Kaolin Application Modulates Grapevine Photochemistry and Defence Responses in Distinct Mediterranean-Type Climate Vineyards
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Plant Material, Treatments, and Sampling
2.3. Weather Conditions and Characterisation of the Study Areas
2.4. Gene Expression by RT-qPCR
2.5. Leaf Temperature and Chlorophyll Measurements
2.6. Determination of Foliar Metabolites
2.7. Chlorophyll a Fluorescence Measurements
2.8. Analysis of Fluorescence Transients Using JIP Test Parameters
2.9. Statistical Analysis
3. Results
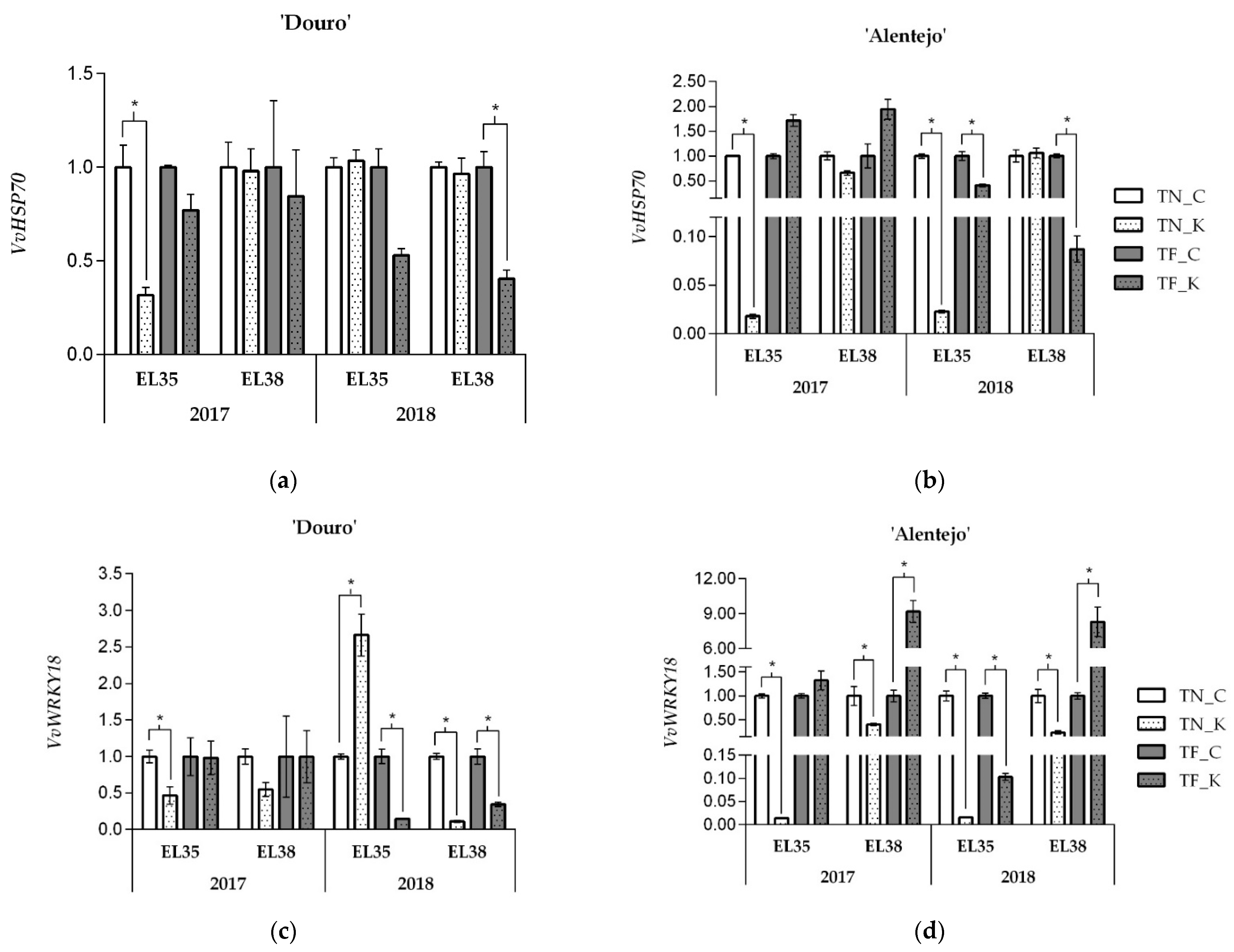
3.1. Kaolin Effects onVvHSP70 and VvWRKY18 Gene Expression
3.2. Leaf Temperature and Chlorophyll Content
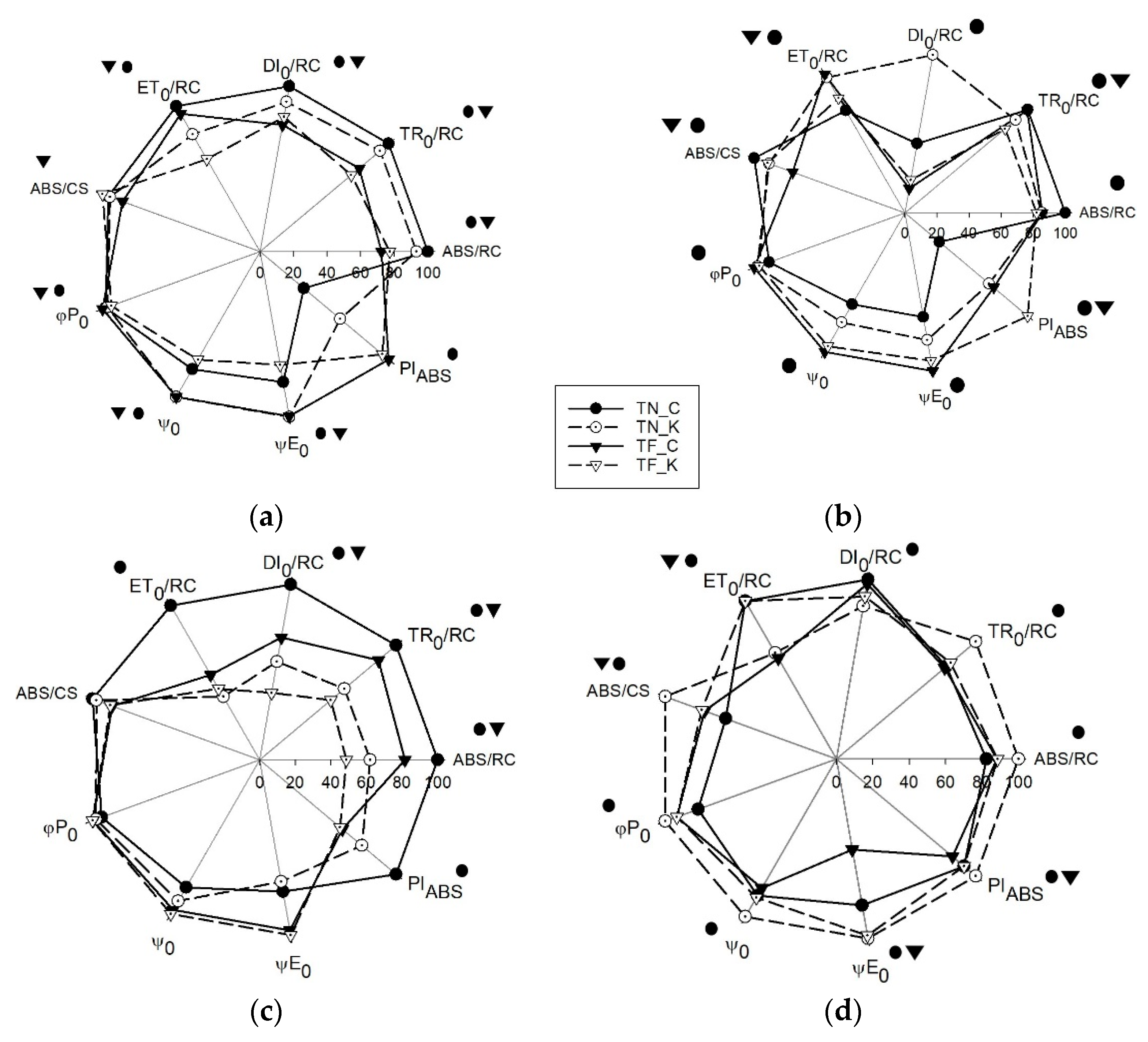
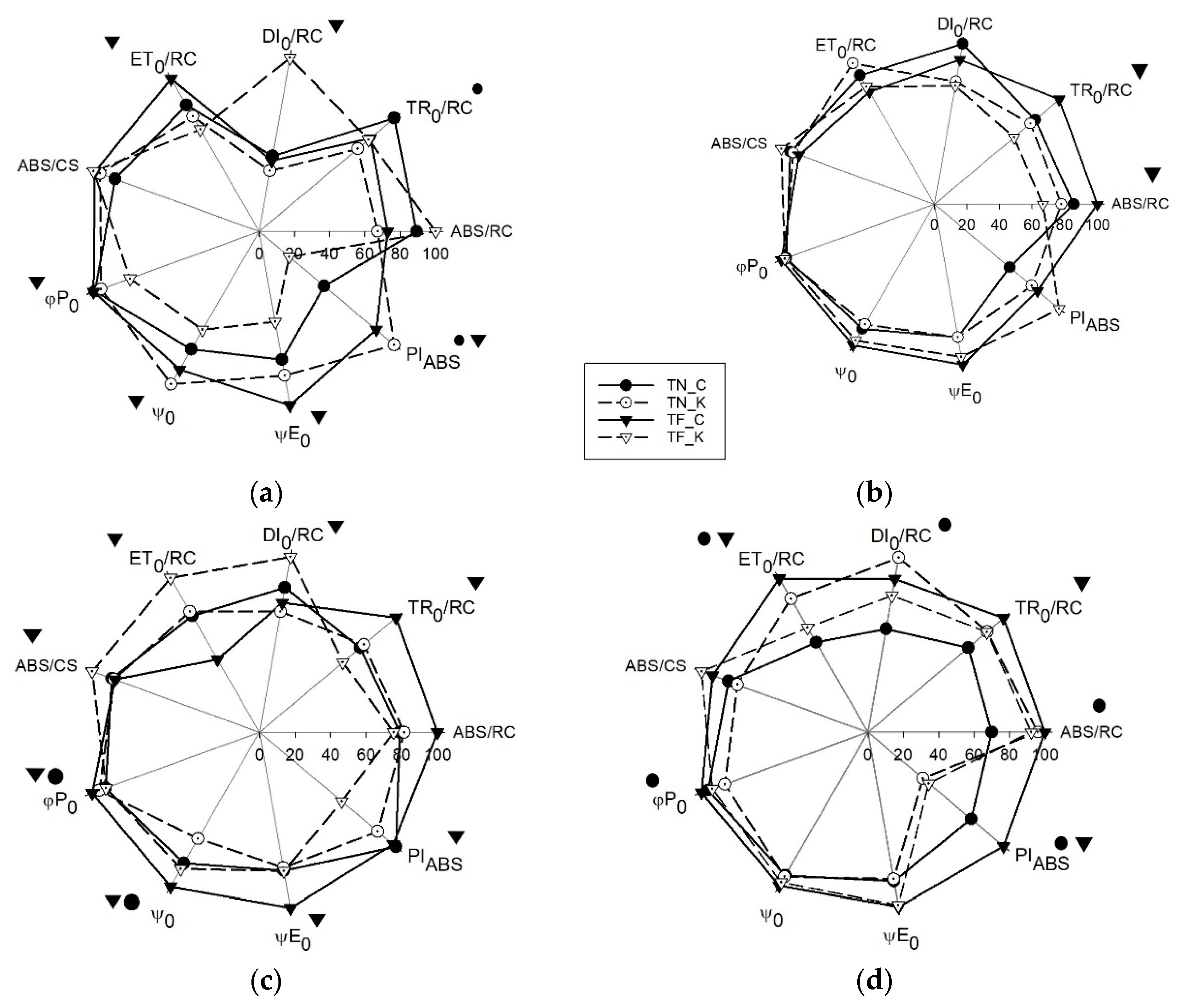
3.3. Transient Chlorophyll a Fluorescence Analysis by JIP-Test
3.4. Kaolin Effects on Lipid Peroxidation, Proline, and Ascorbate Accumulation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Gene | Gene Unique ID | Primer (5′–3’) | Size (bp) | |
|---|---|---|---|---|
| VvHSP70 | GSVIVT01008331001 | Fw Rv | GACCTTGGGGGTGGTACTTT GCCAACCAATCCACAACTCT | 131 |
| VvWRKY18 | GSVIVT01035885001 | Fw Rv | GAAGCCAAGAGAAAGCACCA GGCTCTGGGAGAAGGGTTAT | 145 |
| VvACT2 | GSVIVT01026580001 | Fw Rv | GCCATCCAAGCTGTTCTCTC CAGTAAGGTCACGTCCAGCA | 157 |
| VvTUB2 | GSVIVT01037405001 | Fw Rv | CAACTCTGACCTCCGAAAGC CTTGGAGTCCCACATTTGCT | 154 |
References
- Giorgi, F. Climate change hot-spots. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Santos, J.A.; Fraga, H.; Malheiro, A.C.; Moutinho-Pereira, J.; Dinis, L.-T.; Correia, C.; Moriondo, M.; Leolini, L.; Dibari, C.; Costafreda-Aumedes, S.; et al. A review of the potential climate change impacts and adaptation options for European viticulture. Appl. Sci. 2020, 10, 3092. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.-T.; Machado, N.; Moutinho-Pereira, J. Grapevine abiotic stress assessment and search for sustainable adaptation strategies in Mediterranean-like climates. A review. Agron. Sustain. Dev. 2018, 38. [Google Scholar] [CrossRef]
- Zandalinas, S.I.; Mittler, R.; Balfagón, D.; Arbona, V.; Gómez-Cadenas, A. Plant adaptations to the combination of drought and high temperatures. Physiol. Plant. 2018, 162, 2–12. [Google Scholar] [CrossRef]
- Carvalho, L.C.; Coito, J.L.; Colaço, S.; Sangiogo, M.; Amâncio, S. Heat stress in grapevine: The pros and cons of acclimation. Plant Cell Environ. 2015, 38, 777–789. [Google Scholar] [CrossRef]
- Vives-Peris, V.; Marmaneu, D.; Gomez-Cadenas, A.; Perez-Clemente, R.M. Characterization of Citrus WRKY transcription factors and their responses to phytohormones and abiotic stresses. Biol. Plant. 2018, 62, 33–44. [Google Scholar] [CrossRef]
- Akram, N.A.; Shafiq, F.; Ashraf, M. Ascorbic Acid-A Potential Oxidant Scavenger and Its Role in Plant Development and Abiotic Stress Tolerance. Front. Plant Sci. 2017, 8, 613. [Google Scholar] [CrossRef]
- Masouleh, S.S.S.; Aldine, N.J.; Sassine, Y.N. The role of organic solutes in the osmotic adjustment of chilling-stressed plants (vegetable, ornamental and crop plants). Ornam. Hortic. 2019, 25, 434–442. [Google Scholar] [CrossRef]
- Mittler, R. Abiotic stress, the field environment and stress combination. Trends Plant Sci. 2006, 11, 15–19. [Google Scholar] [CrossRef]
- Brillante, L.; Belfiore, N.; Gaiotti, F.; Lovat, L.; Sansone, L.; Poni, S.; Tomasi, D. Comparing kaolin and pinolene to improve sustainable grapevine production during drought. PLoS ONE 2016, 11, e0156631. [Google Scholar] [CrossRef] [PubMed]
- Dinis, L.T.; Malheiro, A.C.; Luzio, A.; Fraga, H.; Ferreira, H.; Goncalves, I.; Pinto, G.; Correia, C.M.; Moutinho-Pereira, J. Improvement of grapevine physiology and yield under summer stress by kaolin-foliar application: Water relations, photosynthesis and oxidative damage. Photosynthetica 2018, 56, 641–651. [Google Scholar] [CrossRef]
- Frioni, T.; Tombesi, S.; Sabbatini, P.; Squeri, C.; Lavado Rodas, N.; Palliotti, A.; Poni, S. Kaolin reduces ABA biosynthesis through the inhibition of neoxanthin synthesis in grapevines under water deficit. Int. J. Mol. Sci. 2020, 21, 4950. [Google Scholar] [CrossRef]
- Glenn, D.M.; Cooley, N.; Walker, R.; Clingeleffer, P.; Shellie, K. Impact of kaolin particle film and water deficit on wine grape water use efficiency and plant water relations. HortScience 2010, 45, 1178–1187. [Google Scholar] [CrossRef]
- Shellie, K.C.; King, B.A. Kaolin particle film and water deficit influence Malbec leaf and berry temperature, pigments, and photosynthesis. Am. J. Enol. Vitic. 2013, 64, 223–230. [Google Scholar] [CrossRef]
- FAO. International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports; IUSS Working Group WRB, World Reference Base for Soil Resources: Rome, Italy, 2015. [Google Scholar]
- Coombe, B.G. Growth stages of the grapevine: Adoption of a system for identifying grapevine growth stages. Aust. J. Grape Wine Res. 1995, 1, 104–110. [Google Scholar] [CrossRef]
- Kottek, M.; Grieser, J.; Beck, C.; Rudolf, B.; Rubel, F. World map of the Köppen-Geiger climate classification updated. Meteorol. Z. 2006, 15, 259–263. [Google Scholar] [CrossRef]
- Tonietto, J.; Carbonneau, A. A multicriteria climatic classification system for grape-growing regions worldwide. Agric. For. Meteorol. 2004, 124, 81–97. [Google Scholar] [CrossRef]
- Fraga, H.; Molitor, D.; Leolini, L.; Santos, J.A. What Is the Impact of Heatwaves on European Viticulture? A Modelling Assessment. Appl. Sci. 2020, 10, 3030. [Google Scholar] [CrossRef]
- Gambino, G.; Perrone, I.; Gribaudo, I. A rapid and effective method for RNA extraction from different tissues of grapevine and other woody plants. Phytochem. Anal. 2008, 19, 520–525. [Google Scholar] [CrossRef]
- Pfaffl, M.W. A new mathematical model for relative quantification in real-time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef]
- Pfaffl, M.W. Relative expression software tool (REST(C)) for group-wise comparison and statistical analysis of relative expression results in real-time PCR. Nucleic Acids Res. 2002, 30, e36. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid-reactive-substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Bates, L.S.; Waldren, R.P.; Teare, I.D. Rapid determination of free proline for water-stress studies. Plant Soil 1973, 39, 205–207. [Google Scholar] [CrossRef]
- Okamura, M. An improved method for determination of l-ascorbic acid and l-dehydroascorbic acid in blood plasma. Clin. Chim. Acta 1980, 103, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Strasser, B.J.; Strasser, R.J. Measuring Fast Fluorescence Transients to Address Environmental Questions: The JIP-Test. In Photosynthesis: From Light to Biosphere; KAP Press: Dordrecht, The Netherlands, 1995; pp. 4869–4872. [Google Scholar] [CrossRef]
- Strasser, R.J.; Srivastava, A.; Tsimilli-Michael, M. The fluorescence transient as a tool to characterize and screen photosynthetic samples. In Probing Photosynthesis: Mechanisms, Regulation and Adaptation; Yunus, M., Pathre, U., Mohanty, P., Eds.; Taylor & Francis Publishers: London, UK, 2000; pp. 445–483. [Google Scholar]
- Strasser, R.J.; Tsimilli-Michael, M.; Srivastava, A. Analysis of the Chlorophyll a Fluorescence Transient. In Chlorophyll a Fluorescence; Springer: Dordrecht, The Netherlands, 2004; Volume 19, pp. 321–362. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Ferreira, H.; Rocha, L.; Pavia, I.; Moutinho-Pereira, J.; Correia, C.M. Kaolin particle film modulates morphological, physiological and biochemical olive tree responses to drought and rewatering. Plant Physiol. Biochem. 2018, 133, 29–39. [Google Scholar] [CrossRef] [PubMed]
- Conde, A.; Neves, A.; Breia, R.; Pimentel, D.; Dinis, L.-T.; Bernardo, S.; Correia, C.M.; Cunha, A.; Gerós, H.; Moutinho-Pereira, J. Kaolin particle film application stimulates photoassimilate synthesis and modifies the primary metabolome of grape leaves. J. Plant Physiol. 2018, 223, 47–56. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, M.; Katoh, H.; Takayanagi, T.; Suzuki, S. Characterization of thermotolerance-related genes in grapevine (Vitis vinifera). J. Plant Physiol. 2010, 167, 812–819. [Google Scholar] [CrossRef] [PubMed]
- Frioni, T.; Saracino, S.; Squeri, C.; Tombesi, S.; Palliotti, A.; Sabbatini, P.; Magnanini, E.; Poni, S. Understanding kaolin effects on grapevine leaf and whole-canopy physiology during water stress and re-watering. J. Plant Physiol. 2019, 242, 153020. [Google Scholar] [CrossRef] [PubMed]
- Costa, R.; Fraga, H.; Fonseca, A.; García de Cortázar-Atauri, I.; Val, M.C.; Carlos, C.; Reis, S.; Santos, J.A. Grapevine phenology of cv. Touriga Franca and Touriga Nacional in the Douro wine region: Modelling and climate change projections. Agronomy 2019, 9, 210. [Google Scholar] [CrossRef]
- García de Cortázar-Atauri, I.; Duchêne, E.; Destrac-Irvine, A.; Barbeau, G.; De Rességuier, L.; Lacombe, T.; Parker, A.K.; Saurin, N.; Van Leeuwen, C. Grapevine phenology in France: From past observations to future evolutions in the context of climate change. OENO One 2017, 51, 115–126. [Google Scholar] [CrossRef]
- Reis, S.; Fraga, H.; Carlos, C.; Silvestre, J.; Eiras-Dias, J.; Rodrigues, P.; Santos, J.A. Grapevine Phenology in Four Portuguese Wine Regions: Modeling and Predictions. Appl. Sci. 2020, 10, 3708. [Google Scholar] [CrossRef]
- Abou-Khaled, A.; Hagan, R.M.; Davenport, D.C. Effects of kaolinite as a reflective antitranspirant on leaf temperature, transpiration, photosynthesis, and water-use efficiency. Water Resour. Res. 1970, 6, 280–289. [Google Scholar] [CrossRef]
- Brito, C.; Dinis, L.-T.; Moutinho-Pereira, J.; Correia, C. Kaolin, an emerging tool to alleviate the effects of abiotic stresses on crop performance. Sci. Hortic. 2019, 250, 310–316. [Google Scholar] [CrossRef]
- Dinis, L.T.; Ferreira, H.; Pinto, G.; Bernardo, S.; Correia, C.M.; Moutinho-Pereira, J. Kaolin-based, foliar reflective film protects photosystem II structure and function in grapevine leaves exposed to heat and high solar radiation. Photosynthetica 2016, 54, 47–55. [Google Scholar] [CrossRef]
- Abdallah, M.M.S.; El-Bassiouny, H.M.S.; AbouSeeda, M.A. Potential role of kaolin or potassium sulfate as anti-transpirant on improving physiological, biochemical aspects and yield of wheat plants under different watering regimes. Bull. Natl. Res. Cent. 2019, 43. [Google Scholar] [CrossRef]
- Faghih, S.; Zamani, Z.; Fatahi, R.; Liaghat, A. Effects of deficit irrigation and kaolin application on vegetative growth and fruit traits of two early ripening apple cultivars. Biol. Res. 2019, 52. [Google Scholar] [CrossRef] [PubMed]
- Beneragama, C.K.; Balasooriya, B.L.H.N.; Perera, T.M.R.S. Use of O-J-I-P chlorophyll fluorescence transients to probe multiple effects of UV-C radiation on the photosynthetic apparatus of Euglena. Int. J. Appl. Sci. Biotechnol. 2014, 2, 553–558. [Google Scholar] [CrossRef]
- Strasser, B.J. Donor side capacity of Photosystem II probed by chlorophyll a fluorescence transients. Photosyn. Res. 1997, 52, 147–155. [Google Scholar] [CrossRef]
- Bernardo, S.; Dinis, L.T.; Luzio, A.; Pinto, G.; Meijón, M.; Valledor, L.; Conde, A.; Gerós, H.; Correia, C.M.; Moutinho-Pereira, J. Kaolin particle film application lowers oxidative damage and DNA methylation on grapevine (Vitis vinifera L.). Environ. Exp. Bot. 2017, 139, 39–47. [Google Scholar] [CrossRef]
- Dinis, L.T.; Bernardo, S.; Luzio, A.; Pinto, G.; Meijón, M.; Pintó-Marijuan, M.; Cotado, A.; Correia, C.; Moutinho-Pereira, J. Kaolin modulates ABA and IAA dynamics and physiology of grapevine under Mediterranean summer stress. J. Plant Physiol. 2018, 220, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Rizhsky, L.; Liang, H.; Shuman, J.; Shulaev, V.; Davletova, S.; Mittler, R. When Defense Pathways Collide. The Response of Arabidopsis to a Combination of Drought and Heat Stress. Plant Physiol. 2004, 134, 1683–1696. [Google Scholar] [CrossRef] [PubMed]
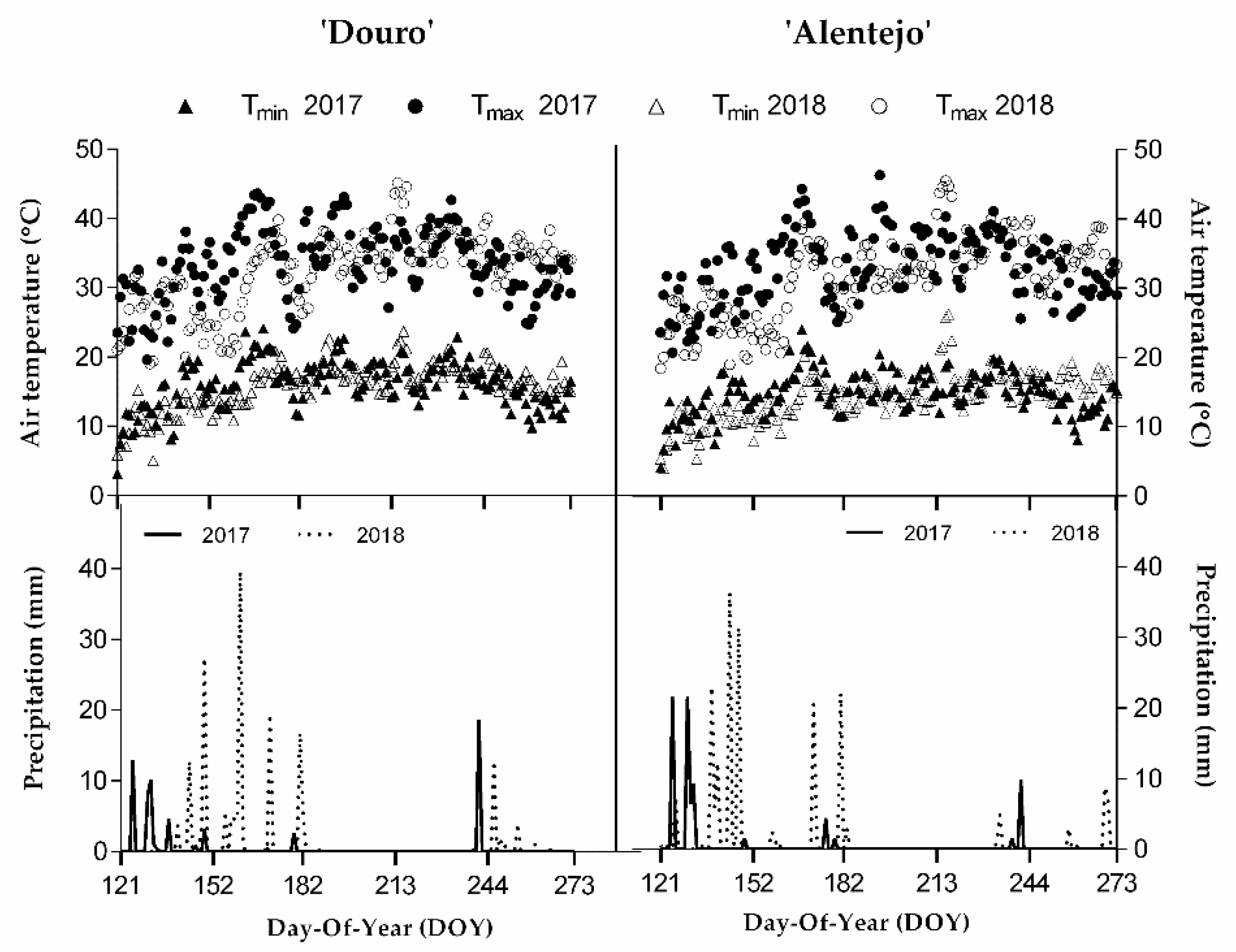
| Region | Season | HI | Class | CI | Class | DI | Class |
|---|---|---|---|---|---|---|---|
| Douro | 2017 | 3283 | Very warm | 14.2 | Temperate nights | −147.1 | Very dry |
| 2018 | 3022 | Very warm | 16.5 | Temperate nights | −47.0 | Moderately dry | |
| Alentejo | 2017 | 3148 | Very warm | 13.5 | Cool nights | −207.6 | Very dry |
| 2018 | 2887 | Warm | 16.4 | Temperate nights | −119.5 | Very dry |
| Year | Stage | Douro | Alentejo | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Touriga Nacional | Touriga Franca | Touriga Nacional | Touriga Franca | |||||||
| Control | Kaolin | Control | Kaolin | Control | Kaolin | Control | Kaolin | |||
| Leaf Temperature | 2017 | EL35 | 45.33 ± 1.78 a | 40.33 ± 0.99 c | 41.78 ± 1.30 b | 39.94 ± 0.92 c | 31.20 ± 1.29 ab | 30.56 ± 1.17 b | 33.24 ± 2.26 a | 31.94 ± 1.20 ab |
| EL38 | 37.31 ± 0.82 b * | 37.78 ± 0.70 ab * | 39.35 ± 1.55 a * | 38.70 ± 0.76 ab | 32.22 ± 0.93 c | 32.69 ± 1.55 cb * | 36.20 ± 2.18 a * | 34.72 ± 1.35 ab * | ||
| 2018 | EL35 | 31.39 ± 1.18 | 31.38 ± 0.80 | 31.17 ± 1.12 | 30.89 ± 1.72 | 30.93 ± 0.91 b | 30.65 ± 1.29 b | 35.89 ± 1.68 a | 32.78 ± 1.36 b | |
| EL38 | 37.33 ± 1.43 * | 36.61 ± 1.97 * | 36.61 ± 2.07 * | 35.89 ± 2.24 * | 36.50 ± 1.87 b * | 35.28 ± 1.98 b * | 39.61 ± 1.93 a * | 37.06 ± 1.91 b * | ||
| Total Chlorophyll | 2017 | EL35 | 285.3 ± 20.2 c | 316.0 ± 17.8 c | 386.0 ± 26.9 b | 453.2 ± 21.8 a | 316.0 ± 22.7 b | 431.3 ± 10.0 a | 328.0 ± 32.2 b | 460.7 ± 11.2 a |
| EL38 | 353.0 ± 24.0 b * | 344.7 ± 27.4 b | 380.6 ± 30.7 b | 425.5 ± 34.5 b | 298.7 ± 16.5 c | 393.5 ± 27.5 a * | 365.5 ± 26.3 ab | 351.8 ± 27.1 b * | ||
| 2018 | EL35 | 333.8 ± 32.4 c | 392.2 ± 24.0 b | 283.7 ± 14.3 d | 450.5 ± 38.1 a | 445.0 ± 26.2 | 438.2 ± 54.7 | 439.8 ± 34.0 | 409 ± 20.4 | |
| EL38 | 241.3 ± 23.1 b * | 247.8 ± 27.2 b * | 297.8 ± 8.0 a | 309.0 ± 16.4 a * | 401.0 ± 24.1 * | 410.3 ± 10.7 | 409.0 ± 48.5 | 406.8 ± 35.6 | ||
| Douro Trial | Season | Stage | Touriga Nacional | Touriga Franca | ||
|---|---|---|---|---|---|---|
| Control | Kaolin | Control | Kaolin | |||
| TBARS | 2017 | EL35 | 13.14 ± 1.37 a | 10.87 ± 2.17 a | 6.17 ± 1.06 b | 13.36 ± 0.95 a |
| EL38 | 23.68 ± 2.03 a * | 15.20 ± 0.27 b * | 1.67 ± 0.26 c * | 2.07 ± 1.03 c * | ||
| 2018 | EL35 | 12.35 ± 2.52 a | 4.99 ± 1.09 bc | 2.75 ± 0.56 c | 6.96 ± 1.62 b | |
| EL38 | 9.58 ± 0.72 b * | 13.18 ± 2.34 a * | 12.62 ± 1.02 a * | 4.93 ± 0.31 c | ||
| Proline | 2017 | EL35 | 8.20 ± 0.85 | 8.35 ± 0.77 | 7.20 ± 0.57 | 7.53 ± 0.40 |
| EL38 | 6.73 ± 0.61 b * | 6.32 ± 0.56 b * | 9.85 ± 0.40 a * | 7.09 ± 0.73 b | ||
| 2018 | EL35 | 16.79 ± 1.64 b | 10.94 ± 0.79 c | 13.26 ± 0.34 c | 21.16 ± 2.29 a | |
| EL38 | 16.25 ± 1.61 a | 8.76 ± 0.90 b | 10.80 ± 0.37 b * | 15.34 ± 1.42 a * | ||
| AsA | 2017 | EL35 | 2.70 ± 0.16 a | 1.50 ± 0.10 c | 0.67 ± 0.07 d | 1.84 ± 0.08 b |
| EL38 | 2.35 ± 0.25 a * | 1.59 ± 0.16 b | 0.73 ± 0.06 c | 1.54 ± 0.19 b * | ||
| 2018 | EL35 | 4.50 ± 0.26 a | 2.45 ± 0.15 b | 1.66 ± 0.12 c | 2.52 ± 0.32 b | |
| EL38 | 3.44 ± 0.31 a * | 3.23 ± 0.21 ab * | 2.87 ± 0.27 a * | 2.49 ± 0.19 b * | ||
| DAsA | 2017 | EL35 | 1.23 ± 0.06 c | 1.43 ± 0.02 b | 0.34 ± 0.01 d | 1.64 ± 0.06 a |
| EL38 | 1.58 ± 0.14 a * | 0.20 ± 0.02 d * | 0.56 ± 0.05 c * | 0.89 ± 0.08 b * | ||
| 2018 | EL35 | 1.90 ± 0.30 ab | 1.77 ± 0.05 ab | 1.61 ± 0.16 b | 1.92 ± 0.18 a | |
| EL38 | 1.49 ± 0.17 bc * | 2.57 ± 0.12 a * | 1.77 ± 0.11 b | 1.21 ± 0.04 c * | ||
| % reduction ascorbate | 2017 | EL35 | 81.65 ± 3.72 a | 31.15 ± 0.73 c | 52.53 ± 2.42 b | 12.88 ± 1.76 d |
| EL38 | 78.73 ± 1.93 b | 89.28 ± 3.24 a * | 33.25 ± 1.65 d * | 59.66 ± 8.88 c * | ||
| 2018 | EL35 | 49.81 ± 2.53 a | 30.68 ± 2.73 b | 42.52 ± 5.95 a | 24.05 ± 1.97 b | |
| EL38 | 53.80 ± 2.21 b | 67.51 ± 4.90 a * | 27.17 ± 4.96 c * | 55.52 ± 2.40 b * | ||
| Alentejo Trial | Season | Stage | Touriga Nacional | Touriga Franca | ||
|---|---|---|---|---|---|---|
| Control | Kaolin | Control | Kaolin | |||
| TBARS | 2017 | EL35 | 43.11 ± 2.09 a | 28.52 ± 3.30 b | 13.17 ± 1.82 d | 21.85 ± 1.66 c |
| EL38 | 18.17 ± 0.84 a * | 14.41 ± 1.03 ab * | 13.61 ± 0.45 b | 13.48 ± 1.29 b * | ||
| 2018 | EL35 | 30.86 ± 4.27 a | 20.94 ± 2.52 b | 12.41 ± 0.88 c | 6.80 ± 0.42 d | |
| EL38 | 20.30 ± 3.17 a * | 11.07 ± 3.08 b * | 11.36 ± 1.72 b | 10.00 ± 2.48 b | ||
| Proline | 2017 | EL35 | 4.73 ± 0.50 b | 4.29 ± 0.08 b | 7.47 ± 0.82 a | 7.84 ± 0.52 a |
| EL38 | 6.86 ± 0.51 b * | 8.13 ± 0.66 a * | 7.51 ± 0.50 ab | 8.00 ± 0.85 ab | ||
| 2018 | EL35 | 8.65 ± 1.08 b | 8.46 ± 0.79 b | 12.04 ± 0.88 a | 13.65 ± 0.86 a | |
| EL38 | 11.10 ± 0.27 a * | 10.29 ± 0.47 a * | 9.86 ± 0.85 a * | 17.28 ± 2.23 b * | ||
| AsA | 2017 | EL35 | 4.65 ± 0.40 a | 2.45 ± 0.27 b | 1.21 ± 0.07 d | 1.47 ± 0.15 c |
| EL38 | 1.41 ± 0.06 a * | 1.39 ± 0.12 a * | 0.65 ± 0.03 b * | 0.54 ± 0.05 b * | ||
| 2018 | EL35 | 3.82 ± 0.40 b | 4.98 ± 0.55 a | 2.55 ± 0.18 c | 3.03 ± 0.14 c | |
| EL38 | 3.64 ± 0.38 b | 4.16 ± 0.34 a * | 1.51 ± 0.12 d * | 2.46 ± 0.14 c * | ||
| DAsA | 2017 | EL35 | 2.22 ± 0.26 a | 1.10 ± 0.08 b | 0.65 ± 0.07 c | 0.69 ± 0.04 c |
| EL38 | 0.70 ± 0.09 a * | 0.75 ± 0.04 a * | 0.27 ± 0.02 b * | 0.18 ± 0.02 b * | ||
| 2018 | EL35 | 1.29 ± 0.07 c | 1.46 ± 0.15 c | 2.66 ± 0.21 a | 1.91 ± 0.25 b | |
| EL38 | 1.98 ± 0.18 a * | 1.32 ± 0.12 b | 1.42 ± 0.07 b * | 1.42 ± 0.20 b * | ||
| % reduction ascorbate | 2017 | EL35 | 58.05 ± 4.63 b | 59.58 ± 4.14 b | 71.63 ± 6.60 a | 60.42 ± 1.79 b |
| EL38 | 70.38 ± 4.05 b * | 71.32 ± 5.96 b * | 59.31 ± 1.78 c * | 92.17 ± 8.90 a * | ||
| 2018 | EL35 | 70.14 ± 3.14 a | 70.38 ± 3.04 a | 7.08 ± 1.66 c | 28.15 ± 3.52 b | |
| EL38 | 44.12 ± 1.30 b * | 69.14 ± 2.64 a | 29.08 ± 6.13 c * | 68.45 ± 1.78 a * | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bernardo, S.; Luzio, A.; Machado, N.; Ferreira, H.; Vives-Peris, V.; Malheiro, A.C.; Correia, C.; Gómez-Cadenas, A.; Moutinho-Pereira, J.; Dinis, L.-T. Kaolin Application Modulates Grapevine Photochemistry and Defence Responses in Distinct Mediterranean-Type Climate Vineyards. Agronomy 2021, 11, 477. https://doi.org/10.3390/agronomy11030477
Bernardo S, Luzio A, Machado N, Ferreira H, Vives-Peris V, Malheiro AC, Correia C, Gómez-Cadenas A, Moutinho-Pereira J, Dinis L-T. Kaolin Application Modulates Grapevine Photochemistry and Defence Responses in Distinct Mediterranean-Type Climate Vineyards. Agronomy. 2021; 11(3):477. https://doi.org/10.3390/agronomy11030477
Chicago/Turabian StyleBernardo, Sara, Ana Luzio, Nelson Machado, Helena Ferreira, Vicente Vives-Peris, Aureliano C. Malheiro, Carlos Correia, Aurelio Gómez-Cadenas, José Moutinho-Pereira, and Lia-Tânia Dinis. 2021. "Kaolin Application Modulates Grapevine Photochemistry and Defence Responses in Distinct Mediterranean-Type Climate Vineyards" Agronomy 11, no. 3: 477. https://doi.org/10.3390/agronomy11030477
APA StyleBernardo, S., Luzio, A., Machado, N., Ferreira, H., Vives-Peris, V., Malheiro, A. C., Correia, C., Gómez-Cadenas, A., Moutinho-Pereira, J., & Dinis, L.-T. (2021). Kaolin Application Modulates Grapevine Photochemistry and Defence Responses in Distinct Mediterranean-Type Climate Vineyards. Agronomy, 11(3), 477. https://doi.org/10.3390/agronomy11030477










