N presented. Overall, results showed that the two soil types responded differently to the addition of the digestates, and the benefits depended on soil characteristics as well as on the type of the digestate applied. There was a higher effect on soil microbial activity in sandy loam soil compared to that of loam soil. Chicken manure digestate had the highest value of dehydrogenase activity and soil microbial biomass C of 9.23 µg TPFg−1 h−1 and 175.6 µg g−1 across the two soil types. CO2 and N2O emissions were moderately higher in loam soil when compared to that of sandy loam soil. The highest CO2 peak emission at 0.0107 µg ha−1 h−1 occurred in pig manure digestate in the sandy loam soil, and regular peak patterns observed in loam soil fertilized with pig digestate manure. Chicken manure digestate had the highest peak emissions across both soil types at 0.007950 mg ha−1 h−1 and 0.5667 mg ha−1 h−1 in the loam and sandy loam soil, respectively. The biomass yield varied across the soil types irrespective of the digestate applied. The agricultural benefits of digestates in different receiving soil ecosystems supplying essential nutrients for crop productivity, coupled with its environmental benefits, makes it an encouraging prospect in temperate climate zones.
1. Introduction
The sustainable utilization of agricultural wastes continues to receive global attention as it aims to reduce the negative environmental impact of wastes and can contribute to proper waste handling processes [1]. These organic wastes are processed through various methods, but the anaerobic digestion (AD) appears to be one of the most promising [2].
The recycling of animal wastes (manure) holds more benefits when treated anaerobically in biogas plants when compared to that of direct application of untreated animal wastes in agricultural fields [3,4]. The animal waste digestate were known to show better microbial stability and a higher amount of nitrogen in the form of ammonium compared to that of undigested agricultural, animal, or other biological wastes [5]. In addition, the amendment of digestate to agricultural soils facilitates soil mineralization due to their relatively low amount of organic matter and highly stable organic substances [5]. They are compounds with a relatively lower molecular weight and low C:N ratio. The content of nutrients in digestate depends on the composition of the processed feedstock. There are essential features that enable the animal waste digestate to be qualified as fertilizer, and this includes but is not limited to nutrient composition and availability, pH, dry matter and organic dry matter content, homogeneity, and absence of biological and chemical contaminants [6]. However, the use of fertilizers was linked to the environmental issues, presenting more benefits and no damage to the environment. This study was planned to analyze the effect of different types of digestate used for crop fertilization on environmental issues—change of microbial activity, soil quality, and greenhouse gas (GHG) emissions.
Aside from the roles played by the nutrients present in organic fertilizers, soil microbial components play significant roles in nutrient recycling and carbon sequestration. Soil microbial communities are composed of a wide variety of groups that adapt their abundance and activity to environmental factors. The macro and micronutrient content of digestate is not only important for the crops, but also for soil microorganisms, since it contains growth promoters and hormones. Therefore, it could be used for stubble remains to facilitate their decomposition. Jastrow et. al. [7] noted that for reducing soil organic carbon (SOC) turnover and enhancing sequestration, there is a need to modify the soil physicochemical environment to benefit the activities of microbes. The controlled amendment of soil with quality assured digestates would help to provide a conducive environment for the microbes to thrive, and in the long-term, help to attain the desired accumulation of SOC through the harmonization of inputs and outputs from soil organic matter. The biological activity of these communities plays an essential role in the geochemical transformations of organic matter, and consequently, on soil fertility [8]. The use of animal waste digestate to complement the use of mineral fertilizers and the effect of agricultural practices on the different soil types is not deeply consistent [9,10]. However, based on the different soil types and digestate properties, the influence of digestate treatments on soil microbiota can vary depending on the pedoclimate conditions and the agricultural plan employed. Hence, under a well-managed system, digestates can be applied safely to soil [11,12,13,14]; however, some negative effects of digestate application were reported on soil microbial activities [15,16]. The high content of potassium in pig digestate is a good example of this negative effect, since at a higher concentration it is toxic to the microbial cell wall. Digestates may be toxic because they possess inhibitory substances to soil microbes, resulting in lower rates of ammonia oxidation and denitrification. [17,18,19]. Therefore, each type of digestate must be examined due to its effect on soil microbial activity.
The provision of readily available nutrients to plants is encouraging the application of digestate to agricultural soils for crop growth, therefore reducing the use of inorganic fertilizers and GHG emissions during its production [20]. The use of inorganic fertilizers is a major contributor to GHG emissions in agriculture due to very high GHG emissions in synthetic fertilizer production and use of nonrenewable sources; therefore, the use of digestates helps to change this trend by minimizing the use of mineral fertilizers. However, the direct GHG emissions from cropland where organic fertilizers are used are usually higher. The application of digestate in particular, and organic fertilization in general, comes with its own negative consequences on the environment because of its potential to cause higher direct N2O [6] and CO2 emissions compared to that of mineral fertilizers [21,22]. The enhanced release of GHGs due to organic fertilization can be attributed to the fact that the combined supply of easily degradable organic carbon and nitrogen (N) stimulate microbial growth, C-decomposition, and C-mineralization, as well as nitrification and denitrification [23]. Besides, N2O emissions are enhanced by high organic matter content as the availability of carbon and N usually increases denitrification rates. N2O can be produced both during nitrification and denitrification, although increased denitrification rates do not necessarily lead to higher N2O emissions. There is still a knowledge gap in how the relatively high organic matter content contained in the digestates with volatile, high NH4+ and low NO3− affects emissions as it relates to different soil types. Methane emissions would be expected to be low when digestates are applied to aerobic soils, but as they contain methanogenic bacteria, they may still induce emissions. Therefore, more attention must be given to GHG emission factors after application of different types of digestate on different soil types.
Due to the readily available rich nutrient content, digestate application resulted in significantly higher aboveground biomass yields in the case of winter wheat and spring wheat than that of the farmyard manure and undigested slurry treatment [24]. Similarly, regarding the effect of liquid digestate and the equal quantity of water to the yield of sweet corn and silage maize, significantly higher yields were found in the digestate treatment [25,26]. However, usually only long-term use of digestate has a significant effect on productivity. Nevertheless, there is limited information about the possibilities of using digestate for crop fertilization and its influence on crop quality in different soil environments. The insufficient information is mainly observed in northern part of temperate climate zone, where biogas production technologies came later than in the southern part. Some researchers studied the influence of digestates in specific soil types, where positive effects of digestates were reported, ranging from GHG mitigation to improved soil health parameters [23,25]. However, there is a need to bridge the knowledge gap on how the nitrogen sources from the digestates can influence GHG and microbial activities in loam and sandy loam soil types.
We hypothesized that the use of digestate for the fertilization of crops grown in loam and sandy loam will increase soil microbial activity and improve soil quality, resulting in increased crop productivity and no significant differences between digestate type.
The aim of the present research was to understand the underlying mechanisms that distinguish the nitrogen source contained in the digestate by: (i) evaluating the effect of the digestates on microbiological activity in the two different soil types, (ii) determining the influence of the digestates on GHG emissions in the two different soil types, and (iii) understanding the effects of the digestates on selective soil chemical composition, spring wheat growth, and biomass yield.
The results of this study are linked to better understanding of the environmental and economic sustainability of biogas production (sustainable residue treatment) in line with effective digestate utilization. The results could be used for the preparation of recommendations for agricultural as well as environmental specialists. There is also basic information for further research, i.e., analyzing the microbial activity by identifying the microbial communities in the soil and determining their effect on soil and crop quality and the environment.
2. Materials and Methods
2.1. Experimental Design and Treatments
The experiment was conducted in 2020 (January to April) at the Agrobiology laboratory of Lithuanian Research Centre for Agriculture and Forestry. The two soil samples used for the pot trials were collected from the top layer (0–20 cm) of two different fields: one in the experimental site of Vytautas Magnus University, Agricultural Academy (54°53′03.5″ N 23°50′13.5″ E), and the second from a fallow domestic farmyard with no agricultural activities (54°59′09.1″ N 23°29′46.9″ E). The visible plant debris and stones were manually removed from the field moist soil samples. Then, the remaining soil was thoroughly mixed, air-dried at room temperature, passed through a 2-mm sieve, and adjusted to 40% water holding capacity (WHC) by adding deionized water. The experiment was carried out at controlled conditions. Each experimental ten-liter pot was filled with 12 kg of sandy loam textured (SLT) and loam textured (LT) soils, and spring wheat (Triticum aestivum) variety Collada (Einbeck, Germany) was sown using 40 seeds per pot. Pots were randomized, with three replications of each digestate treatment in the two soil types. Additionally, three pots without any fertilizer material were used as control.
The experimental treatments were as follows: nontreated, pig manure digestate, chicken manure digestate, and cow manure digestate applied to the sandy loam and loam soil. The additional control with synthetic nitrogen fertilizer treatment for the loam soil was selected to identify the differences between different types of fertilizers and the nontreated pot (as soils were rich in P and K, no additional synthetic mineral PK fertilizers were added). The digestate application dose for each treatment was 0.16 l for pig manure digestate, 0.16 l for chicken manure digestate, and 0.25 l for cow manure digestate, which corresponded with the total surface area per pot (0.049 m2). For each digestate application, the rate of digestate was calculated according to the respective content of total nitrogen. The experimental pots were stored under controlled climate conditions in laboratory test chambers (CLIMACELL 707, MMM Medcenter Einrichtungen GmbH, Munich, Germany). Chamber parameters were set as appropriate for wheat growth—for a day (12 h) and night (12 h) mode as follows: temperature 23 ± 0.5 °C during the day and 18 ± 0.5 °C during the night, RH 68 ± 2%, fan mode on 100%, light on during the day and light off during the night. Water was regularly added to the pots to maintain the water content at 50% water holding capacity.
2.2. Determination of Soil Physicochemical Properties
The chemical composition of the soil sampled from the field at the beginning of the field trial was determined (as illustrated in Table 1). The content of mobile K2O, mobile P2O5, Ca, and Mg in the soil was determined using ammonium lactate-acetic acid extraction by the Egner, Riehm, and Domingo (A-L) method. The determination of soil acidity (pH) was made in 1:5 (vol−1) soil suspension in the 1 M KCl solution [27]. The same analyses were made after the experiment, and changes in soil chemical composition after the experiment are presented in the results section. To characterize the soil, the analysis of soil texture was made before the experiment using the pipette method (Eijkelkamp equipment) and results are presented in Table 2.

Table 1.
Chemical composition of soil (0–20 cm layer) before fertilization.

Table 2.
Soil texture.
2.3. Digestate Analysis
The digestates were obtained from agro-industrial biogas plants at three locations in Lithuania, with the treatments used serving as their primary feedstocks. The digestates were spread on the soil surface without injection on the treatment pots. The chemical composition of the digestates applied were analyzed, and results presented in Table 3. A chemical analysis of the digestates used in the pot experiments was carried out. A flame photometer (FP) (Sherwood, Cambridge, UK) for mobile potassium K; a UV-VIS spectrophotometer (Shimadzu, Duisburg, Germany) for mobile phosphorus P; total nitrogen (Ntot) was determined using the Kjeldahl nitrogen distiller method, while organic carbon (Corg) was determined using a dry combustion method with total carbon analyzer Liquid TOC II. Electrical conductivity was determined using a thermo Orion stara2150 star A215 Benchtop conductivity meter.

Table 3.
Digestate physicochemical parameters.
2.4. Soil Microbial Activity Analysis
2.4.1. Soil Dehydrogenase Enzyme Activity Measurements
Dehydrogenase activity was determined according to the method [28]. 20 g of air-dried soil and 0.2 g of CaCO3 were thoroughly mixed. Six g each of this mixture was dispensed into the three test tubes (three replicates of each sample). To each tube, 1 mL of 3% 2,3,5-triphenyl tetrazolium chloride (TTC) aqueous solution and 2.5 mL distilled water were added. The contents of each tube were mixed with a glass stirring rod, then the tube was stoppered and incubated at 37 °C for 24 h. After 24 h, the stopper was removed, 10 mL methanol was added, and the tube was shaken for 1 min using a stirrer (IKA, Germany). The filtrate was transferred into a glass funnel and filtered into a 50 mL volumetric flask. The filtrate was diluted through the funnel to a 50 mL volume into the volumetric flask using methanol. The intensity of the red color was measured using a UV-Vis spectrophotometer (Shimadzu, Kyoto, Japan) using a wavelength of 485 nm in a 1 cm cuvette with methanol as a blank reference. The amount of triphenyl formazan (TPF) produced by soil samples was estimated using the calibration curve prepared by 5–50 µg mL−1 of TPF.
2.4.2. Soil Microbial Biomass Carbon (SMB-C) Determination Using Fumigation Extraction Method
Soil Microbial Biomass Carbon was determined using the fumigation-extraction method [29]. 20 g of soil was fumigated via exposing the soil to the alcohol-free CHCl3 vapor in a sealed vacuum desiccator for 24 h. The fumigated soil was evacuated repeatedly in a clean empty desiccator until the odor of CHCl3 was no more detected and then extracted with 80 mL of 0.5 M K2SO4 (soil: K2SO4 = 1:4) for 30 min by oscillating shaking at 200 rpm, and then filtered through a Whatman No. 42 filter paper. Organic carbon content in the extracts was measured using the dichromate digestion method. Two mL of K2Cr2O7 (66.7 mM) and 15 mL of the digestion mixture (2:1 conc. H2SO4:H3PO4 (v/v)) were added to 8 mL of extract in a 250 mL conical flask. The mixture was gently refluxed for 30 min, allowed to cool, and was then diluted with 20 mL distilled water. The excess K2Cr2O7 was measured by titration with ferrous ammonium sulfate (40.0 mM) using a 1.10-phenanthroline-ferrous sulfate complex (25 mM) solution as an indicator. The extraction of nonfumigated soil was the same as that of the fumigated soil. SMB-C was calculated from the differences in extractable organic carbon (OC) between the fumigated and nonfumigated soil sample with a conversion factor (KEC) of 0.38 [29].
SMB-C was calculated as
where Ec = (OC extracted from fumigated soil) − (OC extracted from nonfumigated soil) and KEC = 0.38.
SMB-C = Ec/KEC,
2.5. Measurement of CO2 and N2O
The GHG fluxes from the soil were measured by the static chamber gas chromatography technique with slight modifications [30]. The chamber was a cylindrical-shaped groove with a 19.5 cm diameter that was permanently fixed to the pots. There was rubber top with in-built fan for proper mixture of the air chamber and a small hole for installing a pierceable rubber septum. The gas samples were taken using a well-sealed 20 cc syringe through this pierceable rubber septum. The fluxes of CO2 and N2O were measured at one-week intervals from the start of the cultivating season. For sampling, gas samples were taken through the rubber septum placed on the top of the frame. There was a time interval of 10 min to allow for the proper mixture of the air chamber before expelling the air samples into the vials using a polypropylene 20 mL volume syringe. The samples were analyzed with a gas chromatograph (HP 6890 Series, GC System, Hwelett, Packard, Palo Alto, CA, USA) equipped with flame ionization (FID) and electron capture detectors (ECD), and nickel catalyst was used for converting CO2 to CH4. The temperatures of the GC oven, FID, and ECD were 70 °C, 300 °C, and 350 °C, respectively. The gas chromatography procedures were as described by [30]. Standard temperature and pressure (STP) conditions were assumed, as the temperature had a similar trend in the chamber, and the molar volume of air was assumed as 22.4 L. The flux rate of each GHG was calculated based on the rate of change in GHG concentration within the chamber, estimated as the slope of the linear regression between the GHG concentration and gas sampling time. The CO2 and N2O fluxes were determined from each plot by the following closed-chamber equation [31,32,33]:
where F is the CO2 flux (μg ha−1 h−1), or N2O flux (mg ha−1h−1), ΔC/Δt is the rate of change of CO2 or N2O concentration inside the chamber, A is the surface area of the chamber, VC is the total volume of the chamber corrected for temperature, Mmol is molar mass of CO2 or N2O gas accumulation, and Vmol is the molar volume of N2O inside the chamber corrected for the air temperature using the ideal gas law.
F = ΔC/Δt Vc/A Mmol/Vmol
2.6. Plant Test
Physiological parameters of plants—chlorophyll index and fluorescence—were measured together every two weeks with five SPAD and fluorescence measurements taken per leaf and averaged. The chlorophyll index was measured with a MINOLTA SPAD 502 instrument based on the ability of chlorophyll to absorb blue (400–500 nm) and red (600–700 nm) waves. The level of absorption is converted to a numerical index of chlorophyll content in the leaves. Fluorescence is measured with a chlorophyll fluorimeter OS -30p. Fluorescence shows the photoactivity of chlorophyll—the transfer of electrons in the second photosystem—and shows how efficiently the energy of photosynthesis is used [34]. All the nondestructive measurements were made by placing the equipment on plant leaves (five measures per each pot).
At the end of the experiment (100 days), plants were harvested for the determination of total biomass yield of the crop. The collected samples were weighed. Samples were oven dried at 105 °C until constant weight to determine the dry mass of biomass.
2.7. Statistical Analysis
One way analysis and Two-way analysis of variance (ANOVA) with Duncan’s multiple range tests were calculated using the SAS software package, version 9.3 (SAS Institute Inc., Cary, NC, USA) (p ≤ 0.05) to identify the significance and possible interactions of the soil types and factor treatments. Mean ± SE (standard error of the mean) was used to describe the variability of measurements. The normality of the distribution of gas emissions was tested, and to verify the normality of the data, we used the Shapiro–Wilk test with significance level α = 0.05
3. Results
3.1. The Changes of Soil Selected Chemical Properties after Application of Different Types of Digestate
3.1.1. The Soil Chemical Composition in Loam Soil after the Experiment
In comparing the organic carbon content in all the treatments after harvesting, carbon content decreased from 1.70% before the experiment to 0.93–1.10% after experiment. The highest concentration of organic carbon was observed in treatments with pig manure digestate, while the lowest was in pots applied with synthetic nitrogen fertilizers (as illustrated in Table 4). The nitrogen content in the loam soil before the experiment was 0.164%. As shown in Table 4, the concentration of this element decreased during the experiment in all treatments. The lowest values were observed in control treatment and the one applied with chicken manure digestate. The highest nitrogen content was in the treatment applied with pig manure digestate. For the P2O5, the control treatment was significantly different from the other treatments with the highest P2O5 changes. There was a higher K2O content in treatments applied with all types of manure digestate in the loam soil compared to that of the control (as illustrated in Table 4).

Table 4.
Soil (loam) chemical composition after experiment.
3.1.2. The Influence of Different Types of Digestate on Changes in Loam and Sandy Loam Soil Chemical Composition during the Experimental Period
The pH in the two soil types were generally slightly alkaline and depended on the digesting process during crop cultivation, mainly by the degradation of volatile fatty acids and the production of ammonia (NH3) during the process (as illustrated in Table 1). The alkaline pH of digestate is an effective tool for the problem of soil acidification. The digestate application did not cause any significant changes in the soil pH (data not presented).
Organic carbon content in the loam soil decreased in all the digestate treatments, with the lowest decrease in pig manure digestate. In the sandy loam soil, organic carbon content decreased in cow manure digestate, while there were increases in the pig manure digestate and chicken digestate (as illustrated in Figure 1a).
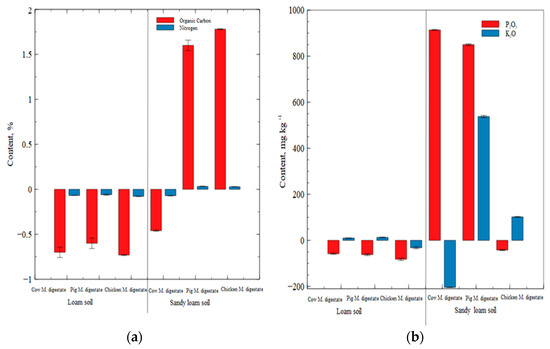
Figure 1.
(a) Changes in soil organic carbon (%) and total nitrogen (%). (b) Changes in soil phosphorus (P2O5, mg kg−1) and potassium K2O, mg kg−1) during experiment. For all variables, significant differences at p < 0.05 were detected when comparing soil types (Factor A), fertilizers (Factor B), and A × B interaction.
During the cultivation period, nitrogen content decreased across the loam soil type irrespective of the digestates applied while in the sandy loam soil; N content increased, except for cow manure digestate treatment which decreased (as illustrated in Figure 1a).
Phosphorus content decreased across all the digestates in the loam soil, while there were significant increased changes in phosphorus in sandy loam fertilized soil, with an exception for chicken manure digestate treatment (as illustrated in Figure 1b). Distribution of potassium slightly increased in the cow manure digestate and pig manure digestate treatment in the loam soil with a decrease in treatment applied with chicken manure digestate. In the sandy loam soil, potassium decreased in pots applied with cow manure digestate, with significant increase after the application of pig manure digestate with the supposedly higher content of potassium, which comes from digestate available for plants (as illustrated in Figure 1b).
3.2. Soil Microbial Activities
Comparing the soil microbial activity between different types of fertilizer in the loam soil, lower SMB-C and DHA values in the nonfertilized control were observed compared to that of other treatments with the highest DHA value observed in chicken manure digestate and highest SMB-C value found in cow manure digestate treatments (as illustrated in Table 5.

Table 5.
Effect of fertilization treatment on soil microbial activities in loam soil: dehydrogenase activity and soil microbial biomass-carbon.
Soil DHA in the synthetic nitrogen treatment was comparable to that of non-fertilized control, while the SMB-C value in the synthetic nitrogen fertilizer was equal to that of the chicken manure digestate treatment, but less than in cow and pig manure digestate treatments.
In comparing the digestate application in the two soil types, SMB-C and DHA values were observed to be higher in the sandy loam soil fertilized with digestates compared to that of loam soil (as illustrated in Table 6). The least soil DHA value was observed in cow manure digestate in the loam soil and highest in chicken manure digestate application in sandy loam soil. The lowest SMB-C values were observed in chicken manure digestate fertilization in loam soil, while the highest was observed in sandy loam soil fertilized with chicken manure digestate (as illustrated in Table 6). There was significant interaction at p < 0.05 between the soil type and the digestate.

Table 6.
Effect of digestate fertilization on soil microbial activities in two soil types: dehydrogenase activity and soil microbial biomass-carbon.
3.3. Soil GHG Emission
3.3.1. Carbon Dioxide Emission
The control soil performed low CO2 instantaneous emissions throughout the incubation period in the loam soil, attaining about 0.00766 µg ha−1 h−1 emissions on average. In this frame, the pig manure digestate showed the highest emission rate immediately after fertilization at day 7. Pig manure digestate showed higher CO2 emissions at the different observed measuring days. Pig manure digestate was significantly different at p < 0.05 from other treatments at days 7, 27, 34, 41, 63, and 76. There were also significant differences (p < 0.05) in CO2 emissions between the synthetic nitrogen and the other treatments in the loam soil at days 7, 56, and 90 over the course of the measuring period (as illustrated in Figure 2).
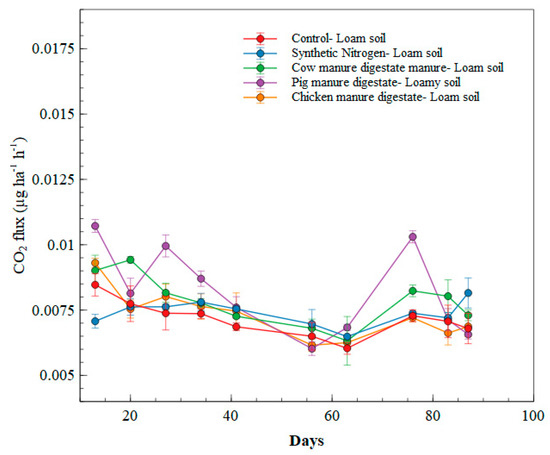
Figure 2.
CO2 emission flux of treatments in loam soil.
In comparing the digestates applied to loam and sandy loam soil, the highest rate of CO2 emissions was observed in the sandy loam soil fertilized with pig manure digestate with irregular peaks observed. In particular, emissions were higher on day 20 in the sandy loam soil fertilized with pig digestate manure at >0.01296 µg ha−1 h−1 (as illustrated in Figure 3). Emissions trends show a similar behavior for the cow digestate treatment in both soil types (as illustrated in Figure 3). The lowest emissions were observed in the loam soil fertilized with pig manure digestate and chicken manure digestate between days 50 and 65 when compared to that of sandy loam soil.
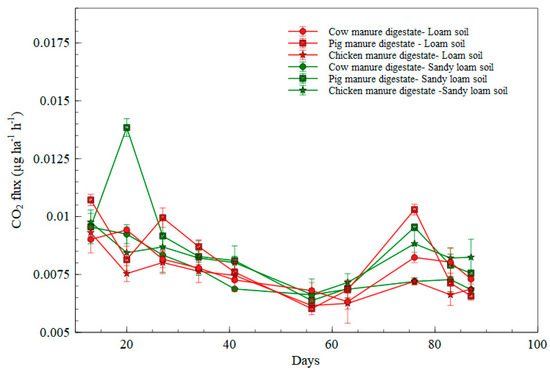
Figure 3.
CO2 emission flux for digestate treatments in loam and sandy loam soil.
3.3.2. N2O Emission
The emission rates of N2O in loam fertilized soil were generally < 0.00589 mg ha−1 h−1, except in the chicken manure digestate which had an emission rate of 0.00813 mg ha−1 h−1 (as illustrated in Figure 4). The N2O emission rates peaked between days 5 and 20 in all the treatments. At day 40, N2O emission rates flattened out till the end of the cultivation period, with emission rates generally < 0.32739 mg ha−1 h−1. Chicken manure digestate was significantly different (p < 0.05) from other treatments at days 20, 27, 34 and 41.
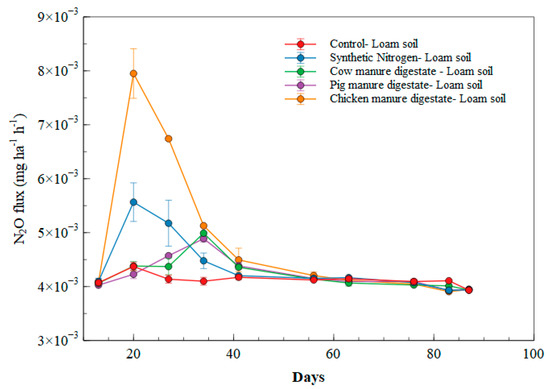
Figure 4.
N2O emissions in loam soil.
In comparing N2O emissions of the digestates treatments in the soil types, there were peaks in all the digestates treatments in both soil types at day 20, with the highest peak observed in the loam soil fertilized with chicken manure digestate treatment which had an emission rate of 0.008135 mg ha−1 h−1 (as illustrated in Figure 5). N2O emissions in all the treatments in sandy loam soil were lower at day 34 when compared to that of emissions in loam soil. N2O emission rates in all the digestates treatment in the loam soil and sandy loam soil flattened out at day 40 to the end of the cultivation period.
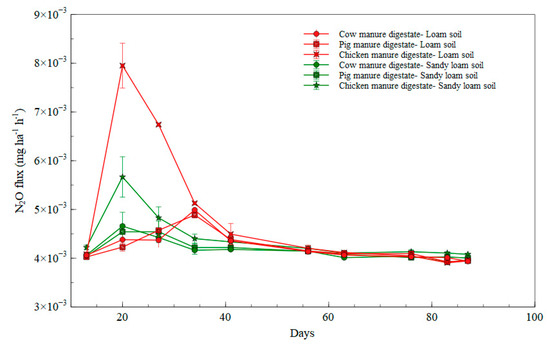
Figure 5.
N2O emissions in digestate treatments in loam and sandy loam soil.
3.4. Results for Plant Measurement
Physiological Parameters in Plant Foliage
In evaluating the chlorophyll index of wheat fertilized with digestates and synthetic nitrogen fertilizer in the loam soil, the obtained results showed a higher chlorophyll index in synthetic nitrogen fertilized treatment and a consistently higher chlorophyll index measurement in the synthetic nitrogen treatment all through the cultivation period than that of the digestate treatments (as illustrated in Figure 6).
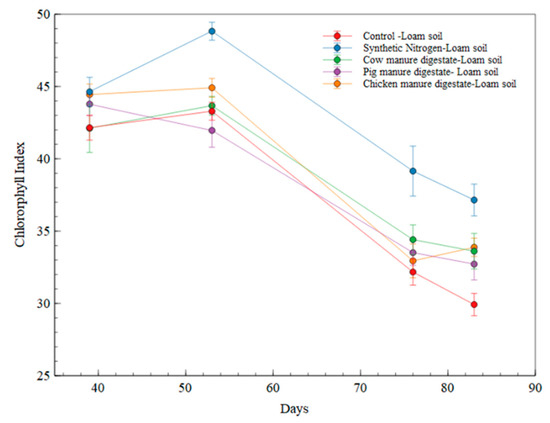
Figure 6.
Chlorophyll Index measurement in loam soil fertilized treatments.
In comparing digestates treatment in the two soil types, chlorophyll index was higher in the loam soil across all the digestates than that of the sandy loam soil. Cow manure digestate and chicken manure digestate in sandy loamy soil had the lowest chlorophyll index throughout the measurement period (as illustrated in Figure 7).
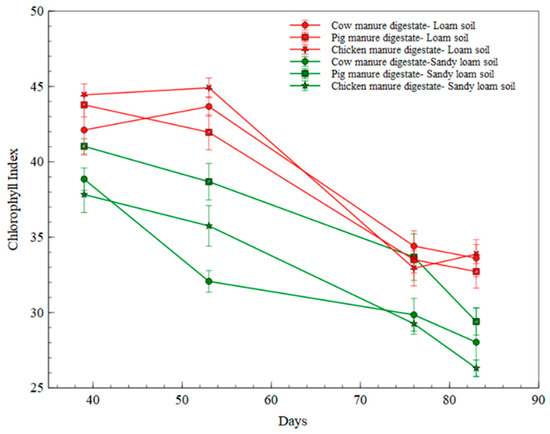
Figure 7.
Chlorophyll Index measurement in digestate treatments in soil types.
The chlorophyll fluorescence in the loam soil was observed to be lower in synthetic nitrogen fertilizer at the early stage of cultivation with a sustained decrease towards the end of the cultivation period (as illustrated in Figure 8). All the digestates showed a consistently higher chlorophyll fluorescence in the cultivation period, with the pig manure digestate fertilized treatment maintaining the high level with control while other treatments decreased towards the harvesting period. Pig manure digestate fertilized loam soil was significantly different (p < 0.05) to that of the control and the other treatments. The unfertilized pot had a higher fluorescence towards the harvest period from day 70 to day 85.
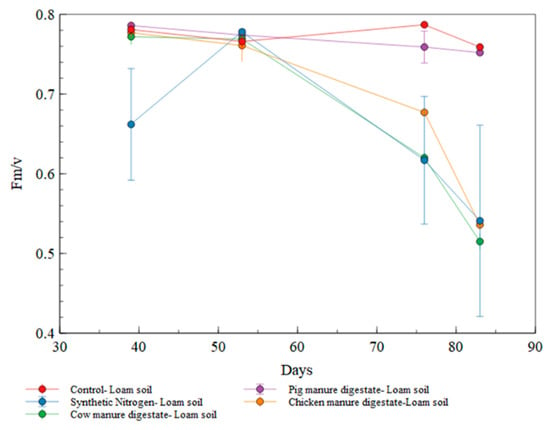
Figure 8.
Florescence measurement for all treatments in loam soil.
In comparing florescence measurements in the digestate-fertilized treatments in the two soil types, the loam soil had higher chlorophyll fluorescence compared with that of the sandy loam soil (as illustrated in Figure 9).
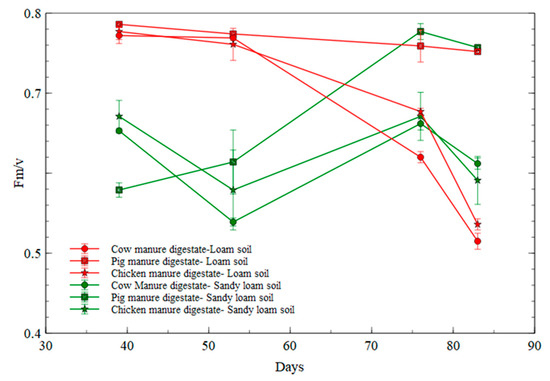
Figure 9.
Florescence measurement in digestate treatments between soil types.
3.5. Biomass Yield
3.5.1. The Comparison of Cultivated Wheat Biomass Yield in Control, Synthetic Nitrogen, and Digestates Treatment
The yield results showed that the highest biomass productivity was observed in the cow manure digestate fertilized treatment in the loam soil, while the lowest rate of biomass productivity was in control. There were significant differences (p < 0.05) between the control and other treatments, while the biomass yield in synthetic nitrogen treatment was significantly different from that of the cow manure digestates (p < 0.05), as shown in Figure 10. All digestate fertilized treatments had higher biomass yield compared to that of the control in the loam soil.
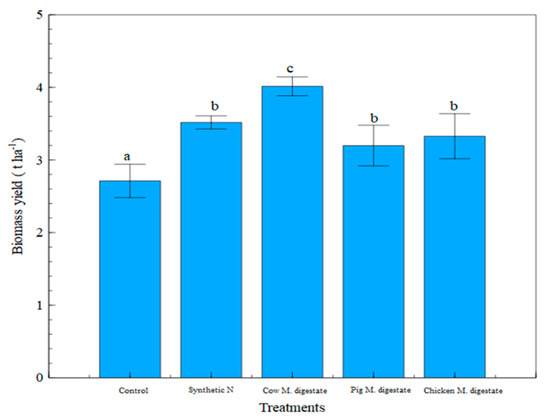
Figure 10.
Biomass yield measurement in all treatments in loam soil. Note: treatments with different letters are significantly different at p < 0.05.
3.5.2. Comparison of Biomass Yield of Digestates between Soils
The yield results showed that the biomass productivity in the cow manure digestate fertilized treatment in the loam soil was the highest, while it was the lowest in sandy loam soil (as illustrated in Figure 11), with chicken manure digestate fertilized treatment having the highest biomass yield in the sandy loam soil. Under statistical consideration, there were significant differences (p < 0.05) in the interaction between the soil type and the digestates, indicating that yield increase is soil type- and digestate-dependent.
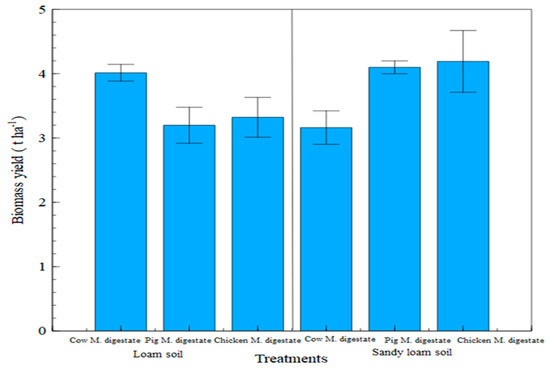
Figure 11.
Biomass yield measurement in digestate treatments between soil types. Factor A (soil type) = *; Factor B (fertilizer) = n.s. A × B = *; * denotes significant differences at p < 0.05.
4. Discussion
4.1. Soil Chemical Properties
The composition of the primary feedstock strongly influences the chemical properties of the digestate. The carbon and nitrogen contents are important elements for plants, and both decreased in the loam soil after the experiment compared to that of sandy loam soil. This is probably due to their consumption by soil microbes, although this did not lead to a higher soil microbial activity when compared to that of the sandy loam. The total N concentration was significant in the loam soil, but it reduced across soil types, except in pig manure digestate and chicken manure digestate in sandy loam soil. The NH4-N were quickly nitrified in soils [5], making both the nitrate and NH4-N to be taken up quickly by plants. These results give credence to earlier reports that composition of soil mineral fraction and soil organic carbon C can impact ammonium absorption [25,35]. Hence, the decreased N content in all the digestate treatments in the loam soil and in the sandy loam soil, except for pig manure digestate and chicken manure digestate, may be impacted by the reduction in nitrifying bacteria activity due to ammonium fixation by soil colloids. The quick mineralization of the nitrogen source is an important factor in the higher microbial activities in the sandy loam soil. This resulted in the utilization and decrease in the carbon source present in the soil. Phosphorus, an important element for plants, decreased in sandy loam digestate fertilized soils. The increased changes in available phosphorus content in the sandy loam digestate applied soil provide further evidence of their utility in the microbial process arising from increasing biological activity. This result correlates with the report by Alburquerque et al. [5], where the addition of digestate is an adequate strategy for alleviating P deficiencies in soils.
4.2. Soil Microbial Activities
Soil microbial biomass is often used as an early indicator of soil quality changes and is susceptible to changes in the soil environment and soil environmental practices [36,37]. Previous studies reported that changes in microbial richness, diversity, N mineralization, and nitrification rates of digestates in soil are more closely related to the organic matter content and microbiological composition of the receiving soil than to its textural properties, which supports our findings in the higher microbial biomass observed in the sandy loam partly attributed to the higher organic matter content [38,39,40]. In essence, the higher availability of organic C and N influenced the microbial activity in the sandy loam soil due to the higher supply of easily degradable organic carbon and nitrogen compared to that of the loam soil. The addition of digestates has varying effects on the soil types driven by the distinct characteristics of the soil types. The higher content of available major nutrients in phosphorus, potassium, and calcium in the sandy loam soil contributed to the higher microbial activities. The amendment with the digestate to the sandy loam soil did not in any way negatively influence the microbial activity.
Dehydrogenase activity (DHA) is often used as a measure of soil microbial activity and serves as an indicator of microbiological redox systems. DHA is closely correlated with respiratory (aerobic) activity in the soil [41]. The increased DHA values in sandy loam soil as compared to that of loam soil is indicative of better aerobic activities. Previous research reported that an increase in the activity of certain soil enzymes, such as B-glucosidase, indicates energy release for microorganisms as an attribute of the type of organic matter added to the soil [42]. This agrees with other studies demonstrating that digestates stimulate microbial activity in soils and increase organic matter mineralization, thus supporting dehydrogenase activity and microorganism growth and activation [1,40]. Contrary to other studies where the microbial activities were found to be higher in loam soil [1], our study showed a lower microbial activity in the loam soil, which might be connected to a moderately higher moisture content in the loam soil (data not reported), creating an environment that made the initial solubility of the digestates slower. Besides the pH that created a suitable environment for the microbes to thrive, the organic carbon present provided an available substrate to be utilized. The higher microbiological activity in the sandy loam soil is an indication of an improved microbial biomass and enzymatic activities, resulting in an improved growth rate of the soil microorganisms.
4.3. GHG Emission
4.3.1. CO2 Emission
The rate of CO2 emissions from control soil was moderate throughout the study, and this demonstrated that a suitable preincubation stage occurred, resulting in a very low background emission throughout the cultivation period. In this context, the CO2 emissions from the soil treated with the digestates showed marked differences, as their application provides a balanced supply of mineral nutrients as well as organic carbon. The activity of soil microorganisms strongly depends on the presence of available organic C and N [24,43,44]. This can be compared with that of the higher microbial activity observed in the digestate amendments and the control, which can be attributed to the quality as well as the complex interaction of the digestate with the soil, accountingfor the higher CO2 emissions observed in the first few weeks of cultivation. The CO2 emissions from treatments with the digestates were moderately higher in the sandy loam soil than that of the loam soil. This can be explained by the higher levels of microbial biomass and plant root respiration in digestate-treated soil, resulting from greater amounts of biogenic materials like mineralizable nitrogen, water soluble carbon, and carbohydrates, although this is in contrast to previous studies of higher emission rates observed in loam soil compared to that of other soil types due to higher pH and soil texture [25]. Another contributing factor is the differences in the biochemical and microbial activity between both soils driven by differences in the soil physical and chemical properties. The difference contributed to the balance in degrading digested material, and therefore, it contains much more available carbon, causing irregular CO2 peak emissions
4.3.2. N2O Emission
Previous studies of soil texture effects on N2O emissions across soil types have yielded mixed conclusions [43,44,45,46]. The loam soil of our study exhibited more moderate emissions of N2O compared to that of the sandy loam soil. This could be explained by several texture-related factors, such as aeration, N availability, and enhanced anaerobic conditions. Factors relating to soil conditions such as soil organic carbon content, soil pH, and texture significantly contributed to N2O emissions [25,47]. The water contents of our experimental units were adjusted to field capacity at weekly intervals, which might have contributed to the higher N2O emissions in the heavy textured loam soils arising from enhanced anaerobic conditions compared to that of the lighter textured sandy loam soils. There were also substantial differences in texture between the two soils, especially a clay content of 16.5% vs. 6.5% (loam vs. sandy soil) (as illustrated in Table 2). The differing texture and structure of the soils could have promoted N2O emissions in the loam soil by creating more microsites with partially anoxic conditions at higher water holding capacity than in the sandy loam soil. NH4+ can be immobilized by microbes (bacteria and fungi) in the soil, and it can be adsorbed to soil particles to a certain extent. Wang and Alva [48] found loam and clay soil to be more capable to sorb NH4+ than sandy soils. Adsorbed NH4+ is immobilized and therefore could mitigate N2O emissions [49].
The differing texture and structure of the soils possibly promoted N2O emissions in the loam soil by creating more microsites with partially anoxic conditions at higher water holding capacity than in that of the sandy loam soil. The higher emissions of N2O in the early stage of the growing season is due to the composition of organic material in the digestates. N2O emissions are stimulated by availability of N sources, which were higher and readily available at the early growth stage. Although denitrification rates were higher, there was sufficient supply of N contributing to keeping plant-available N in the top layer of the soil at the beginning of the growing season while the root system is not yet well developed. The salient effect of sandy loam soil on N2O emission compared to that of loam soil can be attributed to its differing characteristics, arbon and nitrogen contents. It contained 1.5-times more total nitrogen, 2.0-times more organic carbon, and 2.0-times P2O5 than that of the loam soil. Microbial activities observed in the loam soil also possibly contributed to the higher N2O emission rates going by the anoxic conditions, resulting in lower DHA and SMB-C values.
4.4. Physiological Parameters in Plant Foliage
The chlorophyll index shows the amount of chlorophyll in the leaves, and this index can be used to predict plant yields, determine fertilizer demand, and assess plant viability [50]. The treatments improved the physiological parameters in the plants, with no physiological stress arising from the use of the digestates across the two soil types. However, chlorophyll index was higher across all the digestate treatments in the loam soil, which is evidence of a better interaction between the loam soil and the digestates in terms of nutrient availability and soil chemical characteristics. Chlorophyll fluorescence helps to measure plant stress due to environmental stress predicated on the addition of organic amendments [34]. The higher chlorophyll fluorescence observed in the digestate fertilized loam soil provided an indication of the inability of the plants to metabolize properly.
4.5. Plant Biomass Yield
Digestates in general had a similar effect on total biomass yield based on the experiment conducted. The similarity in biomass yield differences between the chicken manure digestate and the pig manure digestate was expected considering there was no difference in the mineral nitrogen content. However, higher biomass yield was observed in the digestate treatments when compared to that of the untreated (control). The better performance from the digestate was due to the availability of mineral nitrogen present in the form of ammonium. The reliance on total mineral nitrogen content available in the digestate on the biomass yields was recognized, and better yields were reported in the application of biogas residues in barley crop and biomass yield [17,51]. Previous studies reported a higher biomass yield in spring wheat and winter wheat fertilized with anaerobic digestate [52,53,54]. Some studies found that biomass yield was not only dependent on digestate fertilization, but also with their management in both short and long-term [55,56]; improved yields were reported when digestate was applied over a period of three years or longer [57]. Other studies found that fertilization increased yield [53,58], especially in cow digestate manure under the application of field conditions. The digestates applied increased the levels of selected minerals in P, K, N, and C, which positively induced the soil chemical nature, and in essence, these changes contributed to the plant productivity and yield. In this study, the digestates were not the sole contributory factor to improved biomass yield, but also the soil types. In comparing the yields in the digestate fertilized treatments in the two soil types, the treatments provided the needed nutrients for plant growth in strong relationship with the conditions provided by the soil types, providing better soil aeration and other soil factors that aided the nutrient uptake and utilization by the plants.
5. Conclusions
The present study provides an overview of how a wide variety of animal waste based digestates affects plant productivity, soil microbial activities, and GHG emissions in two different soil types. Additional N supply from mineralization of the organic material by soil microorganisms stands to benefit plant productivity, particularly in loam and sandy loam soil. The high emissions of N2O in the two soil types provides an insight into finding mitigation routes for digestate application in soil, especially in open agricultural field conditions with variable environmental conditions.
The digestates had a positive effect on the biomass yields and complemented the needed carbon and nitrogen source needed for plant growth, especially in the sandy loam soil due to the retentive potential to maintain the required nutrients needed by the plants at the early stage of plant growth.
Overall, our study showed that digestates from biogas production based on fundamentally different animal feedstocks are promising in fertilizing different soil ecosystems with lower environmental risks and a higher biomass productivity.
Author Contributions
Conceptualization, M.O.D. and V.T.; methodology, M.O.D., V.T.; software, M.O.D. and A.B.; validation, M.O.D., V.T. and S.S.; formal analysis, M.O.D., A.B. and V.T.; investigation, M.O.D. and A.B.; data curation, M.O.D.; writing—original draft preparation, M.O.D.; writing—review and editing, S.S. and V.T.; visualization, M.O.D.; supervision, S.S. and V.T.; project administration, V.T.; funding acquisition, V.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Lithuania (LMTLT), agreement No. S-SIT-20-5, and the APC was funded by Research Council of Lithuania (LMTLT), agreement No. S-SIT-20-5.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
References
- Makádi, M.; Szegi, T.; Tomocsik, A.; Orosz, V.; Michéli, E.; Ferenczy, A.; Posta, K.; Biró, B. Impact of Digestate Application on Chemical and Microbiological Properties of Two Different Textured Soils. Commun. Soil Sci. Plant Anal. 2015, 47, 167–178. [Google Scholar] [CrossRef]
- Muscolo, A.; Settineri, G.; Papalia, T.; Attinà, E.; Basile, C.; Panuccio, M.R.S. Anaerobic co-digestion of recalcitrant agricultural wastes: Characterizing of biochemical parameters of digestate and its impacts on soil ecosystem. Sci. Total. Environ. 2017, 586, 746–752. [Google Scholar] [CrossRef]
- Bhunia, S.; Bhowmik, A.; Mallick, R.; Mukherjee, J. Agronomic Efficiency of Animal-Derived Organic Fertilizers and Their Effects on Biology and Fertility of Soil: A Review. Agronomy 2021, 11, 823. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Urra Potential Benefits and Risks for Soil Health Derived From the Use of Organic Amendments in Agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef] [Green Version]
- Alburquerque, J.A.; de la Fuente, C.; Bernal, M.P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Szymańska, M.; Sosulski, T. Chemical properties and fertilizer value of ten different anaerobic digestates Cradle to cattle farming View project Biogas as Renewable Energy Source View project. Fresenius Environ. Bull. 2018, 27, 3425–3432. [Google Scholar]
- Jastrow, J.D.; Amonette, J.E.; Bailey, V.L. Mechanisms controlling soil carbon turnover and their potential application for enhancing carbon sequestration. Clim. Chang. 2006, 80, 5–23. [Google Scholar] [CrossRef]
- Smith, J.; McNeal, B.; Cheng, H. Estimation of soil microbial biomass: An analysis of the respiratory response of soils. Soil Biol. Biochem. 1985, 17, 11–16. [Google Scholar] [CrossRef]
- Ghosh, S.; Wilson, B.; Ghoshal, S.; Senapati, N.; Mandal, B. Organic amendments influence soil quality and carbon sequestration in the Indo-Gangetic plains of India. Agric. Ecosyst. Environ. 2012, 156, 134–141. [Google Scholar] [CrossRef]
- Barłóg, P.; Hlisnikovský, L.; Kunzová, E. Effect of Digestate on Soil Organic Carbon and Plant-Available Nutrient Content Compared to Cattle Slurry and Mineral Fertilization. Agronomy 2020, 10, 379. [Google Scholar] [CrossRef] [Green Version]
- Risberg, K.; Cederlund, H.; Pell, M.; Arthurson, V.; Schnürer, A. Comparative characterization of digestate versus pig slurry and cow manure—Chemical composition and effects on soil microbial activity. Waste Manag. 2017, 61, 529–538. [Google Scholar] [CrossRef] [PubMed]
- Jaskulska, I.; Lemanowicz, J.; Breza-Boruta, B.; Siwik-Ziomek, A.; Radziemska, M.; Dariusz, J.; Białek, M. Chemical and Biological Properties of Sandy Loam Soil in Response to Long-Term Organic–Mineral Fertilisation in a Warm-Summer Humid Continental Climate. Agronomy 2020, 10, 1610. [Google Scholar] [CrossRef]
- Chowdhury, S.P.; Babin, D.; Sandmann, M.; Jacquiod, S.; Sommermann, L.; Sørensen, S.J.; Fliessbach, A.; Mäder, P.; Geistlinger, J.; Smalla, K.; et al. Effect of long-term organic and mineral fertilization strategies on rhizosphere microbiota assemblage and performance of lettuce. Environ. Microbiol. 2019, 21, 2426–2439. [Google Scholar] [CrossRef] [PubMed]
- Bruhn, A.; Dahl, J.; Nielsen, H.B.; Nikolaisen, L.; Rasmussen, M.B.; Markager, S.; Jensen, P.D. Bioenergy potential of Ulva lactuca: Biomass yield, methane production and combustion. Bioresour. Technol. 2011, 102, 2595–2604. [Google Scholar] [CrossRef] [PubMed]
- Grigatti, M.; Barbanti, L.; Hassan, M.U.; Ciavatta, C. Fertilizing potential and CO2 emissions following the utilization of fresh and composted food-waste anaerobic digestates. Sci. Total. Environ. 2020, 698, 134198. [Google Scholar] [CrossRef]
- Abubaker, J.; Cederlund, H.; Arthurson, V.; Pell, M. Bacterial community structure and microbial activity in different soils amended with biogas residues and cattle slurry. Appl. Soil Ecol. 2013, 72, 171–180. [Google Scholar] [CrossRef]
- Odlare, M.; Arthurson, V.; Pell, M.; Svensson, K.; Nehrenheim, E.; Abubaker, J. Land application of organic waste—Effects on the soil ecosystem. Appl. Energy 2011, 88, 2210–2218. [Google Scholar] [CrossRef]
- Odlare, M.; Pell, M.; Svensson, K. Changes in soil chemical and microbiological properties during 4 years of application of various organic residues. Waste Manag. 2008, 28, 1246–1253. [Google Scholar] [CrossRef]
- Hellström, A.; Nilsson, M.-L.; Kylin, H. Current-use and Organochlorine Pesticides and Polychlorinated Biphenyls in the Biodegradable Fraction of Source Separated Household Waste, Compost, and Anaerobic Digestate. Bull. Environ. Contam. Toxicol. 2010, 86, 60–64. [Google Scholar] [CrossRef] [Green Version]
- Pampillón-González, L.; Luna-Guido, M.; Ruíz-Valdiviezo, V.M.; Franco-Hernández, O.; Fernández-Luqueño, F.; Paredes-López, O.; Hernández, G.; Dendooven, L. Greenhouse Gas Emissions and Growth of Wheat Cultivated in Soil Amended with Digestate from Biogas Production. Pedosphere 2017, 27, 318–327. [Google Scholar] [CrossRef]
- Heintze, G.; Eickenscheidt, T.; Schmidhalter, U.; Drösler, M. Influence of Soil Organic Carbon on Greenhouse Gas Emission Potential After Application of Biogas Residues or Cattle Slurry: Results from a Pot Experiment. Pedosphere 2017, 27, 807–821. [Google Scholar] [CrossRef]
- Köster, J.R.; Cárdenas, L.M.; Bol, R.; Lewicka-Szczebak, D.; Senbayram, M.; Well, R.; Giesemann, A.; Dittert, K. Anaerobic digestates lower N2O emissions compared to cattle slurry by affecting rate and product stoichiometry of denitrification—An N2O isotopomer case study. Soil Biol. Biochem. 2015, 84, 65–74. [Google Scholar] [CrossRef]
- Abubaker, J.; Risberg, K.; Jönsson, E.; Dahlin, A.S.; Cederlund, H.; Pell, M. Short-Term Effects of Biogas Digestates and Pig Slurry Application on Soil Microbial Activity. Appl. Environ. Soil Sci. 2015, 2015, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, M.; Fongen, M.; Foereid, B. Greenhouse gas emissions from digestate in soil. Int. J. Recycl. Org. Waste Agric. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Jarecki, M.K.; Parkin, T.B.; Chan, A.S.K.; Hatfield, J.L.; Jones, R. Greenhouse Gas Emissions from Two Soils Receiving Nitrogen Fertilizer and Swine Manure Slurry. J. Environ. Qual. 2008, 37, 1432–1438. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nielsen, K.; Roß, C.-L.; Hoffmann, M.; Muskolus, A.; Ellmer, F.; Kautz, T. The Chemical Composition of Biogas Digestates Determines Their Effect on Soil Microbial Activity. Agriculture 2020, 10, 244. [Google Scholar] [CrossRef]
- Buneviciene, K.; Drapanauskaite, D.; Mažeika, R.; Tilvikiene, V. Biofuel ash granules as a source of soil and plant nutrients. Zemdirb. Agric. 2021, 108, 19–26. [Google Scholar] [CrossRef]
- Casida, L.E. Microbial Metabolic Activity in Soil as Measured by Dehydrogenase Determinationst. Appl. Environ. Microbiol. 1977, 34, 630–636. [Google Scholar] [CrossRef] [Green Version]
- Vance, E.; Brookes, P.; Jenkinson, D. An extraction method for measuring soil microbial biomass C. Soil Biol. Biochem. 1987, 19, 703–707. [Google Scholar] [CrossRef]
- Kanerva, T.; Regina, K.; Rämö, K.; Ojanperä, K.; Manninen, S. Fluxes of N2O, CH4 and CO2 in a meadow ecosystem exposed to elevated ozone and carbon dioxide for three years. Environ. Pollut. 2007, 145, 818–828. [Google Scholar] [CrossRef]
- Burton, D.L.; Zebarth, B.J.; Gillam, K.M.; Macleod, J.A. Effect of split application of fertilizer nitrogen on N2O emissions from potatoes. Can. J. Soil Sci. 2008, 88, 229–239. [Google Scholar] [CrossRef] [Green Version]
- Sosulski, T.; Szara, E.; Szymańska, M.; Stępień, W.; Rutkowska, B.; Szulc, W. Soil N2O emissions under conventional tillage conditions and from forest soil. Soil Tillage Res. 2019, 190, 86–91. [Google Scholar] [CrossRef]
- Akhtar, K.; Wang, W.; Ren, G.; Khan, A.; Enguang, N.; Khan, A.; Feng, Y.; Yang, G.; Wang, H. Straw mulching with inorganic nitrogen fertilizer reduces soil CO2 and N2O emissions and improves wheat yield. Sci. Total. Environ. 2020, 741, 140488. [Google Scholar] [CrossRef]
- Maxwell, K.; Johnson, G.N. Chlorophyll fluorescence—A practical guide. J. Exp. Bot. 2000, 51, 659–668. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Hernandez-Ramirez, G.; Kryzanowski, L.; Wallace, T.; Grant, R.; Degenhardt, R.; Berger, N.; Lohstraeter, G.; Powers, L.-A. Timing of Manure Injection and Nitrification Inhibitors Impacts on Nitrous Oxide Emissions and Nitrogen Transformations in a Barley Crop. Soil Sci. Soc. Am. J. 2017, 81, 1595–1605. [Google Scholar] [CrossRef] [Green Version]
- Hargreaves, P. Evaluating soil microbial biomass carbon as an indicator of long-term environmental change. Soil Biol. Biochem. 2003, 35, 401–407. [Google Scholar] [CrossRef]
- Li, L.; Xu, M.; Ali, M.E.; Zhang, W.; Duan, Y.; Li, D. Factors affecting soil microbial biomass and functional diversity with the application of organic amendments in three contrasting cropland soils during a field experiment. PLoS ONE 2018, 13, e0203812. [Google Scholar] [CrossRef] [Green Version]
- Sadet-Bourgeteau, S.; Houot, S.; Karimi, B.; Mathieu, O.; Mercier, V.; Montenach, D.; Morvan, T.; Sappin-Didier, V.; Watteau, F.; Nowak, V.; et al. Microbial communities from different soil types respond differently to organic waste input. Appl. Soil Ecol. 2019, 143, 70–79. [Google Scholar] [CrossRef]
- Ho, A.; Ijaz, U.Z.; Janssens, T.K.S.; Ruijs, R.; Kim, S.Y.; De Boer, W.; Termorshuizen, A.; Van Der Putten, W.H.; Bodelier, P. Effects of bio-based residue amendments on greenhouse gas emission from agricultural soil are stronger than effects of soil type with different microbial community composition. GCB Bioenergy 2017, 9, 1707–1720. [Google Scholar] [CrossRef]
- Rigby, H.; Smith, S. Nitrogen availability and indirect measurements of greenhouse gas emissions from aerobic and anaerobic biowaste digestates applied to agricultural soils. Waste Manag. 2013, 33, 2641–2652. [Google Scholar] [CrossRef] [PubMed]
- Wolinska, A.; Stepniewsk, Z. Dehydrogenase Activity in the Soil Environment. In Dehydrogenases; InTech: Vienna, Austria, 2012. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J.; Zhang, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- De la Fuente, C.; Alburquerque, J.A.; Clemente, R.; Bernal, M.P. Soil C and N mineralisation and agricultural value of the products of an anaerobic digestion system. Biol. Fertil. Soils 2013, 49, 313–322. [Google Scholar] [CrossRef]
- Bachmann-Pfabe, S.; Gropp, M.; Eichler-Löbermann, B. Phosphorus availability and soil microbial activity in a 3 year field experiment amended with digested dairy slurry. Biomass Bioenergy 2014, 70, 429–439. [Google Scholar] [CrossRef]
- Signor, D.; Cerri, C.E.P.; Conant, R. N2O emissions due to nitrogen fertilizer applications in two regions of sugarcane cultivation in Brazil. Environ. Res. Lett. 2013, 8, 015013. [Google Scholar] [CrossRef]
- Sosulski, T.; Stępień, W.; Wąs, A.; Szymańska, M. N2O and CO2 Emissions from Bare Soil: Effect of Fertilizer Management. Agriculture 2020, 10, 602. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycles 2002, 16, 6-1–6-13. [Google Scholar] [CrossRef]
- Nkoa, R. Agricultural benefits and environmental risks of soil fertilization with anaerobic digestates: A review. Agron. Sustain. Dev. 2014, 34, 473–492. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.L.; Alva, A.K. Ammonium Adsorption and Desorption in Sandy Soils. Soil Sci. Soc. Am. J. 2000, 64, 1669–1674. [Google Scholar] [CrossRef] [Green Version]
- Gianquinto, G.; Goffart, J.P.; Olivier, M.; Guarda, G.; Colauzzi, M.; Costa, L.D.; Vedove, G.D.; Vos, J.; MacKerron, D.K.L. The use of hand-held chlorophyll meters as a tool to assess the nitrogen status and to guide nitrogen fertilization of potato crop. Potato Res. 2004, 47, 35–80. [Google Scholar] [CrossRef]
- Terhoeven-Urselmans, T.; Scheller, E.; Raubuch, M.; Ludwig, B.; Joergensen, R.G. CO2 evolution and N mineralization after biogas slurry application in the field and its yield effects on spring barley. Appl. Soil Ecol. 2009, 42, 297–302. [Google Scholar] [CrossRef]
- Johansen, A.; Carter, M.S.; Jensen, E.S.; Hauggard-Nielsen, H.; Ambus, P. Effects of digestate from anaerobically digested cattle slurry and plant materials on soil microbial community and emission of CO2 and N2O. Appl. Soil Ecol. 2013, 63, 36–44. [Google Scholar] [CrossRef]
- Simon, T.; Kunzová, E.; Friedlová, M. The effect of digestate, cattle slurry and mineral fertilization on the winter wheat yield and soil quality parameters. Plant Soil Environ. 2016, 61, 522–527. [Google Scholar] [CrossRef] [Green Version]
- Muhmood, A.; Majeed, A.; Niaz, A.; Shah, A.H.; Shah, S.S.H.; Shahid, M.O. Evaluation of Anaerobic Digestate Potential as Organic Fertilizer in Improving Wheat Production and Soil Properties. Int. J. Plant Soil Sci. 2018, 24, 1–10. [Google Scholar] [CrossRef]
- Głowacka, A.; Szostak, B.; Klebaniuk, R. Effect of Biogas Digestate and Mineral Fertilisation on the Soil Properties and Yield and Nutritional Value of Switchgrass Forage. Agronomy 2020, 10, 490. [Google Scholar] [CrossRef] [Green Version]
- Tilvikiene, V.; Šlepetienė, A.; Kadžiulienė, Ž. Effects of 5 years of digestate application on biomass production and quality of cocksfoot (Dactylis glomerata L.). Grass Forage Sci. 2017, 73, 206–217. [Google Scholar] [CrossRef]
- Mortola, N.; Romaniuk, R.; Cosentino, V.; Eiza, M.; Carfagno, P.; Rizzo, P.F.; Bres, P.; Riera, N.; Roba, M.; Butti, M.; et al. Potential Use of a Poultry Manure Digestate as a Biofertiliser: Evaluation of Soil Properties and Lactuca sativa Growth. Pedosphere 2019, 29, 60–69. [Google Scholar] [CrossRef]
- Tampio, E.; Salo, T.; Rintala, J. Agronomic characteristics of five different urban waste digestates. J. Environ. Manag. 2016, 169, 293–302. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).