The Composition and Seasonal Variation of Epigeic Arthropods in Different Types of Agricultural Crops and Their Ecotones
Abstract
:1. Introduction
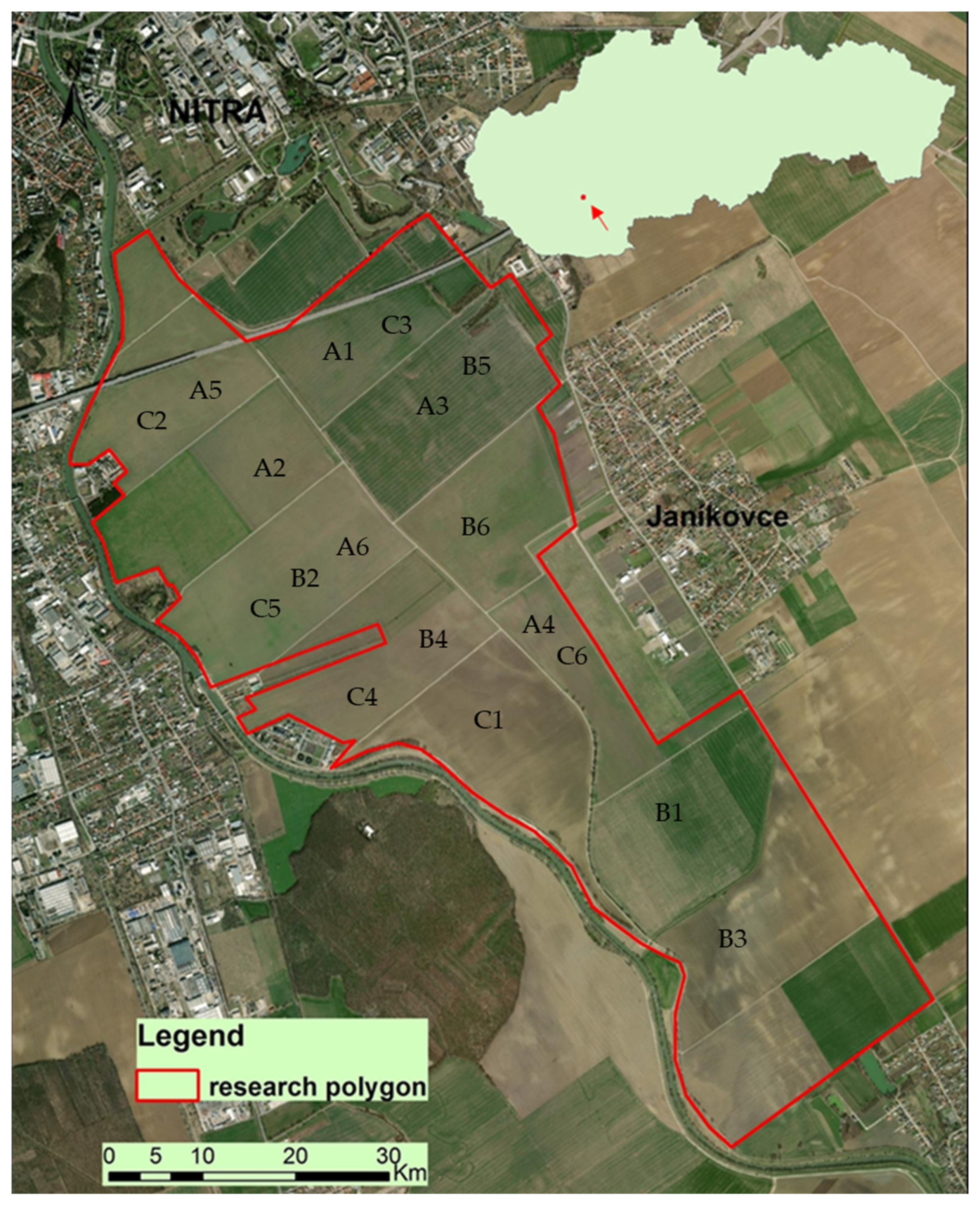
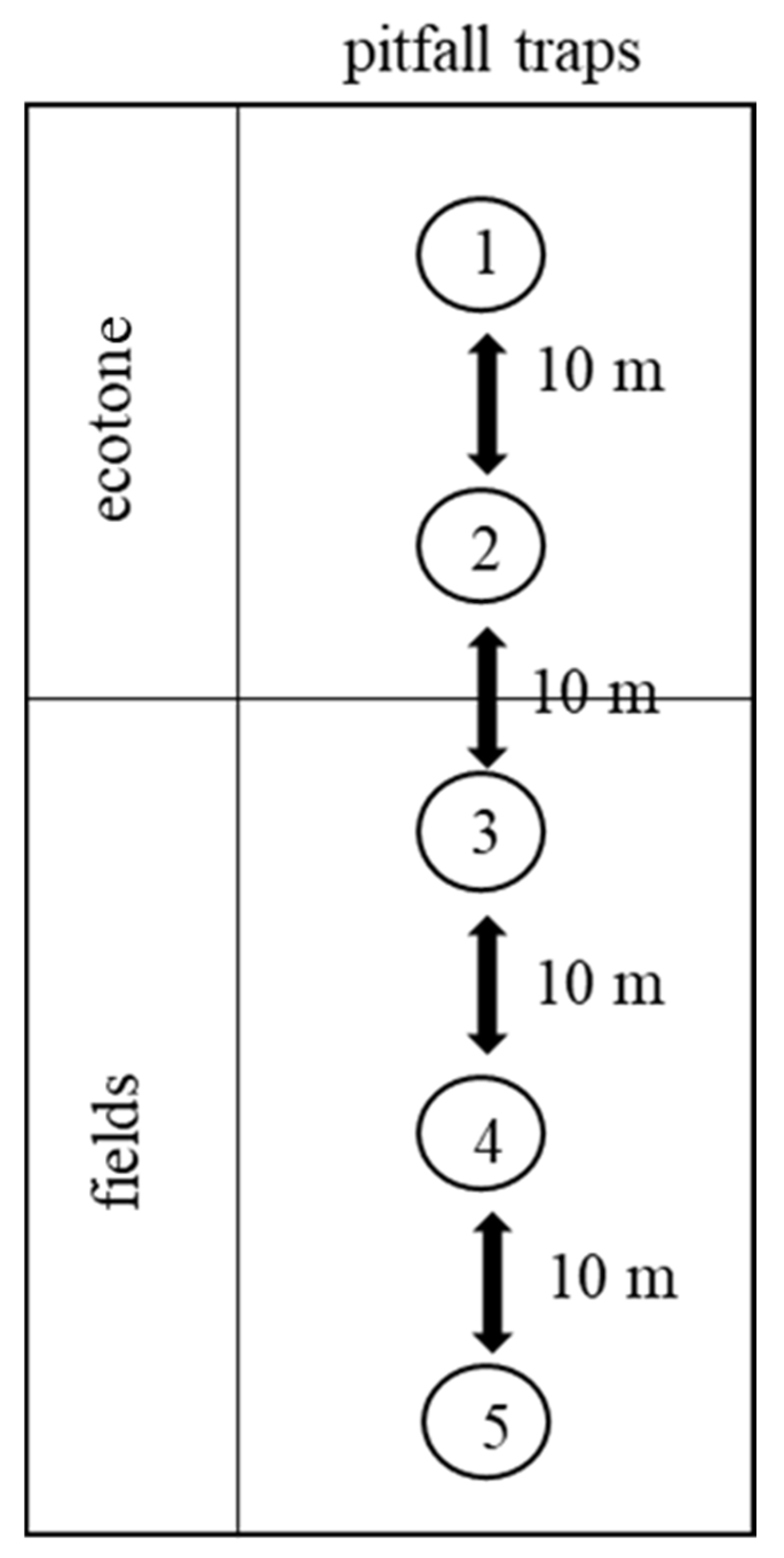
2. Materials and Methods
2.1. Database Quality
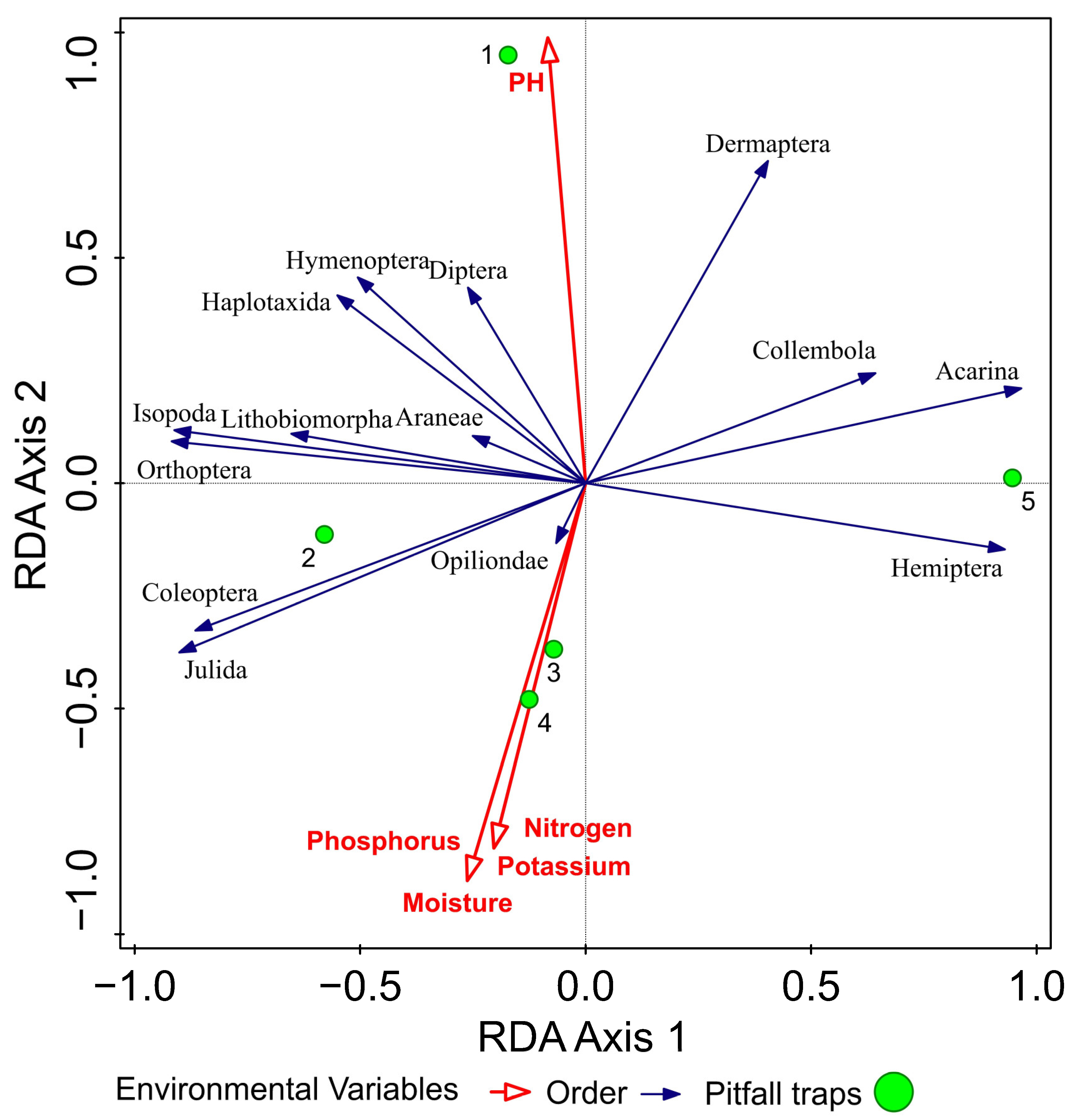
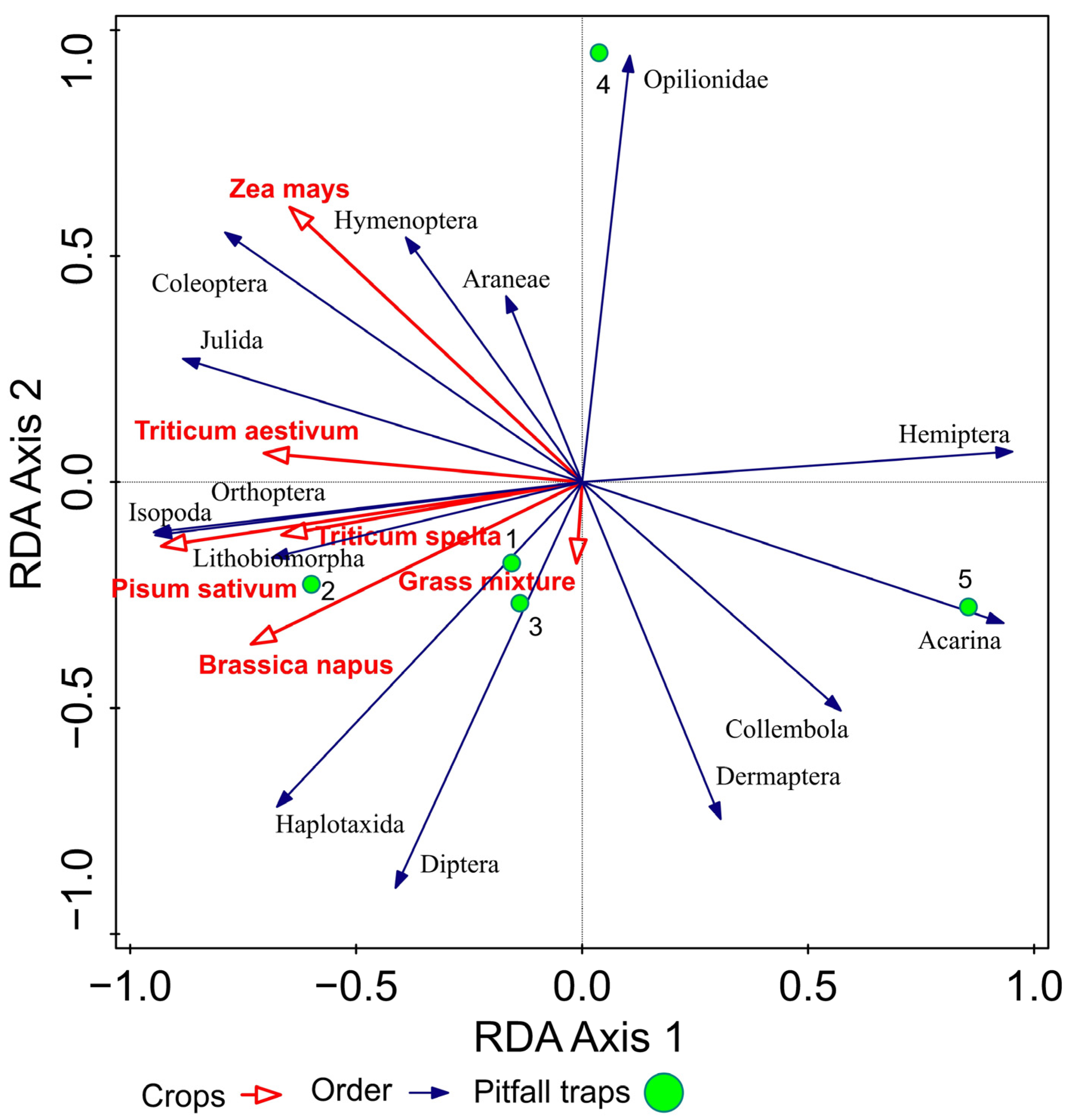
2.2. Statistical Analyses
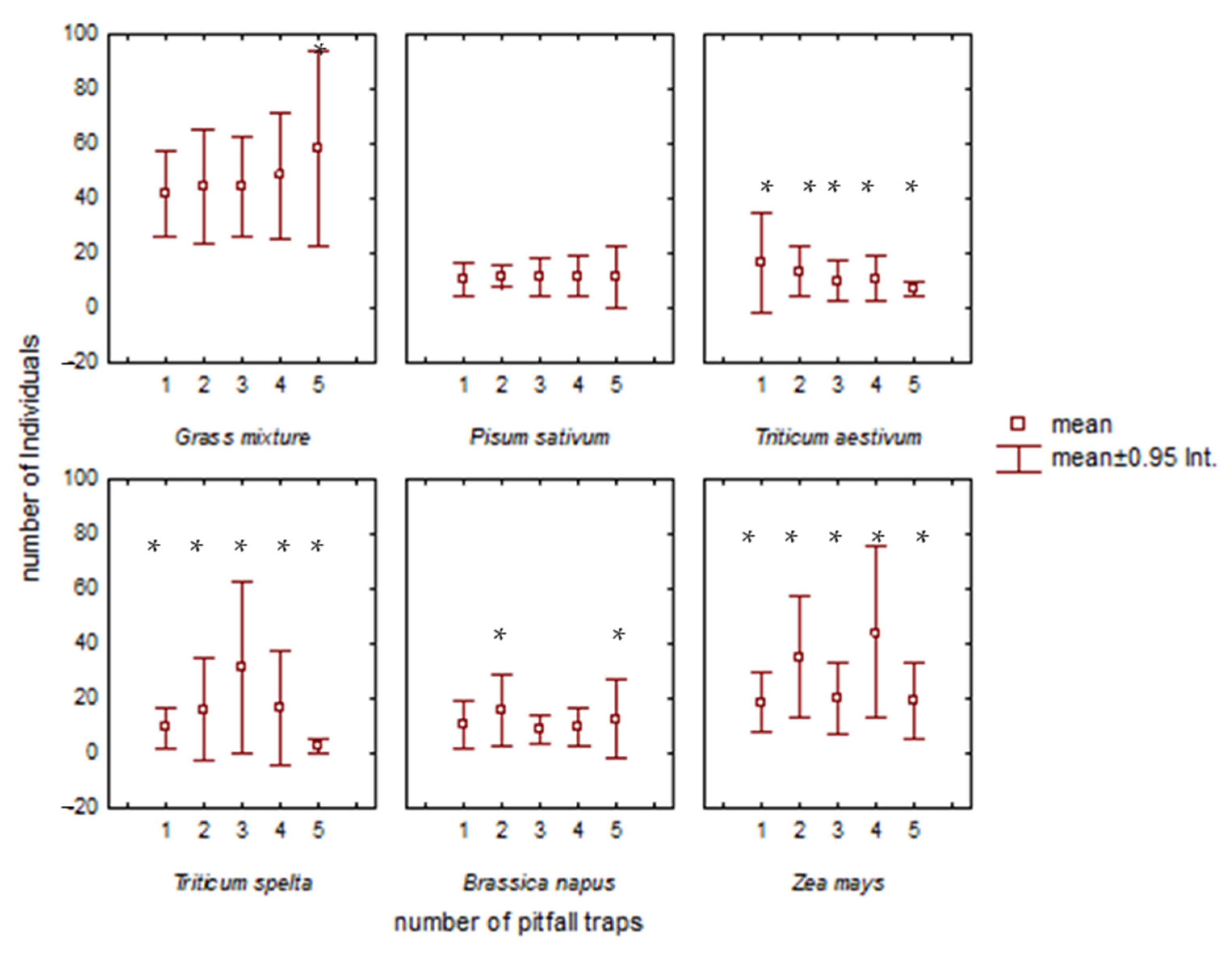
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Dudley, N.; Alexander, S. Agriculture and biodiversity: A review. Biodiversity 2017, 18, 45–49. [Google Scholar] [CrossRef]
- Stoate, C.; Boatman, N.D.; Borralho, R.J.; Carvalho, C.R.; de Snoo, G.R.; Eden, P. Ecological impacts of arable intensification in Europe. J. Environ. Manag. 2001, 63, 337–365. [Google Scholar] [CrossRef]
- Gill, H.K.; McSorley, R.; Branham, M. Effect of organic mulches on soil surface insects and arthropods. Fla. Entomol. 2011, 94, 226–232. [Google Scholar] [CrossRef]
- Haddaway, N.R.; Brown, C.; Eggers, S.; Josefsson, J.; Kronvang, B.; Randall, N.; Uusi-Kämppä, J. The multifunctional roles of vegetated strips around and within agricultural fields. A systematic map protocol. Environ. Evid. 2016, 5, 18. [Google Scholar] [CrossRef] [Green Version]
- Nowakowski, M.; Pywell, R.F. Habitat Creation and Management for Pollinators; Centre for Ecology & Hydrology: Wallingford, UK, 2016; p. 92. [Google Scholar]
- Marshall, E.J.P. Agricultural landscapes: Field margin habitats and their interaction with crop production. J. Crop Improv. 2004, 12, 365–404. [Google Scholar] [CrossRef]
- Brussaard, L.; De Ruiter, P.C.; Brown, G.G. Soil biodiversity for agricultural sustainability. Agric. Ecosyst. Environ. 2007, 121, 233–244. [Google Scholar] [CrossRef]
- Smith, J.; Potts, S.G.; Woodcock, B.A.; Eggleton, P. Can arable field margins be managed to enhance their biodiversity, conservation and functional value for soil macrofauna? J. Appl. Ecol. 2008, 45, 269–278. [Google Scholar] [CrossRef]
- New, T.R. Invertebrate Conservation and Agricultural Ecosystems; Cambridge University Press: Cambridge, UK, 2005; p. 368. [Google Scholar]
- Porhajašová, J.; Noskovič, J.; Rakovská, A.; Babošová, M.; Čeryová, T. Biodiversity and dynamics of occurence of epigeic groups in different types of farming. Acta Hortic. Regiotect. 2015, 18, 5–10. [Google Scholar] [CrossRef] [Green Version]
- Porhajašová, J.; Bartošová, L.M.; Noskovič, J.; Ondrišík, P. Long-term developments and biodiversity in Carabid and Staphylinid (Coleoptera: Carabidae and Staphylinidae) fauna during the application of organic fertilizers under agroecosystem conditions. Pol. J. Environ. Stud. 2018, 27, 2229–2235. [Google Scholar] [CrossRef]
- Krumpálová, Z. Epigeic spiders (Araneae) of one Middle Danube floodplain forest. Biologia 2002, 57, 161–169. [Google Scholar]
- Krumpálová, Z.; Krumpál, M.; Štrbík, I. Classification of epigeic spiders (Araneae) at the western part of the Carpathians (Slovakia). Biologia 2009, 64, 116–123. [Google Scholar] [CrossRef]
- Langraf, V.; David, S.; Babosová, R.; Petrovičová, K.; Schlarmannová, J. Change of ellipsoid biovolume (EV) of ground beetles (Coleoptera, Carabidae) along an urban–suburban–rural gradient of central Slovakia. Diversity 2020, 12, 475. [Google Scholar] [CrossRef]
- Langraf, V.; Petrovičová, K.; David, S.; Svoradová, A.; Schlarmannová, J. Prediction of ecological importance of Carabidae biotopes using community index of the ground beetles (Iks) in the southern part of Central Slovakia. Appl. Ecol. Environ. Res. 2020, 18, 1197–1210. [Google Scholar] [CrossRef]
- Swift, M.J.; Vandermeer, J.; Ramakrishnan, P.S.; Anderson, J.M.; Ong, C.K.; Hawkins, B.A. Biodiversity and agroecosystem function. In Functional Roles of Biodiversity; Mooney, H.A., Cushman, J.H., Medina, E., Sala, O.E., Schulze, E.D., Eds.; Wiley: New York, NY, USA, 1996; pp. 261–297. [Google Scholar]
- Starý, P.; Gerding, M.; Norambunea, H.; Remaudiere, G. Environmental research on aphid parasitoid biocontrol agents in Chile (Hym., Aphidiidae; Hom., Aphidoidea). J. Appl. Entomol. 1993, 115, 292–306. [Google Scholar] [CrossRef]
- Berg, M.P.; Bengtsson, J. Temporal and spatial variability in soil food web structure. Oikos 2007, 116, 1789–1804. [Google Scholar] [CrossRef]
- Schoenholtz, S.H.; Van Miegroet, H.; Burger, J.A. A review of chemical and physical properties as indicators of forest soil quality: Challenges and opportunities. For. Ecol. Manag. 2000, 138, 335–356. [Google Scholar] [CrossRef]
- Li, Y.; Lindstrom, M.J.; Zhang, J.; Yang, J. Spatial variability of soil erosion and soil quality on hillslopes in the Chinese Loess Plateau. Acta Geol. Hisp. 2001, 35, 261–270. [Google Scholar]
- Gilley, J.E.; Doran, J.W.; Eghball, B. Tillage and fallow effects on selected soil quality characteristics of former conservation reserve program sites. J. Soil Water Conserv. 2001, 56, 126–132. [Google Scholar]
- Six, J.; Elliott, E.T.; Paustian, K. Soil structure and soil organic matter. II. A normalized stability index and the effect of mineralogy. Soil Sci. Soc. Am. J. 2000, 64, 1042–1049. [Google Scholar] [CrossRef]
- Turco, R.F.; Kennedy, A.C.; Jawson, M.D. Microbial indicators of soil quality. In Defining Soil Quality for a Sustainable Environment; Doran, J.W., Coleman, D.C., Bezdicek, D.F., Stewart, B.A., Eds.; SSSA: Madison, WI, USA, 1994; pp. 73–90. [Google Scholar]
- Majzlan, O. Bezchordáty a Chordáty; Danubiaprint, A.S.: Bratislava, Slovakia, 2009; p. 286. [Google Scholar]
- Pokorný, V.; Šifner, F. Book of Insecta; Paseka: Prague, Czeck Republic, 2004; p. 221. [Google Scholar]
- Microsoft SQL Server 2017; Microsoft Corporation: Redmond, WA, USA, 2017.
- Ter Braak, C.J.F.; Šmilauer, P. Canoco Reference Manual and User′s Guide: Software for Ordination, version 5.0; Microcomputer Power: Ithaca, NY, USA, 2012. [Google Scholar]
- Statistica Cz (Softwarový Systém na Analýzu Dat); Version 7; Statsoft, Inc: Tulsa, OK, USA, 2004; Available online: www.StatSoft.Cz (accessed on 1 June 2010).
- Kleijn, D.; Snoeijing, G.I.J. Field boundary vegetation and the effects of agrochemical drift: Botanical change caused by low levels of herbicide and fertilizer. J. Appl. Ecol. 1997, 34, 1413–1425. [Google Scholar] [CrossRef]
- Bote, P.J.; Romero, A.J. Epigeic soil arthropod abundance under different agricultural land uses. Span. J. Agric. Res. 2012, 10, 55–61. [Google Scholar] [CrossRef] [Green Version]
- Magura, T.; Ferrante, M.; Lövei, L.G. Only habitat specialists become smaller with advancing urbanization. Glob. Ecol. Biogeogr. 2020, 29, 1978–1987. [Google Scholar] [CrossRef]
- Lenoir, L.; Lennartsson, T. Effects of timing of grazing on arthropod communities in semi-natural grasslands. J. Insect Sci. 2010, 10, 60. [Google Scholar] [CrossRef] [Green Version]
- Holecová, M.; Lukáš, J.; Haraka′ová, E. Mravce (Hymenoptera, Formicidae) dubovo-hrabových lesov v okolí Bratislavy (JZ Slovensko). Folia Faun. Slov. 2003, 8, 63–69. [Google Scholar]
- Morris, T.; Campos, M. Predatory insects in olive-grove soil. Zool. Baetica 1999, 10, 149–160. [Google Scholar]
- Le Coeur, D.; Baudry, J.; Burel, F. Field margins plant assemblages: Variation partitioning between local and landscapefactors. Landsc. Urban Plan. 1997, 37, 57–71. [Google Scholar] [CrossRef]
- Attwood, S.J.; Maron, M.; House, A.P.N.; Zammit, C. Do arthropod assemblages display globally consistent responses to intensified agricultural land use and management? Glob. Ecol. Biogeogr. 2008, 17, 585–599. [Google Scholar] [CrossRef]
- Langellotto, G.A.; Denno, R.F. Responses of invertebrate natural enemies to complex-structured habitats: A meta-analytical synthesis. Oecologia 2004, 139, 1–10. [Google Scholar] [CrossRef]
- Büchs, W.; Harenberg, A.; Zimmermann, J.; Weiβ, B. Biodiversity, the ultimate agri-environmental indicator? Potential and limits for the application of faunistic elements as gradual indicators in agroecosystems. Agric. Ecosyst. Environ. 2003, 98, 99–123. [Google Scholar] [CrossRef]
- Thomas, C.F.G.; Marshall, E.J. Arthropod abundance and diversity in differently vegetated margins of arable fields. Agric. Ecosyst. Environ. 1999, 72, 131–144. [Google Scholar] [CrossRef]
- MacLeod, A. Attraction and retention of Episyrphus balteatus DeGeer (Diptera: Syrphidae) at an arable field margin with rich and poor floral resources. Agric. Ecosyst. Environ. 1999, 73, 237–244. [Google Scholar] [CrossRef]
- Frank, T. Density of adult hoverflies (Dipt. Syrphidae) in sown weed strips and adjacent fields. J. Appl. Entomol. 1999, 123, 351–355. [Google Scholar] [CrossRef]
- Thorbek, P.; Bilde, T. Reduced numbers of generalist arthropod predators after crop management. J. Appl. Ecol. 2004, 41, 526–538. [Google Scholar] [CrossRef]
- Sharley, D.J.; Hoffmann, A.A.; Thomson, L.J. The effects of soil tillage on beneficial invertebrates within the vineyard. Agric. For. Entomol. 2008, 10, 233–243. [Google Scholar] [CrossRef]
- Lionello, P.; Malanotte-Rizzoli, P.; Boscolo, R. Mediterranean Climate Variability; Elsevier Science: San Diego, CA, USA, 2006; p. 438. [Google Scholar]
- Leather, S.R.; Walters, K.F.A.; Bale, J.S. The Ecology of Insect Overwintering; Cambridge University Press: Cambridge, UK, 1995; p. 268. [Google Scholar]
- Simão, F.C.P.; Carretero, M.A.; Amaral, M.J.A.; Soares, A.M.V.M.; Mateos, E. Composition and seasonal ariation of epigeic arthropods in field margins of NW Portugal. Turk. J. Zool. 2015, 39, 404–411. [Google Scholar] [CrossRef]
- Greenberg, C.H.; McGrane, A. A comparison of relative abundance and biomass of ground-dwelling arthropods under different forest management practices. For. Ecol. Manag. 1996, 89, 31–41. [Google Scholar] [CrossRef]
- Faly, L.I.; Kolombar, T.M.; Prokopenko, E.V.; Pakhomov, O.Y.; Brygadyrenko, V.V. Structure of litter macrofauna communities in poplar plantations in an urban ecosystem in Ukraine. Biosyst. Divers. 2017, 25, 29–38. [Google Scholar] [CrossRef] [Green Version]
- Brygadyrenko, V.V. Community structure of litter invertebrates of forest belt ecosystems in the Ukrainian steppe zone. Int. J. Environ. Res. 2015, 9, 1183–1192. [Google Scholar] [CrossRef]

| Crops/Environmental Variables | Pitfall Traps | ||||
|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | |
| Brassica napus | |||||
| potassium | 14.09 | 16.36 | 16 | 16.88 | 14.38 |
| phosphorus | 1.13 | 1.31 | 1.28 | 1.35 | 1.15 |
| nitrogen | 14.09 | 16.36 | 16 | 16.88 | 14.38 |
| pH | 7.01 | 7.10 | 7.11 | 6.98 | 6.98 |
| moisture | 2 | 2.09 | 1.90 | 2.13 | 2.13 |
| Grass mixture | |||||
| potassium | 8 | 13.50 | 13.50 | 14 | 12.50 |
| phosphorus | 0.64 | 1.08 | 1.08 | 1.12 | 1 |
| nitrogen | 8 | 13.50 | 13.50 | 14 | 12.50 |
| pH | 6.99 | 6.93 | 6.82 | 6.90 | 6.92 |
| moisture | 1.30 | 1.70 | 1.70 | 1.60 | 1.60 |
| Pisum sativum | |||||
| potassium | 11.54 | 14.17 | 12.08 | 13.33 | 15.42 |
| phosphorus | 0.92 | 1.13 | 0.97 | 1.07 | 1.23 |
| nitrogen | 11.54 | 14.17 | 12.08 | 13.33 | 15.42 |
| pH | 7.06 | 6.81 | 6.88 | 6.90 | 6.85 |
| moisture | 1.54 | 1.58 | 1.75 | 1.50 | 1.58 |
| Triticum aestivum | |||||
| potassium | 16.67 | 17.50 | 16.67 | 19.17 | 15 |
| phosphorus | 1.33 | 1.40 | 1.33 | 1.53 | 1.20 |
| nitrogen | 16.67 | 17.50 | 16.67 | 19.17 | 15 |
| pH | 7.02 | 6.95 | 7 | 7 | 6.98 |
| moisture | 1.50 | 1.67 | 1.83 | 1.83 | 1.67 |
| Triticum spelta | |||||
| potassium | 14 | 14 | 10 | 14 | 10 |
| phosphorus | 1.12 | 1.12 | 0.80 | 1.12 | 0.80 |
| nitrogen | 14 | 14 | 10 | 14 | 10 |
| pH | 6.96 | 7 | 7 | 7 | 7.13 |
| moisture | 1.40 | 1.60 | 1.40 | 1.80 | 1.50 |
| Zea mays | |||||
| potassium | 10 | 15.45 | 12.73 | 12.73 | 13.18 |
| phosphorus | 0.80 | 1.24 | 1.02 | 1.02 | 1.05 |
| nitrogen | 10 | 15.45 | 12.73 | 12.73 | 13.18 |
| pH | 7 | 7 | 6.86 | 6.89 | 7.02 |
| moisture | 1.45 | 1.91 | 1.55 | 1.55 | 1.73 |
| Arthropods/Crops | Pitfall Traps | ∑ Ind. | ||||
|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | ||
| Grass mixture/∑ individuals | 3494 | 3940 | 3526 | 3622 | 3800 | 18,382 |
| Araneae | 355 | 356 | 253 | 382 | 353 | 1699 |
| Opilionidea | 3 | 9 | 7 | 24 | 14 | 57 |
| Collembola | 1011 | 1161 | 838 | 835 | 1603 | 5448 |
| Isopoda | 60 | 39 | 56 | 32 | 22 | 209 |
| Julida | 773 | 1062 | 1103 | 1013 | 490 | 4441 |
| Lithobiomorpha | 15 | 27 | 15 | 17 | 13 | 87 |
| Coleoptera | 751 | 833 | 806 | 908 | 752 | 4050 |
| Dermaptera | 0 | 0 | 0 | 2 | 0 | 2 |
| Diptera | 144 | 160 | 172 | 110 | 156 | 742 |
| Hemiptera | 36 | 34 | 37 | 44 | 61 | 212 |
| Hymenoptera | 124 | 67 | 57 | 97 | 51 | 396 |
| Orthoptera | 98 | 112 | 70 | 76 | 58 | 414 |
| Haplotaxida | 25 | 29 | 18 | 10 | 9 | 91 |
| Pisum sativum/∑ individuals | 281 | 287 | 229 | 211 | 148 | 1156 |
| Araneae | 23 | 42 | 20 | 13 | 20 | 118 |
| Isopoda | 9 | 9 | 9 | 16 | 0 | 43 |
| Julida | 31 | 54 | 17 | 17 | 12 | 131 |
| Coleoptera | 175 | 145 | 156 | 150 | 96 | 722 |
| Dermaptera | 12 | 15 | 13 | 1 | 14 | 55 |
| Diptera | 2 | 0 | 0 | 0 | 0 | 2 |
| Hymenoptera | 10 | 22 | 1 | 7 | 6 | 46 |
| Orthoptera | 19 | 0 | 13 | 7 | 0 | 39 |
| Zea mays/∑ individuals | 1426 | 2556 | 1398 | 2565 | 1017 | 8962 |
| Araneae | 15 | 24 | 38 | 10 | 19 | 106 |
| Opilionidea | 33 | 24 | 24 | 21 | 20 | 122 |
| Collembola | 0 | 0 | 3 | 0 | 0 | 3 |
| Isopoda | 123 | 144 | 131 | 121 | 120 | 639 |
| Julida | 21 | 25 | 14 | 0 | 0 | 60 |
| Lithobiomorpha | 21 | 29 | 17 | 16 | 20 | 103 |
| Coleoptera | 1126 | 2178 | 1109 | 2329 | 777 | 7519 |
| Dermaptera | 0 | 1 | 0 | 0 | 0 | 1 |
| Diptera | 54 | 65 | 26 | 14 | 21 | 180 |
| Hymenoptera | 16 | 6 | 16 | 23 | 14 | 75 |
| Orthoptera | 11 | 53 | 13 | 31 | 21 | 129 |
| Haplotaxida | 6 | 7 | 7 | 0 | 5 | 25 |
| Triticum aestivum/∑ individuals | 315 | 256 | 192 | 226 | 145 | 1134 |
| Araneae | 2 | 1 | 2 | 11 | 0 | 16 |
| Isopoda | 0 | 0 | 0 | 2 | 0 | 2 |
| Julida | 34 | 52 | 34 | 59 | 59 | 238 |
| Coleoptera | 225 | 156 | 101 | 124 | 56 | 662 |
| Dermaptera | 10 | 4 | 4 | 12 | 9 | 39 |
| Diptera | 20 | 19 | 14 | 2 | 9 | 64 |
| Hymenoptera | 17 | 0 | 4 | 4 | 0 | 25 |
| Orthoptera | 4 | 23 | 25 | 12 | 12 | 76 |
| Haplotaxida | 3 | 1 | 8 | 0 | 0 | 12 |
| Brassica napus/∑ individuals | 210 | 379 | 151 | 125 | 129 | 994 |
| Araneae | 16 | 22 | 26 | 14 | 17 | 95 |
| Isopoda | 1 | 2 | 1 | 1 | 0 | 5 |
| Julida | 0 | 4 | 2 | 0 | 0 | 6 |
| Lithobiomorpha | 2 | 0 | 0 | 2 | 0 | 4 |
| Coleoptera | 171 | 313 | 106 | 80 | 100 | 770 |
| Diptera | 1 | 12 | 1 | 1 | 4 | 19 |
| Hemiptera | 1 | 2 | 0 | 0 | 0 | 3 |
| Hymenoptera | 6 | 2 | 4 | 23 | 1 | 36 |
| Orthoptera | 12 | 21 | 11 | 4 | 7 | 55 |
| Haplotaxida | 0 | 1 | 0 | 0 | 0 | 1 |
| Triticum spelta/∑ individuals | 113 | 162 | 286 | 117 | 9 | 687 |
| Araneae | 7 | 10 | 14 | 14 | 4 | 49 |
| Isopoda | 10 | 16 | 12 | 6 | 0 | 44 |
| Julida | 2 | 0 | 1 | 0 | 2 | 5 |
| Coleoptera | 85 | 132 | 256 | 97 | 3 | 573 |
| Hymenoptera | 4 | 3 | 0 | 0 | 0 | 7 |
| Orthoptera | 5 | 1 | 3 | 0 | 0 | 9 |
| ∑ individuals from all crops | 5839 | 7580 | 5782 | 6866 | 5248 | 31,315 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Langraf, V.; Petrovičová, K.; Schlarmannová, J. The Composition and Seasonal Variation of Epigeic Arthropods in Different Types of Agricultural Crops and Their Ecotones. Agronomy 2021, 11, 2276. https://doi.org/10.3390/agronomy11112276
Langraf V, Petrovičová K, Schlarmannová J. The Composition and Seasonal Variation of Epigeic Arthropods in Different Types of Agricultural Crops and Their Ecotones. Agronomy. 2021; 11(11):2276. https://doi.org/10.3390/agronomy11112276
Chicago/Turabian StyleLangraf, Vladimír, Kornélia Petrovičová, and Janka Schlarmannová. 2021. "The Composition and Seasonal Variation of Epigeic Arthropods in Different Types of Agricultural Crops and Their Ecotones" Agronomy 11, no. 11: 2276. https://doi.org/10.3390/agronomy11112276






