Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review
Abstract
:1. Introduction
2. Amount and Characteristics of Po in Soil and Organic Inputs
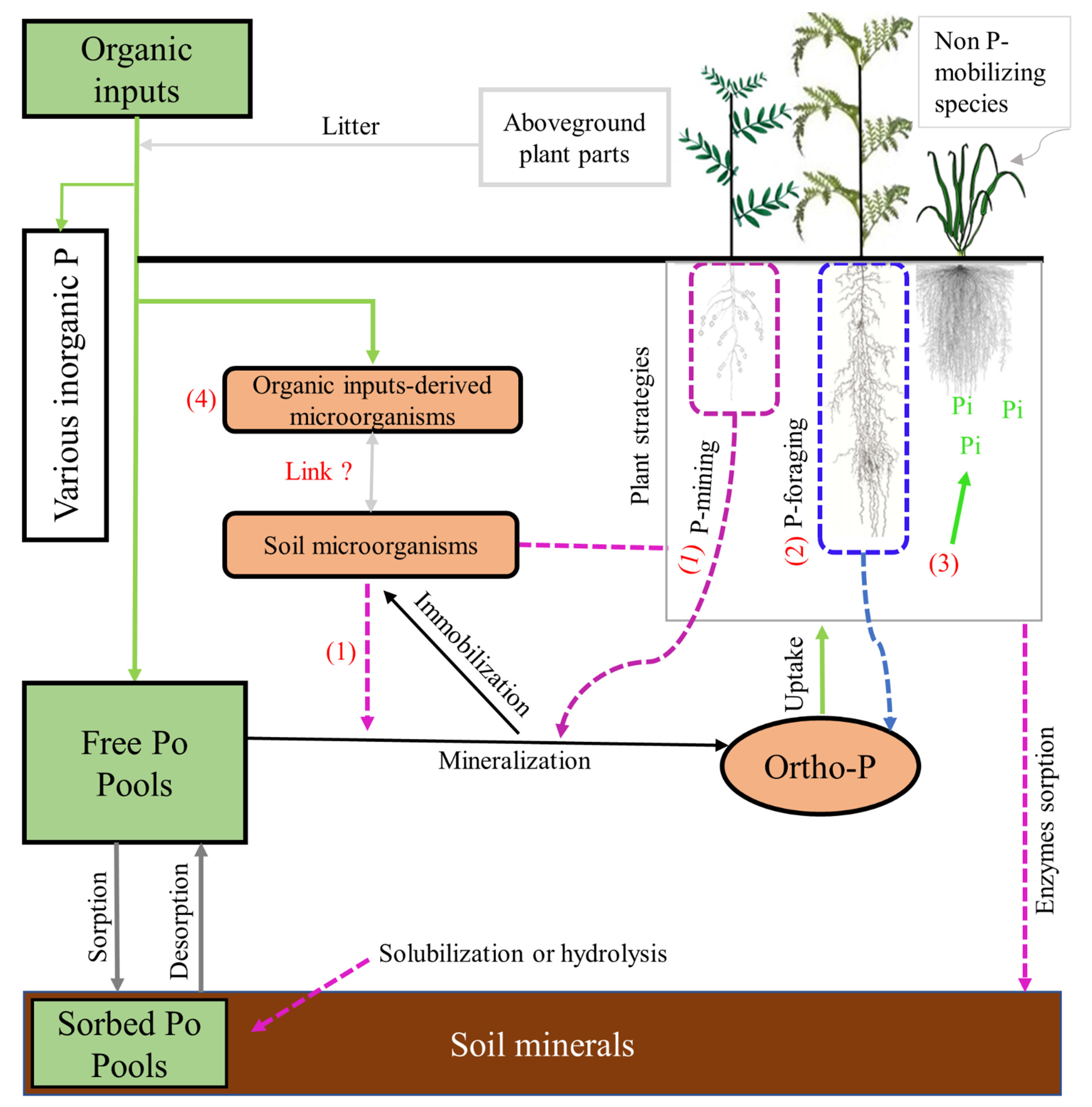
3. Organic Phosphorus Dynamics in Rhizosphere
3.1. Soil Microbial Processes Involved in Po Mobilization
3.2. Root Mechanisms Involved in the Fate of Po Forms
4. Approaches/Strategies to Improve Po-Use Efficiency
4.1. Understand and Manage Plant Traits, Root-Associated Microorganism and Po Pool Interactions to Characterize P Dynamics and P Availability
4.2. Development of New Cropping Systems to Recycle P from Po Pools
4.2.1. Cover Crops and Po Availability
4.2.2. Management of Po Inputs in Crop Rotations
5. Future Prospects
6. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Jarvie, H.P.; Flaten, D.; Sharpley, A.N.; Kleinman, P.J.; Healy, M.G.; King, S.M. Future Phosphorus: Advancing New 2D Phosphorus Allotropes and Growing a Sustainable Bioeconomy. J. Environ. Qual. 2019, 48, 1145–1155. [Google Scholar] [CrossRef] [Green Version]
- Withers, P.J.A.; van Dijk, K.C.; Neset, T.-S.S.; Nesme, T.; Oenema, O.; Rubæk, G.H.; Schoumans, O.F.; Smit, B.; Pellerin, S. Stewardship to Tackle Global Phosphorus Inefficiency: The Case of Europe. AMBIO 2015, 44, 193–206. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippelli, G.M. The Global Phosphorus Cycle: Past, Present, and Future. Elements 2008, 4, 89–95. [Google Scholar] [CrossRef]
- Schoumans, O.F.; Bouraoui, F.; Kabbe, C.; Oenema, O.; van Dijk, K.C. Phosphorus Management in Europe in a Changing World. AMBIO 2015, 44, 180–192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cordell, D.; Drangert, J.-O.; White, S. The Story of Phosphorus: Global Food Security and Food for Thought. Glob. Environ. Chang. 2009, 19, 292–305. [Google Scholar] [CrossRef]
- Bennett, E.M.; Schipanski, M.E. The Phosphorus Cycle. In Fundamentals of Ecosystem Science; Elsevier: Amsterdam, The Netherlands, 2013; pp. 159–178. ISBN 978-0-12-088774-3. [Google Scholar]
- McCormick, K.; Kautto, N. The Bioeconomy in Europe: An Overview. Sustainability 2013, 5, 2589–2608. [Google Scholar] [CrossRef] [Green Version]
- Zulkifli, A.A.; Mohd Yusoff, M.Z.; Abd Manaf, L.; Zakaria, M.R.; Roslan, A.M.; Ariffin, H.; Shirai, Y.; Hassan, M.A. Assessment of Municipal Solid Waste Generation in Universiti Putra Malaysia and Its Potential for Green Energy Production. Sustainability 2019, 11, 3909. [Google Scholar] [CrossRef] [Green Version]
- Bracco, S.; Calicioglu, O.; Gomez San Juan, M.; Flammini, A. Assessing the Contribution of Bioeconomy to the Total Economy: A Review of National Frameworks. Sustainability 2018, 10, 1698. [Google Scholar] [CrossRef] [Green Version]
- Scarlat, N.; Dallemand, J.-F.; Monforti-Ferrario, F.; Nita, V. The Role of Biomass and Bioenergy in a Future Bioeconomy: Policies and Facts. Environ. Dev. 2015, 15, 3–34. [Google Scholar] [CrossRef]
- Mohanty, A.K.; Misra, M.; Drzal, L.T. [Sustainable Bio-Composites from Renewable Resources: Opportunities and Challenges in the Green Materials World]. J. Polym. Environ. 2002, 10, 19–26. [Google Scholar] [CrossRef]
- Houot, S. Intérêts et Limites de La Substitution Par La Fertilisation Organique. In Proceedings of the Fertilisation et Fertilité des Sols, Paris, France, 7 March 2018. [Google Scholar]
- Houot, S.; Pons, M.-N.; Pradel, M.; Tibi, A. Recyclage de Déchets Organiques En Agriculture: Effets Agronomiques et Environnementaux de Leur Épandage; Matière à débattre et décider; QUAE: Paris, France, 2016. [Google Scholar]
- Faucon, M.-P.; Houben, D.; Reynoird, J.-P.; Mercadal-Dulaurent, A.-M.; Armand, R.; Lambers, H. Advances and Perspectives to Improve the Phosphorus Availability in Cropping Systems for Agroecological Phosphorus Management. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2015; Volume 134, pp. 51–79. ISBN 978-0-12-803323-4. [Google Scholar]
- Harrison, A.F. Soil Organic Phosphorus: A Review of World Literature; CAB International ©1987: Wallingford, UK, 1987; ISBN 978-0-85198-589-3. [Google Scholar]
- Andrino, A.; Boy, J.; Mikutta, R.; Sauheitl, L.; Guggenberger, G. Carbon Investment Required for the Mobilization of Inorganic and Organic Phosphorus Bound to Goethite by an Arbuscular Mycorrhiza (Solanum Lycopersicum × Rhizophagus Irregularis). Front. Environ. Sci. 2019, 7, 26. [Google Scholar] [CrossRef] [Green Version]
- Kahiluoto, H.; Kuisma, M.; Ketoja, E.; Salo, T.; Heikkinen, J. Phosphorus in Manure and Sewage Sludge More Recyclable than in Soluble Inorganic Fertilizer. Environ. Sci. Technol. 2015, 49, 2115–2122. [Google Scholar] [CrossRef] [Green Version]
- Sulieman, S.; Mühling, K.H. Utilization of Soil Organic Phosphorus as a Strategic Approach for Sustainable Agriculture. J. Plant Nutr. Soil Sci. 2021, 184, 311–319. [Google Scholar] [CrossRef]
- Sulieman, S.; Tran, L.-S.P. Phosphorus Homeostasis in Legume Nodules as an Adaptive Strategy to Phosphorus Deficiency. Plant Sci. 2015, 239, 36–43. [Google Scholar] [CrossRef]
- Zogli, P.; Pingault, L.; Libault, M. Physiological and Molecular Mechanisms and Adaptation Strategies in Soybean (Glycine max) Under Phosphate Deficiency. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability: Adaptation and Regulatory Implication; Sulieman, S., Tran, L.-S.P., Eds.; Springer International Publishing: Cham, Switzerland, 2017; pp. 219–242. ISBN 978-3-319-55729-8. [Google Scholar]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Dixon, L.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Haygarth, P.M. Land Use and Soil Factors Affecting Accumulation of Phosphorus Species in Temperate Soils. Geoderma 2015, 257–258, 29–39. [Google Scholar] [CrossRef]
- Nash, D.M.; Haygarth, P.M.; Turner, B.L.; Condron, L.M.; McDowell, R.W.; Richardson, A.E.; Watkins, M.; Heaven, M.W. Using Organic Phosphorus to Sustain Pasture Productivity: A Perspective. Geoderma 2014, 221–222, 11–19. [Google Scholar] [CrossRef]
- Turner, B.L.; Papházy, M.J.; Haygarth, P.M.; Mckelvie, I.D. Inositol Phosphates in the Environment. Phil. Trans. R. Soc. Lond. B 2002, 357, 449–469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vincent, A.G.; Vestergren, J.; Grobner, G.; Persson, P.; Schleucher, J.; Giesler, R. Soil Organic Phosphorus Transformations in a Boreal Forest Chronosequence. Plant Soil 2013, 367, 149–162. [Google Scholar] [CrossRef]
- Vestergren, J.; Vincent, A.G.; Jansson, M.; Persson, P.; Ilstedt, U.; Gröbner, G.; Giesler, R.; Schleucher, J. High-Resolution Characterization of Organic Phosphorus in Soil Extracts Using 2D 1H–31P NMR Correlation Spectroscopy. Environ. Sci. Technol. 2012, 46, 3950–3956. [Google Scholar] [CrossRef]
- Newman, R.H.; Tate, K.R. Soil Phosphorus Characterisation by 31 p Nuclear Magnetic Resonance. Commun. Soil Sci. Plant Anal. 1980, 11, 835–842. [Google Scholar] [CrossRef]
- Doolette, A.; Smernik, R.J.; Dougherty, W. Spiking Improved Solution Phosphorus31 Nuclear Magnetic Resonance Identification of Soil Phosphorus Compounds. Soil Sci. Soc. Am. J. 2009, 73, 919–927. [Google Scholar] [CrossRef]
- Doolette, A.L.; Smernik, R.J.; Dougherty, W.J. Overestimation of the Importance of Phytate in NaOH–EDTA Soil Extracts as Assessed by 31P NMR Analyses. Org. Geochem. 2011, 42, 955–964. [Google Scholar] [CrossRef]
- Cui, Y.; Barford, J.P.; Renneberg, R. Development of a Glucose-6-Phosphate Biosensor Based on Coimmobilized p-Hydroxybenzoate Hydroxylase and Glucose-6-Phosphate Dehydrogenase. Biosens. Bioelectron. 2007, 22, 2754–2758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hogema, B.M.; Arents, J.C.; Inada, T.; Aiba, H.; van Dam, K.; Postma, P.W. Catabolite Repression by Glucose 6-Phosphate, Gluconate and Lactose in Escherichia Coli. Mol. Microbiol. 1997, 24, 857–867. [Google Scholar] [CrossRef] [Green Version]
- Richardson, A.E.; Simpson, R.J. Soil Microorganisms Mediating Phosphorus Availability: Phosphorus plant physiology. Plant Physiol. 2011, 156, 989–996. [Google Scholar] [CrossRef] [Green Version]
- Bünemann, E.K.; Condron, L.M. Phosphorus and sulphur cycling in terrestrial ecosystems. In Nutrient Cycling in Terrestrial Ecosystems; Springer: Berlin/Heidelberg, Germany, 2007; pp. 65–92. [Google Scholar]
- Lazali, M.; Brahimi, S.; Benadis, C.; Drevon, J.J. Stratégies et mécanismes d’adaptation des légumineuses à la faible disponibilité des sols en phosphore. Rev. Maroc. Sci. Agron. Vétérinaires 2020, 8, 294–300. [Google Scholar]
- Richardson, A.E. Utilization of Soil Organic Phosphorus by Higher Plants. Org. Phosphorus Environ. 2005, 139, 165–184. [Google Scholar] [CrossRef]
- Margenot, A.J.; Sommer, R.; Mukalama, J.; Parikh, S.J. Biological P Cycling Is Influenced by the Form of P Fertilizer in an Oxisol. Biol. Fertil. Soils 2017, 53, 899–909. [Google Scholar] [CrossRef]
- Skene, K.R. Pattern Formation in Cluster Roots: Some Developmental and Evolutionary Considerations. Ann. Bot. 2000, 85, 901–908. [Google Scholar] [CrossRef] [Green Version]
- Jain, A.; Vasconcelos, M.J.; Raghothama, K.G.; Sahi, S.V. Molecular Mechanisms of Plant Adaptation to Phosphate Deficiency. Plant Breed. Rev. 2007, 29, 359. [Google Scholar]
- Jones, D.L.; Hodge, A.; Kuzyakov, Y. Plant and Mycorrhizal Regulation of Rhizodeposition. New Phytol. 2004, 163, 459–480. [Google Scholar] [CrossRef] [PubMed]
- Lazali, M.; Bargaz, A. Examples of belowground mechanisms enabling legumes to mitigate phosphorus deficiency. In Legume Nitrogen Fixation in Soils with Low Phosphorus Availability; Springer: Berlin/Heidelberg, Germany, 2017; pp. 135–152. [Google Scholar]
- Fuentes, B.; de la Luz Mora, M.; Bolan, N.S.; Naidu, R. Chapter 16 Assessment of phosphorus bioavailability from organic wastes in soil. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2008; Volume 32, pp. 363–411. ISBN 978-0-444-52169-9. [Google Scholar]
- Scherer, H.; Sharma, S. Phosphorus Fractions and Phosphorus Delivery Potential of a Luvisol Derived from Loess Amended with Organic Materials. Biol. Fertil. Soils 2002, 35, 414–419. [Google Scholar]
- Monbet, P.; McKelvie, I.D.; Worsfold, P.J. Dissolved Organic Phosphorus Speciation in the Waters of the Tamar Estuary (SW England). Geochim. Cosmochim. Acta 2009, 73, 1027–1038. [Google Scholar] [CrossRef]
- Ruttenberg, K.C.; Sulak, D.J. Sorption and Desorption of Dissolved Organic Phosphorus onto Iron (Oxyhydr)Oxides in Seawater. Geochim. Cosmochim. Acta 2011, 75, 4095–4112. [Google Scholar] [CrossRef]
- Anderson, G.; Williams, E.G.; Moir, J.O. A Comparison of the Sorption of Inorganic Orthophosphate and Inositol Hexaphosphate by Six Acid Soils. J. Soil Sci. 1974, 25, 51–62. [Google Scholar] [CrossRef]
- Anderson, G. Assessing Organic Phosphorus in Soils. Role Phosphorus Agric. 1980, 234, 411–431. [Google Scholar] [CrossRef]
- Dalai, R.C. Soil Organic Phosphorus. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 1977; Volume 29, pp. 83–117. ISBN 978-0-12-000729-5. [Google Scholar]
- Turner, B.L.; McKelvie, I.D.; Haygarth, P.M. Characterisation of Water-Extractable Soil Organic Phosphorus by Phosphatase Hydrolysis. Soil Biol. Biochem. 2002, 34, 27–35. [Google Scholar] [CrossRef]
- Hu, Z.; Jaisi, D.P.; Yan, Y.; Chen, H.; Wang, X.; Wan, B.; Liu, F.; Tan, W.; Huang, Q.; Feng, X. Adsorption and Precipitation of Myo -Inositol Hexakisphosphate onto Kaolinite. Eur. J. Soil Sci. 2020, 71, 226–235. [Google Scholar] [CrossRef]
- Darch, T.; Blackwell, M.S.A.; Hawkins, J.M.B.; Haygarth, P.M.; Chadwick, D. A Meta-Analysis of Organic and Inorganic Phosphorus in Organic Fertilizers, Soils, and Water: Implications for Water Quality. Crit. Rev. Environ. Sci. Technol. 2014, 44, 2172–2202. [Google Scholar] [CrossRef]
- Ganta, P.B.; Kühn, O.; Ahmed, A.A. QM/MM Simulations of Organic Phosphorus Adsorption at the Diaspore–Water Interface. Phys. Chem. Chem. Phys. 2019, 21, 24316–24325. [Google Scholar] [CrossRef]
- Granger, S.J.; Bol, R.; Anthony, S.; Owens, P.N.; White, S.M.; Haygarth, P.M. Towards a Holistic Classification of Diffuse Agricultural Water Pollution from Intensively Managed Grasslands on Heavy Soils. Adv. Agron. 2010, 105, 83–115. [Google Scholar]
- Ignatiades, L.; Gotsis-Skretas, O. A Review on Toxic and Harmful Algae in Greek Coastal Waters (E. Mediterranean Sea). Toxins 2010, 2, 1019–1037. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jarosch, K.A.; Doolette, A.L.; Smernik, R.J.; Tamburini, F.; Frossard, E.; Bünemann, E.K. Characterisation of Soil Organic Phosphorus in NaOH-EDTA Extracts: A Comparison of 31P NMR Spectroscopy and Enzyme Addition Assays. Soil Biol. Biochem. 2015, 91, 298–309. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, W.; Liu, S.; He, Z.; Zhao, X.; Liu, Y.; Guo, J.; Giesy, J.P.; Wu, F. Bioavailability and Preservation of Organic Phosphorus in Lake Sediments: Insights from Enzymatic Hydrolysis and 31P Nuclear Magnetic Resonance. Chemosphere 2018, 211, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L.; Cade-Menun, B.J.; Westermann, D.T. Organic Phosphorus Composition and Potential Bioavailability in Semi-Arid Arable Soils of the Western United States. Soil Sci. Soc. Am. J. 2003, 67, 1168–1179. [Google Scholar] [CrossRef] [Green Version]
- Guggenberger, G.; Christensen, B.T.; Rubaek, G.; Zech, W. Land-Use and Fertilization Effects on P Forms in Two European Soils: Resin Extraction and 31P-NMR Analysis. Eur. J. Soil Sci. 1996, 47, 605–614. [Google Scholar] [CrossRef]
- Toor, G.S.; Hunger, S.; Peak, J.D.; Sims, J.T.; Sparks, D.L. Advances in the Characterization of Phosphorus in Organic Wastes: Environmental and Agronomic Applications. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2006; Volume 89, pp. 1–72. ISBN 978-0-12-000807-0. [Google Scholar]
- Toor, G.S.; Cade-Menun, B.J.; Sims, J.T. Establishing a Linkage between Phosphorus Forms in Dairy Diets, Feces, and Manures. J. Environ. Qual. 2005, 34, 1380–1391. [Google Scholar] [CrossRef]
- He, Z.; Cade-Menun, B.J.; Toor, G.S.; Fortuna, A.-M.; Honeycutt, C.W.; Sims, J.T. Comparison of Phosphorus Forms in Wet and Dried Animal Manures by Solution Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy and Enzymatic Hydrolysis. J. Environ. Qual. 2007, 36, 1086–1095. [Google Scholar] [CrossRef] [PubMed]
- He, Z.; Honeycutt, C.W.; Griffin, T.S.; Cade-Menun, B.J.; Pellechia, P.J.; Dou, Z. Phosphorus Forms in Conventional and Organic Dairy Manure Identified by Solution and Solid State P-31 NMR Spectroscopy. J. Environ. Qual. 2009, 38, 1909–1918. [Google Scholar] [CrossRef]
- Bol, R.; Amelung, W.; Haumaier, L. Phosphorus-31–Nuclear Magnetic–Resonance Spectroscopy to Trace Organic Dung Phosphorus in a Temperate Grassland Soil. J. Plant Nutr. Soil Sci. 2006, 169, 69–75. [Google Scholar] [CrossRef]
- Hansen, J.; Cade-Menun, B.; Strawn, D. Phosphorus Speciation in Manure-Amended Alkaline Soils. J. Environ. Qual. 2004, 33, 1521–1527. [Google Scholar] [CrossRef] [PubMed]
- Turner, B.L. Optimizing Phosphorus Characterization in Animal Manures by Solution Phosphorus-31 Nuclear Magnetic Resonance Spectroscopy. J. Environ. Qual. 2004, 33, 10. [Google Scholar] [CrossRef]
- Pagliari, P.H.; Laboski, C.A.M. Investigation of the Inorganic and Organic Phosphorus Forms in Animal Manure. J. Environ. Qual. 2012, 41, 901–910. [Google Scholar] [CrossRef]
- Li, G.; Li, H.; Leffelaar, P.A.; Shen, J.; Zhang, F. Characterization of Phosphorus in Animal Manures Collected from Three (Dairy, Swine, and Broiler) Farms in China. PLoS ONE 2014, 9, e102698. [Google Scholar] [CrossRef] [Green Version]
- McDowell, R.W.; Dou, Z.; Toth, J.D.; Cade-Menun, B.J.; Kleinman, P.J.A.; Soder, K.; Saporito, L. A Comparison of Phosphorus Speciation and Potential Bioavailability in Feed and Feces of Different Dairy Herds Using 31P Nuclear Magnetic Resonance Spectroscopy. J. Environ. Qual. 2008, 37, 741–752. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bünemann, E.K.; Heenan, D.P.; Marschner, P.; McNeill, A.M. Long-Term Effects of Crop Rotation, Stubble Management and Tillage on Soil Phosphorus Dynamics. Soil Res. 2006, 44, 611. [Google Scholar] [CrossRef]
- Koopmans, G.F.; Chardon, W.J.; McDowell, R.W. Phosphorus Movement and Speciation in a Sandy Soil Profile after Long-Term Animal Manure Applications. J. Environ. Qual. 2007, 36, 305–315. [Google Scholar] [CrossRef]
- Murphy, P.N.C.; Bell, A.; Turner, B.L. Phosphorus Speciation in Temperate Basaltic Grassland Soils by Solution 31P NMR Spectroscopy. Eur. J. Soil Sci. 2009, 60, 638–651. [Google Scholar] [CrossRef]
- Turner, B.L. Storage-Induced Changes in Phosphorus Solubility of Air-Dried Soils. Soil Sci. Soc. Am. J. 2005, 69, 630–633. [Google Scholar] [CrossRef] [Green Version]
- McDowell, R.W.; Condron, L.M.; Stewart, I.; Cave, V. Chemical Nature and Diversity of Phosphorus in New Zealand Pasture Soils Using 31 P Nuclear Magnetic Resonance Spectroscopy and Sequential Fractionation. Nutr. Cycl. Agroecosyst. 2005, 72, 241–254. [Google Scholar] [CrossRef]
- Hawkes, G.E.; Powlson, D.S.; Randall, E.W.; Tate, K.R. A 31P Nuclear Magnetic Resonance Study of the Phosphorus Species in Alkali Extracts of Soils from Long-Term Field Experiments. J. Soil Sci. 1984, 35, 35–45. [Google Scholar] [CrossRef]
- Condron, L.M.; Turner, B.L.; Cade-Menun, B.J. Chemistry and Dynamics of Soil Organic Phosphorus. In Phosphorus: Agriculture and the Environment; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2005; pp. 87–121. ISBN 978-0-89118-269-6. [Google Scholar]
- Cheesman, A.W.; Turner, B.L.; Reddy, K.R. Forms of Organic Phosphorus in Wetland Soils. Biogeosci. Discuss. 2014, 11, 8569–8605. [Google Scholar] [CrossRef] [Green Version]
- Cade-Menun, B.J. Characterizing Phosphorus Forms in Cropland Soils with Solution 31P-NMR: Past Studies and Future Research Needs. Chem. Biol. Technol. Agric. 2017, 4, 19. [Google Scholar] [CrossRef]
- Roberts, T.L.; Johnston, A.E. Phosphorus Use Efficiency and Management in Agriculture. Resour. Conserv. Recycl. 2015, 105, 275–281. [Google Scholar] [CrossRef]
- He, Z.; Griffin, T.S.; Honeycutt, C.W. Enzymatic Hydrolysis of Organic Phosphorus in Swine Manure and Soil. J. Environ. Qual. 2004, 33, 367–372. [Google Scholar] [CrossRef] [PubMed]
- Gahoonia, T.S.; Nielsen, N.E. Root Traits as Tools for Creating Phosphorus Efficient Crop Varieties. Plant Soil 2004, 260, 47–57. [Google Scholar] [CrossRef]
- Chen, C.R.; Condron, L.M.; Davis, M.R.; Sherlock, R.R. Effects of Plant Species on Microbial Biomass Phosphorus and Phosphatase Activity in a Range of Grassland Soils. Biol. Fertil. Soils 2004, 40, 313–322. [Google Scholar] [CrossRef]
- Jakobsen, I.; Leggett, M.E.; Richardson, A.E. Rhizosphere Microorganisms and Plant Phosphorus Uptake. In Agronomy Monographs; Thomas Sims, J., Sharpley, A.N., Eds.; American Society of Agronomy; Crop Science Society of America; Soil Science Society of America: Madison, WI, USA, 2015; pp. 437–494. ISBN 978-0-89118-269-6. [Google Scholar]
- Hinsinger, P.; Herrmann, L.; Lesueur, D.; Robin, A.; Trap, J.; Waithaisong, K.; Plassard, C. Impact of roots, microorganisms and microfauna on the fate of soil phosphorus in the rhizosphere. In Annual Plant Reviews; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; Volume 48, pp. 375–407. ISBN 978-1-118-95884-1. [Google Scholar]
- Tian, J.; Ge, F.; Zhang, D.; Deng, S.; Liu, X. Roles of Phosphate Solubilizing Microorganisms from Managing Soil Phosphorus Deficiency to Mediating Biogeochemical P Cycle. Biology 2021, 10, 158. [Google Scholar] [CrossRef]
- Gaiero, J.R.; Bent, E.; Fraser, T.D.; Condron, L.M.; Dunfield, K.E. Validating Novel Oligonucleotide Primers Targeting Three Classes of Bacterial Non-Specific Acid Phosphatase Genes in Grassland Soils. Plant Soil 2018, 427, 39–51. [Google Scholar] [CrossRef]
- Annaheim, E.; Frossar, E.; Bünemann, E.K. Characterisation of Organic Phosphorus Compounds in Soil by Phosphatase Hydrolysis. In Proceedings of the 19th World Congress of Soil Science, Soil Solutions for a Changing World, Brisbane, Australia, 1–6 August 2010; pp. 9–11. [Google Scholar]
- Richardson, A.E.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; Smith, S.E.; Harvey, P.R.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; et al. Plant and Microbial Strategies to Improve the Phosphorus Efficiency of Agriculture. Plant Soil 2011, 349, 121–156. [Google Scholar] [CrossRef]
- Garcia-Lopez, A.M.; Aviles, M.; Delgado, A. Plant Uptake of Phosphorus from Sparingly Available P- Sources as Affected by Trichoderma Asperellum T34. Agric. Food Sci. 2015, 24, 249–260. [Google Scholar] [CrossRef]
- Garcia-Lopez, A.M.; Delgado, A. Effect of Bacillus Subtilis on Phosphorus Uptake by Cucumber as Affected by Iron Oxides and the Solubility of the Phosphorus Source. Agric. Food Sci. 2016, 25, 216–224. [Google Scholar] [CrossRef]
- García-López, A.M.; Recena, R.; Delgado, A. The Adsorbent Capacity of Growing Media Does Not Constrain Myo-Inositol Hexakiphosphate Hydrolysis but Its Use as a Phosphorus Source by Plants. Plant Soil 2020. [Google Scholar] [CrossRef]
- Stutter, M.I.; Shand, C.A.; George, T.S.; Blackwell, M.S.A.; Bol, R.; MacKay, R.L.; Richardson, A.E.; Condron, L.M.; Turner, B.L.; Haygarth, P.M. Recovering Phosphorus from Soil: A Root Solution? Environ. Sci. Technol. 2012, 46, 1977–1978. [Google Scholar] [CrossRef]
- Giles, C.; Cade-Menun, B.; Hill, J. The Inositol Phosphates in Soils and Manures: Abundance, Cycling, and Measurement. Can. J. Soil. Sci. 2011, 91, 397–416. [Google Scholar] [CrossRef]
- Celi, L.; Prati, M.; Magnacca, G.; Santoro, V.; Martin, M. Role of Crystalline Iron Oxides on Stabilization of Inositol Phosphates in Soil. Geoderma 2020, 374, 114442. [Google Scholar] [CrossRef]
- George, T.S.; Gregory, P.J.; Hocking, P.; Richardson, A.E. Variation in Root-Associated Phosphatase Activities in Wheat Contributes to the Utilization of Organic P Substrates in Vitro, but Does Not Explain Differences in the P-Nutrition of Plants When Grown in Soils. Environ. Exp. Bot. 2008, 64, 239–249. [Google Scholar] [CrossRef]
- Cosgrove, D.J. The Chemical Nature of Soil Organic Phosphorus. I. Inositol Phosphates. Soil Res. 1963, 1, 203–214. [Google Scholar] [CrossRef]
- Lung, S.-C.; Leung, A.; Kuang, R.; Wang, Y.; Leung, P.; Lim, B.-L. Phytase Activity in Tobacco (Nicotiana tabacum) Root Exudates Is Exhibited by a Purple Acid Phosphatase. Phytochemistry 2008, 69, 365–373. [Google Scholar] [CrossRef]
- George, T.S.; Simpson, R.J.; Gregory, P.J.; Richardson, A.E. Differential Interaction of Aspergillus Niger and Peniophora Lycii Phytases with Soil Particles Affects the Hydrolysis of Inositol Phosphates. Soil Biol. Biochem. 2007, 39, 793–803. [Google Scholar] [CrossRef]
- Annaheim, K.E.; Rufener, C.B.; Frossard, E.; Bünemann, E.K. Hydrolysis of Organic Phosphorus in Soil Water Suspensions after Addition of Phosphatase Enzymes. Biol. Fertil. Soils 2013, 49, 1203–1213. [Google Scholar] [CrossRef] [Green Version]
- Annaheim, K.E.; Doolette, A.L.; Smernik, R.J.; Mayer, J.; Oberson, A.; Frossard, E.; Bünemann, E.K. Long-term addition of organic fertilizers has little effect on soil organic phosphorus as characterized by 31P NMR spectroscopy and enzyme additions. Geoderma 2015, 257, 67–77. [Google Scholar]
- He, Z.; Honeycutt, C.W. Enzymatic Characterization of Organic Phosphorus in Animal Manure. J. Environ. Qual. 2001, 30, 1685–1692. [Google Scholar] [CrossRef] [PubMed]
- Wyss, M.; Brugger, R.; Kronenberger, A.; Rémy, R.; Fimbel, R.; Oesterhelt, G.; Lehmann, M.; van Loon, A.P.G.M. Biochemical Characterization of Fungal Phytases (Myo-Inositol Hexakisphosphate Phosphohydrolases): Catalytic Properties. Appl. Environ. Microbiol. 1999, 65, 367–373. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marlida, Y.; Delfita, R.; Adnadi, P.; Ciptaan, G. Isolation, Characterization and Production of Phytase from Endophytic Fungus Its Application for Feed. Pak. J. Nutr. 2010, 9, 471–474. [Google Scholar] [CrossRef] [Green Version]
- Kim, Y.-O.; Lee, J.-K.; Kim, H.-K.; Yu, J.-H.; Oh, T.-K. Cloning of the Thermostable Phytase Gene (Phy) from Bacillus sp. DS11 and Its Overexpression in Escherichia coli. FEMS Microbiol. Lett. 1998, 162, 185–191. [Google Scholar] [CrossRef] [Green Version]
- Konietzny, U.; Greiner, R. Molecular and Catalytic Properties of Phytate-Degrading Enzymes (Phytases). Int. J. Food Sci. Technol. 2002, 37, 791–812. [Google Scholar] [CrossRef] [Green Version]
- Scott, J.J. Alkaline Phytase Activity in Nonionic Detergent Extracts of Legume Seeds. Plant Physiol. 1991, 95, 1298–1301. [Google Scholar] [CrossRef]
- Azeem, M.; Riaz, A.; Chaudhary, A.N.; Hayat, R.; Hussain, Q.; Tahir, M.I.; Imran, M. Microbial Phytase Activity and Their Role in Organic P Mineralization. Arch. Agron. Soil Sci. 2015, 61, 751–766. [Google Scholar] [CrossRef]
- White, C.; Sayer, J.A.; Gadd, G.M. Microbial Solubilization and Immobilization of Toxic Metals: Key Biogeochemical Processes for Treatment of Contamination. FEMS Microbiol. Rev. 1997, 20, 503–516. [Google Scholar] [CrossRef]
- Chauhan, B.S.; Mahajan, G.; Randhawa, R.K.; Singh, H.; Kang, M.S. Global Warming and Its Possible Impact on Agriculture in India. In Advances in Agronomy; Elsevier: Amsterdam, The Netherlands, 2014; Volume 123, pp. 65–121. ISBN 978-0-12-420225-2. [Google Scholar]
- Alori, E.T.; Glick, B.R.; Babalola, O.O. Microbial Phosphorus Solubilization and Its Potential for Use in Sustainable Agriculture. Front. Microbiol. 2017, 8, 971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fasim, F.; Ahmed, N.; Parsons, R.; Gadd, G.M. Solubilization of Zinc Salts by a Bacterium Isolated from the Air Environment of a Tannery. FEMS Microbiol. Lett. 2002, 213, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Nautiyal, C.S.; Bhadauria, S.; Kumar, P.; Lal, H.; Mondal, R.; Verma, D. Stress Induced Phosphate Solubilization in Bacteria Isolated from Alkaline Soils. FEMS Microbiol. Lett. 2000, 182, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Johri, A.K.; Oelmüller, R.; Dua, M.; Yadav, V.; Kumar, M.; Tuteja, N.; Varma, A.; Bonfante, P.; Persson, B.L.; Stroud, R.M. Fungal Association and Utilization of Phosphate by Plants: Success, Limitations, and Future Prospects. Front. Microbiol. 2015, 6. [Google Scholar] [CrossRef] [Green Version]
- Park, I.; Lee, J.; Cho, J. Degradation of Phytate Pentamagnesium Salt by Bacillus sp. T4 Phytase as a Potential Eco-Friendly Feed Additive. Asian-Australas. J. Anim. Sci. 2012, 25, 1466–1472. [Google Scholar] [CrossRef] [Green Version]
- Johnson, S.C.; Yang, M.; Murthy, P.P.N. Heterologous Expression and Functional Characterization of a Plant Alkaline Phytase in Pichia Pastoris. Protein Expr. Purif. 2010, 74, 196–203. [Google Scholar] [CrossRef]
- Tang, J.; Leung, A.; Leung, C.; Lim, B.L. Hydrolysis of Precipitated Phytate by Three Distinct Families of Phytases. Soil Biol. Biochem. 2006, 38, 1316–1324. [Google Scholar] [CrossRef] [Green Version]
- Lim, B.L.; Yeung, P.; Cheng, C.; Hill, J.E. Distribution and Diversity of Phytate-Mineralizing Bacteria. ISME J. 2007, 1, 321–330. [Google Scholar] [CrossRef]
- Adhya, T.K.; Kumar, N.; Reddy, G.; Podile, A.R.; Bee, H.; Samantaray, B. Microbial Mobilization of Soil Phosphorus and Sustainable P Management in Agricultural Soils. Sustain. Phosphorus Manag. 2015, 108, 8. [Google Scholar]
- Zimmermann, P.; Zardi, G.; Lehmann, M.; Zeder, C.; Amrhein, N.; Frossard, E.; Bucher, M. Engineering the Root-Soil Interface via Targeted Expression of a Synthetic Phytase Gene in Trichoblasts. Plant Biotechnol. J. 2003, 1, 353–360. [Google Scholar] [CrossRef]
- Lung, S.-C.; Lim, B. Assimilation of Phytate-Phosphorus by the Extracellular Phytase Activity of Tobacco (Nicotiana tabacum) Is Affected by the Availability of Soluble Phytate. Plant Soil 2006, 279, 187–199. [Google Scholar] [CrossRef] [Green Version]
- Allison, V.J.; Condron, L.M.; Peltzer, D.A.; Richardson, S.J.; Turner, B.L. Changes in Enzyme Activities and Soil Microbial Community Composition along Carbon and Nutrient Gradients at the Franz Josef Chronosequence, New Zealand. Soil Biol. Biochem. 2007, 39, 1770–1781. [Google Scholar] [CrossRef]
- Wallenstein, M.D.; McMahon, S.K.; Schimel, J.P. Seasonal Variation in Enzyme Activities and Temperature Sensitivities in Arctic Tundra Soils. Glob. Chang. Biol. 2009, 15, 1631–1639. [Google Scholar] [CrossRef]
- Fitriatin, B.N.; Joy, B.; Subroto, T. The Influence of Organic Phosphorous Substrate on Phosphatase Activity of Soil Microbes. In Proceedings of the International Seminar of Chemistry, Jatinangor, Indonesia, 30–31 October 2008; pp. 30–31. [Google Scholar]
- Sarapatka, B. Phosphatase Activity of Eutric Cambisols (Uppland, Sweden) in Relation to Soil Properties and Farming Systems. Sci. Agric. Bohem. 2002, 33, 18–24. [Google Scholar]
- Qu, Y.; Tang, J.; Li, Z.; Zhou, Z.; Wang, J.; Wang, S.; Cao, Y. Soil Enzyme Activity and Microbial Metabolic Function Diversity in Soda Saline–Alkali Rice Paddy Fields of Northeast China. Sustainability 2020, 12, 10095. [Google Scholar] [CrossRef]
- Fierer, N. Embracing the Unknown: Disentangling the Complexities of the Soil Microbiome. Nat. Rev. Microbiol. 2017, 15, 579–590. [Google Scholar] [CrossRef] [PubMed]
- Olsson, R.; Giesler, R.; Loring, J.S.; Persson, P. Enzymatic Hydrolysis of Organic Phosphates Adsorbed on Mineral Surfaces. Environ. Sci. Technol. 2012, 46, 285–291. [Google Scholar] [CrossRef]
- Bian, J.; Tang, J.; Zhang, L.; Ma, H.; Zhao, J. Arsenic Distribution and Geological Factors in the Western Jilin Province, China. J. Geochem. Explor. 2012, 112, 347–356. [Google Scholar] [CrossRef]
- Francioli, D.; Schulz, E.; Lentendu, G.; Wubet, T.; Buscot, F.; Reitz, T. Mineral vs. Organic Amendments: Microbial Community Structure, Activity and Abundance of Agriculturally Relevant Microbes Are Driven by Long-Term Fertilization Strategies. Front. Microbiol. 2016, 7, 1446. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Feng, J.; Kwon, K.D.; Kubicki, J.D.; Phillips, B.L. Surface Speciation of Phosphate on Boehmite (γ-AlOOH) Determined from NMR Spectroscopy. Langmuir 2010, 26, 4753–4761. [Google Scholar] [CrossRef]
- Liang, J.-L.; Liu, J.; Jia, P.; Yang, T.; Zeng, Q.; Zhang, S.; Liao, B.; Shu, W.; Li, J. Novel Phosphate-Solubilizing Bacteria Enhance Soil Phosphorus Cycling Following Ecological Restoration of Land Degraded by Mining. ISME J. 2020, 14, 1600–1613. [Google Scholar] [CrossRef] [Green Version]
- Yadav, R.S.; Meena, S.C.; Patel, S.I.; Patel, K.I.; Akhtar, M.S.; Yadav, B.K.; Panwar, J. Bioavailability of Soil P for Plant Nutrition. In Farming for Food and Water Security; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2012; Volume 10, pp. 177–200. ISBN 978-94-007-4499-8. [Google Scholar]
- Yue, Z.; Shen, Y.; Chen, Y.; Liang, A.; Chu, C.; Chen, C.; Sun, Z. Microbiological Insights into the Stress-Alleviating Property of an Endophytic Bacillus Altitudinis WR10 in Wheat under Low-Phosphorus and High-Salinity Stresses. Microorganisms 2019, 7, 508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kageyama, H.; Tripathi, K.; Rai, A.K.; Cha-um, S.; Waditee-Sirisattha, R.; Takabe, T. An Alkaline Phosphatase/Phosphodiesterase, PhoD, Induced by Salt Stress and Secreted Out of the Cells of Aphanothece Halophytica, a Halotolerant Cyanobacterium. Appl. Environ. Microbiol. 2011, 77, 5178–5183. [Google Scholar] [CrossRef] [Green Version]
- Kumar, V.; Singh, P.; Jorquera, M.A.; Sangwan, P.; Kumar, P.; Verma, A.K.; Agrawal, S. Isolation of Phytase-Producing Bacteria from Himalayan Soils and Their Effect on Growth and Phosphorus Uptake of Indian Mustard (Brassica Juncea). World J. Microbiol. Biotechnol. 2013, 29, 1361–1369. [Google Scholar] [CrossRef] [PubMed]
- Boukhris, I.; Farhat-Khemakhem, A.; Blibech, M.; Bouchaala, K.; Chouayekh, H. Characterization of an Extremely Salt-Tolerant and Thermostable Phytase from Bacillus Amyloliquefaciens US573. Int. J. Biol. Macromol. 2015, 80, 581–587. [Google Scholar] [CrossRef]
- Kumar, A.; Singh, S.; Mukherjee, A.; Rastogi, R.P.; Verma, J.P. Salt-Tolerant Plant Growth-Promoting Bacillus Pumilus Strain JPVS11 to Enhance Plant Growth Attributes of Rice and Improve Soil Health under Salinity Stress. Microbiol. Res. 2021, 242, 126616. [Google Scholar] [CrossRef] [PubMed]
- Barua, S.; Tripathi, S.; Chakraborty, A.; Ghosh, S.; Chakrabarti, K. Characterization and Crop Production Efficiency of Diazotrophic Bacterial Isolates from Coastal Saline Soils. Microbiol. Res. 2012, 167, 95–102. [Google Scholar] [CrossRef]
- Malboobi, M.A.; Owlia, P.; Behbahani, M.; Sarokhani, E.; Moradi, S.; Yakhchali, B.; Deljou, A.; Morabbi Heravi, K. Solubilization of Organic and Inorganic Phosphates by Three Highly Efficient Soil Bacterial Isolates. World J. Microbiol. Biotechnol. 2009, 25, 1471–1477. [Google Scholar] [CrossRef]
- Noskova, Y.; Likhatskaya, G.; Terentieva, N.; Son, O.; Tekutyeva, L.; Balabanova, L. A Novel Alkaline Phosphatase/Phosphodiesterase, CamPhoD, from Marine Bacterium Cobetia Amphilecti KMM 296. Mar. Drugs 2019, 17, 657. [Google Scholar] [CrossRef] [Green Version]
- Xiang, W.; Liang, H.; Liu, S.; Luo, F.; Tang, J.; Li, M.; Che, Z. Isolation and Performance Evaluation of Halotolerant Phosphate Solubilizing Bacteria from the Rhizospheric Soils of Historic Dagong Brine Well in China. World J. Microbiol. Biotechnol. 2011, 27, 2629–2637. [Google Scholar] [CrossRef]
- Zhu, F.; Qu, L.; Hong, X.; Sun, X. Isolation and Characterization of a Phosphate-Solubilizing Halophilic Bacterium Kushneria Sp. YCWA18 from Daqiao Saltern on the Coast of Yellow Sea of China. Evid.-Based Complement. Altern. Med. 2011, 2011, e615032. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Sethi, B.K.; Mishra, R.R.; Kumari, S.; Thatoi, H. Alkaline Phosphatase Activity of a Phosphate Solubilizing Alcaligenes Faecalis, Isolated from Mangrove Soil. Biotechnol. Res. Innov. 2017, 1, 101–111. [Google Scholar] [CrossRef]
- Behera, B.C.; Yadav, H.; Singh, S.K.; Mishra, R.R.; Sethi, B.K.; Dutta, S.K.; Thatoi, H.N. Phosphate Solubilization and Acid Phosphatase Activity of Serratia Sp. Isolated from Mangrove Soil of Mahanadi River Delta, Odisha, India. J. Genet. Eng. Biotechnol. 2017, 15, 169–178. [Google Scholar] [CrossRef]
- Jiang, H.; Wang, T.; Chi, X.; Wang, M.; Chen, N.; Chen, M.; Pan, L.; Qi, P. Isolation and Characterization of Halotolerant Phosphate Solubilizing Bacteria Naturally Colonizing the Peanut Rhizosphere in Salt-Affected Soil. Geomicrobiol. J. 2020, 37, 110–118. [Google Scholar] [CrossRef]
- Simpson, R.J.; Oberson, A.; Culvenor, R.A.; Ryan, M.H.; Veneklaas, E.J.; Lambers, H.; Lynch, J.P.; Ryan, P.R.; Delhaize, E.; Smith, F.A.; et al. Strategies and Agronomic Interventions to Improve the Phosphorus-Use Efficiency of Farming Systems. Plant Soil 2011, 349, 89–120. [Google Scholar] [CrossRef]
- Robles-Aguilar, A.A.; Pang, J.; Postma, J.A.; Schrey, S.D.; Lambers, H.; Jablonowski, N.D. The Effect of PH on Morphological and Physiological Root Traits of Lupinus Angustifolius Treated with Struvite as a Recycled Phosphorus Source. Plant Soil 2019, 434, 65–78. [Google Scholar] [CrossRef] [Green Version]
- White, P.J.; Hammond, J.P. Phosphorus nutrition of terrestrial plants. In The Ecophysiology of Plant-Phosphorus Interactions; Springer: Berlin/Heidelberg, Germany, 2008; pp. 51–81. [Google Scholar]
- Fang, D.; Wei, S.; Xu, Y.; Xiong, J.; Tan, W. Impact of Low-Molecular Weight Organic Acids on Selenite Immobilization by Goethite: Understanding a Competitive-Synergistic Coupling Effect and Speciation Transformation. Sci. Total Environ. 2019, 684, 694–704. [Google Scholar] [CrossRef]
- Haling, R.E.; Brown, L.K.; Stefanski, A.; Kidd, D.R.; Ryan, M.H.; Sandral, G.A.; George, T.S.; Lambers, H.; Simpson, R.J. Differences in Nutrient Foraging among Trifolium Subterraneum Cultivars Deliver Improved P-Acquisition Efficiency. Plant Soil 2018, 424, 539–554. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Rubio, G.; Lynch, J.P. The Importance of Root Gravitropism for Inter-Root Competition and Phosphorus Acquisition Efficiency: Results from a Geometric Simulation Model. Plant Soil 2000, 218, 159–171. [Google Scholar] [CrossRef]
- Rubio, G.; Liao, H.; Yan, X.; Lynch, J.P. Topsoil Foraging and Its Role in Plant Competitiveness for Phosphorus in Common Bean. Crop Sci. 2003, 43, 598–607. [Google Scholar] [CrossRef]
- Honvault, N.; Houben, D.; Nobile, C.; Firmin, S.; Lambers, H.; Faucon, M.-P. Tradeoffs among Phosphorus-Acquisition Root Traits of Crop Species for Agroecological Intensification. Plant Soil 2020, 461, 137–150. [Google Scholar] [CrossRef]
- Hammond, J.P.; Broadley, M.R.; White, P.J.; King, G.J.; Bowen, H.C.; Hayden, R.; Meacham, M.C.; Mead, A.; Overs, T.; Spracklen, W.P. Shoot Yield Drives Phosphorus Use Efficiency in Brassica Oleracea and Correlates with Root Architecture Traits. J. Exp. Bot. 2009, 60, 1953–1968. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lynch, J.P. Root Phenes That Reduce the Metabolic Costs of Soil Exploration: Opportunities for 21st Century Agriculture: New Roots for Agriculture. Plant Cell Environ. 2015, 38, 1775–1784. [Google Scholar] [CrossRef]
- Wen, Z.; Li, H.; Shen, Q.; Tang, X.; Xiong, C.; Li, H.; Pang, J.; Ryan, M.H.; Lambers, H.; Shen, J. Tradeoffs among Root Morphology, Exudation and Mycorrhizal Symbioses for Phosphorus-acquisition Strategies of 16 Crop Species. New Phytol. 2019, 223, 882–895. [Google Scholar] [CrossRef] [PubMed]
- Lambers, H.; Shane, M.W.; Cramer, M.D.; Pearse, S.J.; Veneklaas, E.J. Root Structure and Functioning for Efficient Acquisition of Phosphorus: Matching Morphological and Physiological Traits. Ann. Bot. 2006, 98, 693–713. [Google Scholar] [CrossRef] [Green Version]
- Pearse, S.J.; Veneklaas, E.J.; Cawthray, G.; Bolland, M.D.A.; Lambers, H. Triticum aestivum Shows a Greater Biomass Response to a Supply of Aluminium Phosphate than Lupinus Albus, despite Releasing Fewer Carboxylates into the Rhizosphere. New Phytol. 2006, 169, 515–524. [Google Scholar] [CrossRef]
- Wang, L.; Liao, H.; Yan, X.; Zhuang, B.; Dong, Y. Genetic Variability for Root Hair Traits as Related to Phosphorus Status in Soybean. Plant Soil 2004, 261, 77–84. [Google Scholar] [CrossRef]
- Zhu, J.; Kaeppler, S.M.; Lynch, J.P. Topsoil Foraging and Phosphorus Acquisition Efficiency in Maize (Zea mays). Funct. Plant Biol. 2005, 32, 749. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lamont, B.B. Structure, Ecology and Physiology of Root Clusters—A Review. Plant Soil 2003, 248, 1–19. [Google Scholar] [CrossRef]
- Trachsel, S.; Kaeppler, S.M.; Brown, K.M.; Lynch, J.P. Shovelomics: High Throughput Phenotyping of Maize (Zea mays L.) Root Architecture in the Field. Plant Soil 2011, 341, 75–87. [Google Scholar] [CrossRef]
- Fort, F.; Freschet, G.T. Plant Ecological Indicator Values as Predictors of Fine-root Trait Variations. J. Ecol. 2020, 108, 1565–1577. [Google Scholar] [CrossRef]
- Parker, J.S.; Cavell, A.C.; Dolan, L.; Roberts, K.; Grierson, C.S. Genetic Interactions during Root Hair Morphogenesis in Arabidopsis. Plant Cell 2000, 12, 1961–1974. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Raghothama, K.G. Phosphate Acquisition. Annu. Rev. Plant Physiol. Plant Mol. Biol. 1999, 50, 665–693. [Google Scholar] [CrossRef] [PubMed]
- Smith, F.W. The phosphate uptake mechanism. In Food Security in Nutrient-Stressed Environments: Exploiting Plants’ Genetic Capabilities; Adu-Gyamfi, J.J., Ed.; Developments in Plant and Soil Sciences; Springer: Dordrecht, The Netherlands, 2002; pp. 235–244. ISBN 978-94-017-1570-6. [Google Scholar]
- Ma, Z.; Guo, D.; Xu, X.; Lu, M.; Bardgett, R.D.; Eissenstat, D.M.; McCormack, M.L.; Hedin, L.O. Evolutionary History Resolves Global Organization of Root Functional Traits. Nature 2018, 555, 94–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ryan, P.; Delhaize, E.; Jones, D. Function and mechanism of organic anion exudation from plant to roots. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 2001, 52, 527–560. [Google Scholar] [CrossRef] [PubMed]
- Nobile, C.; Houben, D.; Michel, E.; Firmin, S.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Phosphorus-Acquisition Strategies of Canola, Wheat and Barley in Soil Amended with Sewage Sludges. Sci. Rep. 2019, 9, 14878. [Google Scholar] [CrossRef] [PubMed]
- Weisskopf, L.; Abou-Mansour, E.; Fromin, N.; Tomasi, N.; Santelia, D.; Edelkott, I.; Neumann, G.; Aragno, M.; Tabacchi, R.; Martinoia, E. White Lupin Has Developed a Complex Strategy to Limit Microbial Degradation of Secreted Citrate Required for Phosphate Acquisition. Plant Cell Environ. 2006, 29, 919–927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guppy, C.N.; Menzies, N.W.; Moody, P.W.; Blamey, F.P.C. Competitive Sorption Reactions between Phosphorus and Organic Matter in Soil: A Review. Soil Res. 2005, 43, 189. [Google Scholar] [CrossRef]
- Carminati, A.; Vetterlein, D.; Koebernick, N.; Blaser, S.; Weller, U.; Vogel, H.-J. Do Roots Mind the Gap? Plant Soil 2013, 367, 651–661. [Google Scholar] [CrossRef] [Green Version]
- Li, S.M.; Li, L.; Zhang, F.S.; Tang, C. Acid Phosphatase Role in Chickpea/Maize Intercropping. Ann. Bot. 2004, 94, 297–303. [Google Scholar] [CrossRef] [Green Version]
- Neumann, G.; Massonneau, A.; Martinoia, E.; Römheld, V. Physiological Adaptations to Phosphorus Deficiency during Proteoid Root Development in White Lupin. Planta 1999, 208, 373–382. [Google Scholar] [CrossRef]
- Damon, P.M.; Bowden, B.; Rose, T.; Rengel, Z. Crop Residue Contributions to Phosphorus Pools in Agricultural Soils: A Review. Soil Biol. Biochem. 2014, 74, 127–137. [Google Scholar] [CrossRef] [Green Version]
- Aziz, T.; Rahmatullah; Maqsood, M.A.; Sabir, M.; Kanwal, S. Categorization of brassica cultivars for phosphorus acquisition from phosphate rock on basis of growth and ionic parameters. J. Plant Nutr. 2011, 34, 522–533. [Google Scholar] [CrossRef]
- Oburger, E.; Jones, D.L.; Wenzel, W.W. Phosphorus Saturation and PH Differentially Regulate the Efficiency of Organic Acid Anion-Mediated P Solubilization Mechanisms in Soil. Plant Soil 2011, 341, 363–382. [Google Scholar] [CrossRef]
- Ryan, J.; Curtin, D.; Cheema, M.A. Significance of Iron Oxides and Calcium Carbonate Particle Size in Phosphate Sorption by Calcareous Soils. Soil Sci. Soc. Am. J. 1985, 49, 74–76. [Google Scholar] [CrossRef]
- Courty, P.-E.; Franc, A.; Garbaye, J. Temporal and Functional Pattern of Secreted Enzyme Activities in an Ectomycorrhizal Community. Soil Biol. Biochem. 2010, 42, 2022–2025. [Google Scholar] [CrossRef]
- Kirk, G.J.D.; Santos, E.E.; Santos, M.B. Phosphate Solubilization by Organic Anion Excretion from Rice Growing in Aerobic Soil: Rates of Excretion and Decomposition, Effects on Rhizosphere PH and Effects on Phosphate Solubility and Uptake. New Phytol. 1999, 142, 185–200. [Google Scholar] [CrossRef]
- Hallama, M.; Pekrun, C.; Lambers, H.; Kandeler, E. Hidden Miners—The Roles of Cover Crops and Soil Microorganisms in Phosphorus Cycling through Agroecosystems. Plant Soil 2019, 434, 7–45. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Simpson, R.; Richardson, A. The Growth and Phosphorus Utilisation of Plants in Sterile Media When Supplied with Inositol Hexaphosphate, Glucose 1-Phosphate or Inorganic Phosphate. Plant Soil 2000, 220, 165–174. [Google Scholar] [CrossRef]
- Darch, T.; Giles, C.D.; Blackwell, M.S.A.; George, T.S.; Brown, L.K.; Menezes-Blackburn, D.; Shand, C.A.; Stutter, M.I.; Lumsdon, D.G.; Mezeli, M.M.; et al. Inter- and Intra-Species Intercropping of Barley Cultivars and Legume Species, as Affected by Soil Phosphorus Availability. Plant Soil 2018, 427, 125–138. [Google Scholar] [CrossRef]
- Pang, J.; Bansal, R.; Zhao, H.; Bohuon, E.; Lambers, H.; Ryan, M.H.; Ranathunge, K.; Siddique, K.H.M. The Carboxylate-Releasing Phosphorus-Mobilizing Strategy Can Be Proxied by Foliar Manganese Concentration in a Large Set of Chickpea Germplasm under Low Phosphorus Supply. New Phytol. 2018, 219, 518–529. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tinker, P.B. The role of microorganisms in mediating and facilitating the uptake of plant nutrients from soil. In Biológical Processes and Soil Fertility; Springer: Berlin/Heidelberg, Germany, 1984; pp. 77–91. [Google Scholar]
- Gashaw Deressa, T.; Schenk, M.K. Contribution of Roots and Hyphae to Phosphorus Uptake of Mycorrhizal Onion (Allium cepa L.)-A Mechanistic Modeling Approach. Z. Pflanzenernähr. Bodenk. 2008, 171, 810–820. [Google Scholar] [CrossRef]
- Graham, J.H.; Eissenstat, D.M. Host Genotype and the Formation and Function of VA Mycorrhizae. Plant Soil 1994, 159, 179–185. [Google Scholar] [CrossRef]
- Sattelmacher, B.; Horst, W.J.; Becker, H.C. Factors that contribute to genetic variation for nutrient efficiency of crop plants. Z. Pflanzenernaehr. Bodenk. 1994, 157, 215–224. [Google Scholar] [CrossRef]
- Smith, S.E.; Gianinazzi-Pearson, V. Physiological Interactions Between Symbionts in Vesicular-Arbuscular Mycorrhizal Plants. Annu. Rev. Plant. Physiol. Plant. Mol. Biol. 1988, 39, 221–244. [Google Scholar] [CrossRef]
- Gerke, J. Phytate (Inositol Hexakisphosphate) in Soil and Phosphate Acquisition from Inositol Phosphates by Higher Plants. A Review. Plants 2015, 4, 253–266. [Google Scholar] [CrossRef] [Green Version]
- Nielsen, K.L.; Bouma, T.J.; Lynch, J.P.; Eissenstat, D.M. Effects of Phosphorus Availability and Vesicular-Arbuscular Mycorrhizas on the Carbon Budget of Common Bean (Phaseolus Vulgaris). New Phytol. 1998, 139, 647–656. [Google Scholar] [CrossRef] [Green Version]
- Lynch, J.P. Root architecture and nutrient acquisition. In Nutrient Acquisition by Plants; Springer: Berlin/Heidelberg, Germany, 2005; pp. 147–183. [Google Scholar]
- Zhu, J.; Lynch, J.P. The Contribution of Lateral Rooting to Phosphorus Acquisition Efficiency in Maize (Zea mays) Seedlings. Funct. Plant Biol. 2004, 31, 949. [Google Scholar] [CrossRef]
- Bates, T.R.; Lynch, J.P. Plant Growth and Phosphorus Accumulation of Wild Type and Two Root Hair Mutants of Arabidopsis thaliana (Brassicaceae). Am. J. Bot. 2000, 87, 958–963. [Google Scholar] [CrossRef] [Green Version]
- Schneider, K.D.; Thiessen Martens, J.R.; Zvomuya, F.; Reid, D.K.; Fraser, T.D.; Lynch, D.H.; O’Halloran, I.P.; Wilson, H.F. Options for Improved Phosphorus Cycling and Use in Agriculture at the Field and Regional Scales. J. Environ. Qual. 2019, 48, 1247–1264. [Google Scholar] [CrossRef] [Green Version]
- Yadav, B.K.; Tarafdar, J.C. Availability of Unavailable Phosphate Compounds as a Phosphorus Source for Clusterbean (Cyamopsis tetragonoloba (L.) Taub.) through the Activity of Phosphatase and Phytase Produced by Actinomycetes. J. Arid. Legum. 2007, 4, 110–116. [Google Scholar]
- Yadav, R.S.; Tarafdar, J.C. Phytase and Phosphatase Producing Fungi in Arid and Semi-Arid Soils and Their Efficiency in Hydrolyzing Different Organic P Compounds. Soil Biol. Biochem. 2003, 35, 745–751. [Google Scholar] [CrossRef]
- Li, C.; Kuyper, T.W.; van der Werf, W.; Zhang, J.; Li, H.; Zhang, F.; Hoffland, E. Testing for Complementarity in Phosphorus Resource Use by Mixtures of Crop Species. Plant Soil 2019, 439, 163–177. [Google Scholar] [CrossRef]
- Strock, C.F.; Morrow de la Riva, L.; Lynch, J.P. Reduction in Root Secondary Growth as a Strategy for Phosphorus Acquisition. Plant Physiol. 2018, 176, 691–703. [Google Scholar] [CrossRef] [Green Version]
- Raven, J.A.; Lambers, H.; Smith, S.E.; Westoby, M. Costs of Acquiring Phosphorus by Vascular Land Plants: Patterns and Implications for Plant Coexistence. New Phytol. 2018, 217, 1420–1427. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houben, D.; Michel, E.; Nobile, C.; Lambers, H.; Kandeler, E.; Faucon, M.-P. Response of Phosphorus Dynamics to Sewage Sludge Application in an Agroecosystem in Northern France. Appl. Soil Ecol. 2019, 137, 178–186. [Google Scholar] [CrossRef]
- Trouillefou, C.M.; Le Cadre, E.; Cacciaguerra, T.; Cunin, F.; Plassard, C.; Belamie, E. Protected Activity of a Phytase Immobilized in Mesoporous Silica with Benefits to Plant Phosphorus Nutrition. J. Sol-Gel Sci. Technol. 2015, 74, 55–65. [Google Scholar] [CrossRef]
- Findenegg, G.R.; Nelemans, J.A. The Effect of Phytase on the Availability of P from Myo-Inositol Hexaphosphate (Phytate) for Maize Roots. Plant Soil 1993, 154, 189–196. [Google Scholar] [CrossRef]
- Richardson, A.E. Prospects for Using Soil Microorganisms to Improve the Acquisition of Phosphorus by Plants. Funct. Plant Biol. 2001, 28, 897–906. [Google Scholar] [CrossRef]
- Irshad, U.; Brauman, A.; Villenave, C.; Plassard, C. Phosphorus Acquisition from Phytate Depends on Efficient Bacterial Grazing, Irrespective of the Mycorrhizal Status of Pinus Pinaster. Plant Soil 2012, 358, 155–168. [Google Scholar] [CrossRef]
- Anderson, C.R.; Condron, L.M.; Clough, T.J.; Fiers, M.; Stewart, A.; Hill, R.A.; Sherlock, R.R. Biochar Induced Soil Microbial Community Change: Implications for Biogeochemical Cycling of Carbon, Nitrogen and Phosphorus. Pedobiologia 2011, 54, 309–320. [Google Scholar] [CrossRef]
- Mudge, S.R.; Smith, F.W.; Richardson, A.E. Root-Specific and Phosphate-Regulated Expression of Phytase under the Control of a Phosphate Transporter Promoter Enables Arabidopsis to Grow on Phytate as a Sole P Source. Plant Sci. 2003, 165, 871–878. [Google Scholar] [CrossRef]
- Huang, W.; Wang, S.; Zhu, Z.; Li, L.; Yao, X.; Rudolph, V.; Haghseresht, F. Phosphate Removal from Wastewater Using Red Mud. J. Hazard. Mater. 2008, 158, 35–42. [Google Scholar] [CrossRef]
- Menezes-Blackburn, D.; Zhang, H.; Stutter, M.; Giles, C.D.; Darch, T.; George, T.S.; Shand, C.; Lumsdon, D.; Blackwell, M.; Wearing, C.; et al. A Holistic Approach to Understanding the Desorption of Phosphorus in Soils. Environ. Sci. Technol. 2016, 50, 3371–3381. [Google Scholar] [CrossRef] [Green Version]
- Franchini, J.C.; Pavan, M.A.; Miyazawa, M. Redistribution of Phosphorus in Soil through Cover Crop Roots. Braz. Arch. Biol. Technol. 2004, 47, 381–386. [Google Scholar] [CrossRef]
- Pavinato, P.S.; Merlin, A.; Rosolem, C.A. Organic Compounds from Plant Extracts and Their Effect on Soil Phosphorus Availability. Pesq. Agropec. Bras. 2008, 43, 1379–1388. [Google Scholar] [CrossRef]
- Soltangheisi, A.; Teles, A.P.B.; Sartor, L.R.; Pavinato, P.S. Cover Cropping May Alter Legacy Phosphorus Dynamics Under Long-Term Fertilizer Addition. Front. Environ. Sci. 2020, 8, 13. [Google Scholar] [CrossRef] [Green Version]
- Calegari, A.; Tiecher, T.; Hargrove, W.L.; Ralisch, R.; Tessier, D.; de Tourdonnet, S.; de Fátima Guimarães, M.; dos Santos, D.R. Long-Term Effect of Different Soil Management Systems and Winter Crops on Soil Acidity and Vertical Distribution of Nutrients in a Brazilian Oxisol. Soil Tillage Res. 2013, 133, 32–39. [Google Scholar] [CrossRef]
- Cui, H.; Zhou, Y.; Gu, Z.; Zhu, H.; Fu, S.; Yao, Q. The Combined Effects of Cover Crops and Symbiotic Microbes on Phosphatase Gene and Organic Phosphorus Hydrolysis in Subtropical Orchard Soils. Soil Biol. Biochem. 2015, 82, 119–126. [Google Scholar] [CrossRef]
- Bowman, R.A.; Cole, C.V. An exploratory method for fractionation of organic phosphorus from grassland soils. Soil Sci. 1978, 125, 95–101. [Google Scholar] [CrossRef]
- Slazak, A.; Freese, D.; da Silva Matos, E.; Hüttl, R.F. Soil Organic Phosphorus Fraction in Pine–Oak Forest Stands in Northeastern Germany. Geoderma 2010, 158, 156–162. [Google Scholar] [CrossRef]
- Wang, X.; Hu, Y.; Tang, Y.; Yang, P.; Feng, X.; Xu, W.; Zhu, M. Phosphate and Phytate Adsorption and Precipitation on Ferrihydrite Surfaces. Environ. Sci. Nano 2017, 4, 2193–2204. [Google Scholar] [CrossRef]
- Pang, J.; Ryan, M.H.; Tibbett, M.; Cawthray, G.R.; Siddique, K.H.M.; Bolland, M.D.A.; Denton, M.D.; Lambers, H. Variation in Morphological and Physiological Parameters in Herbaceous Perennial Legumes in Response to Phosphorus Supply. Plant Soil 2010, 331, 241–255. [Google Scholar] [CrossRef]
- Lemming, C.; Bruun, S.; Jensen, L.S.; Magid, J. Plant Availability of Phosphorus from Dewatered Sewage Sludge, Untreated Incineration Ashes, and Other Products Recovered from a Wastewater Treatment System. J. Plant Nutr. Soil Sci. 2017, 180, 779–787. [Google Scholar] [CrossRef]
- Glæsner, N.; van der Bom, F.; Bruun, S.; McLaren, T.; Larsen, F.H.; Magid, J. Phosphorus Characterization and Plant Availability in Soil Profiles after Long-Term Urban Waste Application. Geoderma 2019, 338, 136–144. [Google Scholar] [CrossRef]
| N° | Organic Input | Extractant | Analysis | Total Pi | Total Po | References |
|---|---|---|---|---|---|---|
| 1 | Feces (dairy) | NaOH-EDTA | 31PNMR | 4961 mg·kg−1 (64) | 2650 mg·kg−1 (36) | [57] |
| 2 | Manure (dairy) | NaOH-EDTA | 31PNMR | 4231 mg·kg−1 (75) | 1396 mg·kg−1 (26) | [57,58] |
| 3 | Dairy manure–dry | NaOH-EDTA | 31PNMR | 4736 mg·kg−1 (67) | 2342 mg·kg−1 (30) | [59] |
| 4 | Dairy manure–wet | NaOH-EDTA | Enzymatic hydrolysis | 3840 mg·kg−1 (57) | 2957 mg·kg−1 (49) | [60] |
| 5 | Dung | NaOH-EDTA | 31PNMR | 58 mg·kg−1 (22) | 399 mg·kg−1 (40) | [61] |
| 6 | Solid manure (dairy) | NaOH-EDTA | 31PNMR | 5731 mg·kg−1 (67) | 2848 mg·kg−1 (33) | [62] |
| 7 | Lagoon manure (dairy) | NaOH-EDTA | 31PNMR | 19 mg·kg−1 (66) | 10 mg·kg−1 (34) | [62] |
| 8 | Cattle manure | NaOH-EDTA | 31PNMR | 3120 mg·kg−1 (74) | 1080 mg·kg−1 (25) | [63] |
| 9 | Dairy manure | Water | 31PNMR | 1870 mg·kg−1 (85) | 221 mg·kg−1 (15) | [60] |
| 10 | Dairy manure | NaAcNa2S2O4 | 31PNMR | 3680 mg·kg−1 (79) | 944 mg·kg−1 (21) | [60] |
| 11 | Dairy manure | NaOH-EDTA | 31PNMR | 3637 mg·kg−1 (77) | 964 mg·kg−1 (23) | [60] |
| 12 | Animal species (beef and dairy cattle, swine, chicken, turkey, dairy goat, horse, and sheep) | H2O, NaHCO3, NaOH | - | - | 500–8800 mg·kg−1 | [64] |
| 13 | Broiler litter | NaHCO3 | 31PNMR | 1300 mg·kg−1 (12) | 2800 mg·kg−1 (25) | [65] |
| 14 | Dairy manure | NaHCO3 | 31PNMR | 2400 mg·kg−1 (35) | 890 mg·kg−1 (13) | [65] |
| 15 | Swine manure | NaHCO3 | 31PNMR | 6500 mg·kg−1 (21) | 1600 mg·kg−1 (5) | [65] |
| N° | Land Use | Extractant | Analysis | Total Pi | Total Po | References |
|---|---|---|---|---|---|---|
| 1 | Semi-arid irrigated arable soils (U.S.) | EDTA | 31PNMR | 141 mg·kg−1 (72) | 57 mg·kg−1 (27) | [55] |
| 2 | Semi-arid irrigated arable soils (U.S.) | Bicarbonate | 31PNMR | 31 mg·kg−1 (76) | 7 mg·kg−1 (26) | [55] |
| 3 | Grassland (Australia) | Deionised water | Enzymatic hydrolysis | 1.3 mg·kg−1 (36) | 1.5 mg·kg−1 (48) | [47] |
| 4 | Grassland (New Zealand) | NaOH-EDTA | Enzymatic hydrolysis | 454 mg·kg−1 (56) | 23 mg·kg−1 (4) | [66] |
| 5 | Clover and arable plots (Australia) | NaOH-EDTA | 31PNMR | 345 mg·kg−1 (76) | 6 mg·kg−1 (2.5) | [67] |
| 6 | Grassland (U.S.) | NaOH-EDTA | 31PNMR | 221 mg·kg−1 (82) | 54 mg·kg−1 (19) | [62] |
| 7 | Grassland (Netherlands) | NaOH-EDTA | 31PNMR | 406 mg·kg−1 (56) | 207 mg·kg−1 (44) | [68] |
| 8 | Grassland (Ireland) | NaOH-EDTA | 31PNMR | 358 mg·kg−1 (47) | 373 mg·kg−1 (54) | [69] |
| 9 | Grassland (New Zealand) | NaOH-EDTA | 31PNMR | (32) | (68) | [26] |
| 10 | Grassland (England and Wales) | NaOH-EDTA | 31PNMR | 275 mg·kg−1 (40) | 480 mg·kg−1 (70) | [55] |
| 11 | Grassland (England and Wales) | NaHCO3 | 31PNMR | 18 mg·kg−1 (25) | 70 mg·kg−1 (80) | [70] |
| 12 | Grassland (New Zealand) | NaOH-EDTA | 31PNMR | 500 mg·kg−1 (50) | 20 mg·kg−1 (3) | [71] |
| 13 | Forest, grassland and arable (Germany) | NaOH | 31PNMR | 52 mg·kg−1 (30) | 149 mg·kg−1 (56) | [56] |
| 14 | Grassland (England) | NaOH | 31PNMR | 224 mg·kg−1 (50) | 140 mg·kg−1 (40) | [72] |
| 15 | Semi-arid grassland and arable (Canada) | H2O and NaOH | 31PNMR | 175 mg·kg−1 (58) | 117 mg·kg−1 (42) | [73] |
| 16 | Grassland (U.S.) | NaOH-EDTA | 31PNMR | 23 mg·kg−1 (20) | 66 mg·kg−1 (67) | [74] |
| 17 | Arable (Canada) | NaOH-EDTA | 31PNMR | 504 mg·kg−1 (73) | 370 mg·kg−1 (48) | [75] |
| 18 | Grassland (New Zealand) | NaOH-EDTA | 31PNMR | 620 mg·kg−1 (54) | 465 mg·kg−1 (45) | [71] |
| 19 | Burkina Faso Arable Lixisol | NaOH-EDTA | 31PNMR | 19 mg·kg−1 (23) | - | [53] |
| 20 | Madagascar Arable Ferralsol | NaOH-EDTA | 31PNMR | 50 mg·kg−1 (54) | 15 mg·kg−1 (12) | [53] |
| 21 | Australia Grassland Alfisol | NaOH-EDTA | 31PNMR | 35 mg·kg−1 (39) | 16 mg·kg−1 (17) | [53] |
| 22 | Germany Arable Luvisol | NaOH-EDTA | 31PNMR | 249 mg·kg−1 (74) | 35 mg·kg−1 (13) | [53] |
| 23 | Switzerland 2 Arable Luvisol | NaOH-EDTA | 31PNMR | 228 mg·kg−1 (66) | 36 mg·kg−1 (12) | [53] |
| 24 | Switzerland 3 Grassland Cambisol | NaOH-EDTA | 31PNMR | 121–396 mg·kg−1 (36–66) | 8–206 mg·kg−1 (1–17) | [53] |
| 25 | Different soils | - | - | - | (>55) | [76] |
| 26 | Caribou soil with conventional cultivation history | NaOH | Enzymatic hydrolysis | 1162 mg·kg−1 | 330 mg·kg−1 | [77] |
| 27 | Caribou soil with manure application history | NaOH | Enzymatic hydrolysis | 966 mg·kg−1 | 249 mg·kg−1 | [77] |
| 28 | Swine manure | Enzymatic hydrolysis | 173 mg·kg−1 | 235 mg·kg−1 | [77] |
| Pi Forms | Po Forms | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Phosphomonoesters | Phosphodiesters | ||||||||
| N° | Organic Input | Orthophosphate Pyrophosphate Polyphosphate | IHP | Labile | Phospholipids | DNA/ Polynaucleotide | Other | Phosphanate | Unidentified |
| 1 | Feces (dairy) | 4961 mg·kg−1 | 1325 mg·kg−1 | 624 mg·kg−1 | 423 mg·kg−1 | 154 mg·kg−1 | 113 mg·kg−1 | 73 mg·kg−1 | - |
| 2 | Manure (dairy) | 4231 mg·kg−1 | 496 mg·kg−1 | 503 mg·kg−1 | 210 mg·kg−1 | 108 mg·kg−1 | 83 mg·kg−1 | 55 mg·kg−1 | - |
| 3 | Dairy manure dry | 4736 mg·kg−1 | 268 mg·kg−1 | 204 mg·kg−1 | - | 24 mg·kg−1 | - | - | 1842 mg·kg−1 |
| 4 | Dairy manure wet | 3840 mg·kg−1 | 678 mg·kg−1 | 608 mg·kg−1 | - | 434 mg·kg−1 | - | - | 1237 mg·kg−1 |
| 5 | Dung | 5–24 mg·kg−1 | 61–106 mg·kg−1 | - | 40–103 mg·kg−1 | 58–90 mg·kg−1 | - | 0–2 mg·kg−1 | - |
| 6 | Solid manure (dairy) | 5731 mg·kg−1 | 1338 mg·kg−1 | 1236 mg·kg−1 | 154 mg·kg−1 | 77 mg·kg−1 | 231 mg·kg−1 | 43 mg·kg−1 | |
| 7 | Lagoon manure (dairy) | 19.1 mg·kg−1 | 3.2 mg·kg−1 | 5.9 mg·kg−1 | 0.3 mg·kg−1 | 0.3 mg·kg−1 | - | - | - |
| 8 | Cattle manure | 3120 mg·kg−1 | 500 mg·kg−1 | 140 mg·kg−1 | 220 mg·kg−1 | 220 mg·kg−1 | - | - | - |
| 9 | Dairy manure | 1870 mg·kg−1 | 0 | 136 mg·kg−1 | - | 18 mg·kg−1 | 67 mg·kg−1 | 85 mg·kg−1 | - |
| 10 | Dairy manure | 3680 mg·kg−1 | 444 mg·kg−1 | 369 mg·kg−1 | - | 51 mg·kg−1 | 81 mg·kg−1 | 131 mg·kg−1 | - |
| 11 | Dairy manure | 3637 mg·kg−1 | 444 mg·kg−1 | 385 mg·kg−1 | - | 45 mg·kg−1 | 90 mg·kg−1 | - | - |
| Pi Forms | Po Forms | |||||||
|---|---|---|---|---|---|---|---|---|
| Phosphomonoesters | Phosphodiesters | Other P Forms | ||||||
| Soil Types | Orthophosphate Pyrophosphate Polyphosphate | IHP | Labile | Phospholipids | DNA/ Polynaucleotide | Phosphanate | Unidentified | |
| 1 | Semi-arid irrigated arable soils (U.S.) | 150 mg·kg−1 | 45 mg·kg−1 | 10 mg·kg−1 | 1 mg·kg−1 | 0.3 mg·kg−1 | 1.3 mg·kg−1 | - |
| 2 | Semi-arid irrigated arable soils (U.S.) | 31 mg·kg−1 | 1.6 mg·kg−1 | 1.4 mg·kg−1 | 0.1 mg·kg−1 | 0.9 mg·kg−1 | 1 mg·kg−1 | 2.2 mg·kg−1 |
| 3 | Grassland (Australia) | 1.3 mg·kg−1 | 0.28 mg·kg−1 | 0.04 mg·kg−1 | 0.2 mg·kg−1 | 0.03 mg·kg−1 | 0.23 mg·kg−1 | 0.8 mg·kg−1 |
| 4 | Grassland (New Zealand) | 454 mg·kg−1 | 221 mg·kg−1 | 100 mg·kg−1 | 5 mg·kg−1 | 5 mg·kg−1 | 9.5 mg·kg−1 | . |
| 5 | Clover and arable plots (Australia) | 345 mg·kg−1 | 40 mg·kg−1 | 14 mg·kg−1 | 3 mg·kg−1 | 2.5 mg·kg−1 | - | - |
| 6 | Grassland (U.S.) | 221 mg·kg−1 | 37 mg·kg−1 | 11 mg·kg−1 | 0.1 mg·kg−1 | 1.8 mg·kg−1 | 0 | - |
| 7 | Grassland (Netherlands) | 406 mg·kg−1 | 150 mg·kg−1 | 56 mg·kg−1 | 0.3 mg·kg−1 | 0.1 mg·kg−1 | - | - |
| 8 | Grassland (Ireland) | 353 mg·kg−1 | 239 mg·kg-1 | 100 mg·kg−1 | - | 16.4 mg·kg−1 | 3.2 mg·kg−1 | - |
| 9 | Grassland (New Zealand) | - | - | - | - | - | - | - |
| 10 | Grassland (England and Wales) | 271 mg·kg−1 | 142 mg·kg−1 | 102 mg·kg−1 | 21 mg·kg−1 | 17 mg·kg−1 | 6.8 mg·kg−1 | 22.6 mg·kg−1 |
| 11 | Grassland (England and Wales) | 18 mg·kg−1 | - | 12.4 mg·kg−1 | 0.8 mg·kg−1 | 3.6 mg·kg−1 | - | 39 mg·kg−1 |
| Organic Phosphorus Forms | Mode of Action That Root Traits and Microbes Act to Mobilize the Po | Associated Microorganisms | Reference |
|---|---|---|---|
| Glycerophosphate and phytate | Alkaline phosphatase and acid phosphatase; phytase | Bacillus coagulans | [129] |
| Ca-phytate | pH reduced; phytase | Bacillus altitudinis WR10 | [130] |
| Po pools | Alkaline phosphatase | Aphanothece halophytica | [131] |
| Na-phytate | pH reduced; phytase | Tetrathiobacter sp. PB-03 and Bacillus sp. PB-13 | [132] |
| Phytic acid | Phytase | Bacillus amyloliquefaciens US573 Acromobacter sp. PB-01 | [133] |
| Total Po pools | Alkaline phosphatase and acid phosphatase | Bacillus pumilus strain JPVS11 | [134] |
| beta-Glycerophosphate | pH reduced; acid phosphatase | Agrobacterium sp. and Bacillus sp. | [135] |
| 5-bromo-4-chloro-3-indolyl phosphate (BCIP) | pH reduced; phosphatase | Pantoea agglomerans strain P5 Microbacterium laevaniformans strain P7 and Pseudomonas putida strain P13 | [136] |
| p-nitrophenyl phosphate (pNPP) and guanosine 5-triphosphate (GTP) | Alkaline phosphatase/phosphodiesterase activity | Cobetia amphilecti | [137] |
| Lecithin | pH reduced; organic acid | Kushneria sp. YCWA18, Bacillus megaterium | [138,139] |
| Total Po pools | pH reduced, oxalic acid, citric acid, malic acid, succinic acid and acetic acid; alkaline phosphatase | Alcaligenes faecalis | [140] |
| p-nitrophenyl phosphate | Malic acid, lactic acid and acetic acid; acid phosphatase, pH reduced, oxalic acid, citric | Serratia sp., Alcaligenes faecalis | [141] |
| Fe-Po, and lecithin | pH reduced | Ensifer sesbaniae, Gordonia terrae, Bacillus sp., Acinetobacter sp. | [142] |
| Case Studies | Observation/Concept | Po Forms | Test Plant | Reference |
|---|---|---|---|---|
| Sewage sludge application in an agroecosystem (long-term (>20 years) cropland field in northern France | Sludge is as effective as TSP to improve soil P availability. Sludge promotes soil enzymatic activities (phosphatases) for Po hydrolysis | Po forms, Apatite-P, Nonapatite-P | Oilseed rape–winter wheat–winter barley rotation | [198] |
| Measured of ten morphological and physiological traits involved in P acquisition across species in two contrasting soils with moderate P limitation. | There is Tradeoffs between thicker and thinner roots. Thicker roots exhibiting greater carboxylate release or phosphatase activity in the rhizosheath | Total P | Thirteen species of diverse phylogenetic lineages | [150] |
| Placement of phytase in the vicinity of roots using mesoporous silica nanoparticle materials. | Phytases are stable and resistant to soil degradation | Phytate/IHP | Medicago truncatula | [199] |
| Intercropping of P-mobilizing and non-P-mobilizing crop species | P-mobilizing crop species (legume) improve Po utilization for non-P-mobilizing species (non-legume) | Phytate | Wheat/chickpea | [170] |
| Contribution of phytate to plant nutrition is affected by Fe oxides and phosphohydrolases releasing microorganisms in the growing medium. | Phytase activity and organic anions concentration increased with increased Fe oxides in the media. Phytate supplied was recovered as inorganic P at the highest Fe oxide concentration. Inoculants of B. subtilis promoted an enhanced hydrolytic activity at the highest Fe oxide concentration. | Phytate/myo-IHP | Cucumber plants | [86] |
| Application of phytase to the root medium of plants | Phytase increases Po hydrolysis | myo-IHP (phytin) | Maize | [200] |
| Inoculation of plants with soil isolates/microorganisms that possess efficient phytase activity | Mineralization of complex organic substrates by phytases | myo-IHP | Pasture legume (subterranean clover, white clover, alfalfa) and pasture grass (wallaby grass, Phalaris) species | [201] |
| P-acquisition strategies of three main crops are affected by the application of sewage sludges, compared with a mineral P fertilizer. | Wheat and barley had a greater root carboxylate release. Canola had higher root released acid phosphatase activity which promoted the mineralization of sludge-derived Po | Po forms, Apatite-P, Nonapatite-P | wheat, barley and canola | [166] |
| Inoculation of plants with arbuscular mycorrhizal fungi | Mycorrhizal colonization contributes to Po cycling and plant Pi acquisition | Phytate, RNA, lecithin | Red clover | [146] |
| Application of bacterial grazer (nematodes) together with mycorrhiza and P-solubilizing bacteria | Interaction of bacterial grazers with mycorrhiza and phosphobacteria promotes P org solubilization | Phytate | Maritime pine | [202] |
| Biochar addition to agricultural soils | Biochar enhances Pi-solubilizing bacteria | Po and Pi forms | Ryegrass | [203] |
| Genetic transformation of plants to overexpress extracellular phytases in root cells | Transgenic lines display better Pi nutrition owing to the efficient release of extracellular root phytases | Phytate | Arabidopsis Subterranean clover Potato Tobacco | [116,201,204] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Amadou, I.; Houben, D.; Faucon, M.-P. Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review. Agronomy 2021, 11, 2267. https://doi.org/10.3390/agronomy11112267
Amadou I, Houben D, Faucon M-P. Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review. Agronomy. 2021; 11(11):2267. https://doi.org/10.3390/agronomy11112267
Chicago/Turabian StyleAmadou, Issifou, David Houben, and Michel-Pierre Faucon. 2021. "Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review" Agronomy 11, no. 11: 2267. https://doi.org/10.3390/agronomy11112267
APA StyleAmadou, I., Houben, D., & Faucon, M.-P. (2021). Unravelling the Role of Rhizosphere Microbiome and Root Traits in Organic Phosphorus Mobilization for Sustainable Phosphorus Fertilization. A Review. Agronomy, 11(11), 2267. https://doi.org/10.3390/agronomy11112267








