The Impact of Harvesting Time on Fusarium Mycotoxins in Spring Wheat Grain and Their Interaction with Grain Quality
Abstract
1. Introduction
2. Materials and Methods
2.1. Field Plantings
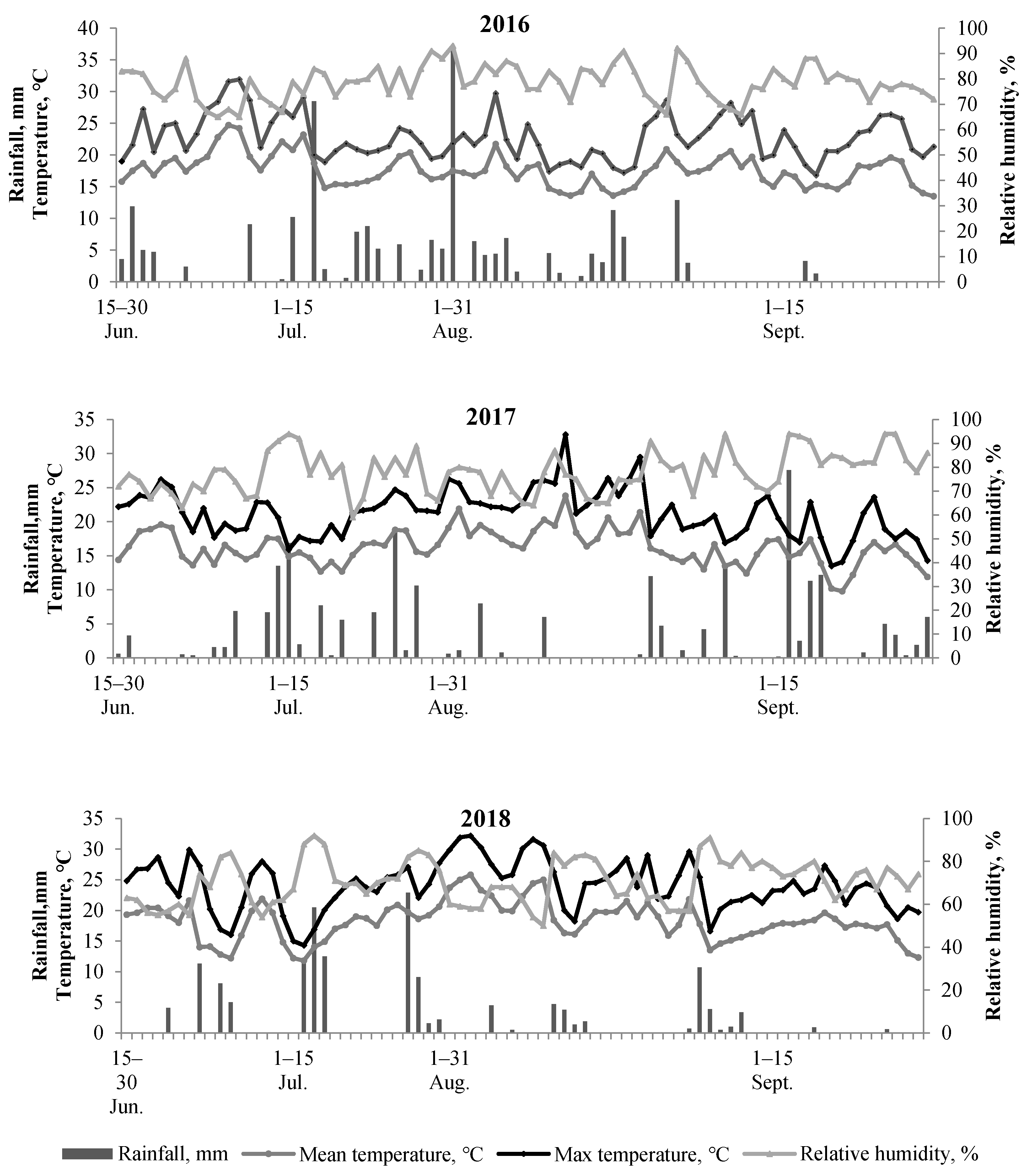
Weather Conditions
2.2. Quantification of Mycotoxins
2.2.1. Reagents
2.2.2. Preparation of Samples and Test Procedure
2.3. Grain Infection with Fusarium Spp. Fungi
2.4. Determination of Wheat Quality Parameters
2.5. Statistical Analyses
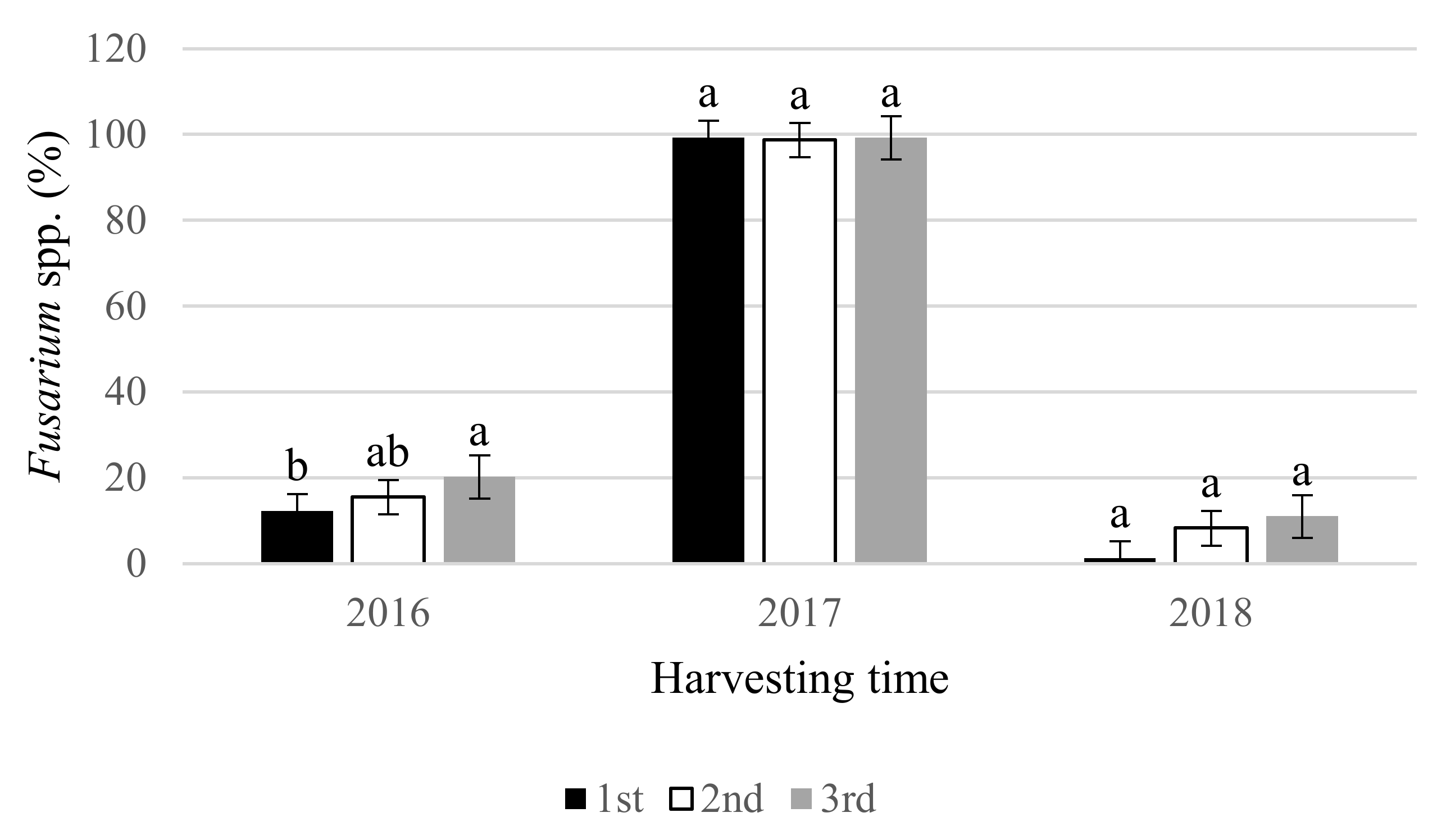
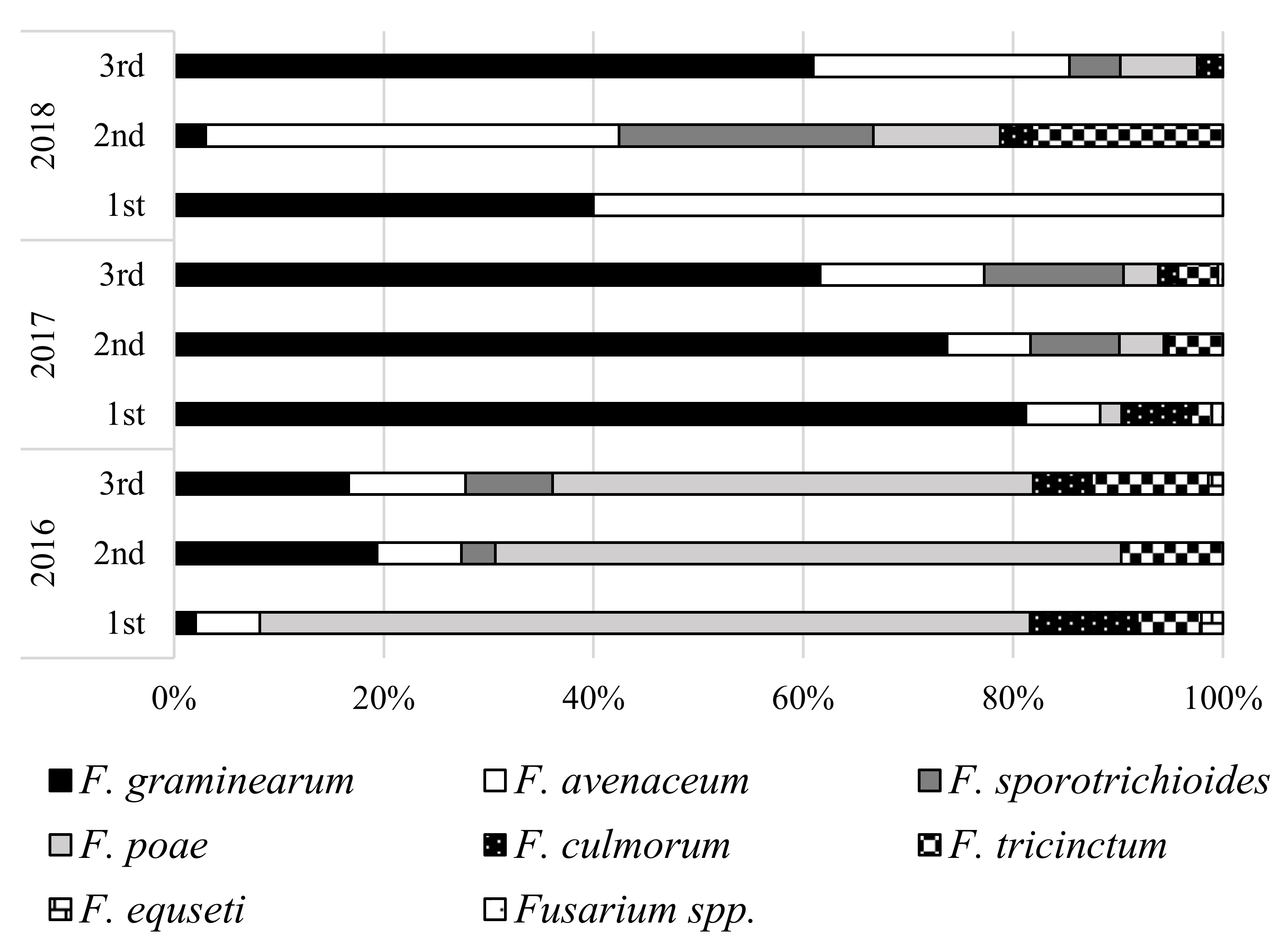
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matarese, F.; Sarrocco, S.; Gruber, S.; Seidl-Seiboth, V.; Vannacci, G. Biocontrol of Fusarium head blight: Interactions between Trichoderma and mycotoxigenic Fusarium. Microbiology 2012, 158, 98–106. [Google Scholar] [CrossRef] [PubMed]
- Gilbert, J.; Haber, S. Overview of some recent research developments in fusarium head blight of wheat. Can. J. Plant Pathol. 2013, 35, 149–174. [Google Scholar] [CrossRef]
- Cirlini, M.; Generotti, S.; Dall’Erta, A.; Lancioni, P.; Ferrazzano, G.; Massi, A.; Galaverna, G.; Dall’Asta, C. Durum Wheat (Triticum Durum Desf.) Lines Show Different Abilities to Form Masked Mycotoxins under Greenhouse Conditions. Toxins 2013, 6, 81–95. [Google Scholar] [CrossRef] [PubMed]
- Mankevičienė, A.; Semaškienė, R.; Dabkevičius, Z.; Kochiieru, Y.; Janavičienė, S.; Jonavičienė, A. Do black dots on wheat grains have an impact on deoxynivalenol accumulation? Zemdirbyste Agric. 2019, 106, 249–256. [Google Scholar] [CrossRef]
- Xu, X.-M.; Nicholson, P.; Thomsett, M.A.; Simpson, D.; Cooke, B.M.; Doohan, F.M.; Brennan, J.; Monaghan, S.; Moretti, A.; Mule, G.; et al. Relationship Between the Fungal Complex Causing Fusarium Head Blight of Wheat and Environmental Conditions. Phytopathology 2008, 98, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Edwards, S.G. Impact of agronomic and climatic factors on the mycotoxin content of harvested oats in the United Kingdom. Food Addit. Contam. Part A 2017, 34, 2230–2241. [Google Scholar] [CrossRef]
- Mateo, J.J.; Mateo, R.; Jimenez, M. Accumulation of type A trichotecenes in maize, wheat and rice by Fusarium sporotrichoides isolates under diverse culture conditions. Int. J. Food Microbiol. 2002, 72, 115–123. [Google Scholar] [CrossRef]
- Sforza, S.; Dall’Asta, C.; Marchelli, R. Recent advances in mycotoxin determination in food and feed by hyphenated chromatographic techniques/mass spectrometry. Mass Spectrom. Rev. 2005, 25, 54–76. [Google Scholar] [CrossRef] [PubMed]
- Schöneberg, T.; Jenny, E.; Wettstein, F.E.; Bucheli, T.D.; Mascher, F.; Bertossa, M.; Musa, T.; Seifert, K.; Gräfenhan, T.; Keller, B.; et al. Occurrence of Fusarium species and mycotoxins in Swiss oats—Impact of cropping factors. Eur. J. Agron. 2018, 92, 123–132. [Google Scholar] [CrossRef]
- Xue, A.G.; Frégeau-Reid, J.; Rowsell, J.; Babcock, C.; Hoekstra, G.J.; Sparry, E. Effect of harvesting time on incidence of seed-borne Fusarium spp. in spring wheat in eastern Ontario. Can. J. Plant Sci. 2004, 84, 757–763. [Google Scholar] [CrossRef]
- Trienekens, J.; Zuurbier, P. Quality and safety standards in the food industry, developments and challenges. Int. J. Prod. Econ. 2008, 113, 107–122. [Google Scholar] [CrossRef]
- Igrejas, G.; Ikeda, T.M.; Guzmán, C. Wheat Quality for Improving Processing and Human Health; Springer: Berlin, Germany, 2020; p. 542. [Google Scholar]
- Kreuzberger, M.; Limsuwan, S.; Eggert, K.; Karlovsky, P.; Pawelzik, E. Impact of Fusarium spp. infection of bread wheat (Triticum aestivum L.) on composition and quality of flour in association with EU maximum level for deoxynivalenol. J. Appl. Bot. Food Qual. 2015, 88, 177–185. [Google Scholar]
- Wang, J.H.; Pawelzik, E.; Weinert, J.; Wolf, G.A. Impact of Fusarium culmorum on the polysaccharides of wheat flour. J. Agric.Food Chem. 2005, 53, 5818–5823. [Google Scholar] [CrossRef] [PubMed]
- Siuda, R.; Grabowski, A.; Lenc, L.; Ralcewicz, M.; Spychaj-Fabisiak, E. Influence of the degree of fusariosis on technological traits of wheat grain. Int. J. Food Sci. Technol. 2010, 45, 2596–2604. [Google Scholar] [CrossRef]
- Lancova, K.; Hajslova, J.; Kostelanska, M.; Kohoutkova, J.; Nedelnik, J.; Moravcova, H.; Vanova, M. Fate of trichothecene mycotoxins during the processing: Milling and baking. Food Addit. Contam. Part A 2008, 25, 650–659. [Google Scholar] [CrossRef]
- Wang, J.; Wieser, H.; Pawelzik, E.; Weinert, J.; Keutgen, A.J.; Wolf, G.A. Impact of the fungal protease produced by Fusarium culmorum on the protein quality and breadmaking properties of winter wheat. Eur. Food Res. Technol. 2005, 220, 552–559. [Google Scholar] [CrossRef]
- Eggert, K.; Rawel, H.M.; Pawelzik, E. In vitro degradation of wheat gluten fractions by Fusarium graminearum proteases. Eur. Food Res. Technol. 2011, 233, 697–705. [Google Scholar] [CrossRef]
- Edwards, S.G. Influence of agricultural practices on fusarium infection of cereals and subsequent contamination of grain by trichothecene mycotoxins. Toxicol. Lett. 2004, 153, 29–35. [Google Scholar] [CrossRef]
- Del Ponte, E.M.; Fernandes, J.M.C.; Bergstrom, G.C. Influence of Growth Stage on Fusarium Head Blight and Deoxynivalenol Production in Wheat. J. Phytopathol. 2007, 155, 577–581. [Google Scholar] [CrossRef]
- López, A.M.M.; Manthey, F.A.; Simsek, S. Wet milling technique applied to deoxynivalenol-contaminated wheat dry-milled fractions. Cereal Chem. J. 2019, 96, 487–496. [Google Scholar] [CrossRef]
- Matthäus, K.; Dänicke, S.; Vahjen, W.; Simon, O.; Wang, J.; Valenta, H.; Meyer, K.; Strumpf, A.; Ziesenib, H.; Flachowsky, G. Progression of mycotoxin and nutrient concentrations in wheat after inoculation with Fusarium culmorum. Arch. Anim. Nutr. 2004, 58, 19–35. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Pawelzik, E.; Weinert, J.; Zhao, Q.; Wolf, G.A. Factors influencing falling number in winter wheat. Eur. Food Res. Technol. 2008, 226, 1365–1371. [Google Scholar] [CrossRef]
- EC. Commission regulation (EC) 1881/2006. Off. J. Eur. Un. 2006, 364, 5–24. [Google Scholar]
- EC. Commission recommendation (EC) 165/2013. Off. J. Eur. Un. 2013, 91, 12–15. [Google Scholar]
- Mathur, S.B.; Kongsdal, O. Common Laboratory Seed Health Testing Methods for Detecting Fungi, 1st ed.; International Seed Testing Association (ISTA): Bassersdorf, Switzerland, 2003; pp. 393–399. [Google Scholar]
- Nelson, P.E.; Toussoun, T.A.; Marasas, W.F.O. Fusarium Species: An illustrated Manual for Identification; Penn State University Press: Pennsylvania, PA, USA, 1983; p. 206. [Google Scholar]
- Leslie, J.F.; Summerell, B.A. Fusarium laboratory workshops—A recent history. Mycotoxin Res. 2006, 22, 73–74. [Google Scholar] [CrossRef]
- International Organization for Standardization. ISO 7971–3:2019 Cereals. Determination of Bulk Density, Called Mass per Hectolitre. Part 3: Routine Method; International Organization for Standardization: Geneva, Switzerland, 2019; p. 19. [Google Scholar]
- International Organization for Standardization. ISO 712:2009 Cereals and Cereal Products. Determination of Moisture Content. Reference Method; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- International Organization for Standardization. ISO 20483:2013 Cereals and Pulses—Determination of the Nitrogen Content and Calculation of the Crude Protein Content—Kjeldahl Method; International Organization for Standardization: Geneva, Switzerland, 2013. [Google Scholar]
- Butkutė, B.; Padarauskas, A.; Cesevičienė, J.; Pavilonis, A.; Taujenis, L.; Lemežienė, N. Perennial legumes as a source of ingredients for healthy food: Proximate, mineral and phytoestrogen composition and antibacterial activity. J. Food Sci. Technol. 2017, 54, 2661–2669. [Google Scholar] [CrossRef] [PubMed]
- ICC Standard No 155, 1994. Determination of Wet Gluten Quantity and Quality (Gluten Index ac. to Perten) of Whole Wheat Meal and Wheat Flour (Triticum aestivum). Available online: https://icc.or.at/publications/icc-standards/standards-overview/155-standard-method (accessed on 4 February 2021).
- ICC Standard No 123/1, 1994. Determination of Starch Content by Hydrochloric Acid Dissolution, International Association for Cereal Science and Technology. Available online: https://icc.or.at/publications/icc-standards/standards-overview/123-1-standard-method (accessed on 4 February 2021).
- International Organization for Standardization. ISO 5529:2007 Wheat—Determination of the Sedimentation Index—Zeleny Test; International Organization for Standardization: Geneva, Switzerland, 2007. [Google Scholar]
- International Organization for Standardization. ISO 3093:2009 Wheat, Rye and Their Flours, Durum Wheat and Durum Wheat Semolina—Determination of the Falling Number According to Hagberg-Perten; International Organization for Standardization: Geneva, Switzerland, 2009. [Google Scholar]
- AACC International. AACC Method 55-30.01 Particle Size Index for Wheat Hardness; AACC International: St. Paul, MN, USA.
- West, J.S.; Holdgate, S.; Townsend, J.A.; Edwards, S.G.; Jennings, P.; Fitt, B.D. Impacts of changing climate and agronomic factors on fusarium ear blight of wheat in the UK. Fungal Ecol. 2012, 5, 53–61. [Google Scholar] [CrossRef]
- Ferrigo, D.; Raiola, A.; Causin, R. Fusarium Toxins in Cereals: Occurrence, Legislation, Factors Promoting the Appearance and Their Management. Molecules 2016, 21, 627. [Google Scholar] [CrossRef]
- Góral, T.; Wiśniewska, H.; Ochodzki, P.; Nielsen, L.K.; Walentyn-Góral, D.; Stępień, Ł. Relationship between Fusarium head blight, kernel damage, concentration of Fusarium biomass, and Fusarium toxins in grain of winter wheat inoculated with Fusarium culmorum. Toxins 2019, 11, 2. [Google Scholar] [CrossRef]
- Giorni, P.; Bertuzzi, T.; Battilani, P. Impact of Fungi Co-occurrence on Mycotoxin Contamination in Maize During the Growing Season. Front. Microbiol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Nordkvist, E.; Häggblom, P. Fusarium mycotoxin contamination of cereals and bedding straw at Swedish pig farms. Anim. Feed. Sci. Technol. 2014, 198, 231–237. [Google Scholar] [CrossRef]
- Birr, T.; Hasler, M.; Verreet, J.-A.; Klink, H. Composition and Predominance of Fusarium Species Causing Fusarium Head Blight in Winter Wheat Grain Depending on Cultivar Susceptibility and Meteorological Factors. Microorganisms 2020, 8, 617. [Google Scholar] [CrossRef] [PubMed]
- Bianchini, A.; Bullerman, L.B. Mycotoxins classification. Encyclopedia of Food Microbiology, 2nd ed.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 854–861. [Google Scholar]
- Cesevičienė, J.; Mašauskienė, A. The effect of harvest time on winter wheat grain protein content and sedimentation index. Žemdirbystė Agric. 2008, 95, 58–72. [Google Scholar]
- Official Journal of the European Union. Commission Regulation EC No 687/2008 of 18 July 2008. Available online: https://eur-lex.europa.eu/LexUriServ/LexUriServ.do?uri=OJ:L:2008:192:0020:0048:EN:PDF (accessed on 4 February 2021).
- Cesevičienė, J.; Mašauskienė, A. The impact of nitrogen fertilization and harvest time on Hagberg–Perten falling number of winter wheat. Zemès ukio Mokslai 2007, 2, 11–17. [Google Scholar]
- Dexter, J.E.; Clear, R.M.; Preston, K.R. Fusarium head blight: Effect on the milling and baking of some Canadian wheats. Cereal Chem. 1996, 73, 695–701. [Google Scholar]
- Gärtner, B.H.; Munich, M.; Kleijer, G.; Mascher, F. Characterisation of kernel resistance against Fusarium infection in spring wheat by baking quality and mycotoxin assessments. Eur. J. Plant Pathol. 2007, 120, 61–68. [Google Scholar] [CrossRef]
- Seitz, L.M.; Eustace, W.D.; Mohr, H.E.; Shogren, M.D.; Yamazaki, W.T. Cleaning, milling, and baking tests with hard red winter wheat containing deoxynivalenol. Cereal Chem. 1986, 63, 146–150. [Google Scholar]
- Prange, A.; Birzele, B.; Krämer, J.; Meier, A.; Modrow, H.; Köhler, P. Fusarium-inoculated wheat: Deoxynivalenol contents and baking quality in relation to infection time. Food Control. 2005, 16, 739–745. [Google Scholar] [CrossRef]
- Terzi, V.; Morcia, C.; Faccioli, P.; Faccini, N.; Rossi, V.; Cigolini, M.; Corbellini, M.; Scudellari, D.; Delogu, G. Fusarium DNA traceability along the bread production chain. Int. J. Food Sci. Technol. 2007, 42, 1390–1396. [Google Scholar] [CrossRef]
- Nightingale, M.J.; Marchylo, B.A.; Clear, R.M.; Dexter, J.E.; Preston, K.R. Fusarium Head Blight: Effect of Fungal Proteases on Wheat Storage Proteins. Cereal Chem. J. 1999, 76, 150–158. [Google Scholar] [CrossRef]
| Years | Flowering Date | 1st Harvesting Time | 2nd Harvesting Time | 3rd Harvesting Time |
|---|---|---|---|---|
| 22 06 | 04 08 | 16 08 | 24 08 | |
| 2016 | full maturity * | full maturity + 12 days | full maturity + 20 days | |
| 30 06 | 31 08 | 08 09 | 14 09 | |
| 2017 | full maturity * | full maturity + 8 days | full maturity + 14 days | |
| 22 06 | 06 08 | 16 08 | 23 08 | |
| 2018 | full maturity * | full maturity + 10 days | full maturity + 17 days |
| DON (µg kg−1) | T-2 (µg kg−1) | ZEA (µg kg−1) | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Years | Harvesting Time | Harvesting Time | Harvesting Time | ||||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| 2016 | 446.0 c | 573.0 b | 783.0 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| 2017 | 2103.0 b | 10250.0 a | 13809.0 a | 30.6 b | 67.3 a | 74.0 a | 50.5 b | 895.0 a | 1055.4 a |
| 2018 | 381.0 a | 326.0 a | 352.0 a | <LOD | <LOD | <LOD | <LOD | <LOD | <LOD |
| EC, 2006 | 1250.0 | 100.0 | 100.0 | ||||||
| 2016 | 2017 | 2018 | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Harvesting Time | Harvesting Time | Harvesting Time | |||||||
| 1st | 2nd | 3rd | 1st | 2nd | 3rd | 1st | 2nd | 3rd | |
| F. graminearum vs. DON | 0.65 | 0.21 | 0.82 | 0.28 | 0.00 | 0.27 | 0.00 | 0.00 | 0.00 |
| F. graminearum vs. ZEA | 0.17 | 0.00 | 0.00 | 0.90 | 0.21 | 0.27 | 0.00 | 0.00 | 0.00 |
| F. sporotrichioides vs. T-2 | 0.00 | 0.14 | 0.41 | 0.00 | 0.01 | 0.05 | 0.00 | 0.17 | 0.60 |
| F. tricinctum vs. T-2 | 0.33 | 0.00 | 0.07 | 0.55 | 0.10 | 0.64 | 0.00 | 0.01 | 0.21 |
| F. poae vs. T-2 | 0.81 | 0.98 | 0.76 | 0.02 | 0.23 | 0.00 | 0.00 | 0.01 | 0.00 |
| Harvesting Time | Mass Per Hectolitre, kg hl−1 | PSI, % | Sedimentation Zeleny, mL | Falling Number, s | Wet Gluten Content, % | Gluten Index,% | Gluten Water Hydration, % |
|---|---|---|---|---|---|---|---|
| 2016 | |||||||
| 1—full maturity | 80.5 a | 5.46 b | 67.0 a | 399 a | 32.0 b | 91.0 a | 209.1 b |
| 2—full maturity + 12 days | 78.7 b | 6.67 a | 67.0 a | 256 b | 31.2 b | 94.9 a | 207.6 b |
| 3—full maturity + 20 days | 75.9 c | 5.38 b | 67.0 a | 64 c | 34.1 a | 82.0 b | 223.2 a |
| 2017 | |||||||
| 1—full maturity | 78.4 a | 6.85 b | 49.3 b | 351 a | 31.0 a | 91.7 a | 212.7 a |
| 2—full maturity + 8 days | 74.0 b | 7.36 a | 50.4 ab | 191 b | 31.0 a | 90.4 a | 213.1 a |
| 3—full maturity + 14 days | 68.8 c | 7.67 a | 54.4 a | 85 c | 30.3 a | 91.4 a | 214.7 a |
| 2018 | |||||||
| 1—full maturity | 81.9 a | 5.71 b | 53.0 a | 313 c | 30.3 a | 96.9 b | 195.6 a |
| 2—full maturity + 10 days | 72.2 c | 6.64 a | 44.5 b | 378 a | 27.5 c | 98.8 a | 184.3 b |
| 3—full maturity + 17 days | 78.8 b | 6.61 a | 43.8 c | 370 b | 28.3 b | 98.5 a | 192.4 a |
| Harvesting Time | Protein, % | Starch, % | Ash, % | Fat, % |
|---|---|---|---|---|
| 2016 | ||||
| 1—full maturity | 11.6 b | 68.3 a | 2.27 ab | 1.07 b |
| 2—full maturity + 12 days | 11.3 c | 67.9 b | 2.24 b | 1.28 a |
| 3—full maturity + 20 days | 12.2 a | 68.1 ab | 2.48 a | 1.06 b |
| 2017 | ||||
| 1—full maturity | 14.1 a | 65.8 a | 2.51 a | 2.40 b |
| 2—full maturity + 8 days | 14.4 a | 65.6 a | 2.38 a | 2.55 ab |
| 3—full maturity + 14 days | 14.3 a | 65.4 a | 2.16 a | 2.71 a |
| 2018 | ||||
| 1—full maturity | 15.5 a | 64.1 b | 1.87 a | 2.53 b |
| 2—full maturity + 10 days | 15.5 a | 64.2 b | 1.84 a | 2.38 c |
| 3—full maturity + 17 days | 15.5 a | 64.5 a | 1.91 a | 2.81 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kochiieru, Y.; Mankevičienė, A.; Cesevičienė, J.; Semaškienė, R.; Ramanauskienė, J.; Gorash, A.; Janavičienė, S.; Venslovas, E. The Impact of Harvesting Time on Fusarium Mycotoxins in Spring Wheat Grain and Their Interaction with Grain Quality. Agronomy 2021, 11, 642. https://doi.org/10.3390/agronomy11040642
Kochiieru Y, Mankevičienė A, Cesevičienė J, Semaškienė R, Ramanauskienė J, Gorash A, Janavičienė S, Venslovas E. The Impact of Harvesting Time on Fusarium Mycotoxins in Spring Wheat Grain and Their Interaction with Grain Quality. Agronomy. 2021; 11(4):642. https://doi.org/10.3390/agronomy11040642
Chicago/Turabian StyleKochiieru, Yuliia, Audronė Mankevičienė, Jurgita Cesevičienė, Roma Semaškienė, Jūratė Ramanauskienė, Andrii Gorash, Sigita Janavičienė, and Eimantas Venslovas. 2021. "The Impact of Harvesting Time on Fusarium Mycotoxins in Spring Wheat Grain and Their Interaction with Grain Quality" Agronomy 11, no. 4: 642. https://doi.org/10.3390/agronomy11040642
APA StyleKochiieru, Y., Mankevičienė, A., Cesevičienė, J., Semaškienė, R., Ramanauskienė, J., Gorash, A., Janavičienė, S., & Venslovas, E. (2021). The Impact of Harvesting Time on Fusarium Mycotoxins in Spring Wheat Grain and Their Interaction with Grain Quality. Agronomy, 11(4), 642. https://doi.org/10.3390/agronomy11040642








