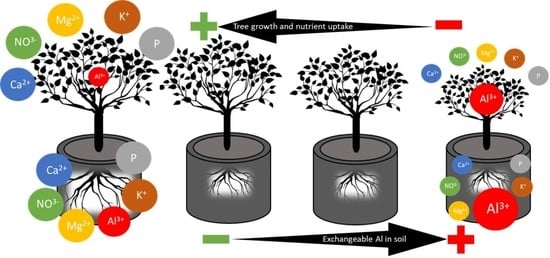
Aluminum Toxicity in Sweet Cherry Trees Grown in an Acidic Volcanic Soil
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Plant Material
2.2. Experimental Design and Cultivation Management
2.3. Tree Measurements
2.4. Statistical Analysis
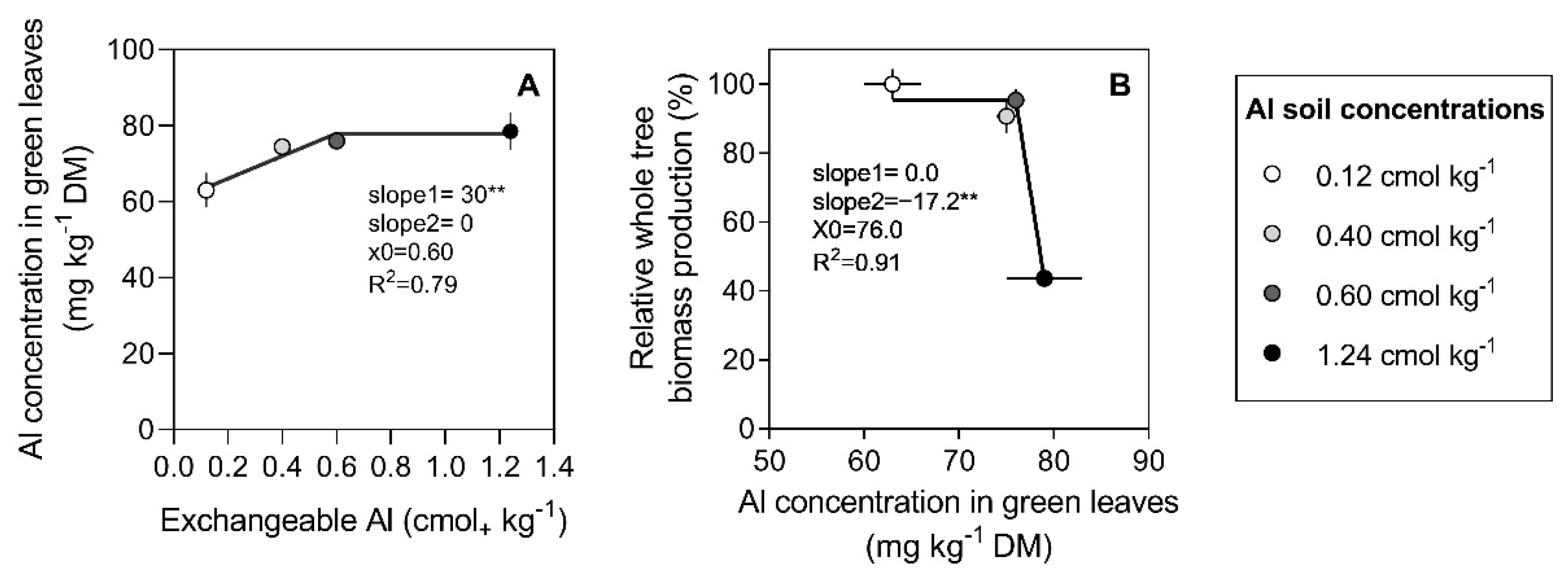
3. Results
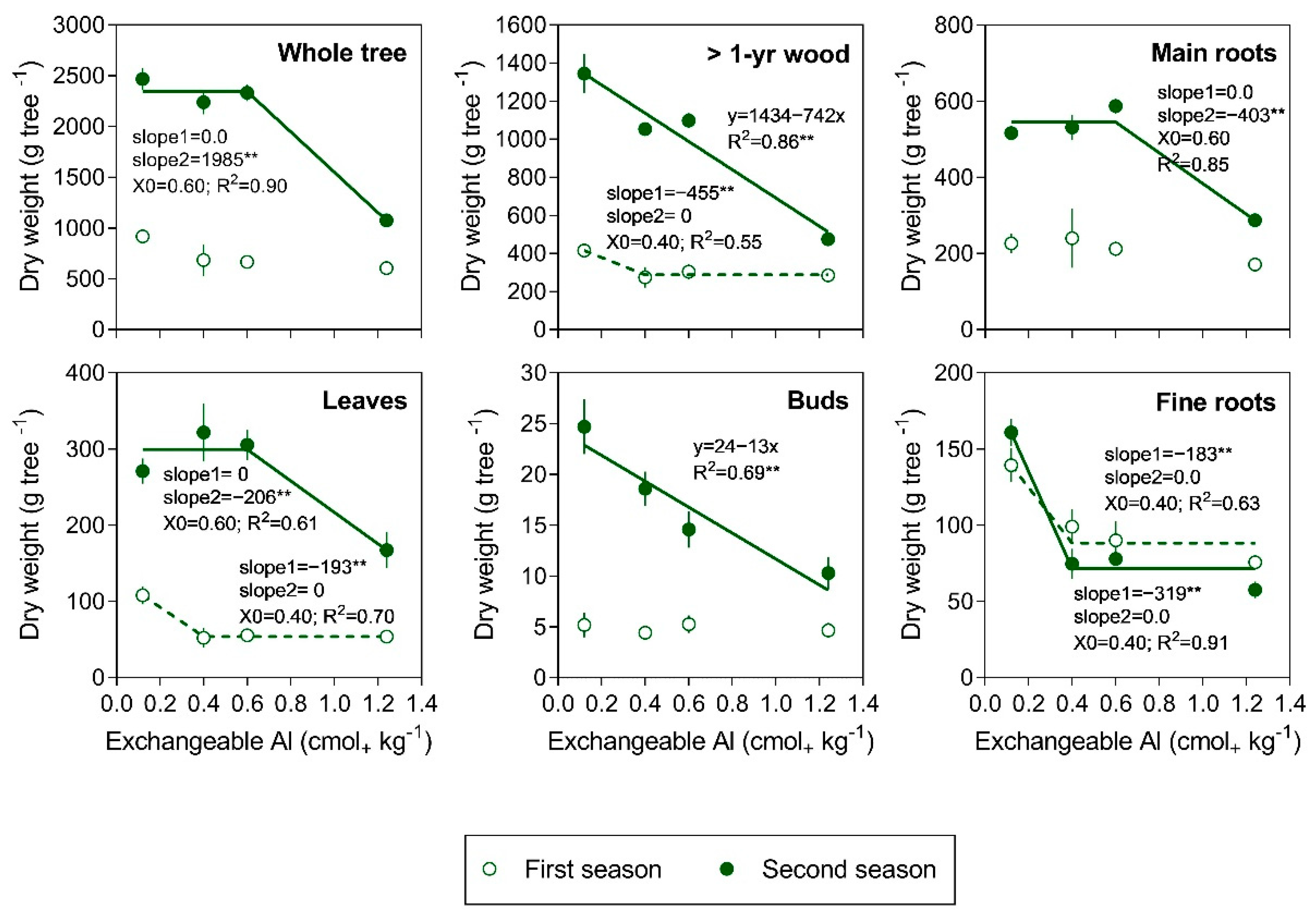
3.1. Tree Growth and Biomass Production
3.2. Al Concentration in Tree Organs
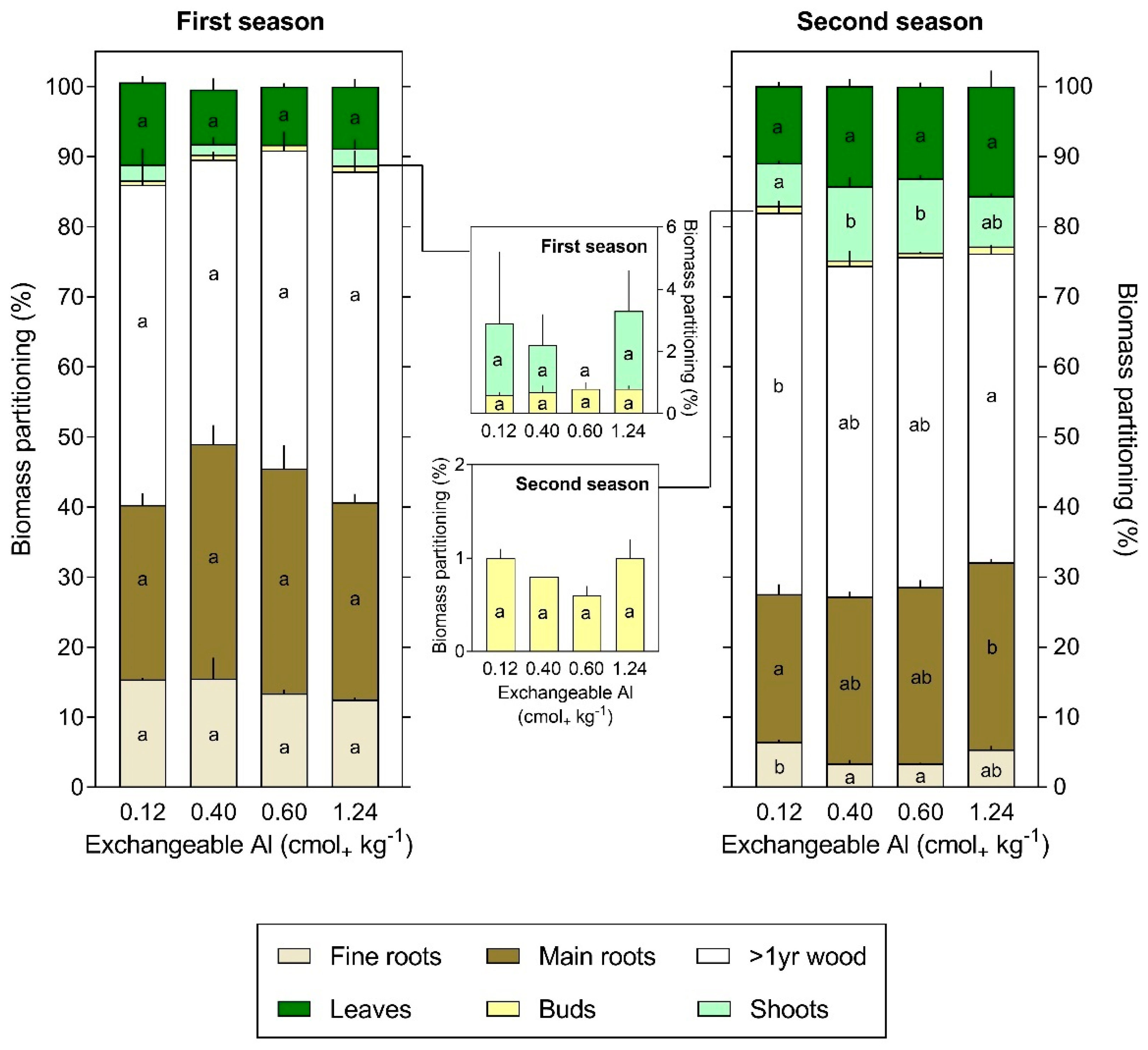
3.3. Al Content and Partitioning
3.4. Nutrient Content in Trees
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- iQonsulting. Anuario Cereza 2020–2021 Desafíos En El Mercado Chino Ante El Aumento de La Oferta. Available online: http://www.iqonsulting.com/yb/# (accessed on 21 April 2021).
- Dahlgren, R.A.; Saigusa, M.; Ugolini, F.C. The Nature, Properties and Management of Volcanic Soils. Adv. Agron. 2004, 82, 113–182. [Google Scholar] [CrossRef]
- Borie, F.; Aguilera, P.; Castillo, C.; Valentine, A.; Seguel, A.; Barea, J.M.; Cornejo, P. Revisiting the Nature of Phosphorus Pools in Chilean Volcanic Soils as a Basis for Arbuscular Mycorrhizal Management in Plant P Acquisition. J. Soil Sci. Plant Nutr. 2019, 19, 390–401. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef]
- Kinraide, T.B. Identity of the Rhizotoxic Aluminium Species. Plant Soil 1991, 134, 167–178. [Google Scholar] [CrossRef]
- Delhaize, E.; Ryan, P.R. Aluminum Toxicity and Tolerance in Plants. Plant Physiol. 1995, 107, 315–321. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- von Uexküll, H.R.; Mutert, E. Global Extent, Development and Economic Impact of Acid Soils. Plant Soil 1995, 171, 1–15. [Google Scholar] [CrossRef]
- Matsumoto, H. Cell Biology of Aluminum Toxicity Tolerance in Higher Plants. Int. Rev. Cytol. 2000, 200, 1–46. [Google Scholar] [CrossRef]
- Čiamporová, M. Morphological and Structural Responses of Plant Roots to Aluminium at Organ, Tissue, and Cellular Levels. Biol. Plant. 2002, 45, 161–171. [Google Scholar] [CrossRef]
- Scott, B.J.; Ridley, A.M.; Conyers, M.K. Management of Soil Acidity in Long-Term Pastures of South-Eastern Australia: A Review. Aust. J. Exp. Agric. 2000, 40, 1173–1198. [Google Scholar] [CrossRef]
- Zhao, X.Q.; Shen, R.F. Aluminum—Nitrogen Interactions in the Soil—Plant System. Front. Plant Sci. 2018, 9, 807. [Google Scholar] [CrossRef] [Green Version]
- Graham, C.J. The Influence of Nitrogen Source and Aluminum on Growth and Elemental Composition of Nemaguard Peach Seedlings. J. Plant Nutr. 2001, 24, 423–439. [Google Scholar] [CrossRef]
- Liao, X.Y.; Yang, L.T.; Lu, Y.B.; Ye, X.; Chen, L.S. Roles of Rootstocks and Scions in Aluminum-Tolerance of Citrus. Acta Physiol. Plant. 2015, 37, 1743. [Google Scholar] [CrossRef]
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.; Suravajhala, P.; Kavi Kishor, P. Toxicity and Tolerance of Aluminum in Plants: Tailoring Plants to Suit to Acid Soils. BioMetals 2016, 29, 187–210. [Google Scholar] [CrossRef] [PubMed]
- Dos Santos Neto, J.; Delfini, J.; Silva, T.W.; Hirose, A.A.; Novais, J.M.; Azeredo Gonçalves, L.S.; Moda-Cirino, V. Response of Common Bean Cultivars and Lines to Aluminum Toxicity. Agronomy 2020, 10, 296. [Google Scholar] [CrossRef] [Green Version]
- Barceló, J.; Poschenrieder, C. Fast Root Growth Responses, Root Exudates, and Internal Detoxification as Clues to the Mechanisms of Aluminium Toxicity and Resistance: A Review. Environ. Exp. Bot. 2002, 48, 75–92. [Google Scholar] [CrossRef]
- Kochian, L.V.; Hoekenga, O.A.; Piñeros, M.A. How Do Crop Plants Tolerate Acid Soils? Mechanisms of Aluminum Tolerance and Phosphorous Efficiency. Annu. Rev. Plant Biol. 2004, 55, 459–493. [Google Scholar] [CrossRef] [PubMed]
- Sivaguru, M.; Fujiwara, T.; Samaj, J.; Baluska, F.; Yang, Z.; Osawa, H.; Maeda, T.; Mori, T.; Volkmann, D.; Matsumoto, H. Aluminum-Induced 1→3-β-D-Glucan Inhibits Cell-to-Cell Trafficking of Molecules through Plasmodesmata. A New Mechanism of Aluminum Toxicity in Plants. Plant Physiol. 2000, 124, 991–1005. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Panda, S.K.; Matsumoto, H. Molecular Physiology of Aluminum Toxicity and Tolerance in Plants. Bot. Rev. 2007, 73, 326–347. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Pax, F.; Blamey, C. Theoretical and Experimental Assessment of Nutrient Solution Composition in Short-Term Studies of Aluminium Rhizotoxicity. Plant Soil 2016, 40, 311–326. [Google Scholar] [CrossRef]
- Chandra, J.; Keshavkant, S. Mechanisms Underlying the Phytotoxicity and Genotoxicity of Aluminum and Their Alleviation Strategies: A Review. Chemosphere 2021, 278, 130384. [Google Scholar] [CrossRef]
- Hirano, Y.; Kaneko, S.; Isomura, A. Root Morphology and Nutritional Status of Japanese Red Cedar Saplings Subjected to in Situ Levels of Aluminum in Forest Soil Solution. J. Res. 2003, 8, 209–214. [Google Scholar] [CrossRef]
- Kopittke, P.M.; Blamey, F.P.C.; Menzies, N.W. Toxicities of Soluble Al, Cu, and La Include Ruptures to Rhizodermal and Root Cortical Cells of Cowpea. Plant Soil 2007, 303, 217–227. [Google Scholar] [CrossRef]
- Ryan, P.R.; Ditomaso, J.M.; Kochian, L.V. Aluminium Toxicity in Roots: An Investigation of Spatial Sensitivity and the Role of the Root Cap. J. Exp. Bot. 1993, 44, 437–446. [Google Scholar] [CrossRef]
- Rahman, M.A.; Lee, S.H.; Ji, H.C.; Kabir, A.H.; Jones, C.S.; Lee, K.W. Importance of Mineral Nutrition for Mitigating Aluminum Toxicity in Plants on Acidic Soils: Current Status and Opportunities. Int. J. Mol. Sci. 2018, 19, 3073. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivaguru, M.; Horst, W.J. The Distal Part of the Transition Zone Is the Most Aluminum-Sensitive Apical Root Zone of Maize. Plant Physiol. 1998, 116, 155–163. [Google Scholar] [CrossRef] [Green Version]
- Blair, M.W.; López-Marín, H.D.; Rao, I.M. Identification of Aluminum Resistant Andean Common Bean (Phaseolus vulgaris L.) Genotypes. Braz. J. Plant Physiol. 2009, 21, 291–300. [Google Scholar] [CrossRef]
- Kochian, L.V. Cellular Mechanisms of Aluminum Toxicity and Resistance in Plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1995, 46, 237–260. [Google Scholar] [CrossRef]
- Vitorello, V.A.; Capaldi, F.R.; Stefanuto, V.A. Recent Advances in Aluminum Toxicity and Resistance in Higher Plants. Braz. J. Plant Physiol. 2005, 17, 129–143. [Google Scholar] [CrossRef]
- Horst, W.J.; Wang, Y.; Eticha, D. The Role of the Root Apoplast in Aluminium-Induced Inhibition of Root Elongation and in Aluminium Resistance of Plants: A Review. Ann. Bot. 2010, 106, 185–197. [Google Scholar] [CrossRef]
- Rahman, R.; Upadhyaya, H. Aluminium Toxicity and Its Tolerance in Plant: A Review. J. Plant Biol. 2021, 64, 101–121. [Google Scholar] [CrossRef]
- Kochian, L.V.; Piñeros, M.A.; Hoekenga, O.A. The Physiology, Genetics and Molecular Biology of Plant Aluminum Resistance and Toxicity. Plant Soil 2005, 274, 175–195. [Google Scholar] [CrossRef]
- Grisel, N.; Zoller, S.; Künzli-Gontarczyk, M.; Lampart, T.; Münsterkötter, M.; Brunner, I.; Bovet, L.; Métraux, J.P.; Sperisen, C. Transcriptome Responses to Aluminum Stress in Roots of Aspen (Populus Tremula). BMC Plant Biol. 2010, 10, 1–15. [Google Scholar] [CrossRef] [Green Version]
- Cárcamo, M.P.; Reyes-Díaz, M.; Rengel, Z.; Alberdi, M.; Omena-Garcia, R.P.; Nunes-Nesi, A.; Inostroza-Blancheteau, C. Aluminum Stress Differentially Affects Physiological Performance and Metabolic Compounds in Cultivars of Highbush Blueberry. Sci. Rep. 2019, 9, 11275. [Google Scholar] [CrossRef] [Green Version]
- Kochian, L.V.; Piñeros, M.A.; Liu, J.; Magalhaes, J.V. Plant Adaptation to Acid Soils: The Molecular Basis for Crop Aluminum Resistance. Annu. Rev. Plant Biol. 2015, 66, 571–598. [Google Scholar] [CrossRef]
- Bano, C.; Amist, N.; Singh, N.B. Transporters Regulating Aluminum Uptake, Accumulation, and Toxicity in Plants; Elsevier Inc.: Amsterdam, The Netherlands, 2021. [Google Scholar] [CrossRef]
- Rao, N.K.S.; Shivashankara, K.S.; Laxman, R.H. Abiotic Stress Physiology of Horticultural Crops. In Abiotic Stress Physiology of Horticultural Crops; Springer: New Delhi, India, 2016; pp. 1–368. [Google Scholar] [CrossRef]
- Chen, L.S.; Yang, L.T.; Guo, P.; Jiang, H.X.; Tang, N. Aluminum Toxicity and Fruit Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2020. [Google Scholar] [CrossRef]
- Neilsen, G.H.; Neilsen, D.; Atkinson, D. Top and Root Growth and Nutrient Absorption of Prunus avium L. at Two Soil PH and P Levels. Plant Soil 1990, 121, 137–144. [Google Scholar] [CrossRef]
- Borkowska, B. Experimental System for Investigations of Aluminium Toxicity in Fruit Trees. In Plant-Soil Interactions at Low pH; Kluwer Academic Publishers: Dordrecht, The Netherlands, 1991; pp. 999–1004. [Google Scholar] [CrossRef]
- Melakeberhan, H.; Bird, G.W.; Jones, A.L. Soil pH Affects Nutrient Balance in Cherry Rootstock Leaves. HortScience 2001, 36, 916–917. [Google Scholar] [CrossRef] [Green Version]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World Map of the Köppen-Geiger Climate Classification Updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Soil Survey Staff. Soil Taxonomy: A Basic System of Soil Classification for Making and Interpreting Soil Surveys, 2nd ed.; US Department of Agriculture: Washington, DC, USA, 1999.
- Valle, S.R.; Carrasco, J.; Pinochet, D.; Calderini, D.F. Grain Yield, above-Ground and Root Biomass of Al-Tolerant and Al-Sensitive Wheat Cultivars under Different Soil Aluminum Concentrations at Field Conditions. Plant Soil 2009, 318, 299–310. [Google Scholar] [CrossRef]
- Bonomelli, C.; Artacho, P. Nitrogen Application to Non-Bearing “Bing” Sweet Cherry Trees on Gisela®6 Rootstock: Effects on Accumulation and Partitioning of Biomass and Nitrogen. Sci. Hortic. 2013, 162, 293–304. [Google Scholar] [CrossRef]
- Newman, E.I. A Method of Estimating the Total Length of Root in a Sample. J. Appl. Ecol. 1966, 3, 139–145. [Google Scholar] [CrossRef]
- Westwood, M.N. Temperate-Zone Pomology: Physiology and Culture, 3rd ed.; Timber Press: Portland, OR, USA, 1993. [Google Scholar]
- Pinochet, D.; Artacho, P.; Maraboli, A. Manual de Fertilización de Arándanos Cultivados En El Sur de Chile; Imprenta América: Valdivia, Chile, 2014. [Google Scholar]
- Poschenrieder, C.; Gunsé, B.; Corrales, I.; Barceló, J. A Glance into Aluminum Toxicity and Resistance in Plants. Sci. Total Environ. 2008, 400, 356–368. [Google Scholar] [CrossRef]
- Abdulaha-Al Baquy, M.; Li, J.Y.; Xu, C.Y.; Mehmood, K.; Xu, R.K. Determination of Critical pH and Al Concentration of Acidic Ultisols for Wheat and Canola Crops. Solid Earth 2017, 8, 149–159. [Google Scholar] [CrossRef] [Green Version]
- Martini, J.A.; Kochhann, R.A.; Siqueira, O.J.; Borkert, C.M. Response of Soybeans to Liming as Related to Soil Acidity, Al and Mn Toxicities, and P in Some Oxisols of Brazil. Soil Sci. Soc. Am. J. 1974, 38, 616–620. [Google Scholar] [CrossRef]
- Hirano, Y.; Mizoguchi, T.; Brunner, I. Root Parameters of Forest Trees as Sensitive Indicators of Acidifying Pollutants: A Review of Research of Japanese Forest Trees. J. For. Res. 2007, 12, 134–142. [Google Scholar] [CrossRef]
- Vogt, K.A.; Publicover, D.A.; Bloomfield, J.; Perez, J.M.; Vogt, D.J.; Silver, W.L. Belowground Responses as Indicators of Enviromental Change. Environ. Exp. Bot. 1993, 33, 189–205. [Google Scholar] [CrossRef]
- Foy, C.D. Physiological Effects of Hydrogen, Aluminum, and Manganese Toxicities in Acid Soil. Soil Acidity Liming 1984, 12, 57–97. [Google Scholar] [CrossRef]
- Azarenko, A.N.; Chozinski, A.; Brewer, L.J. Fruit Growth Curve Analysis of Seven Sweet Cherry Cultivars. Acta Horticult. 2008, 795, 561–565. [Google Scholar] [CrossRef]
- Signorelli, S.; Shaw, J.; Hermawaty, D.; Wang, Z.; Verboven, P.; Considine, J.A.; Considine, M.J. The Initiation of Bud Burst in Grapevine Features Dynamic Regulation of the Apoplastic Pore Size. J. Exp. Bot. 2020, 71, 719–729. [Google Scholar] [CrossRef] [PubMed]
- Rinne, P.L.H.; Welling, A.; Vahala, J.; Ripel, L.; Ruonala, R.; Kangasjärvi, J.; van der Schoot, C. Chilling of Dormant Buds Hyperinduces FLOWERING LOCUS T and Recruits GA-Inducible 1,3-β-Glucanases to Reopen Signal Conduits and Release Dormancy in Populus. Plant Cell 2011, 23, 130–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud Dormancy in Perennial Fruit Tree Species: A Pivotal Role for Oxidative Cues. Front. Plant Sci. 2018, 9, 1–13. [Google Scholar] [CrossRef] [PubMed]
- Choi, W.G.; Hilleary, R.; Swanson, S.J.; Kim, S.H.; Gilroy, S. Rapid, Long-Distance Electrical and Calcium Signaling in Plants. Annu. Rev. Plant Biol. 2016, 67, 287–307. [Google Scholar] [CrossRef] [PubMed]
- Roy, A.K.; Sharma, A.; Talukder, G. Some Aspects of Aluminum Toxicity in Plants. Bot. Rev. 1988, 54, 145–178. [Google Scholar] [CrossRef]
- Brunner, I.; Sperisen, C. Aluminum Exclusion and Aluminum Tolerance in Woody Plants. Front. Plant Sci. 2013, 4, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, L.T.; Jiang, H.X.; Tang, N.; Chen, L.S. Mechanisms of Aluminum-Tolerance in Two Species of Citrus: Secretion of Organic Acid Anions and Immobilization of Aluminum by Phosphorus in Roots. Plant Sci. 2011, 180, 521–530. [Google Scholar] [CrossRef]
- Jiménez, S.; Ollat, N.; Deborde, C.; Maucourt, M.; Rellán-Álvarez, R.; Moreno, M.Á.; Gogorcena, Y. Metabolic Response in Roots of Prunus Rootstocks Submitted to Iron Chlorosis. J. Plant Physiol. 2011, 168, 415–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferreira, L.; Antunez, L.E. Plant Growth and Organic Acids in Root Sap, Roots and Root Exudates of Prunus Rootstocks in the Function of Nitrogen Fertilization. Ciência Agrícola 2017, 15, 11–18. [Google Scholar]
- Edwards, J.H.; Horton, B.D. Aluminum-Induced Calcium Deficiency in Peach Seedlings. J. Am. Soc. Hortic. Sci. 1977, 102, 459–461. [Google Scholar]
- Reuter, D.; Robinson, J. Plant Analysis: An Interpretation Manual, 2nd ed.; Csiro Publishing: Collingwood, ON, Canada, 1997. [Google Scholar]
- Kar, D.; Pradhan, A.A.; Datta, S. The Role of Solute Transporters in Aluminum Toxicity and Tolerance. Physiol. Plant. 2021, 171, 638–652. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Plaxton, W.C. Phosphorus: Back to The Roots. In Phosphorus Metabolism in Plants; Wiley Blackwell: Chichester, UK, 2015; Volume 48, pp. 3–22. [Google Scholar] [CrossRef]
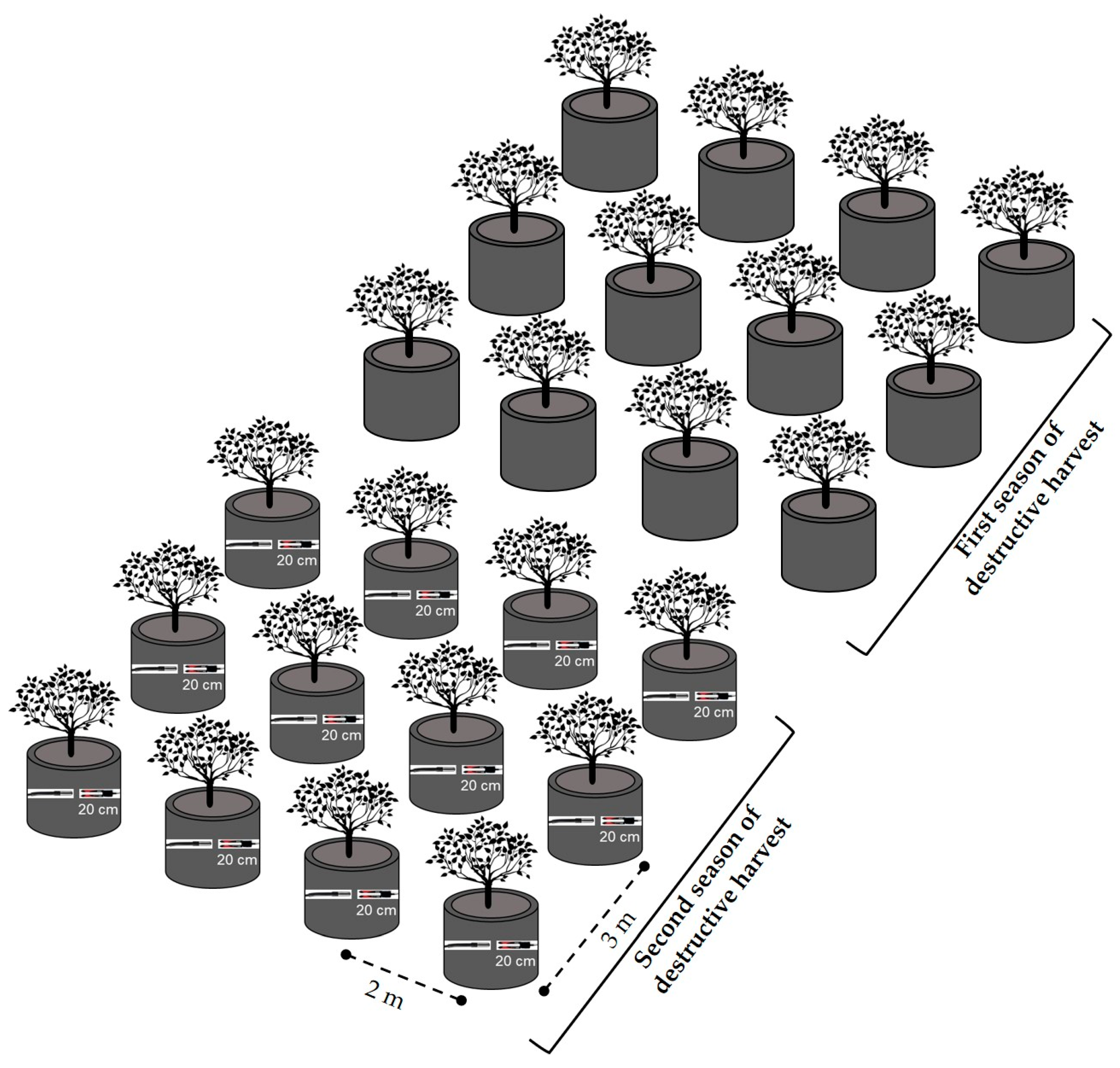
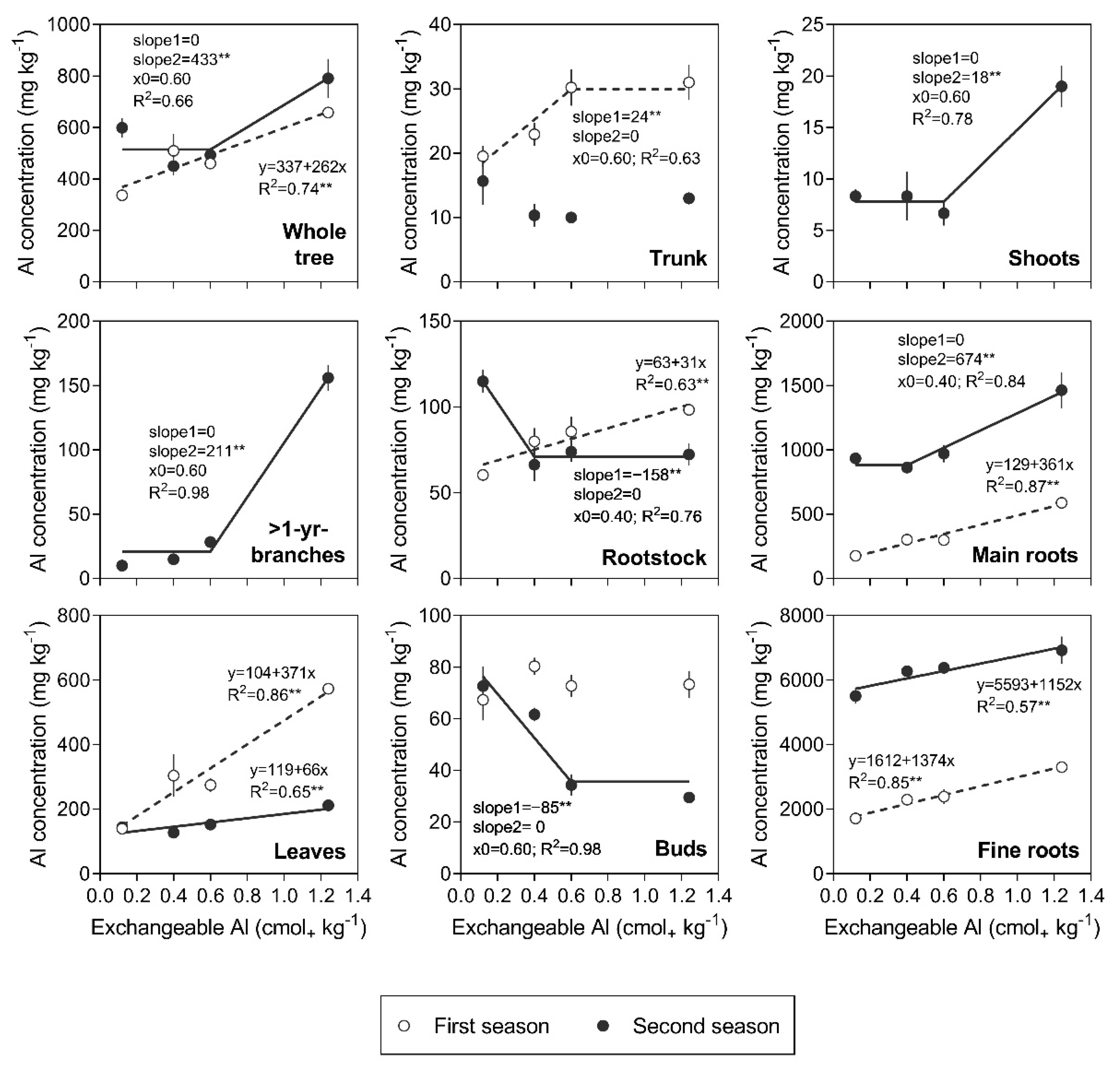
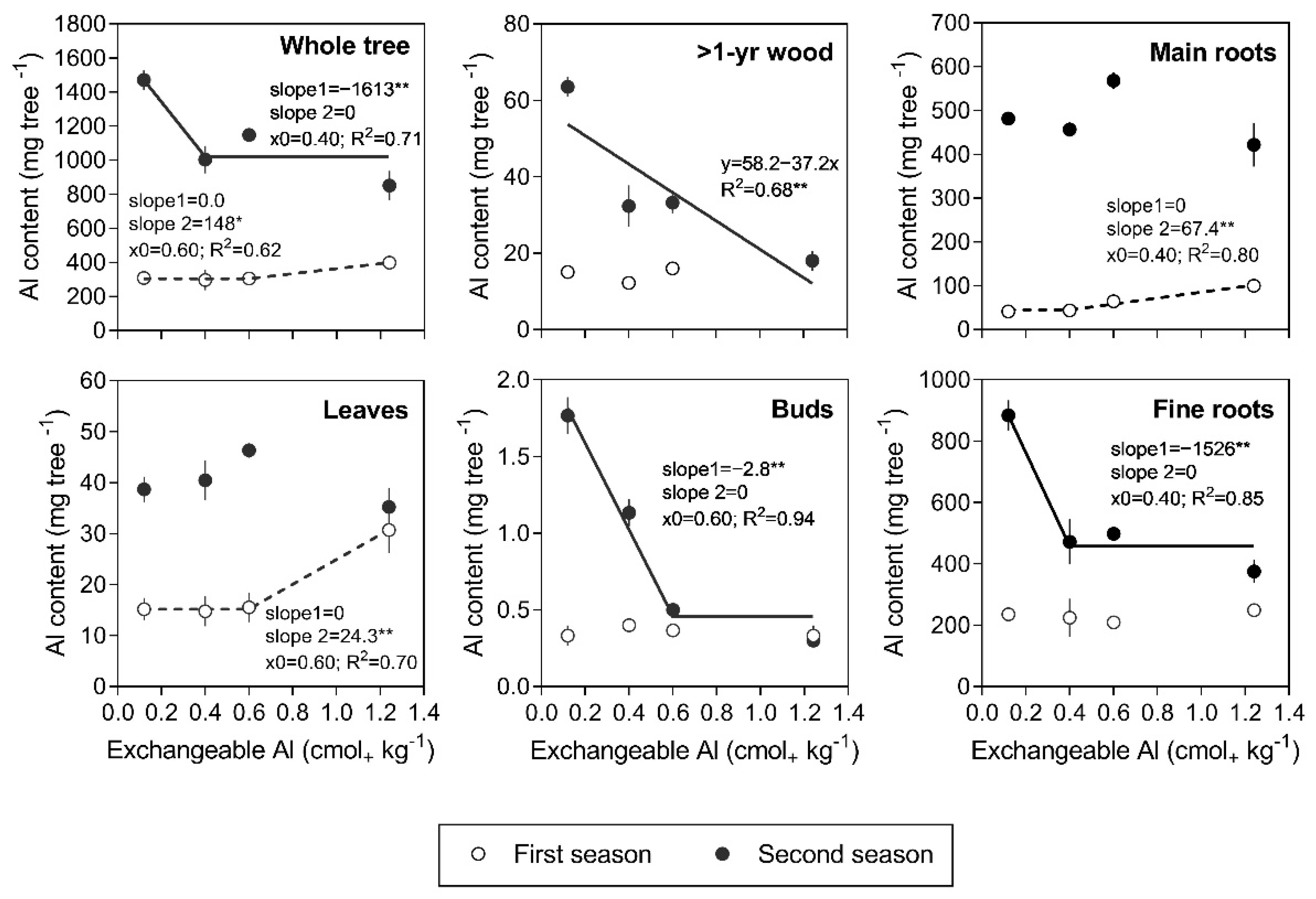
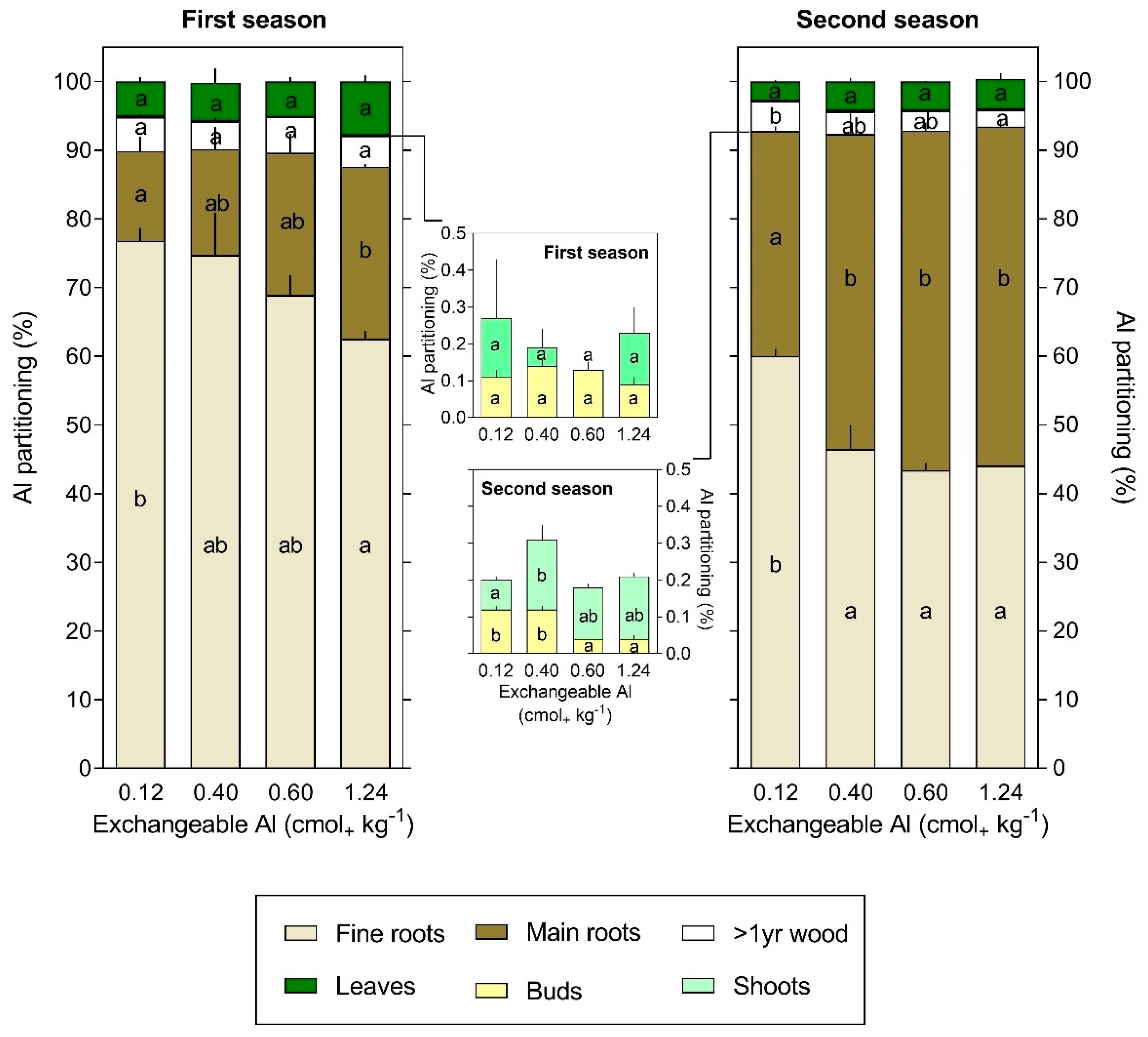
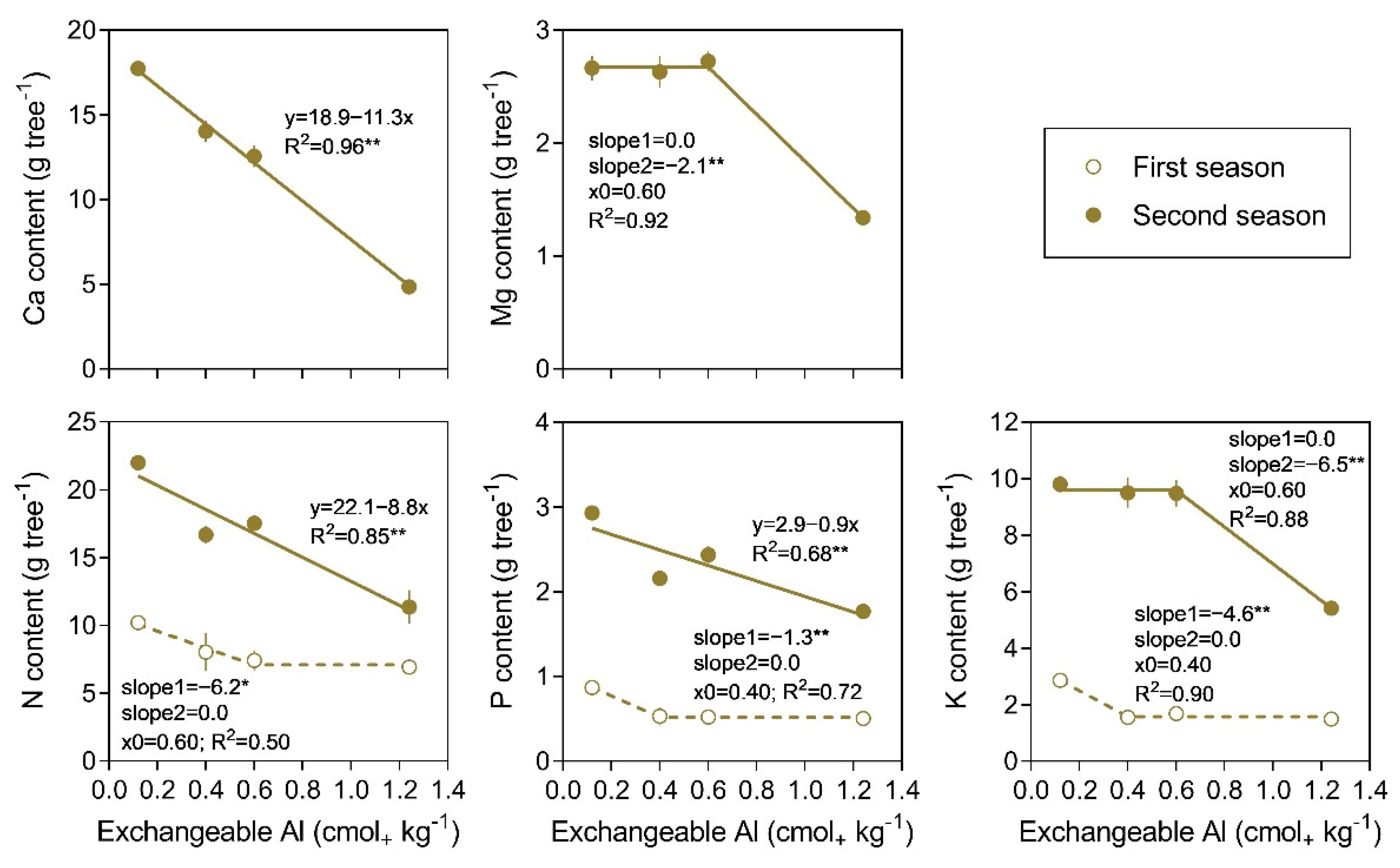
| Treatment | pH Water (1:2.5) | pH CaCl2 0.01 M | KCl-Exchangeable Al (cmol kg−1) | Al Saturation (%) |
|---|---|---|---|---|
| Al 1 | 5.82 (0.06) | 5.11 (0.06) | 0.12 (0.02) | 2.9 (0.5) |
| Al 2 | 5.31 (0.04) | 4.68 (0.02) | 0.40 (0.03) | 19.0 (0.8) |
| Al 3 | 5.17 (0.01) | 4.56 (0.02) | 0.60 (0.03) | 33.2 (1.9) |
| Al 4 | 4.64 (0.01) | 4.25 (0.01) | 1.24 (0.03) | 63.6 (1.0) |
| Soil-Exchangeable Al (cmol kg−1) | TCSA Increment 1 (cm2 Tree−1) | Total Shoot Length (cm Tree−1) | Total Leaf Area 2 (cm2 Tree−1) | Fine-Root Length 2 (m Tree−1) |
|---|---|---|---|---|
| First season | ||||
| 0.12 | 4.24 (0.80) b | 48.5 (48.5) | 0.63 (0.05) | 997.3 (95.4) b |
| 0.40 | 3.68 (0.21) ab | 53.3 (37.0) | 0.30 (0.10) | 628.3 (68.9) a |
| 0.60 | 3.09 (0.10) ab | 27.3 (17.3) | 0.33 (0.05) | 612.0 (58.6) a |
| 1.24 | 1.52 (0.09) a | 17.7 (17.7) | 0.38 (0.08) | 649.6 (48.3) a |
| p-Value | 0.046 | n.s. | n.s | 0.009 |
| Second season | ||||
| 0.12 | 8.99 (1.50) b | 519.2 (6.4) ab | 2.09 (0.09) ab | 1148.8 (72.8) b |
| 0.40 | 8.84 (1.30) b | 704.7 (58.9) b | 2.61 (0.35) b | 487.0 (99.3) a |
| 0.60 | 9.01 (0.62) b | 718.5 (41.0) b | 2.46 (0.23) ab | 536.0 (21.8) a |
| 1.24 | 4.76 (0.39) a | 346.8 (21.8) a | 1.25 (0.13) a | 494.7 (30.4) a |
| p-Value | 0.097 | 0.092 | 0.034 | 0.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bonomelli, C.; Artacho, P. Aluminum Toxicity in Sweet Cherry Trees Grown in an Acidic Volcanic Soil. Agronomy 2021, 11, 1259. https://doi.org/10.3390/agronomy11061259
Bonomelli C, Artacho P. Aluminum Toxicity in Sweet Cherry Trees Grown in an Acidic Volcanic Soil. Agronomy. 2021; 11(6):1259. https://doi.org/10.3390/agronomy11061259
Chicago/Turabian StyleBonomelli, Claudia, and Pamela Artacho. 2021. "Aluminum Toxicity in Sweet Cherry Trees Grown in an Acidic Volcanic Soil" Agronomy 11, no. 6: 1259. https://doi.org/10.3390/agronomy11061259
APA StyleBonomelli, C., & Artacho, P. (2021). Aluminum Toxicity in Sweet Cherry Trees Grown in an Acidic Volcanic Soil. Agronomy, 11(6), 1259. https://doi.org/10.3390/agronomy11061259