Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Essential Oil Obtention
2.3. Essential Oil Analysis
2.3.1. Equipment and Sample Preparation
2.3.2. Qualitative Analysis
2.3.3. Quantitative Analysis
2.4. Termite Collection and Identification
2.5. Mortality and Repellency Tests Experimental Design
2.5.1. One-Factor Response Surface Methodology
2.5.2. Model Validation
2.6. Mortality Test
2.7. Repellency Test
3. Results
3.1. Termiticidal and Repellent Activity of EO
3.2. Essential Oil Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jørgensen, P.M.; León-Yánez, S. Catalogue of the Vascular Plants of Ecuador; Missouri Botanical Garden: St. Louis, MO, USA, 1999; Volume 75. [Google Scholar]
- Friedman, J.; Bolotin, D.; Rios, M.; Mendosa, P.; Cohen, Y.; Balick, M.J. A Novel Method for Identification and Domestication of indigenous Useful Plants in Amazonian Ecuador; Janick, J., Simon, J.E., Eds.; John Wiley and Sons Inc.: New York, NY, USA, 1993; pp. 167–174. [Google Scholar]
- Naranjo, P.; Kijjoa, A.; Giesbrecht, A.M.; Gottlieb, O.R. Ocotea quixos, American cinnamon. J. Ethnopharmacol. 1981, 4, 233–236. [Google Scholar] [CrossRef]
- Sacchetti, G.; Guerrini, A.; Noriega, P.; Bianchi, A.; Bruni, R. Essential oil of wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) leaves from Amazonian Ecuador. Flavour Fragr. J. 2006, 21, 674–676. [Google Scholar] [CrossRef]
- Gavahian, M.; Chu, Y.H.; Lorenzo, J.M.; Mousavi Khaneghah, A.; Barba, F.J. Essential oils as natural preservatives for bakery products: Understanding the mechanisms of action, recent findings, and applications. Crit. Rev. Food Sci. Nutr. 2020, 60, 310–321. [Google Scholar] [CrossRef] [PubMed]
- Olmedo, R.H.; Grosso, N.R. Oxidative stability, affective and descriptive sensory properties of roasted peanut flavored with oregano, laurel, and rosemary essential oils as natural preservatives of food lipids. Eur. J. Lipid Sci. Technol. 2019, 121, 1800428. [Google Scholar] [CrossRef]
- Prakash, B.; Kedia, A.; Mishra, P.K.; Dubey, N.K. Plant essential oils as food preservatives to control moulds, mycotoxin contamination and oxidative deterioration of agri-food commodities—Potentials and challenges. Food Control. 2015, 47, 381–391. [Google Scholar] [CrossRef]
- Smith-Palmer, A.; Stewart, J.; Fyfe, L. The potential application of plant essential oils as natural food preservatives in soft cheese. Food Microbiol. 2001, 18, 463–470. [Google Scholar] [CrossRef]
- Carvalho, I.T.; Estevinho, B.N.; Santos, L. Application of microencapsulated essential oils in cosmetic and personal healthcare products—A review. Int. J. Cosmet. Sci. 2016, 38, 109–119. [Google Scholar] [CrossRef] [PubMed]
- Muyima, N.Y.O.; Zulu, G.; Bhengu, T.; Popplewell, D. The potential application of some novel essential oils as natural cosmetic preservatives in an aqueous cream formulation. Flavour Fragr. J. 2002, 17, 258–266. [Google Scholar] [CrossRef]
- Herman, A. Antimicrobial ingredients as preservative booster and components of self-preserving cosmetic products. Curr. Microbiol. 2019, 76, 744–754. [Google Scholar] [CrossRef]
- Deng, W.; Liu, K.; Cao, S.; Sun, J.; Zhong, B.; Chun, J. Chemical composition, antimicrobial, antioxidant, and antiproliferative properties of grapefruit essential oil prepared by molecular distillation. Molecules 2020, 25, 217. [Google Scholar] [CrossRef]
- Yin, C.; Sun, F.; Rao, Q.; Zhang, Y. Chemical compositions and antimicrobial activities of the essential oil from Pterocarya stenoptera C. DC. Nat. Prod. Res. 2019, 34, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Camele, I.; Elshafie, H.S.; De Feo, V.; Caputo, L. Anti-quorum sensing and antimicrobial effect of Mediterranean plant essential Oils against phytopathogenic bacteria. Front. Microbiol. 2019, 10, 2619. [Google Scholar] [CrossRef] [PubMed]
- Espinoza, G.J.T. El Aprovechamiento del Ishpink (Ocotea quixos). Manual de Buenas Prácticas de Recolección. Available online: http://chankuap.org/wp-content/uploads/2014/03/Manual-de-buenas-practicas-de-la-Ishpink.pdf (accessed on 20 May 2020).
- Noriega, P.; Dacarro, C. Aceite foliar de Ocotea quixos (Lam.) Kosterm.: Actividad antimicrobiana y antifúngica. Granja 2008, 7, 3–8. [Google Scholar] [CrossRef][Green Version]
- Figueiredo, A.C.; Barroso, J.G.; Pedro, L.G.; Scheffer, J.J.C. Factors affecting secondary metabolite production in plants: Volatile components and essential oils. Flavour Fragr. J. 2008, 23, 213–226. [Google Scholar] [CrossRef]
- Tognolini, M.; Barocelli, E.; Ballabeni, V.; Bruni, R.; Bianchi, A.; Chiavarini, M.; Impicciatore, M. Comparative screening of plant essential oils: Phenylpropanoid moiety as basic core for antiplatelet activity. Life Sci. 2006, 78, 1419–1432. [Google Scholar] [CrossRef]
- Ballabeni, V.; Tognolini, M.; Bertoni, S.; Bruni, R.; Guerrini, A.; Rueda, G.M.; Barocelli, E. Antiplatelet and antithrombotic activities of essential oil from wild Ocotea quixos (Lam.) Kosterm. (Lauraceae) calices from Amazonian Ecuador. Pharmacol. Res. 2007, 55, 23–30. [Google Scholar] [CrossRef] [PubMed]
- Scalvenzi, L.; Yaguache-Camacho, B.; Cabrera-Martinez, P.; Guerrini, A. In vitro antifungal activity of essential oils of Ocotea quixos (Lam.) Kosterm. and Piper aduncum L. Bioagro 2016, 28, 39–46. [Google Scholar]
- Pino, J.A.; Fon-Fay, F.M.; Falco, A.S.; Pérez, J.C.; Hernández, I.; Rodeiro, I.; Fernández, M.D. Chemical composition and biological activities of essential oil from Ocotea quixos (Lam.) Kosterm. leaves grown wild in Ecuador. Am. J. Essent. Oils Nat. Prod. 2018, 6, 31–34. [Google Scholar]
- Calderón, F.G.O.; García, P.L.G.; Ortiz, Y.L.S. Chemical composition and antioxidant and antibacterial activity of Ocotea quixos. Rev. Cuba. Plant. Med. 2018, 23, 1–15. [Google Scholar]
- Bruni, R.; Medici, A.; Andreotti, E.; Fantin, C.; Muzzoli, M.; Dehesa, M.; Romagnoli, C.; Sacchetti, G. Chemical composition and biological activities of Ishpingo essential oil, a traditional Ecuadorian spice from Ocotea quixos (Lam.) Kosterm. (Lauraceae) flower calices. Food Chem. 2004, 85, 415–421. [Google Scholar] [CrossRef]
- Ballabeni, V.; Tognolini, M.; Giorgio, C.; Bertoni, S.; Bruni, R.; Barocelli, E. Ocotea quixos Lam. essential oil: In vitro and in vivo investigation on its anti-inflammatory properties. Fitoterapia 2010, 81, 289–295. [Google Scholar] [CrossRef]
- Radice, M.; Pietrantoni, A.; Guerrini, A.; Tacchini, M.; Sacchetti, G.; Chiurato, M.; Venturi, G.; Fortuna, C. Inhibitory effect of Ocotea quixos (Lam.) Kosterm. and Piper aduncum L. essential oils from Ecuador on west Nile virus infection. Plant Biosyst. Int. J. Deal. Asp. Plant Biol. 2019, 153, 344–351. [Google Scholar] [CrossRef]
- Pandey, A.; Chattopadhyay, P.; Banerjee, S.; Pakshirajan, K.; Singh, L. Antitermitic activity of plant essential oils and their major constituents against termite Odontotermes assamensis Holmgren (Isoptera: Termitidae) of north east India. Int. Biodeterior. Biodegrad. 2012, 75, 63–67. [Google Scholar] [CrossRef]
- Santos, A.A.; de Oliveira, B.M.S.; Melo, C.R.; Lima, A.P.S.; Santana, E.D.R.; Blank, A.F.; Picanço, M.C.; Araújo, A.P.A.; Cristaldo, P.F.; Bacci, L. Sub-lethal effects of essential oil of Lippia sidoides on drywood termite Cryptotermes brevis (Blattodea: Termitoidea). Ecotoxicol. Environ. Saf. 2017, 145, 436–441. [Google Scholar] [CrossRef]
- Siramon, P.; Ohtani, Y.; Ichiura, H. Biological performance of Eucalyptus camaldulensis leaf oils from Thailand against the subterranean termite Coptotermes formosanus Shiraki. J. Wood Sci. 2009, 55, 41–46. [Google Scholar] [CrossRef]
- Verma, M.; Sharma, S.; Prasad, R. Biological alternatives for termite control: A review. Int. Biodeterior. Biodegrad. 2009, 63, 959–972. [Google Scholar] [CrossRef]
- Sakasegawa, M.; Hori, K.; Yatagai, M. Composition and antitermite activities of essential oils from Melaleuca species. J. Wood Sci. 2003, 49, 181–187. [Google Scholar] [CrossRef]
- Park, I.L.K.; Shin, S.C. Fumigant activity of plant essential oils and components from garlic (Allium sativum) and clove bud (Eugenia caryophyllata) oils against the Japanese termite (Reticulitermes speratus Kolbe). J. Agric. Food Chem. 2005, 53, 4388–4392. [Google Scholar] [CrossRef] [PubMed]
- Ramírez-López, C.B.; Beltrán-Sánchez, R.; Hernández-Izquierdo, A.; Salvador-Hernández, J.L.; Salcedo-Pérez, E.; Del Río, R.E.; Martínez Pacheco, M.M. Antifeedant activity of caesalpinia coriaria essential oil against Incisitermes marginipennis (Latreille). Phyton 2021, 90, 907–920. [Google Scholar] [CrossRef]
- Zhang, H.; Chen, G.; Lü, S.; Zhang, L.; Guo, M. Insecticidal activities against Odontotermes formosanus and Plutella xylostella and corresponding constituents of tung meal from Vernicia fordii. Insects 2021, 12, 425. [Google Scholar] [CrossRef]
- Mishra, T.; Gangoo, S.A.; Azad, A.; Kumar, A.; Pal, M. Chemical composition and antitermite activity of essential oil from Artemisia absinthium growing in Kashmir valley of India. J. Essent. Oil Bear. Plants 2020, 23, 397–404. [Google Scholar] [CrossRef]
- Salem, M.Z.M.; Ali, M.F.; Mansour, M.M.A.; Ali, H.M.; Moneim, E.M.A.; Abdel-Megeed, A. Anti-termitic activity of three plant extracts, chlorpyrifos, and a bioagent compound (protecto) against termite Microcerotermes eugnathus silvestri (Blattodea: Termitidae) in Egypt. Insects 2020, 11, 756. [Google Scholar] [CrossRef]
- Akbar, M.S.; Sajjad, F.; Afzal, M.; Luqman, M.; Riaz, M.A.; Majeed, M.Z. Field evaluation of promising botanical extracts, plant essential oils and differential chemistry insecticides against subterranean termites Odontotermes obesus (Isoptera: Termitidae). Sarhad J. Agric. 2021, 37, 120–127. [Google Scholar] [CrossRef]
- Khanikor, B.; Barman, J.; Sarma, R.; Mahanta, S.; Adhikari, K. Evaluation of efficacy of three essential oils against Odontotermes feae (Isoptera: Termitidae). J. Environ. Pollut. Human Health 2018, 6, 68–76. [Google Scholar] [CrossRef]
- Almeida, M.L.S.; Oliveira, A.S.; Rodrigues, A.A.; Carvalho, G.S.; Silva, L.B.; Lago, J.H.G.; Casarin, F.E. Antitermitic activity of plant essential oils and their major constituents against termite Heterotermes sulcatus (Isoptera: Rhinotermitidae). J. Med. Plants Res. 2015, 9, 97–103. [Google Scholar] [CrossRef]
- Manzoor, F.; Asma Malik, S.; Naz, N.; Naz, S. Potential of antitermitic activities of eucalyptus oil. Pak. J. Zool. 2012, 44, 2. [Google Scholar]
- Maistrello, L.; Laine, R.A. Nootkatone is a repellent for formosan subterranean termite (Coptotermes formosanus). Artic. J. Chem. Ecol. 2001, 27, 523–531. [Google Scholar] [CrossRef]
- Isman, M.B. Botanical insecticides, deterrents, and repellents in modern agriculture and an increasingly regulated world. Annu. Rev. Entomol. 2006, 51, 45–66. [Google Scholar] [CrossRef] [PubMed]
- Lima, R.K.; Cardoso, M.G.; Moraes, J.C.; Andrade, M.A.; Melo, B.A.; Rodrigues, V.G. Caracterização química e atividade inseticida do óleo essencial de Ageratum conyzoides l. sobre a lagarta-do-cartucho do milho Spodoptera frugiperda (smith, 1797) (Lepidoptera: Noctuidae). Biosci. J. 2010, 26, 1–5. [Google Scholar]
- Anderson, M.J.; Whitcomb, P.J. RSM Simplified: Optimizing Processes Using Response Surface Methods for Design of Experiments; CRC Press: Boca Raton, FL, USA, 2016; ISBN 1315351722. [Google Scholar]
- Jeirani, Z.; Jan, B.M.; Ali, B.S.; Noor, I.M.; See, C.H.; Saphanuchart, W. Prediction of water and oil percolation thresholds of a microemulsion by modeling of dynamic viscosity using response surface methodology. J. Ind. Eng. Chem. 2013, 19, 554–560. [Google Scholar] [CrossRef]
- Stanley, H.; Ogbonna, C.; Abu, G. Exploration of one-factor rsm to optimize the concentration of organic fraction of municipal solid waste (OFMSW) for biogas production. Int. J. Waste Resour. 2017, 7, 1–12. [Google Scholar]
- Crespo, Y.A.; Sánchez, L.R.B.; Quintana, Y.G.; Cabrera, A.S.T.; del Sol, A.B.; Mayancha, D.M.G. Evaluation of the synergistic effects of antioxidant activity on mixtures of the essential oil from Apium graveolens L., Thymus vulgaris L. and Coriandrum sativum L. using simplex-lattice design. Heliyon 2019, 5, e01942. [Google Scholar] [CrossRef] [PubMed]
- Van den Dool, H. A generalization of the retention index system including linear temperature programmed gas-liquid partition chromatography. J. Chromatogr. A 1963, 11, 463–471. [Google Scholar] [CrossRef]
- Adams, R.P. Identification of Essential Oil Components by Gas Chromatography/Mass Spectrometry; Allured Publishing Corporation: Carol Stream, IL, USA, 2007; Volume 456, ISBN 10-1932633219. [Google Scholar]
- De Saint Laumer, J.Y.; Cicchetti, E.; Merle, P.; Egger, J.; Chaintreau, A. Quantification in gas chromatography: Prediction of flame ionization detector response factors from combustion enthalpies and molecular structures. Anal. Chem. 2010, 82, 6457–6462. [Google Scholar] [CrossRef] [PubMed]
- Tissot, E.; Rochat, S.; Debonneville, C.; Chaintreau, A. Rapid GC-FID quantification technique without authentic samples using predicted response factors. Flavour Fragr. J. 2012, 27, 290–296. [Google Scholar] [CrossRef]
- Pal, M.; Kumar, R.; Tewari, S.K. Anti-termite activity of essential oil and its components from Myristica fragrans against Microcerotermes beesoni. J. Appl. Sci. Environ. Manag. 2011, 15, 559–561. [Google Scholar]
- Bakaruddin, N.H.; Dieng, H.; Sulaiman, S.F.; Ab Majid, A.H. Evaluation of the toxicity and repellency of tropical plant extract against subterranean termites, Globitermes sulphureus and Coptotermes gestroi. Inf. Process. Agric. 2018, 5, 298–307. [Google Scholar] [CrossRef]
- Arteaga-Crespo, Y.; Radice, M.; Bravo-Sanchez, L.R.; García-Quintana, Y.; Scalvenzi, L. Optimisation of ultrasound-assisted extraction of phenolic antioxidants from Ilex guayusa Loes. leaves using response surface methodology. Heliyon 2020, 6, e03043. [Google Scholar] [CrossRef]
- Gupta, A.; Sharma, S.; Naik, S.N. Biopesticidal value of selected essential oils against pathogenic fungus, termites, and nematodes. Int. Biodeterior. Biodegrad. 2011, 65, 703–707. [Google Scholar] [CrossRef]
- Maia, J.G.S.; Andrade, E.H.A.; Maria das Graças, B.Z. Aroma volatiles from two fruit varieties of jackfruit (Artocarpus heterophyllus Lam.). Food Chem. 2004, 85, 195–197. [Google Scholar] [CrossRef]
- Chang, S.T.; Cheng, S.S. Antitermitic activity of leaf essential oils and components from Cinnamomum osmophleum. J. Agric. Food Chem. 2002, 50, 1389–1392. [Google Scholar] [CrossRef]
- Cheng, S.S.; Wu, C.L.; Chang, H.T.; Kao, Y.T.; Chang, S.T. Antitermitic and antifungal activities of essential oil of Calocedrus formosana leaf and its composition. J. Chem. Ecol. 2004, 30, 1957–1967. [Google Scholar] [CrossRef] [PubMed]
- Jain, N.; Sharma, M.; Joshi, S.C.; Kaushik, U. Chemical composition, toxicity and antidermatophytic activity of essential oil of Trachyspermum ammi. Indian J. Pharm. Sci. 2018, 80. [Google Scholar] [CrossRef]
- Adams, R.P. Cedar wood oil—Analyses and properties. In Essential Oils and Waxes; Springer: Amsterdam, The Netherlands, 1991; pp. 159–173. [Google Scholar]
- Lin, T.; Yin, H. The effects of Cinnamomum spp. oils on the control of the termite Coptotermes formosanus Shiraki. Bull. Taiwan For. Res. Inst. 1995, 10, 459–464. [Google Scholar]
- Zhu, B.C.R.; Henderson, G.; Chen, F.; Fei, H.; Laine, R.A. Evaluation of vetiver oil and seven insect-active essential oils against the Formosan subterranean termite. J. Chem. Ecol. 2001, 27, 1617–1625. [Google Scholar] [CrossRef]
- Subekti, N. Toxicity of essential oils against termite Macrotermes gilvus Hagen (Blattodea: Termitidae). J. Phys. 2020, 1567, 32053. [Google Scholar] [CrossRef]

| Experiment | Concentration of Essential Oil (%; v/v) | Termite Mortality (%) | Concentration of Essential Oil (%; v/v) | Termite Repellency (%) | ||
|---|---|---|---|---|---|---|
| Experimental * | Predicted | Experimental * | Predicted | |||
| 1 | 0.24 | 100 ± 0.0 | 100 | 0.06 | 58.9 ± 1.9 | 62.0 |
| 2 | 0.05 | 20.0 ± 3.3 | 20.5 | 0.12 | 100 ± 0.0 | 99.2 |
| 3 | 0.30 | 100 ± 0.0 | 100 | 0.06 | 60.0 ± 3.3 | 62.0 |
| 4 | 0.18 | 100 ± 0.0 | 99.67 | 0.01 | 50.0 ± 3.3 | 49.5 |
| 5 | 0.30 | 100 ± 0.0 | 100 | 0.03 | 54.4 ± 3.8 | 52.7 |
| 6 | 0.11 | 96.7 ± 1.9 | 96.6 | 0.11 | 97.8 ± 1.9 | 99.2 |
| 7 | 0.05 | 22.2 ± 1.9 | 20.5 | 0.09 | 80.0 ± 3.3 | 77.5 |
| 8 | 0.18 | 100 ± 0.0 | 99.7 | 0.01 | 48.9 ± 1.9 | 49.5 |
| 9 | 0.18 | 98.9 ± 1.9 | 99.7 | 0.06 | 62.2 ± 1.9 | 62.0 |
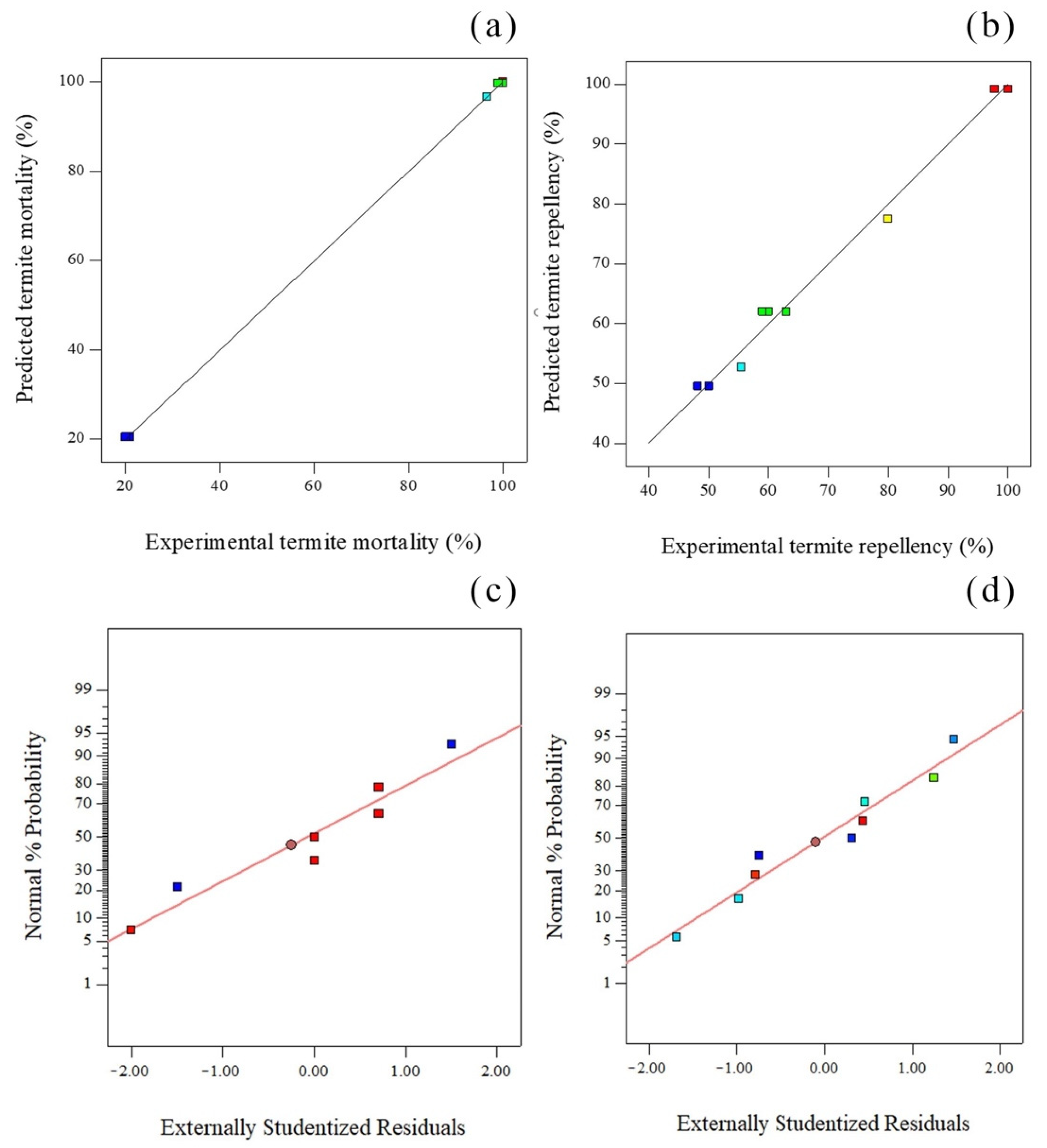
| Mortality | Sequential p-Value | Lack of Fit p-Value | Adjusted R-Squared | Predicted R-Squared | |
|---|---|---|---|---|---|
| Linear | 0.0153 | <0.0001 | 0.5342 | 0.2688 | |
| Quadratic | 0.0018 | <0.0001 | 0.9049 | 0.8743 | |
| Cubic | 0.0031 | <0.0001 | 0.9829 | 0.9343 | |
| Quartic | <0.0001 | 0.9998 | Suggested | ||
| Fifth | Aliased | ||||
| Repellency | Sequential p-value | Lack of Fit p-value | Adjusted R-Squared | Predicted R-Squared | |
| Linear | 0.0001 | 0.0030 | 0.8820 | 0.8244 | |
| Quadratic | 0.0004 | 0.1540 | 0.9858 | 0.9796 | Suggested |
| Cubic | 0.9266 | 0.0679 | 0.9830 | 0.9499 | |
| Quartic | 0.0679 | 0.9917 | |||
| Fifth | Aliased |
| Mortality | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
|---|---|---|---|---|---|---|
| Quartic model | 9685.78 | 4 | 2421.44 | 8302.09 | <0.0001 | Significant |
| A—concentration | 21.20 | 1 | 21.20 | 72.70 | 0.0010 | |
| A2 | 1.52 | 1 | 1.52 | 5.20 | 0.0848 | |
| A3 | 587.25 | 1 | 587.25 | 2013.44 | <0.0001 | |
| A4 | 102.45 | 1 | 102.45 | 351.27 | <0.0001 | |
| Pure error | 1.17 | 4 | 0.29 | |||
| Total corr. | 9686.94 | 8 | ||||
| Repellency | Sum of Squares | df | Mean Square | F-Value | p-Value Prob > F | |
| Quadratic model | 3061.19 | 2 | 1530.60 | 279.14 | <0.0001 | Significant |
| A—concentration | 2774.63 | 1 | 2774.63 | 506.02 | <0.0001 | |
| A2 | 286.57 | 1 | 286.57 | 52.26 | 0.0004 | |
| Pure error | 12.91 | 4 | 3.23 | |||
| Total corr. | 3094.09 | 8 |
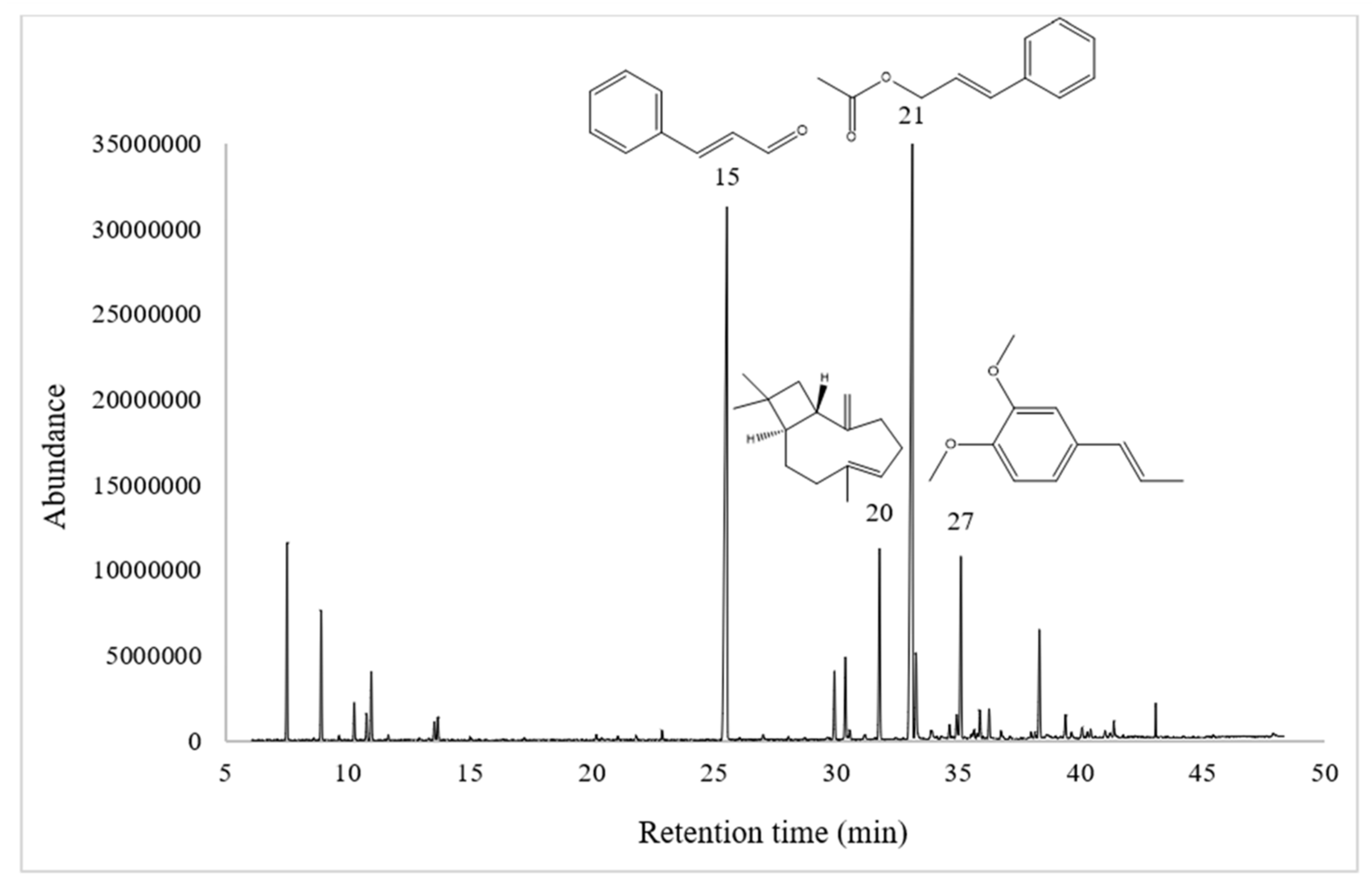
| No. | RT (min) | LIRcal | LIRref A | Compound | % |
|---|---|---|---|---|---|
| 1 | 8.93 | 930 | 923 | α-pinene | 3.09 |
| 2 | 9.66 | 945 | 946 | camphene | 0.13 |
| 3 | 10.28 | 958 | 952 | benzaldehyde | 0.79 |
| 4 | 10.79 | 969 | 975 | sabinene | 0.70 |
| 5 | 10.98 | 973 | 979 | β-pinene | 1.91 |
| 6 | 11.68 | 988 | 988 | β-myrcene | 0.21 |
| 7 | 13.56 | 1026 | 1024 | limonene | 0.55 |
| 8 | 13.70 | 1029 | 1026 | 1,8-cineole | 0.68 |
| 9 | 15.04 | 1056 | 1054 | γ-terpinene | 0.08 |
| 10 | 17.25 | 1100 | 1095 | linalool | 0.13 |
| 11 | 20.19 | 1160 | 1160 B | hydrocinnamaldehyde | 0.12 |
| 12 | 21.07 | 1178 | 1174 | 4-terpineol | 0.16 |
| 13 | 21.82 | 1193 | 1186 | α-terpineol | 0.20 |
| 14 | 22.89 | 1216 | 1217 | (Z)-cinnamaldehyde | 0.13 |
| 15 | 25.53 | 1272 | 1267 | (E)-cinnamaldehyde | 27.03 |
| 16 | 29.94 | 1371 | 1374 | α-copaene | 1.46 |
| 17 | 30.39 | 1381 | 1376 | (E)-methyl cinnamate | 2.45 * |
| 18 | 30.49 | 1384 | 1376 | β-cubebene | |
| 19 | 30.57 | 1385 | 1389 | β-elemene | |
| 20 | 31.78 | 1414 | 1417 | (E)-β-caryophyllene | 5.21 |
| 21 | 33.12 | 1446 | 1443 | (E)-cinnamyl acetate | 36.44 |
| 22 | 33.27 | 1450 | 1452 | α-humulene | 0.29 |
| 23 | 33.90 | 1465 | 1471 | 4,5-di-epi-aristolochene | 0.34 |
| 24 | 34.31 | 1475 | 1480 | germacrene D | 0.48 |
| 25 | 34.65 | 1483 | 1489 | β-selinene | 0.61 |
| 26 | 34.93 | 1490 | 1500 | bicyclogermacrene | 1.81 |
| 27 | 35.11 | 1494 | 1491 | (E)-methyl isoeugenol | 4.18 |
| 28 | 35.54 | 1505 | 1505 | β-bisabolene | 0.46 |
| 29 | 35.78 | 1511 | 1514 | cubebol | 0.23 |
| 30 | 35.89 | 1514 | 1522 | δ-cadinene | 0.66 |
| 31 | 36.27 | 1523 | 1529 | (E)-γ-bisabolene | 0.77 |
| 32 | 36.76 | 1536 | 1544 | α-calacorene | 0.23 |
| 33 | 38.15 | 1571 | 1577 | spathulenol | 0.24 |
| 34 | 38.32 | 1576 | 1582 | caryophyllene oxide | 3.70 |
| 35 | 39.39 | 1604 | 1608 | humulene epoxide II | 0.77 ** |
| 36 | 40.07 | 1629 | 1630 | β-muurola-4,10(14)-dien-1-ol | |
| 37 | 40.30 | 1637 | undetermined (MW 220) | 0.23 *** | |
| 38 | 40.42 | 1641 | 1639 | β-caryophylla-4(12),8(13)-dien-5-ol | |
| 39 | 41.02 | 1663 | undetermined (MW 222) | 0.50 | |
| 40 | 41.22 | 1670 | undetermined (MW 220) | 0.35 | |
| 41 | 41.37 | 1675 | undetermined (MW 220) | 0.36 | |
| 42 | 43.09 | 1769 | 1759 | benzyl benzoate | 0.36 |
| Monoterpenes | 6.67 | ||||
| Oxygenated monoterpenes | 1.16 | ||||
| Aromatic aldehydes | 28.07 | ||||
| Sesquiterpenes | 13.95 | ||||
| Oxygenated sesquiterpenes | 6.38 | ||||
| Esters | 37.62 | ||||
| Others | 4.18 | ||||
| Total | 98.02 | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Arteaga-Crespo, Y.; Ureta-Leones, D.; García-Quintana, Y.; Montalván, M.; Gilardoni, G.; Malagón, O. Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design. Agronomy 2021, 11, 1249. https://doi.org/10.3390/agronomy11061249
Arteaga-Crespo Y, Ureta-Leones D, García-Quintana Y, Montalván M, Gilardoni G, Malagón O. Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design. Agronomy. 2021; 11(6):1249. https://doi.org/10.3390/agronomy11061249
Chicago/Turabian StyleArteaga-Crespo, Yasiel, Diego Ureta-Leones, Yudel García-Quintana, Mayra Montalván, Gianluca Gilardoni, and Omar Malagón. 2021. "Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design" Agronomy 11, no. 6: 1249. https://doi.org/10.3390/agronomy11061249
APA StyleArteaga-Crespo, Y., Ureta-Leones, D., García-Quintana, Y., Montalván, M., Gilardoni, G., & Malagón, O. (2021). Preliminary Predictive Model of Termiticidal and Repellent Activities of Essential Oil Extracted from Ocotea quixos Leaves against Nasutitermes corniger (Isoptera: Termitidae) Using One-Factor Response Surface Methodology Design. Agronomy, 11(6), 1249. https://doi.org/10.3390/agronomy11061249