Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites and Sampling
2.2. Soil Analysis
2.3. Quantification of Fungal Colonization
2.4. Isolation of Endophytic Fungi
2.5. Molecular Identification of Endophytic Fungi
2.6. DSE Species Diversity
2.7. Statistical Analysis
3. Results
3.1. Spatial–Temporal Distribution of DSE Colonization
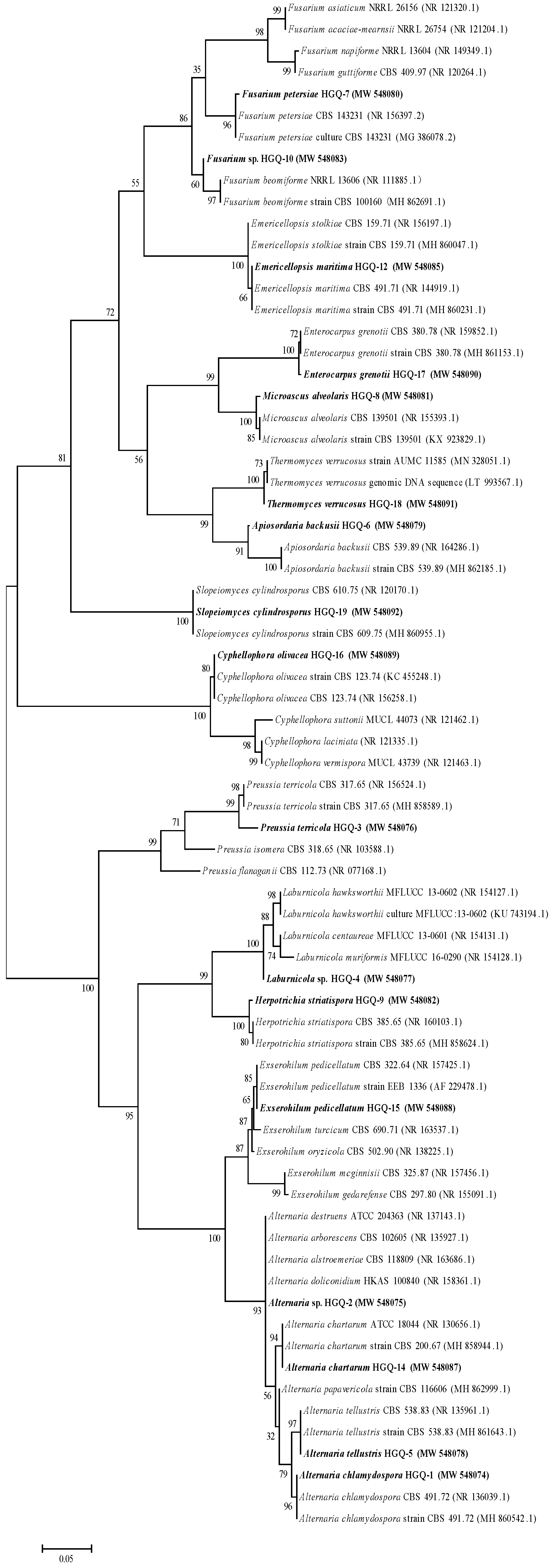
3.2. Morphological Characteristics and Identification of Endophytic Fungi
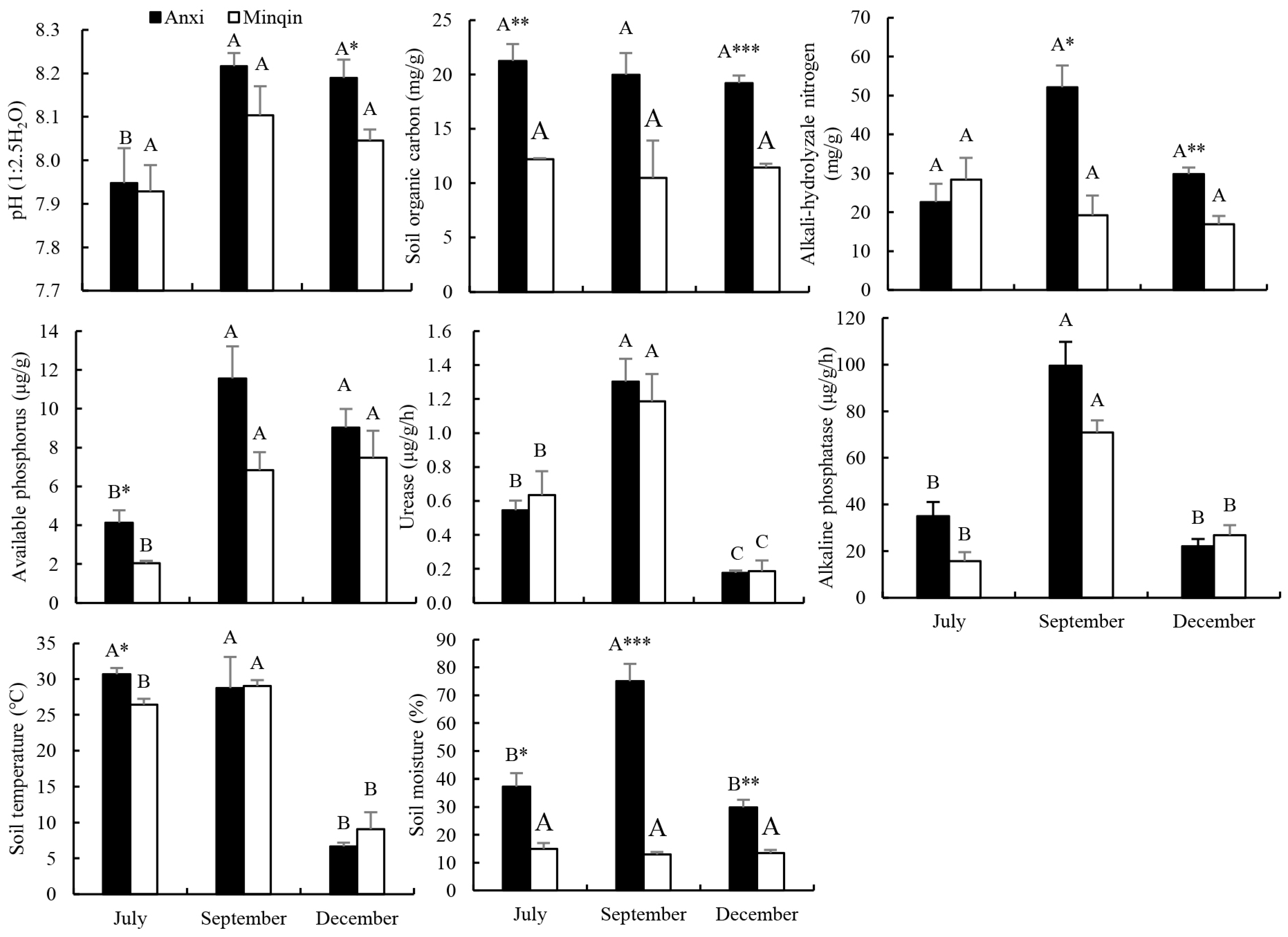
3.3. Spatial–Temporal Distribution of Soil Factors
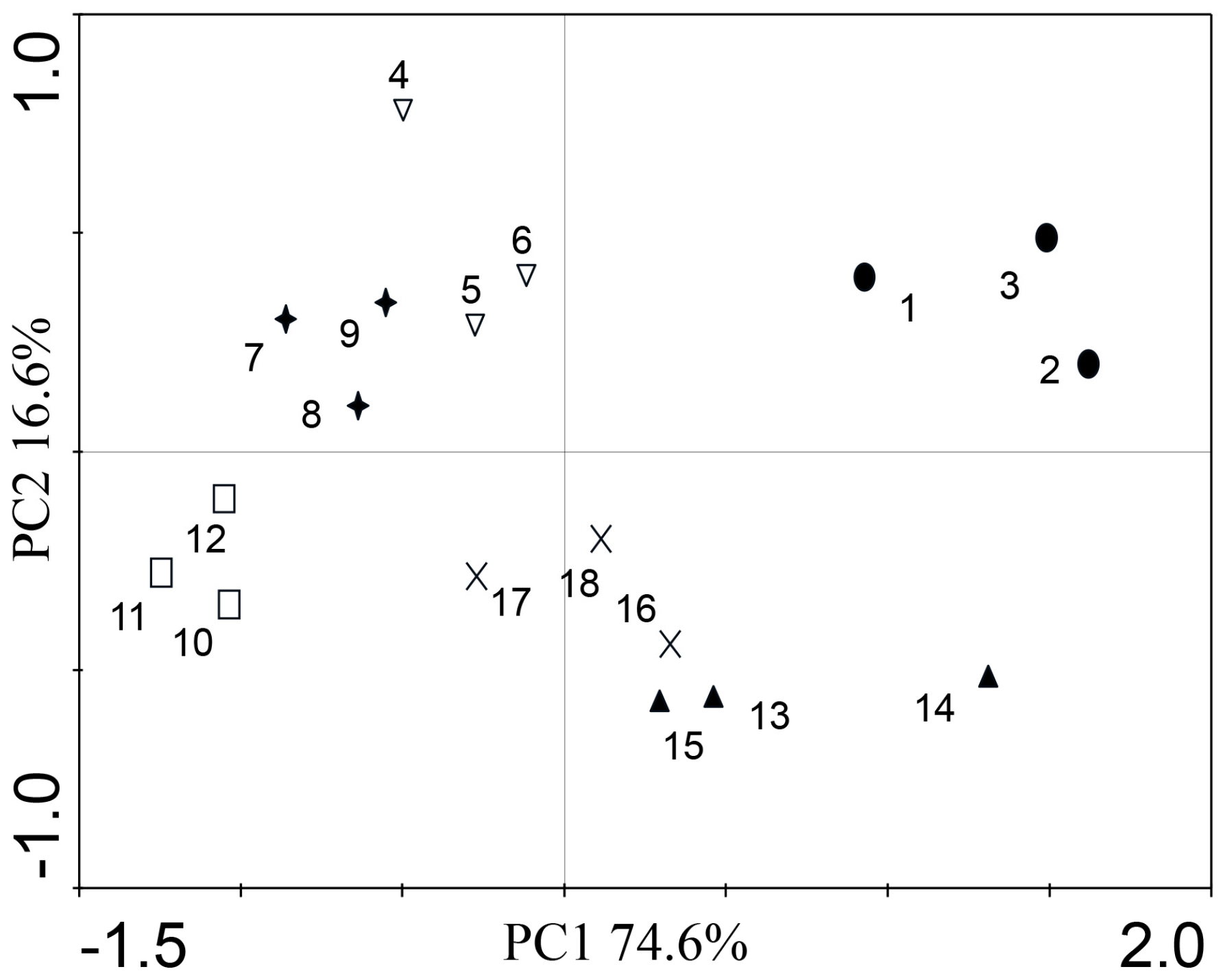
3.4. Principal Component Analysis of DSE Colonization
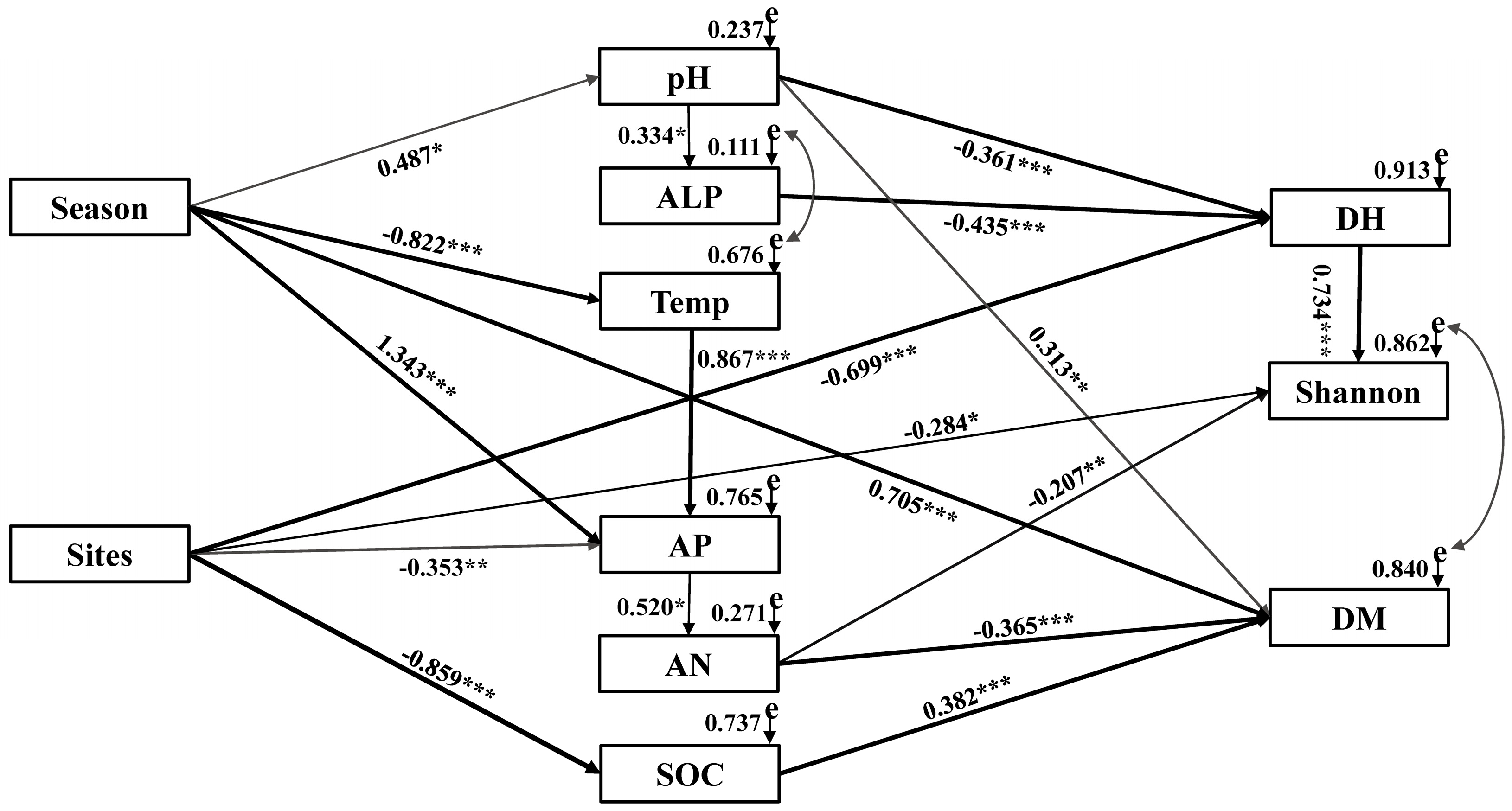
3.5. Correlation Analyses
4. Discussion
4.1. DSE Colonisation Status
4.2. DSE Species Diversity
4.3. DSE and Soil Factors
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tang, Z.; An, H.; Deng, L.; Wang, Y.; Zhu, G.; Shangguan, Z. Effect of desertification on productivity in a desert steppe. Sci. Rep. 2016, 6, 27839. [Google Scholar] [CrossRef]
- Chaves, M.M.; Maroco, J.P.; Pereira, J.S. Understanding plant responses to drought-from genes to the whole plant. Funct. Plant Biol. 2003, 30, 239–264. [Google Scholar] [CrossRef]
- Niu, R.; Liu, J.; Zhao, X.; Qin, Y. Ecological benefit of different revegetated covers in the middle of Hexi corridor, northwestern China. Environ. Earth Sci. 2015, 74, 5699–5710. [Google Scholar] [CrossRef]
- Lioubimtseva, E.; Henebry, G.M. Climate and environmental change in arid Central Asia: Impacts, vulnerability, and adaptations. J. Arid Environ. 2009, 73, 963–977. [Google Scholar] [CrossRef]
- Brooker, R.W.; Maestre, F.T.; Callaway, R.M.; Lortie, C.L.; Cavieres, L.A.; Kunstler, G.; Michalet, R. Facilitation in plant communities: The past, the present, and the future. J. Ecol. 2008, 96, 18–34. [Google Scholar] [CrossRef]
- Jalali, G.A.; Akbarian, H.; Rhoades, C.; Yousefzadeh, H. The effect of the halophytic shrub lycium ruthenicum (mutt) on selected soil properties of a desert ecosystem in central iran. Pol. J. Ecol. 2012, 845–850. [Google Scholar] [CrossRef]
- Kivlin, S.N.; Emery, S.M.; Rudgers, J.A. Fungal symbionts alter plant responses to global change. Am. J. Bot. 2013, 100, 1445–1457. [Google Scholar] [CrossRef]
- Li, X.; He, X.L.; Hou, L.F.; Ren, Y.; Wang, S.J.; Su, F. Dark septate endophytes isolated from a xerophyte plant promote the growth of Ammopiptanthus mongolicus under drought condition. Sci. Rep. 2018, 8, 239–264. [Google Scholar] [CrossRef]
- Jumpponen, A.; Trappe, J.M. Dark septate endophytes: A review of facultative biotrophic root-colonizing fungi. New Phytol. 1998, 140, 295–310. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seeking the elusive function of the root-colonising dark septate endophytic fungi. Stud. Mycol. 2005, 53, 173–189. [Google Scholar] [CrossRef]
- Barrow, J.R. A typical morphology of dark septate fungal root endophytes of Bouteloua in arid southwestern USA rangelands. Mycorrhiza 2003, 13, 239–247. [Google Scholar] [CrossRef]
- Knapp, D.G.; Pintye, A.; Kovács, G.M. The dark side is not fastidious-dark septate endophytic fungi of native and invasive plants of semiarid sandy areas. PLoS ONE 2012, 7, e32570. [Google Scholar] [CrossRef]
- Hou, L.F.; He, X.L.; Li, X.; Wang, S.J.; Zhao, L.L. Species composition and colonization of dark septate endophytes are affected by host plant species and soil depth in the Mu Us sandland, northwest China. Fungal Ecol. 2019, 39, 276–284. [Google Scholar] [CrossRef]
- Van der Heij, M.G.; Martin, F.M.; Selosse, M.A.; Sanders, I.R. Mycorrhizal ecology and evolution: The past, the present, and the future. New Phytol. 2015, 205, 1406–1423. [Google Scholar] [CrossRef] [PubMed]
- Newsham, K.K. A meta-analysis of plant responses to dark septate root endophytes. New Phytol. 2011, 190, 783–793. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.H.; Tang, M.; Chen, H.; Wang, Y.J.; Ban, Y. Arbuscular mycorrhizas and dark septate endophytes colonization status in medicinal plant Lycium barbarum L. in arid Northwestern China. Afr. J. Microbiol. Res. 2010, 4, 1914–1920. [Google Scholar] [CrossRef]
- Zhang, H.H.; Tang, M.; Chen, H.; Wang, Y.J. Effects of a dark-septate endophytic isolate LBF-2 on the medicinal plant Lycium barbarum L. J. Microbiol. 2012, 50, 91–96. [Google Scholar] [CrossRef]
- Li, B.K.; He, X.L.; He, C.; Chen, Y.; Wang, X. Spatial dynamics of dark septate endophytes and soil factors in the rhizosphere of Ammopiptanthus mongolicus in Inner Mongolia, China. Symbiosis 2015, 65, 75–84. [Google Scholar] [CrossRef]
- Xie, L.L.; He, X.L.; Wang, K.; Hou, L.F.; Sun, Q. Spatial dynamics of dark septate endophytes in the roots and rhizospheres of Hedysarum scoparium in northwest China and the influence of edaphic variables. Fungal Ecol. 2017, 26, 135–143. [Google Scholar] [CrossRef]
- Li, X.; He, C.; He, X.L.; Su, F.; Hou, L.F.; Ren, Y.; Hou, Y.T. Dark septate endophytes improve the growth of host and non-host plants under drought stress through altered root development. Plant Soil 2019, 439, 259–272. [Google Scholar] [CrossRef]
- Zuo, Y.L.; Su, F.; He, X.L.; Li, M. Colonization by dark septate endophytes improves the growth of Hedysarum scoparium under multiple inoculum levels. Symbiosis 2020, 82, 201–214. [Google Scholar] [CrossRef]
- Likar, M.; Regvar, M. Isolates of dark septate endophytes reduce metal uptake and improve physiology of Salix caprea L. Plant Soil 2013, 370, 593–604. [Google Scholar] [CrossRef]
- Dos Santos, S.G.D.; Silva, P.R.A.D.; Garcia, A.C.; Zilli, J.É.; Berbara, R.L.L. Dark septate endophyte decreases stress on rice plants. Braz. J. Microbiol. 2017, 48, 333–341. [Google Scholar] [CrossRef]
- Butler, M.; Day, A. Fungal melanins: A review. Can. J. Microbiol. 1998, 44, 1115–1136. [Google Scholar] [CrossRef]
- Zhan, F.; He, Y.; Zu, Y.; Li, T.; Zhao, Z. Characterization of melanin isolated from a dark septate endophyte (DSE), Exophiala pisciphila. World J. Microbiol. Biotechnol. 2011, 27, 2483–2489. [Google Scholar] [CrossRef]
- Mandyam, K.; Jumpponen, A. Seasonal and temporal dynamics of arbuscular mycorrhizal and dark septate endophytic fungi in a tallgrass prairie ecosystem are minimally affected by nitrogen enrichment. Mycorrhiza 2008, 18, 145–155. [Google Scholar] [CrossRef]
- Hazard, C.; Gosling, P.; Mitchell, D.T.; Doohan, F.M.; Bending, G.D. The role of local environment and geographical distance in determining community composition of arbuscular mycorrhizal fungi at the landscape scale. ISME J. 2013, 7, 498–508. [Google Scholar] [CrossRef] [PubMed]
- Dasila, K.; Pandey, A.; Samant, S.; Pande, V. Endophytes associated with Himalayan silver birch (Betula-utilis D. Don) roots in relation to season and soil parameters. Appl. Soil Ecol. 2020, 149, 103513. [Google Scholar] [CrossRef]
- Ruotsalainen, A.; Väre, H.; Vestberg, M. Seasonality of root fungal colonization in low-alpine herbs. Mycorrhiza 2002, 12, 29–36. [Google Scholar] [CrossRef] [PubMed]
- Rayment, J.T.; Jones, S.; French, K. Seasonal patterns of fungal colonisation in Australian native plants of different ages. Symbiosis 2020, 80, 169–182. [Google Scholar] [CrossRef]
- Huusko, K.; Ruotsalainen, A.L.; Markkola, A.M. A shift from arbuscular mycorrhizal to dark septate endophytic colonization in Deschampsia flexuosa roots occurs along primary successional gradient. Mycorrhiza 2017, 27, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Soil Survey Staff. Keys to Soil Taxonomy, 12th ed.; USDA-Natural Resources Conservation Service: Washington, DC, USA, 2014. [Google Scholar]
- Heiri, O.; Lotter, A.F.; Lemcke, G. Loss on ignition as a method for estimating organic and carbonate content in sediments: Reproducibility and comparability of results. J. Paleolimnol. 2001, 25, 101–110. [Google Scholar] [CrossRef]
- Bever, J.D.; Morton, J.B.; Antonovics, J.; Schultz, P.A. Host-dependent sporulation and species diversity of arbuscular mycorrhizal fungi in a mown grassland. J. Ecol. 1996, 84, 71–82. [Google Scholar] [CrossRef]
- Tarafdar, J.C.; Marschner, H. Phosphatase activity in the rhizosphere and hyphosphere of VA mycorrhizal wheat supplied with inorganic and organic phosphorus. Soil Biol. Biochem. 1994, 26, 387–395. [Google Scholar] [CrossRef]
- Hoffmann, G.G.; Teicher, K. A colorimetric technique for determining urease activity in soil. Dung Boden 1961, 95, 55–63. [Google Scholar] [CrossRef]
- Berch, S.M.; Kendrick, B. Vesicular-arbuscular mycorrhizae of southern Ontario ferns and fern-allies. Mycologia 1982, 74, 769–776. [Google Scholar] [CrossRef]
- Biermann, B.; Linderman, R.G. Quantifying vesicular-arbuscular mycorrhizae: A proposed method towards standardization. New Phytol. 1981, 87, 63–67. [Google Scholar] [CrossRef]
- Trouvelot, A.; Kough, J.L.; Gianinazzi-Pearson, V. Mesure du taux demycorhization V A d’un système radiculaire. Recherche de methods d’estimation ayant une signification fonctionnelle. In Physiological and Genetical Aspects of Mycorrhizae; Gianinazzi-Pearson, V., Gianinazzi, S., Eds.; INRA Press: Paris, France, 1986; pp. 217–221. [Google Scholar]
- Maadon, S.N.; Wakid, S.A.; Zainudin, I.I.; Rusli, L.S.; Mohd Zan, M.S.; Hasan, N.A.; Abu Shah, N.A.; Rohani, E.R. Isolation and identification of endophytic fungi from UiTM reserve forest, Negeri Sembilan. Sains Malays. 2018, 47, 3025–3030. [Google Scholar] [CrossRef]
- Tamura, K.; Stecher, G.; Peterson, D.; Filipski, A.; Kumar, S. MEGA6: Molecular evolutionary genetics analysis version 6.0. Mol. Biol. Evol. 2013, 30, 2725–2729. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Wang, Q.; Lu, X.; Okane, I.; Kakishima, M. Endophytic fungal community in stems and leaves of plants from desert areas in China. Mycol. Prog. 2011, 11, 781–790. [Google Scholar] [CrossRef]
- Yuan, Z.L.; Rao, L.B.; Chen, Y.C.; Zhang, C.L.; Wu, Y.G. From pattern to process: Species and functional diversity in fungal endophytes of Abies beshanzuensis. Fungal Biol. 2011, 115, 197–213. [Google Scholar] [CrossRef]
- Shannon, C.E.; Weaver, W. The Mathematical Theory of Communication; University of Illinois Press: Urbana, IL, USA, 1949. [Google Scholar]
- Caruso, T.; Pigino, G.; Bernini, F.; Bargagli, R.; Migliorini, M. The Berger—Parker index as an effective tool for monitoring the biodiversity of disturbed soils: A case study on Mediterranean oribatid (Acari: Oribatida) assemblages. Biodivers. Conserv. 2008, 7, 35–43. [Google Scholar] [CrossRef]
- Samaga, P.V.; Rai, V.R. Diversity and bioactive potential of endophytic fungi from Nothapodytes foetida, Hypericum mysorense and Hypericum japonicum collected from Western Ghats of India. Ann. Microbiol. 2015, 66, 229–244. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Grover, A. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Orłowska, E.; Orłowski, D.; Mesjasz-Przybyłowicz, J.; Turnau, K. Role of mycorrhizal colonization in plant establishment on an alkaline gold mine tailing. Int. J. Phytoremediat. 2010, 13, 185–205. [Google Scholar] [CrossRef]
- Saito, K.; Kuga-Uetake, Y.; Saito, M.; Peterson, R.L. Vacuolar localization of phosphorus in hyphae of Phialocephala fortinii, a dark septate fungal root endophyte. Can. J. Microbiol. 2006, 52, 643–650. [Google Scholar] [CrossRef]
- Wu, L.Q.; Guo, S.X. Interaction between an isolate of dark septate fungi and its host plant Saussurea involucrata. Mycorrhiza 2008, 18, 79–85. [Google Scholar] [CrossRef] [PubMed]
- Yu, T.; Nassuth, A.; Peterson, R.L. Characterization of the interaction between the dark septate fungus Phialocephala fortinii and Asparagus officinalis roots. Can. J. Microbiol. 2001, 47, 741–753. [Google Scholar] [CrossRef]
- U’Ren, J.M.; Lutzoni, F.; Miadlikowska, J.; Laetsch, A.D.; Arnold, E.A. Host and geographic structure of endophytic and endolichenic fungi at a continental scale. Am. J. Bot. 2012, 99, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Penton, C.R.; Derek, S.L.; Amanda, P.; Cole, J.R.; Wu, L.Y.; Schuur, E.A.G.; Zhou, J.Z.; Tiedje, J.M. Denitrifying and diazotrophic community responses to artifcial warming in permafrost and tallgrass prairie soils. Front. Microbiol. 2015, 6, 746. [Google Scholar] [CrossRef] [PubMed]
- Zhang, H.S.; Zhou, M.X.; Zai, X.M.; Zhao, F.G.; Qin, P. Spatio-temporal dynamics of arbuscular mycorrhizal fungi and soil organic carbon in coastal saline soil of China. Sci. Rep. 2020, 10, 9781. [Google Scholar] [CrossRef]
- He, X.L.; Mouratov, S.; Steinberger, Y. Temporal and spatial dynamics of vesicular-arbuscular mycorrhizal fungi under the canopy of Zygophyllum dumosum Boiss. in the Negev Desert. J. Arid Environ. 2002, 52, 379–387. [Google Scholar] [CrossRef]
- Sudová, R.; Kohout, P.; Rydlová, J.; Čtvrtlíková, M.; Suda, J.; Voříšková, J.; Kolaříková, Z. Diverse fungal communities associated with the roots of isoetid plants are structured by host plant identity. Fungal Ecol. 2020, 45, 100914. [Google Scholar] [CrossRef]
- Selim, K.A.; El-Beih, A.A.; Abdel-Rahman, T.M.; El-Diwany, A.I. Biodiversity and antimicrobial activity of endophytes associated with Egyptian medicinal plants. Mycosphere 2011, 6, 669–678. [Google Scholar] [CrossRef]
- Bezerra, J.D.P.; Santos, M.G.S.; Barbosa, R.N.; Svedese, V.M.; Lima, D.M.M. Fungal endophytes from cactus Cereus jamacaru in Brazilian tropical dry forest: A first study. Symbiosis 2013, 60, 53–63. [Google Scholar] [CrossRef]
- Fonseca-García, C.; Devin, C.D.; Etzel, G.; Axel, V.; Tringe, S.G.; Partida-Martínez, L.P. The cacti microbiome: Interplay between habitat-filtering and host-specificity. Front. Microbiol. 2016, 7, 150. [Google Scholar] [CrossRef]
- Pieterse, Z.; Aveling, T.A.S.; Jacobs, A.; Cowan, D.A. Seasonal variability in fungal endophytes from Aizoaceae plants in the Succulent Karoo biodiversity hotspot, South Africa. J. Environ. 2018, 156, 19–26. [Google Scholar] [CrossRef]
- ÖZer, G.; Göre, M.E.; Alkan, M.; Yaman, M.; Dababat, A.A. First report of Exserohilum pedicellatum causing root rot of wheat in azerbaijan. Plant Dis. 2019, 103, 1416. [Google Scholar] [CrossRef]
- Lundberg, D.S.; Lebeis, S.L.; Paredes, S.H.; Yourstone, S.; Gehring, J.; Malfatti, S.; Tremblay, J.; Engelbrektson, A.; Kunin, V.; Rio, T.G.; et al. Defining the core Arabidopsis thaliana root microbiome. Nature 2012, 488, 86–90. [Google Scholar] [CrossRef]
- Qiang, W.; He, X.L.; Wang, J.J.; Zhao, L.L. Temporal and spatial variation of arbuscular mycorrhizal fungi under the canopy of Hedysarum scoparium in the northern desert, China. Appl. Soil Ecol. 2019, 136, 139–147. [Google Scholar] [CrossRef]
- Zhao, D.; Li, T.; Shen, M.; Wang, J.; Zhao, Z. Diverse strategies conferring extreme cadmium (Cd) tolerance in the dark septate endophyte (DSE), Exophiala pisciphila: Evidence from RNA-seq data. Microbiol. Res. 2015, 170, 27–35. [Google Scholar] [CrossRef]
- Ilstedt, U.; Singh, S. Nitrogen and phosphorus limitations of microbial respiration in a tropical phosphorus-fixing acrisol ultisol. compared with organic compost. Soil Biol. Biochem. 2005, 37, 1407–1410. [Google Scholar] [CrossRef]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root structure and functioning for efficient acquisition of phosphorus, matching morphological and physiological traits. Ann. Bot-Lond. 2006, 98, 693–713. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.; Pereira, J.A.; Bota, P.; Bento, A.; Baptista, P. Fungal endophyte communities in above- and belowground olive tree organs and the effect of season and geographic location on their structures. Fungal Ecol. 2016, 20, 193–201. [Google Scholar] [CrossRef]
- Fang, W.; Yang, L.; Zhu, X.; Zeng, L.; Li, X. Seasonal and habitat dependent variations in culturable endophytes of Camellia sinensis. J. Plant Pathol. Microbiol. 2013, 4, 169. [Google Scholar] [CrossRef]
- Peterson, R.L.; Wagg, C.; Pautler, M. Associations between microfungal endophytes and roots: Do structural features indicate function? Botany 2008, 86, 445–456. [Google Scholar] [CrossRef]
- Casazza, G.; Lumini, E.; Ercole, E.; Dovana, F.; Guerrina, M.; Arnulfo, M.; Fusconi, A.; Mucciarelli, M. The abundance and diversity of arbuscular mycorrhizal fungi are linked to the soil chemistry of screes and to slope in the Alpic paleo-endemic Berardia subacaulis. PLoS ONE 2017, 12, e0171866. [Google Scholar] [CrossRef] [PubMed]
- Vries, F.T.; Manning, P.; Tallowin, J.R.B.; Mortimer, S.R.; Pilgrim, E.S.; Harrison, K.A.; Hobbs, P.J.; Quirk, H.; Shipley, B.; Cornelissen, J.H.C.; et al. Abiotic drivers and plant traits explain landscape-scale patterns in soil microbial communities. Ecol. Lett. 2012, 15, 1230–1239. [Google Scholar] [CrossRef]
- Paterson, E.; Gebbing, T.; Abel, C.; Telfer, G. Rhizodeposition shapes rhizosphere microbial community structure in organic soil. New Phytol. 2007, 173, 600–610. [Google Scholar] [CrossRef] [PubMed]
- Ju, T.; Chen, Y.; Chang, C.; An, L. The diversity of soil fungi and its relations with fertility factors in Taxus chinenesis (Pilg.) Rehd community of Xiaolongshan of Tianshui City. Res. Environ. Sci. 2008, 21, 128–132. [Google Scholar] [CrossRef]
- Wang, M.; Shi, S.; Lin, F.; Jiang, P. Response of the soil fungal community to multi-factor environmental changes in a temperate forest. Appl. Soil Ecol. 2014, 81, 45–56. [Google Scholar] [CrossRef]
- Asghar, M.N.; Khan, S.; Mushtaq, S. Management of treated pulp and paper mill effluent to achieve zero discharge. J. Environ. Manag. 2008, 88, 1285–1299. [Google Scholar] [CrossRef]
- Ruotsalainen, A.L.; Eskelinen, A. Root fungal symbionts interact with mammalian herbivory, soil nutrient availability and specific habitat conditions. Oecologia 2011, 166, 807–817. [Google Scholar] [CrossRef] [PubMed]
- Glassman, S.I.; Wang, I.J.; Bruns, T.D. Environmental filtering by pH and soil nutrients drives community assembly in fungi at fine spatial scales. Mol. Biol. 2017, 26, 6960–6973. [Google Scholar] [CrossRef]
- Rajeshkumar, P.P.; Thomas, G.V.; Gupta, A.; Gopal, M. Diversity, richness and degree of colonization of arbuscular mycorrhizal fungi in coconut cultivated along with intercrops in high productive zone of Kerala, India. Symbiosis 2015, 65, 125–141. [Google Scholar] [CrossRef]
- Muthukumar, T.; Sathya, R. Endorhizal fungal association and colonization patterns in Solanaceae. Pol. Bot. J. 2017, 62, 287–299. [Google Scholar] [CrossRef][Green Version]
- Caldwell, B.A.; Jumpponen, A.; Trappe, J.M. Utilization of major detrital substrates by dark septate, root endophytes. Mycologia 2000, 92, 230–232. [Google Scholar] [CrossRef]
- Zuo, Y.L.; He, C.; He, X.L.; Li, X.; Xue, Z.K.; Li, X.M.; Wang, S.J. Plant cover of Ammopiptanthus mongolicus and soil factors shape soil microbial community and catabolic functional diversity in the arid desert in Northwest China. Appl. Soil Ecol. 2019, 147, 103389. [Google Scholar] [CrossRef]
- Dumbrell, A.J.; Ashton, P.D.; Aziz, N.; Feng, G.; Nelson, M.; Dytham, C.; Alastair, H. Distinct seasonal assemblages of arbuscular mycorrhizal fungi revealed by massively parallel pyrosequencing. New Phytol. 2011, 190, 794–804. [Google Scholar] [CrossRef]
- Montero Sommerfeld, H.; Díaz, L.M.; Alvarez, M.; Añazco, V.C.; Matus, F.; Boon, N.; Boeckx, P.; Huygens, D. High winter diversity of arbuscular mycorrhizal fungal communities in shallow and deep grassland soils. Soil Biol. Biochem. 2013, 65, 236–244. [Google Scholar] [CrossRef]


| Item | Season | Sites | Season * Sites | |||
|---|---|---|---|---|---|---|
| F | p | F | p | F | p | |
| Hyphae | 19.023 | <0.001 | 22.6 | <0.001 | 1.995 | 0.179 |
| Microsclerotia | 19.634 | <0.001 | 2.177 | 0.166 | 1.634 | 0.236 |
| Total colonisation | 9.709 | 0.003 | 6.487 | 0.026 | 4.326 | 0.038 |
| Colonization intensity | 33.738 | <0.001 | 9.126 | 0.011 | 21.259 | <0.001 |
| Sampling Time | Sampling Sites | Strain Number | Classification | Number of Strains | IF% |
|---|---|---|---|---|---|
| July | Anxi | HGQ-1 | Alternaria chlamydospora | 12 | 10.53 |
| HGQ-2 | Alternaria sp. | 4 | 3.51 | ||
| HGQ-3 | Preussia terricola | 2 | 1.75 | ||
| HGQ-4 | Laburnicola sp. | 6 | 5.26 | ||
| HGQ-5 | Alternaria tellustris | 4 | 3.51 | ||
| HGQ-6 | Apiosordaria backusii | 2 | 1.75 | ||
| HGQ-7 | Fusarium petersiae | 4 | 3.51 | ||
| Total number of strains | 34 | 29.82 | |||
| Minqin | HGQ-8 | Microascus alveolaris | 6 | 5.26 | |
| HGQ-3 | Preussia terricola | 2 | 1.75 | ||
| HGQ-9 | Herpotrichia striatispora | 4 | 3.51 | ||
| HGQ-10 | Fusarium sp. | 2 | 1.75 | ||
| Total number of strains | 14 | 12.28 | |||
| September | Anxi | HGQ-1 | Alternaria chlamydospora | 2 | 1.75 |
| HGQ-2 | Alternaria sp. | 6 | 5.26 | ||
| HGQ-14 | Alternaria chartarum | 8 | 7.02 | ||
| HGQ-15 | Exserohilum pedicellatum | 2 | 1.75 | ||
| HGQ-3 | Preussia terricola | 8 | 7.02 | ||
| Total number of strains | 26 | 22.81 | |||
| Minqin | HGQ-8 | Microascus alveolaris | 2 | 1.75 | |
| HGQ-16 | Cyphellophora olivacea | 4 | 3.51 | ||
| Total number of strains | 6 | 5.26 | |||
| December | Anxi | HGQ-1 | Alternaria chlamydospora | 8 | 7.02 |
| HGQ-7 | Fusarium petersiae | 2 | 1.75 | ||
| HGQ-12 | Emericellopsis maritima | 4 | 3.51 | ||
| HGQ-17 | Enterocarpus grenotii | 4 | 3.51 | ||
| HGQ-18 | Thermomyces verrucosus | 6 | 5.26 | ||
| Total number of strains | 24 | 21.05 | |||
| Minqin | HGQ-1 | Alternaria chlamydospora | 2 | 1.75 | |
| HGQ-8 | Microascus alveolaris | 4 | 3.51 | ||
| HGQ-19 | Slopeiomyces cylindrosporus | 4 | 3.51 | ||
| Total number of strains | 10 | 8.77 | |||
| Total | 114 | 100 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Han, L.; Shi, J.; He, C.; He, X. Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China. Agronomy 2021, 11, 648. https://doi.org/10.3390/agronomy11040648
Han L, Shi J, He C, He X. Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China. Agronomy. 2021; 11(4):648. https://doi.org/10.3390/agronomy11040648
Chicago/Turabian StyleHan, Li, Jingxin Shi, Chao He, and Xueli He. 2021. "Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China" Agronomy 11, no. 4: 648. https://doi.org/10.3390/agronomy11040648
APA StyleHan, L., Shi, J., He, C., & He, X. (2021). Temporal and Spatial Dynamics of Dark Septate Endophytes in the Roots of Lycium ruthenicum in the Desert Region of Northwest China. Agronomy, 11(4), 648. https://doi.org/10.3390/agronomy11040648






