Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Site and Materials
2.2. Hyperspectral Data Acquisition
2.3. Anatomical Observation
2.4. Plant Traits Examination
2.4.1. Leaf Cover Area (LCA)
2.4.2. Leaf Chlorophyll Content (SPAD)
2.4.3. Leaf Dry Weight (LDW), N Content (LNC), and N Accumulation (LNA)
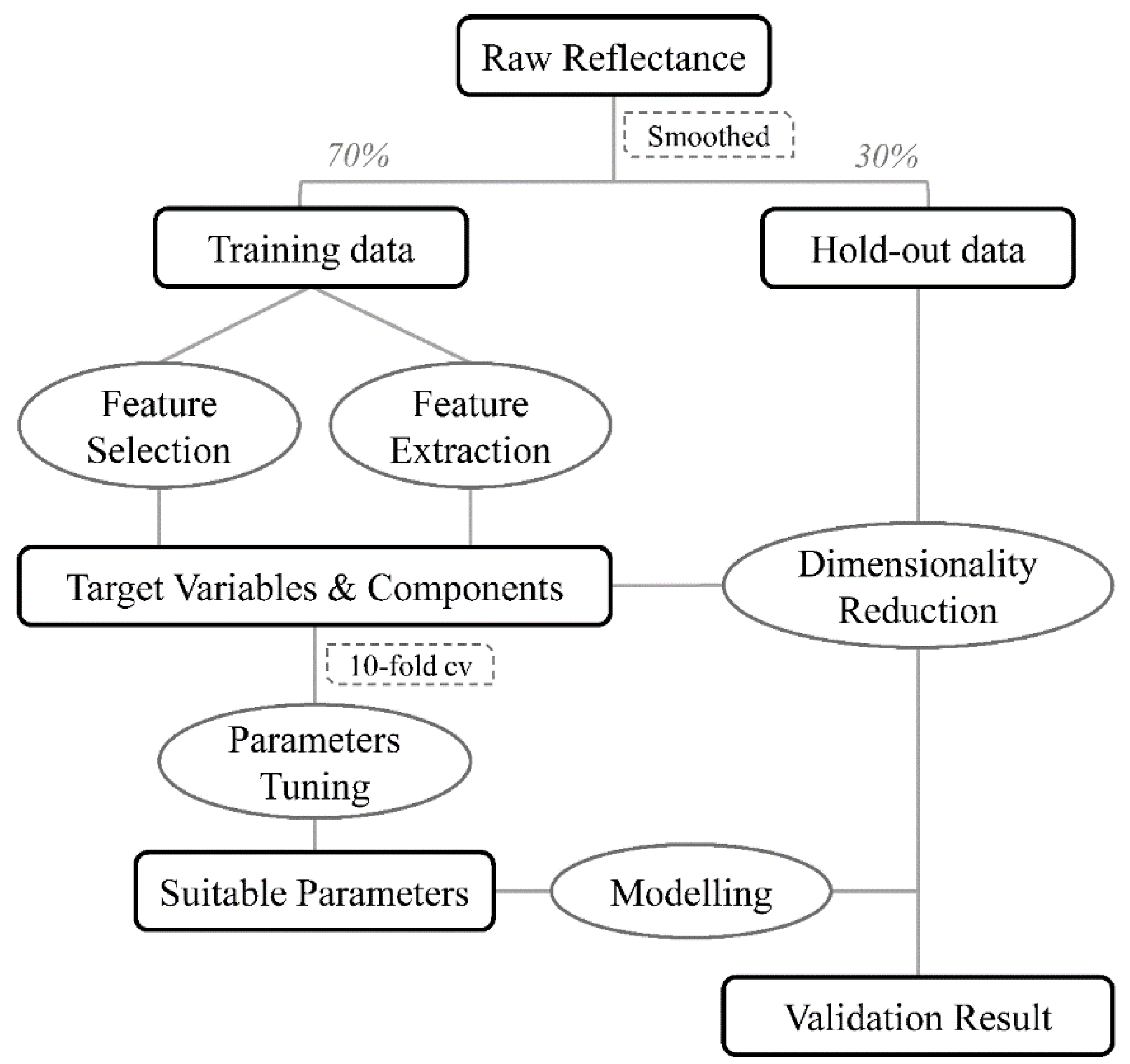
2.5. Data Analysis
2.5.1. Data Pretreatment and Partition
2.5.2. Dimensionality Reduction
2.5.3. Parameters Tuning
2.5.4. Model Performance Evaluation
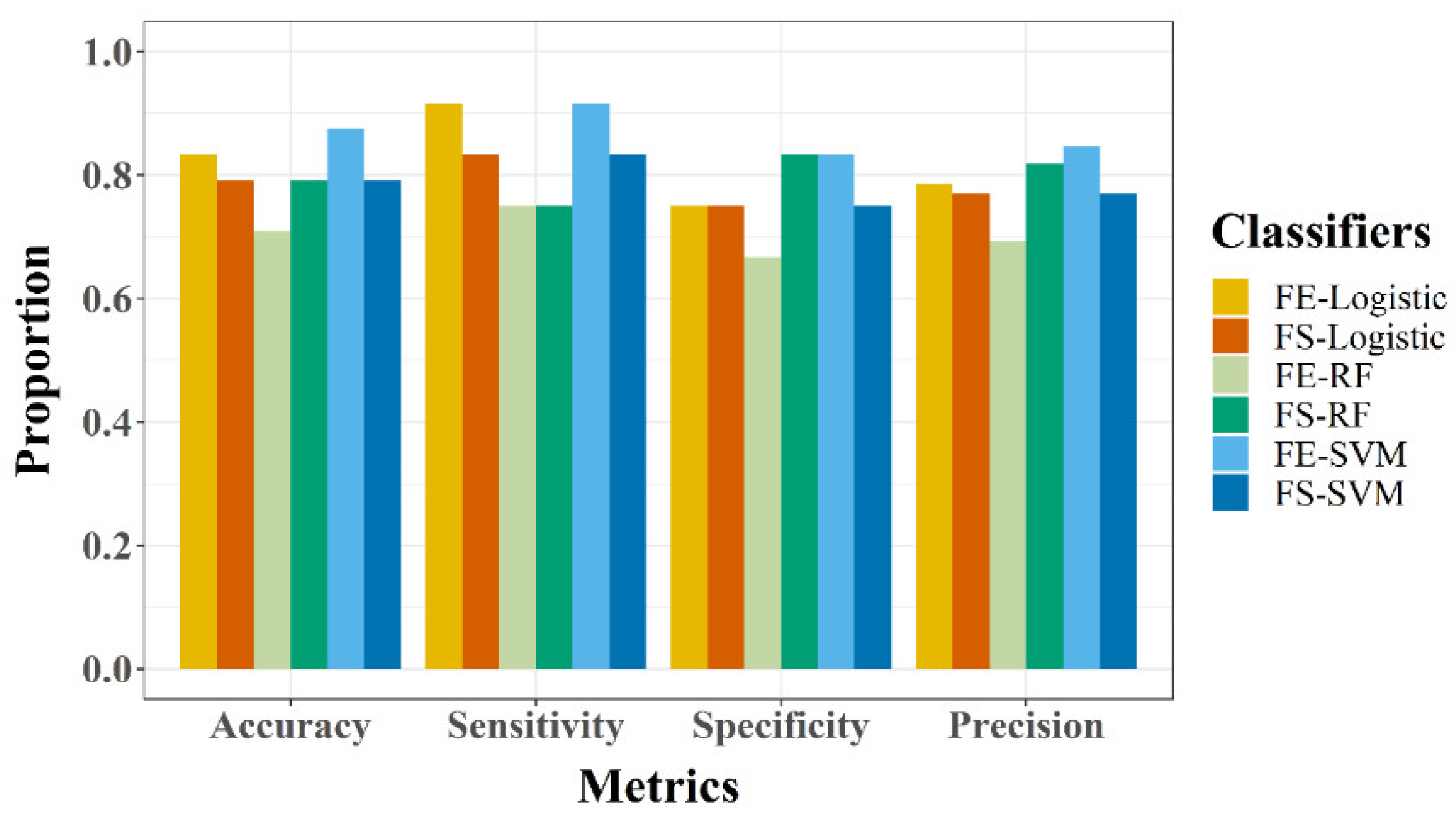
- Accuracy: This represents an overall proportion of accurate classification (correctly classified vegetative and reproductive, respectively).
- Sensitivity: This shows how well the classifiers can identify the reproductive phase when the rice plant enters the reproductive phase.
- Specificity: This shows how well the classifiers can identify vegetative phase when the rice plant has not entered reproductive phase.
- Precision: This computes the probability that a positive prediction is accurate.
3. Results and Discussion
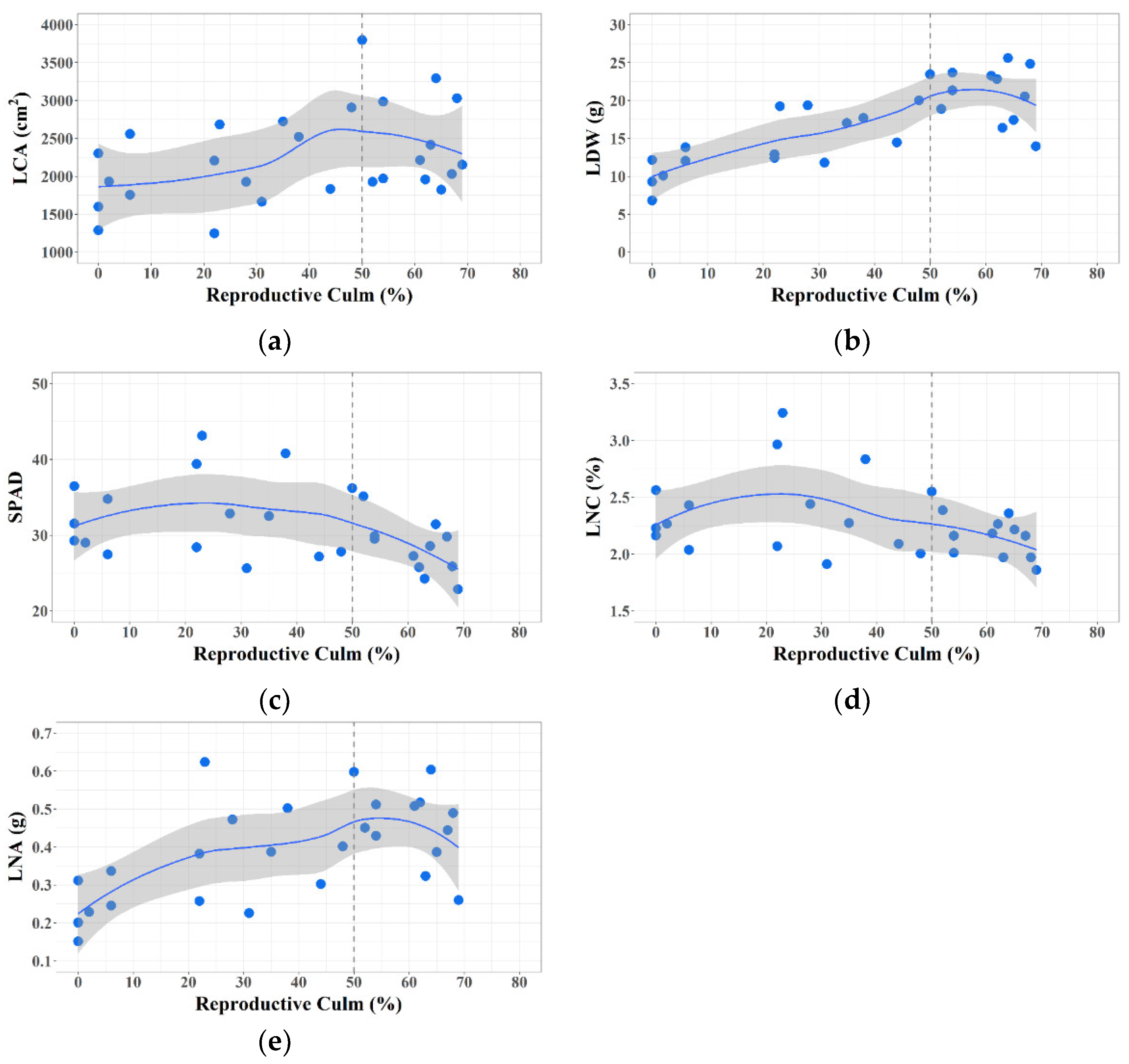
3.1. Plant Trait Changes during Phase Transition
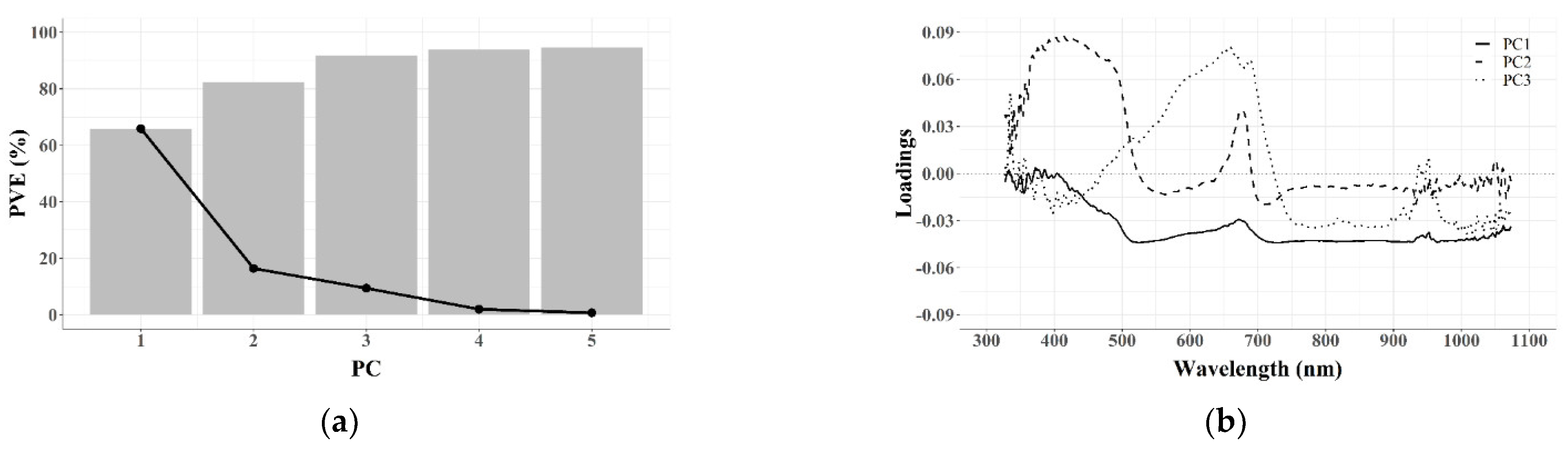
3.2. Dimensionality Reduction
3.2.1. Feature Extraction (FE)
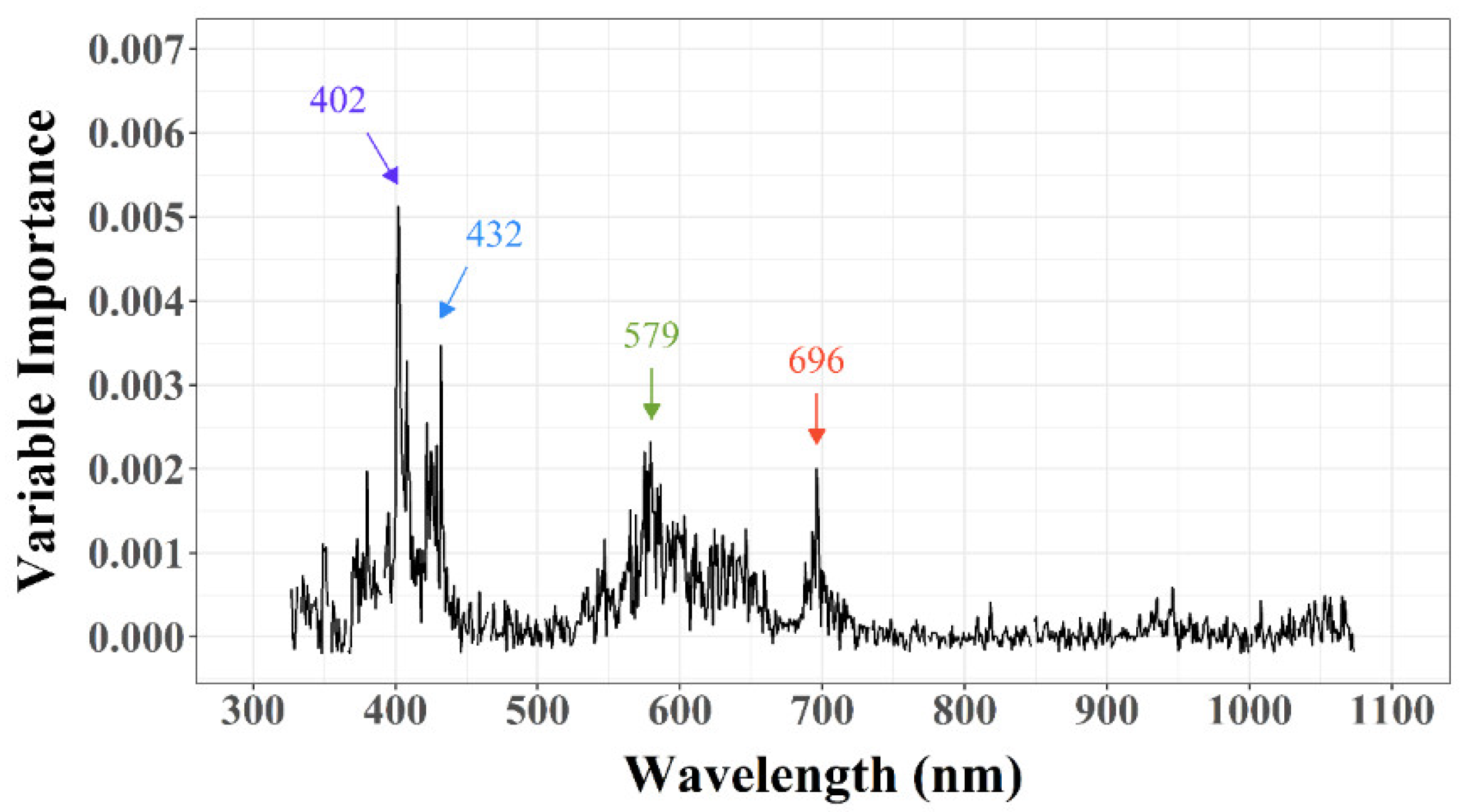
3.2.2. Feature Selection (FS)
3.3. Model Evaluation
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fahad, S.; Adnan, M.; Noor, M.; Arif, M.; Alam, M.; Khan, I.A.; Ullah, H.; Wahid, F.; Mian, I.A.; Jamal, Y.; et al. Chapter 1—Major constraints for global rice production. In Advances in Rice Research for Abiotic Stress Tolerance; Hasanuzzaman, M., Fujita, M., Nahar, K., Biswas, J.K., Eds.; Woodhead Publishing: Sawston, UK, 2019; pp. 1–22. ISBN 978-0-12-814332-2. [Google Scholar]
- Fageria, N.K.; Baligar, V.C. Yield and Yield Components of Lowland Rice as Influenced by Timing of Nitrogen Fertilization. J. Plant Nutr. 1999, 22, 23–32. [Google Scholar] [CrossRef]
- Kamiji, Y.; Yoshida, H.; Palta, J.A.; Sakuratani, T.; Shiraiwa, T. N Applications That Increase Plant N during Panicle Development Are Highly Effective in Increasing Spikelet Number in Rice. Field Crop. Res. 2011, 122, 242–247. [Google Scholar] [CrossRef]
- Zhou, W.; Lv, T.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Ren, W. Morphophysiological Mechanism of Rice Yield Increase in Response to Optimized Nitrogen Management. Sci. Rep. 2017, 7, 17226. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ju, C.; Zhu, Y.; Liu, T.; Sun, C. The Effect of Nitrogen Reduction at Different Stages on Grain Yield and Nitrogen Use Efficiency for Nitrogen Efficient Rice Varieties. Agronomy 2021, 11, 462. [Google Scholar] [CrossRef]
- Ju, C.; Liu, T.; Sun, C. Panicle Nitrogen Strategies for Nitrogen-Efficient Rice Varieties at a Moderate Nitrogen Application Rate in the Lower Reaches of the Yangtze River, China. Agronomy 2021, 11, 192. [Google Scholar] [CrossRef]
- Counce, P.A.; Keisling, T.C.; Mitchell, A.J. A Uniform, Objective, and Adaptive System for Expressing Rice Development. Crop Sci. 2000, 40, 436–443. [Google Scholar] [CrossRef] [Green Version]
- Muhammad, S.; Kim, U.J.; Kumazawa, K. The Uptake, Distribution, and Accumulation of 15N-Labelled Ammonium and Nitrate Nitrogen Top-Dressed at Different Growth Stages of Rice. Soil Sci. Plant Nutr. 1974, 20, 279–286. [Google Scholar] [CrossRef]
- Zhou, W.; Yang, Z.; Wang, T.; Fu, Y.; Chen, Y.; Hu, B.; Yamagishi, J.; Ren, W. Environmental Compensation Effect and Synergistic Mechanism of Optimized Nitrogen Management Increasing Nitrogen Use Efficiency in Indica Hybrid Rice. Front. Plant Sci. 2019, 10, 245. [Google Scholar] [CrossRef]
- Peng, S.; Cassman, K.G. Upper Thresholds of Nitrogen Uptake Rates and Associated Nitrogen Fertilizer Efficiencies in Irrigated Rice. Agron. J. 1998, 90, 178–185. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, F.; Xiao, F.; Tao, Y.; Liu, Z.; Li, G.; Wang, S.; Ding, Y. Contribution of Mineral Nutrients from Source to Sink Organs in Rice under Different Nitrogen Fertilization. Plant Growth Regul. 2018, 86, 159–167. [Google Scholar] [CrossRef] [Green Version]
- Quang Duy, P.; Abe, A.; Hirano, M.; Sagawa, S.; Kuroda, E. Analysis of Lodging-Resistant Characteristics of Different Rice Genotypes Grown under the Standard and Nitrogen-Free Basal Dressing Accompanied with Sparse Planting Density Practices. Plant Prod. Sci. 2004, 7, 243–251. [Google Scholar] [CrossRef]
- Pan, J.; Zhao, J.; Liu, Y.; Huang, N.; Tian, K.; Shah, F.; Liang, K.; Zhong, X.; Liu, B. Optimized Nitrogen Management Enhances Lodging Resistance of Rice and Its Morpho-Anatomical, Mechanical, and Molecular Mechanisms. Sci. Rep. 2019, 9, 20274. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Zhu, X.; He, X.; Li, C.; Chang, T.; Chang, S.; Zhang, H.; Zhang, Y. Scheduling of Nitrogen Fertilizer Topdressing during Panicle Differentiation to Improve Grain Yield of Rice with a Long Growth Duration. Sci. Rep. 2020, 10, 15197. [Google Scholar] [CrossRef] [PubMed]
- Moldenhauer, K.; Counce, P.; Hardke, J. Rice growth and development. In Rice Production Handbook; Hardke, J., Ed.; University of Arkansas Division of Agriculture: Little Rock, AR, USA, 2001; Volume 192, pp. 7–14. [Google Scholar]
- Dunand, R.; Saichuk, J. Rice growth and development. In Louisiana Rice Production Handbook; Saichuk, J., Ed.; Louisiana State University Agricultural Center: Baton Rouge, LA, USA, 2014; Volume 2321, pp. 41–53. [Google Scholar]
- Gilmore, E.C.; Rogers, J.S. Heat Units as a Method of Measuring Maturity in Corn 1. Agron. J. 1958, 50, 611–615. [Google Scholar] [CrossRef]
- Aslam, M.A.; Ahmed, M.; Stöckle, C.O.; Higgins, S.S.; Hayat, R. Can Growing Degree Days and Photoperiod Predict Spring Wheat Phenology? Front. Environ. Sci. 2017, 5, 57. [Google Scholar] [CrossRef]
- Bonhomme, R. Bases and limits to using ‘degree.day’ units. Eur. J. Agron. 2000, 13, 1–10. [Google Scholar] [CrossRef]
- Zeng, L.; Wardlow, B.D.; Xiang, D.; Hu, S.; Li, D. A Review of Vegetation Phenological Metrics Extraction Using Time-Series, Multispectral Satellite Data. Remote Sens. Environ. 2020, 237, 111511. [Google Scholar] [CrossRef]
- Yang, Q.; Shi, L.; Han, J.; Yu, J.; Huang, K. A Near Real-Time Deep Learning Approach for Detecting Rice Phenology Based on UAV Images. Agric. For. Meteorol. 2020, 287, 107938. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, T.; Yao, X.; Deng, X.; Tian, Y.; Cao, W.; Zhu, Y. Detection of Rice Phenology through Time Series Analysis of Ground-Based Spectral Index Data. Field Crop. Res. 2016, 198, 131–139. [Google Scholar] [CrossRef]
- Hufkens, K.; Melaas, E.K.; Mann, M.L.; Foster, T.; Ceballos, F.; Robles, M.; Kramer, B. Monitoring Crop Phenology Using a Smartphone Based Near-Surface Remote Sensing Approach. Agric. For. Meteorol. 2019, 265, 327–337. [Google Scholar] [CrossRef]
- Guo, L.; An, N.; Wang, K. Reconciling the Discrepancy in Ground-and Satellite-observed Trends in the Spring Phenology of Winter Wheat in China from 1993 to 2008. J. Geophys. Res. Atmos. 2016, 121, 1027–1042. [Google Scholar] [CrossRef] [Green Version]
- Zhou, M.; Ma, X.; Wang, K.; Cheng, T.; Tian, Y.; Wang, J.; Zhu, Y.; Hu, Y.; Niu, Q.; Gui, L. Detection of Phenology Using an Improved Shape Model on Time-Series Vegetation Index in Wheat. Comput. Electron. Agric. 2020, 173, 105398. [Google Scholar] [CrossRef]
- Guo, W.; Fukatsu, T.; Ninomiya, S. Automated Characterization of Flowering Dynamics in Rice Using Field-Acquired Time-Series RGB Images. Plant Methods 2015, 11, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, Y.; Cao, Z.; Lu, H.; Li, Y.; Xiao, Y. In-Field Automatic Observation of Wheat Heading Stage Using Computer Vision. Biosyst. Eng. 2016, 143, 28–41. [Google Scholar] [CrossRef]
- Bai, X.; Cao, Z.; Zhao, L.; Zhang, J.; Lv, C.; Li, C.; Xie, J. Rice Heading Stage Automatic Observation by Multi-Classifier Cascade Based Rice Spike Detection Method. Agric. For. Meteorol. 2018, 259, 260–270. [Google Scholar] [CrossRef]
- Tivet, F.; Pinheiro, B.D.S.; Raïssac, M.D.; Dingkuhn, M. Leaf Blade Dimensions of Rice (Oryza sativa L. and Oryza glaberrima Steud.). Relationships between Tillers and the Main Stem. Ann. Bot. 2001, 88, 507–511. [Google Scholar] [CrossRef]
- Xu, X.; Liang, T.; Zhu, J.; Zheng, D.; Sun, T. Review of Classical Dimensionality Reduction and Sample Selection Methods for Large-Scale Data Processing. Neurocomputing 2019, 328, 5–15. [Google Scholar] [CrossRef]
- Asner, G.P. Biophysical and Biochemical Sources of Variability in Canopy Reflectance. Remote Sens. Environ. 1998, 64, 234–253. [Google Scholar] [CrossRef]
- Song, S.; Gong, W.; Zhu, B.; Huang, X. Wavelength Selection and Spectral Discrimination for Paddy Rice, with Laboratory Measurements of Hyperspectral Leaf Reflectance. ISPRS J. Photogramm. Remote Sens. 2011, 66, 672–682. [Google Scholar] [CrossRef]
- Liu, Z.-Y.; Qi, J.-G.; Wang, N.-N.; Zhu, Z.-R.; Luo, J.; Liu, L.-J.; Tang, J.; Cheng, J.-A. Hyperspectral Discrimination of Foliar Biotic Damages in Rice Using Principal Component Analysis and Probabilistic Neural Network. Precis. Agric. 2018, 19, 973–991. [Google Scholar] [CrossRef]
- Almeida, T.I.R.; Filho, D.S. Principal Component Analysis Applied to Feature-Oriented Band Ratios of Hyperspectral Data: A Tool for Vegetation Studies. Int. J. Remote Sens. 2004, 25, 5005–5023. [Google Scholar] [CrossRef]
- Breiman, L. Random Forests. Mach. Learn. 2001, 45, 5–32. [Google Scholar] [CrossRef] [Green Version]
- Liaw, A.; Wiener, M. Classification and Regression by RandomForest. R News 2002, 2, 18–22. [Google Scholar]
- Abdel-Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random Forest Regression and Spectral Band Selection for Estimating Sugarcane Leaf Nitrogen Concentration Using EO-1 Hyperion Hyperspectral Data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Yuan, Z.; Cao, Q.; Zhang, K.; Ata-Ul-Karim, S.T.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Optimal Leaf Positions for SPAD Meter Measurement in Rice. Front. Plant Sci. 2016, 7, 719. [Google Scholar] [CrossRef] [Green Version]
- R Core Team. R: A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2021; Available online: https://www.R-project.org (accessed on 18 May 2021).
- Peng, C.-Y.J.; So, T.-S.H. Logistic Regression Analysis and Reporting: A Primer. Underst. Stat. Stat. Issues Psychol. Educ. Soc. Sci. 2002, 1, 31–70. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Mnzava, M.; Cassman, K.G.; Mitchell, P.L.; Pablico, P.; Robles, R.P.; Samonte, H.P.; Lales, J.S.; Ferrer, A.B. Temporal Origin of Nitrogen in the Grain of Irrigated Rice in the Dry Season: The Outcome of Uptake, Cycling, Senescence and Competition Studied Using a 15N-Point Placement Technique. Field Crop. Res. 2004, 89, 337–348. [Google Scholar] [CrossRef]
- Zur, Y.; Gitelson, A.A.; Chivkunova, O.B.; Merzlyak, M.N. The Spectral Contribution of Carotenoids to Light Absorption and Reflectance in Green Leaves. In Proceedings of the 2nd International Conference Geospatial Information in Agriculture and Forestry, Buena Vista, FL, USA, 10–12 January 2000; Volume 2. [Google Scholar]
- Gitelson, A.A.; Zur, Y.; Chivkunova, O.B.; Merzlyak, M.N. Assessing Carotenoid Content in Plant Leaves with Reflectance Spectroscopy. Photochem. Photobiol. 2002, 75, 272–281. [Google Scholar] [CrossRef]
- Merzlyak, M.N.; Solovchenko, A.E.; Gitelson, A.A. Reflectance Spectral Features and Non-Destructive Estimation of Chlorophyll, Carotenoid and Anthocyanin Content in Apple Fruit. Postharvest Biol. Technol. 2003, 27, 197–211. [Google Scholar] [CrossRef]
- Gamon, J.A.; Penuelas, J.; Field, C.B. A Narrow-Waveband Spectral Index That Tracks Diurnal Changes in Photosynthetic Efficiency. Remote Sens. Environ. 1992, 41, 35–44. [Google Scholar] [CrossRef]
- Horler, D.N.H.; Dockray, M.; Barber, J. The Red Edge of Plant Leaf Reflectance. Int. J. Remote Sens. 1983, 4, 273–288. [Google Scholar] [CrossRef]
- Knipling, E.B. Physical and Physiological Basis for the Reflectance of Visible and Near-Infrared Radiation from Vegetation. Remote Sens. Environ. 1970, 1, 155–159. [Google Scholar] [CrossRef]
- Woolley, J.T. Reflectance and Transmittance of Light by Leaves. Plant Physiol. 1971, 47, 656–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blackburn, G.A. Hyperspectral Remote Sensing of Plant Pigments. J. Exp. Bot. 2007, 58, 855–867. [Google Scholar] [CrossRef] [Green Version]
- Gitelson, A.A.; Merzlyak, M.N. Signature Analysis of Leaf Reflectance Spectra: Algorithm Development for Remote Sensing of Chlorophyll. J. Plant Physiol. 1996, 148, 494–500. [Google Scholar] [CrossRef]
- Sims, D.A.; Gamon, J.A. Relationships between Leaf Pigment Content and Spectral Reflectance across a Wide Range of Species, Leaf Structures and Developmental Stages. Remote Sens. Environ. 2002, 81, 337–354. [Google Scholar] [CrossRef]
- Gitelson, A.; Merzlyak, M.N. Spectral Reflectance Changes Associated with Autumn Senescence of Aesculus Hippocastanum, L. and Acer Platanoides, L. Leaves. Spectral Features and Relation to Chlorophyll Estimation. J. Plant Physiol. 1994, 143, 286–292. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Pandey, R.N.; Sahoo, R.N.; Gupta, V.K.; Datta, S.C.; Kumar, D. Monitoring Nitrogen, Phosphorus and Sulphur in Hybrid Rice (Oryza sativa L.) Using Hyperspectral Remote Sensing. Precis. Agric. 2017, 18, 736–761. [Google Scholar] [CrossRef]
- Wada, S.; Hayashida, Y.; Izumi, M.; Kurusu, T.; Hanamata, S.; Kanno, K.; Kojima, S.; Yamaya, T.; Kuchitsu, K.; Makino, A. Autophagy Supports Biomass Production and Nitrogen Use Efficiency at the Vegetative Stage in Rice. Plant Physiol. 2015, 168, 60–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tkachuk, R.; Mellish, V.J.; Daun, J.K.; Macri, L.J. Determination of Chlorophyll in Ground Rapeseed Using a Modified near Infrared Reflectance Spectrophotometer. J. Am. Oil Chem. Soc. 1988, 65, 381–385. [Google Scholar] [CrossRef]
- Dedeoglu, M. Estimation of Critical Nitrogen Contents in Peach Orchards Using Visible-near Infrared Spectral Mixture Analysis. J. Infrared Spectrosc. 2020, 28, 315–327. [Google Scholar] [CrossRef]
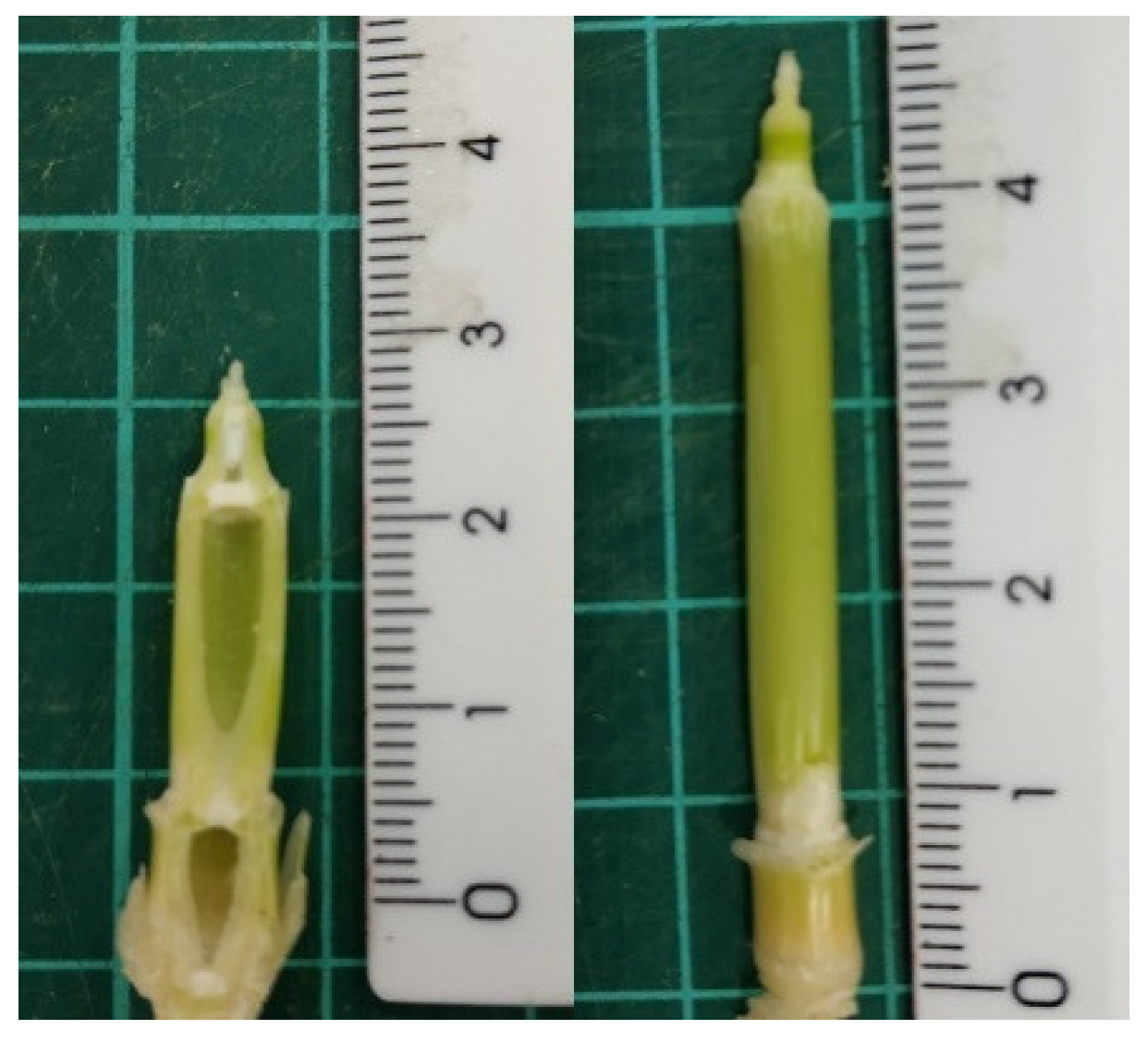

| Models | Parameters |
|---|---|
| FE-logistic | threshold = 0.50 |
| FS-logistic | threshold = 0.60 |
| FE-RF | mtry = 1, ntree = 160 |
| FS-RF | mtry = 1, ntree = 300 |
| FE-SVM | cost = 100, gamma = 0.01 |
| FS-SVM | cost = 10, gamma = 0.01 |
| Predicted | Observed | |
|---|---|---|
| Vegetative | Reproductive | |
| Vegetative | TN | FN |
| Reproductive | FP | TP 1 |
| Traits | PC1 | PC2 | PC3 |
|---|---|---|---|
| LCA | −0.277 | −0.373 | −0.329 |
| LDW | −0.294 | −0.289 | −0.358 |
| SPAD | 0.049 | −0.013 | −0.258 |
| LNC | −0.035 | −0.062 | −0.333 |
| LNA | −0.258 | −0.262 | −0.440 * |
| Traits | 402 nm | 432 nm | 579 nm | 696 nm |
|---|---|---|---|---|
| LCA | −0.346 | −0.274 | −0.019 | −0.044 |
| LDW | −0.067 | −0.016 | −0.028 | −0.124 |
| SPAD | 0.068 | −0.080 | −0.758 *** | −0.777 *** |
| LNC | 0.113 | −0.023 | −0.712 *** | −0.741 *** |
| LNA | 0.000 | −0.014 | −0.332 | −0.427 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lai, J.-K.; Lin, W.-S. Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment. Agronomy 2021, 11, 2465. https://doi.org/10.3390/agronomy11122465
Lai J-K, Lin W-S. Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment. Agronomy. 2021; 11(12):2465. https://doi.org/10.3390/agronomy11122465
Chicago/Turabian StyleLai, Joon-Keat, and Wen-Shin Lin. 2021. "Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment" Agronomy 11, no. 12: 2465. https://doi.org/10.3390/agronomy11122465
APA StyleLai, J.-K., & Lin, W.-S. (2021). Real-Time Detection of Rice Growth Phase Transition for Panicle Nitrogen Application Timing Assessment. Agronomy, 11(12), 2465. https://doi.org/10.3390/agronomy11122465







