Charcoal and Sago Bark Ash on pH Buffering Capacity and Phosphorus Leaching
Abstract
:1. Introduction
2. Materials and Methods
2.1. Soil Sampling and Selected Physico-Chemical Analyses
2.2. Characterization of Charcoal and Sago Bark Ash
2.3. pH Buffering Capacity Determination
- T1:
- Soil only
- T2:
- Charcoal only
- T3:
- TSago bark ash only
- T4:
- 300 g soil + 15.42 g charcoal
- T5:
- 300 g soil + 7.71 g sago bark ash
- T6:
- 300 g soil + 15.42 g charcoal + 7.71 g sago bark ash
2.4. Laboratory Leaching Experiment
2.5. Experimental Design and Statistical Analysis
3. Results and Discussion
3.1. pH Buffering Capacity of Soil, Charcoal, and Sago Bark Ash
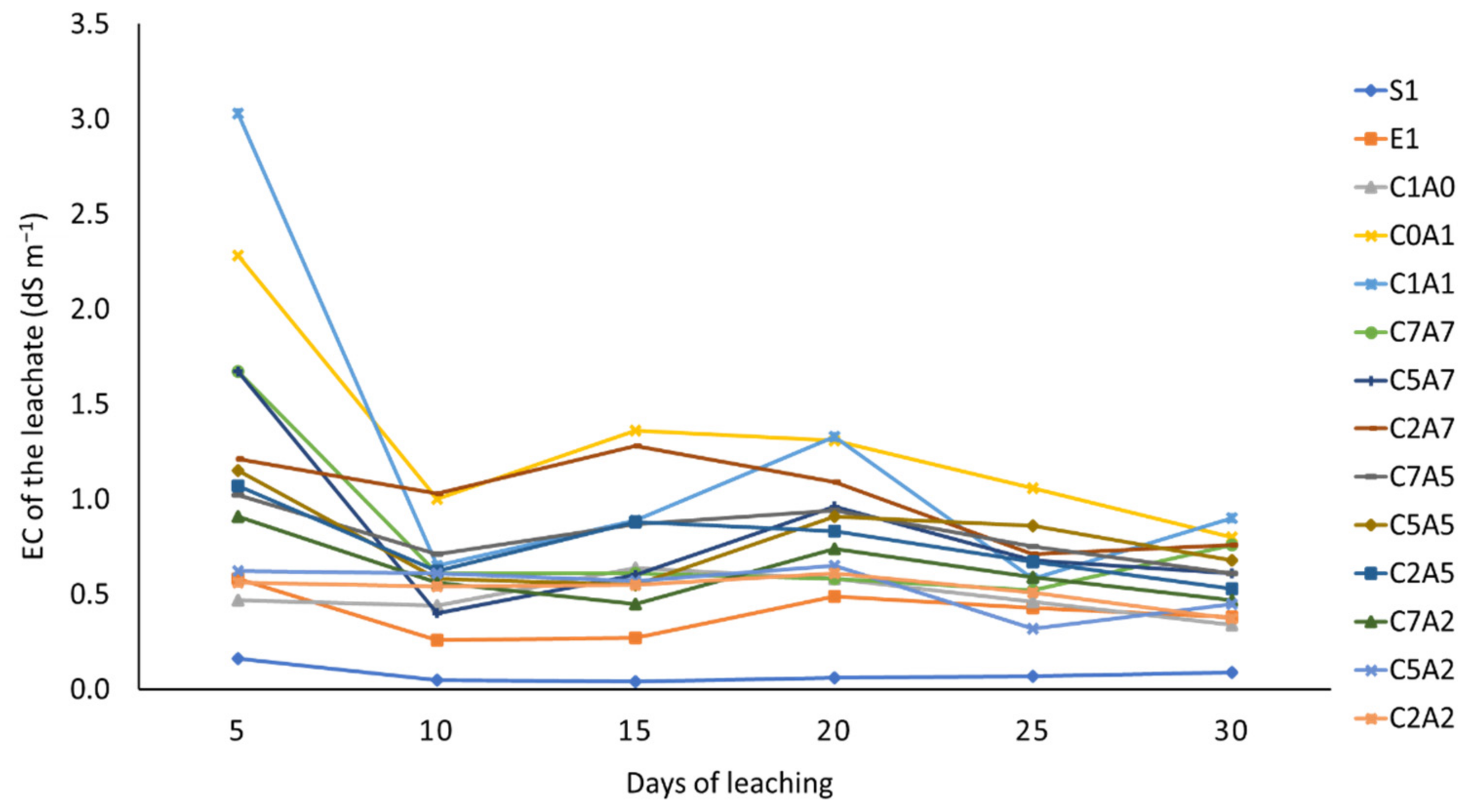
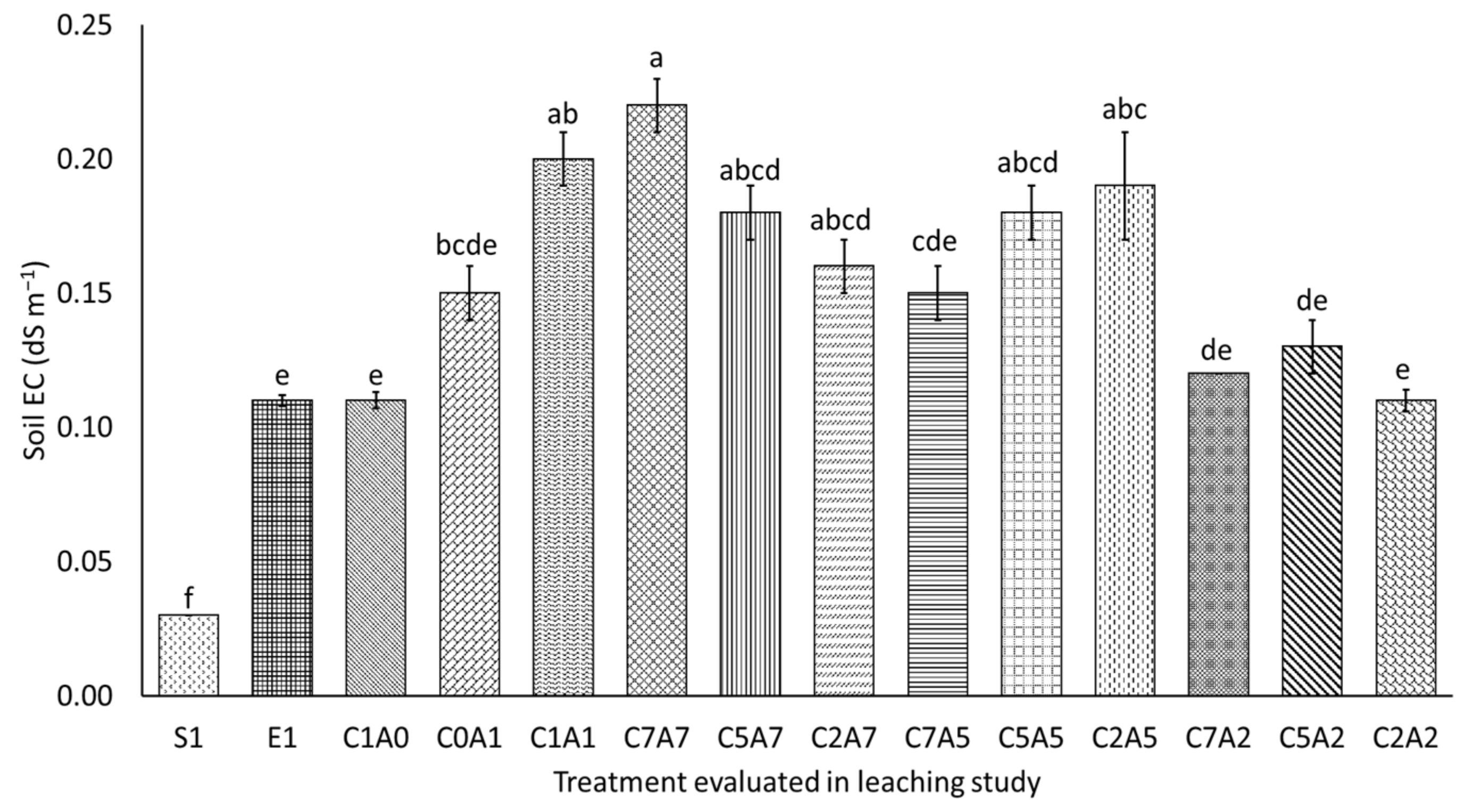
3.2. Treatments on pH and Electrical Conductivity of Leachate over Thirty Days of Leaching
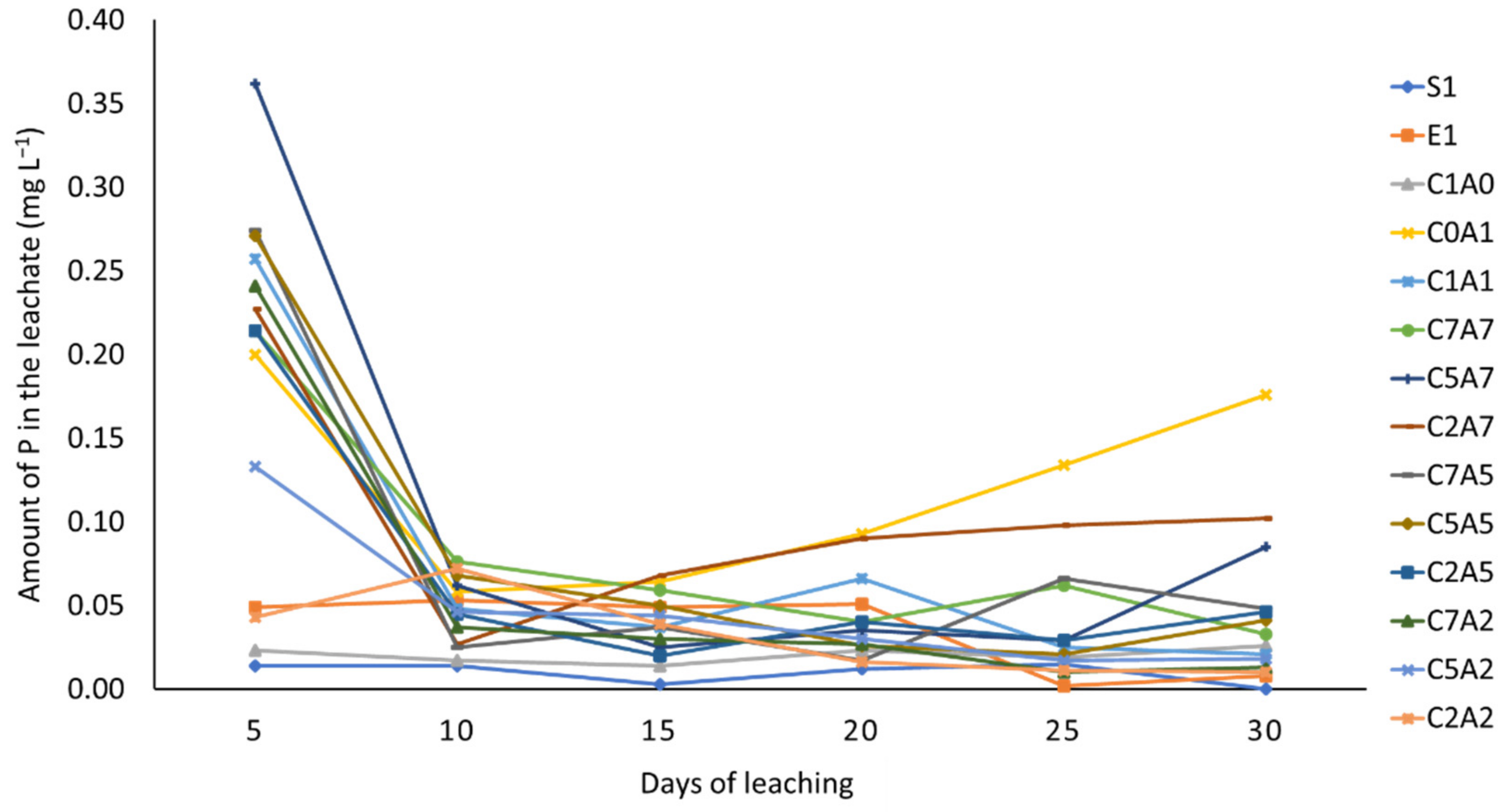
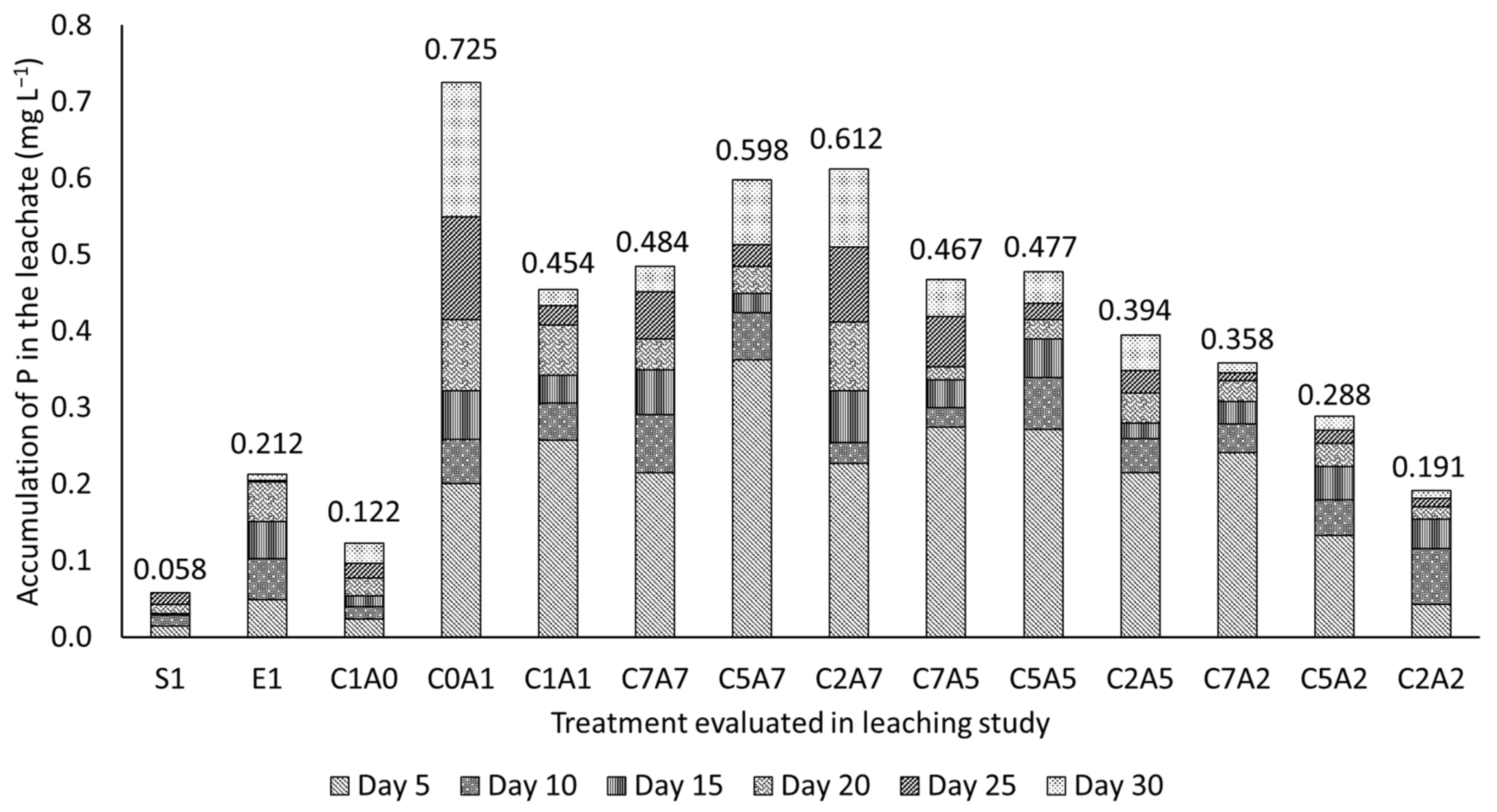
3.3. Effects of Treatments on Phosphorus Availability in Leachate
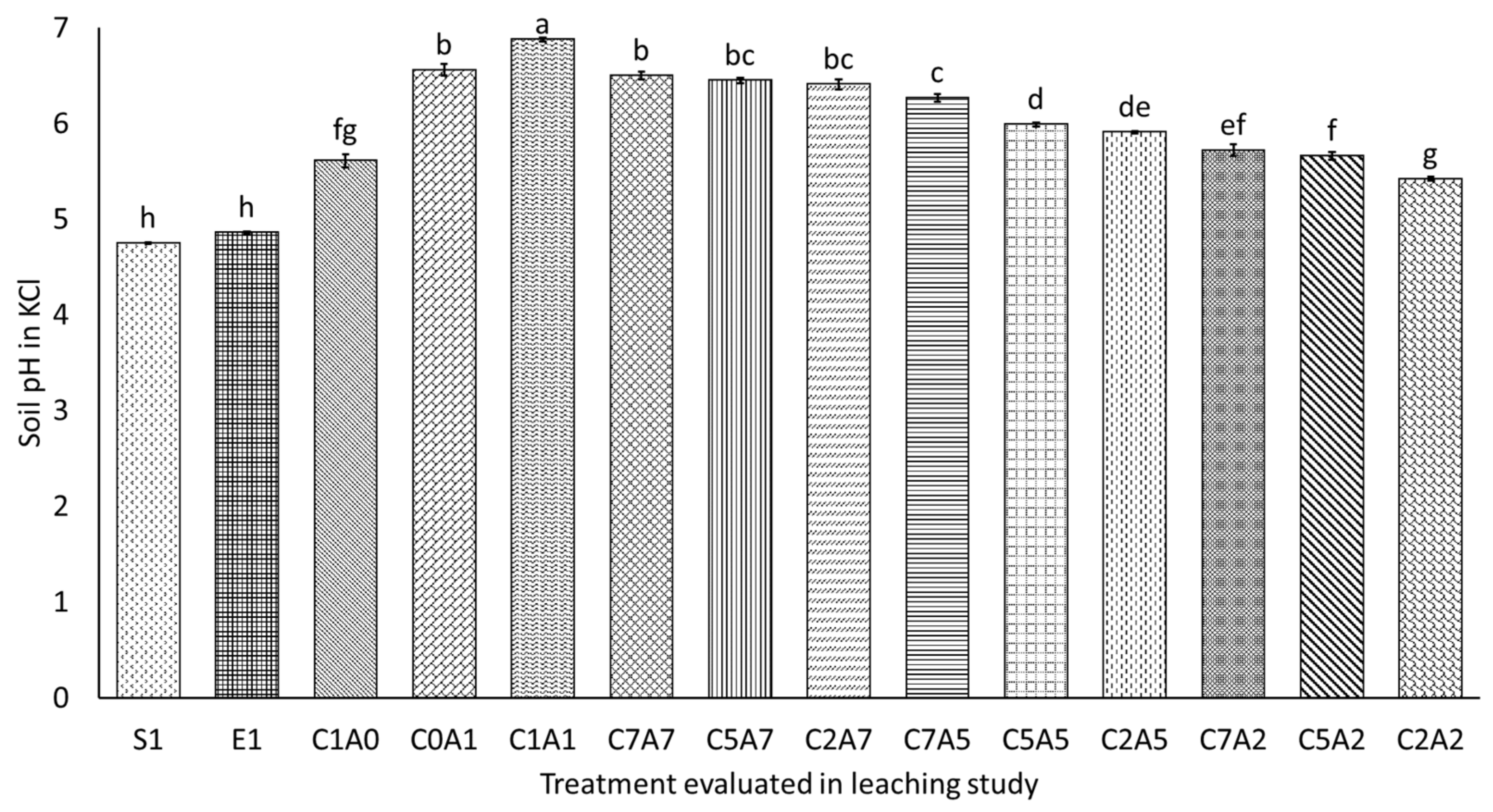
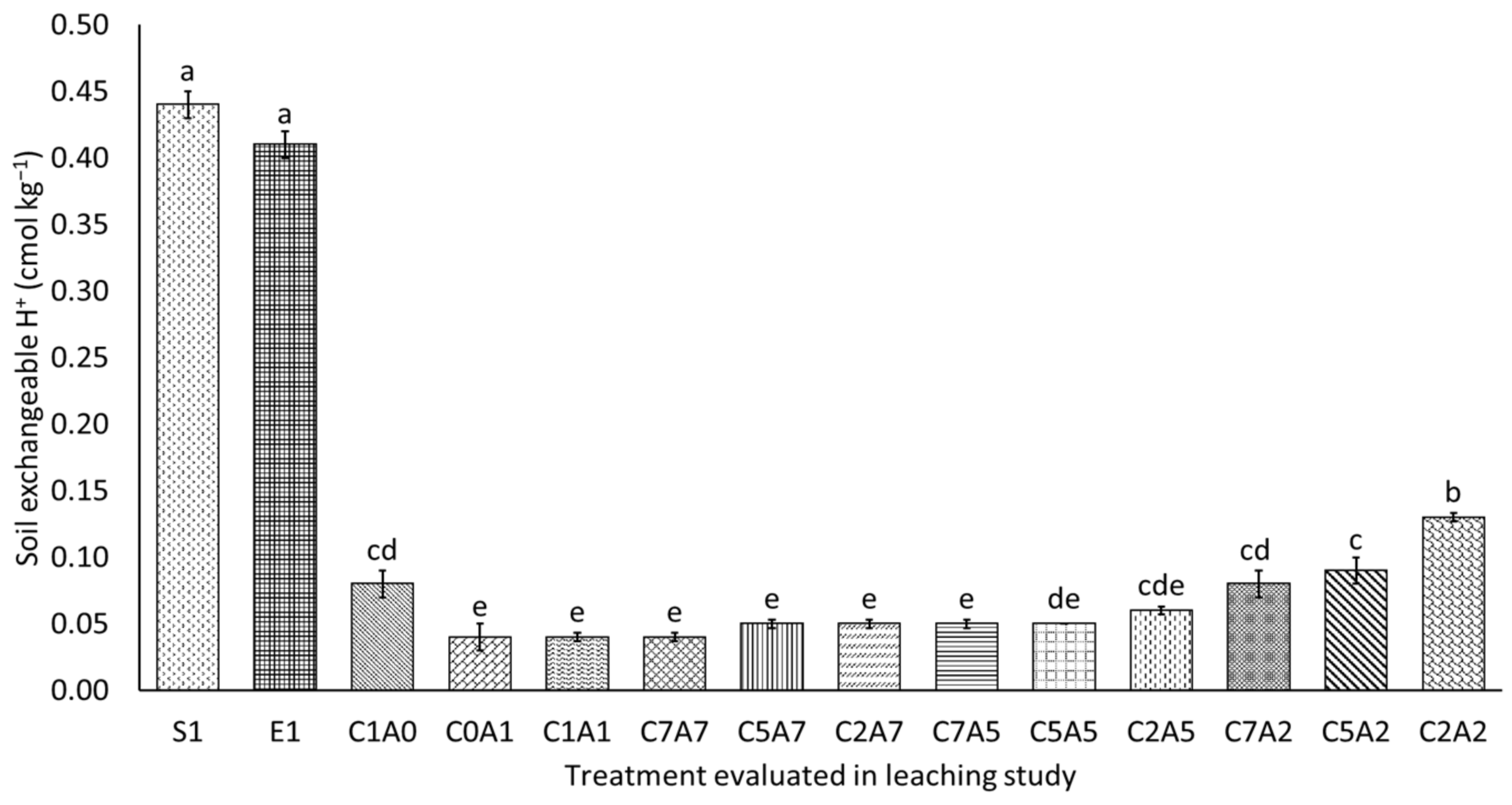
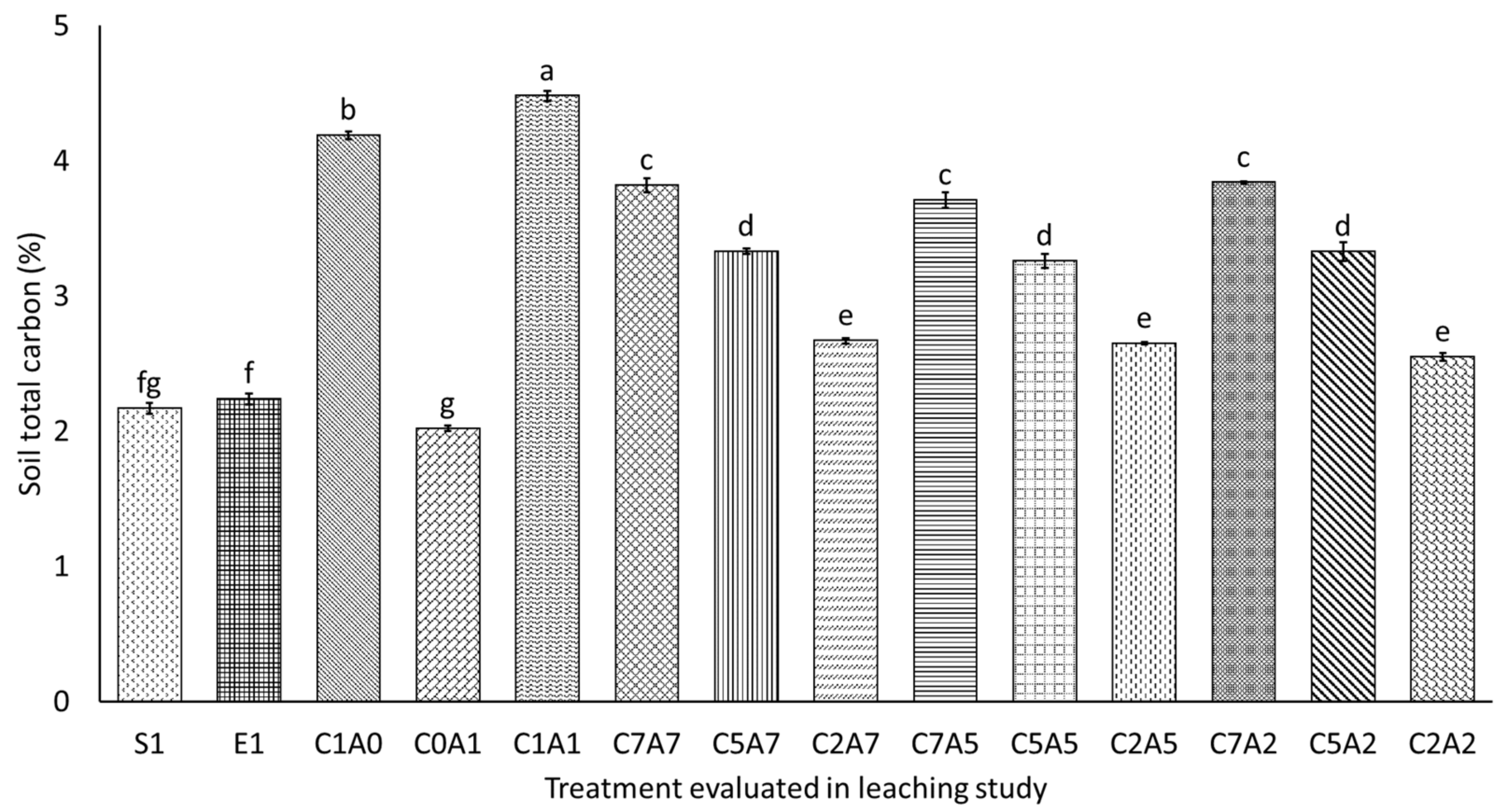
3.4. Selected Soil Chemical Properties after Thirty Days of Leaching
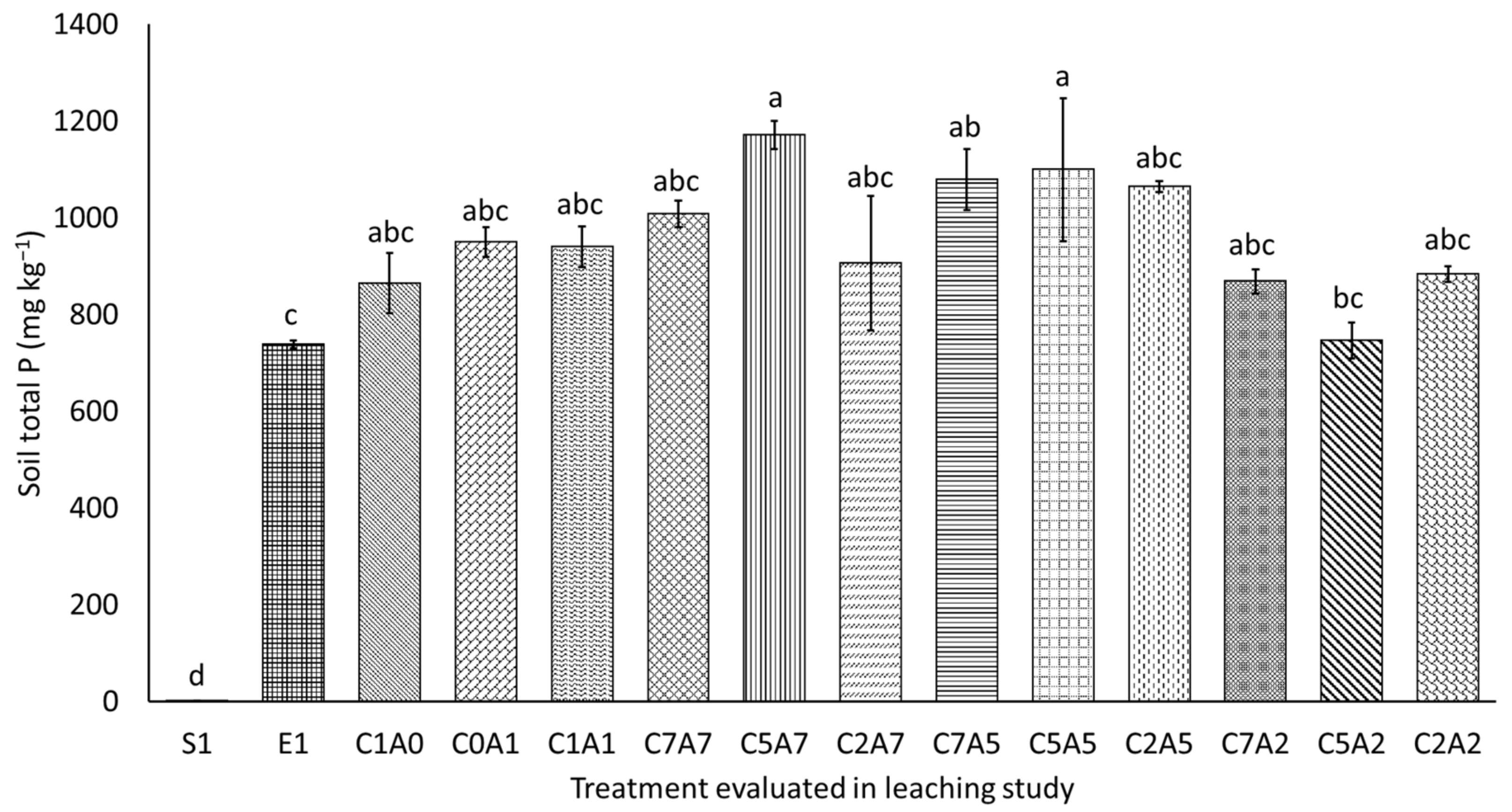
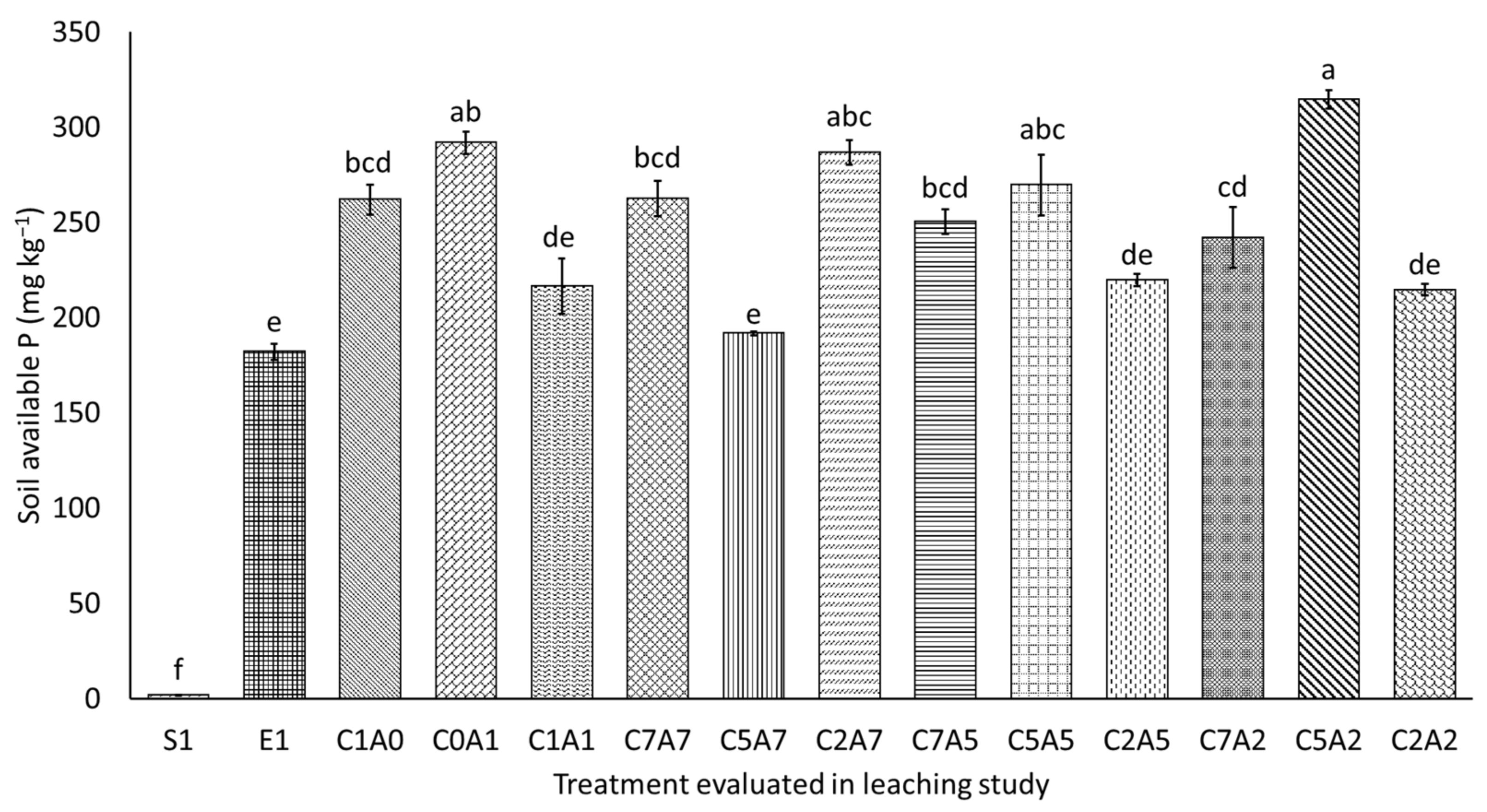
3.5. Soil Total Phosphorus and Available Phosphorus after Thirty Days of Leaching
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Atkinson, C.J.; Fitzgerald, J.D.; Hipps, N.A. Potential mechanisms for achieving agricultural benefits from biochar application to temperate soils: A review. Plant Soil 2010, 337, 1–18. [Google Scholar] [CrossRef]
- Sharma, S.B.; Sayyed, R.Z.; Trivedi, M.H.; Gobi, T.A. Phosphate solubilizing microbes: Sustainable approach for managing phosphorus deficiency in agricultural soils. SpringerPlus 2012, 2, 1–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maguire, R.O.; Foy, R.H.; Bailey, J.S.; Sims, J.T. Estimation of the phosphorus sorption capacity of acidic soils in Ireland. Eur. J. Soil Sci. 2001, 52, 479–487. [Google Scholar] [CrossRef]
- Fink, J.R.; Inda, A.V.; Bavaresco, J.; Barrón, V.; Torrent, J.; Bayer, C. Adsorption and desorption of phosphorus in subtropical soils as affected by management system and mineralogy. Soil Tillage Res. 2016, 155, 62–68. [Google Scholar] [CrossRef]
- Bulmer, D.; Kar, G.; Hamilton, J.; Siciliano, S.; Peak, D. Extent and mechanism of interaction between phosphate and citrate in a calcareous soil. Soil Sci. Soc. Am. J. 2018, 82, 315–322. [Google Scholar] [CrossRef] [Green Version]
- Xu, N.; Chen, M.; Zhou, K.; Wang, Y.; Yin, H.; Chen, Z. Retention of phosphorus on calcite and dolomite: Speciation and modeling. RSC Adv. 2014, 4, 35205–35214. [Google Scholar] [CrossRef]
- Pant, H.K.; Reddy, K.R.; Dierberg, F.E. Bioavailability of organic phosphorus in a submerged aquatic vegetation–dominated treatment wetland. J. Environ. Qual. 2002, 31, 1748–1756. [Google Scholar] [CrossRef]
- Penn, C.J.; Camberato, J.J. A critical review on soil chemical processes that control how soil pH affects phosphorus availability to plants. Agriculture 2019, 9, 120. [Google Scholar] [CrossRef] [Green Version]
- Bolan, N.S.; Adriano, D.C.; Curtin, D. Soil acidification and liming interactions with nutrient and heavy metal transformation and bioavailability. Adv. Agron. 2003, 78, 215–272. [Google Scholar]
- Graham, P.H.; Vance, C.P. Legumes: Importance and constraints to greater use. Plant. Physiol. 2003, 131, 872–877. [Google Scholar] [CrossRef] [Green Version]
- Kunhikrishnan, A.; Thangarajan, R.; Bolan, N.S.; Xu, Y.; Mandal, S.; Gleeson, D.B.; Seshadri, B.; Zaman, M.; Barton, L.; Tang, C.; et al. Functional relationships of soil acidification, liming, and greenhouse gas flux. Adv. Agron. 2016, 139, 1–71. [Google Scholar]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Minimizing phosphorus sorption and leaching in a tropical acid soil using Egypt rock phosphate with organic amendments. Philipp. Agric. Sci. 2016, 99, 176–185. [Google Scholar]
- Latifah, O.; Ahmed, O.H.; Majid, N.M.A. Soil pH buffering capacity and nitrogen availability following compost application in a tropical acid soil. Compost. Sci. Util. 2018, 26, 1–15. [Google Scholar] [CrossRef]
- Garcıa-Gil, J.C.; Ceppi, S.B.; Velasco, M.I.; Polo, A.; Senesi, N. Long-term effects of amendment with municipal solid waste compost on the elemental and acidic functional group composition and pH-buffer capacity of soil humic acids. Geoderma 2004, 121, 135–142. [Google Scholar] [CrossRef]
- Curtin, D.; Trolove, S. Predicting pH buffering capacity of New Zealand soils from organic matter content and mineral characteristics. Soil Res. 2013, 51, 494–502. [Google Scholar] [CrossRef]
- Latifah, O.; Ahmed, O.H.; Abdul Majid, N.M. Enhancing nitrogen availability, ammonium adsorption-desorption, and soil pH buffering capacity using composted paddy husk. Eurasian Soil Sci. 2017, 50, 1483–1493. [Google Scholar] [CrossRef]
- Sharpley, A.; Wang, X. Managing agricultural phosphorus for water quality: Lessons from the USA and China. J. Environ. Sci. 2014, 26, 1770–1782. [Google Scholar] [CrossRef]
- Silveira, M.L.; Vendramini, J.; Sollenberger, L.E. Phosphorus management and water quality problems in grazingland ecosystems. Int. J. Agron. 2010, 2010, 517603. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, R.M.; Parfitt, R.L.; Mackay, A.D.; Ross, D.J.; Budding, P.J. Effects of soil fertility on leaching losses of N, P and C in hill country. N. Z. J. Agric. Res. 2009, 52, 495–497. [Google Scholar] [CrossRef]
- Hoseini, Y.; Taleshmikaiel, R.D. Comparison of phosphorus adsorption isotherms in soil and its relation to soil properties. Int. J. Agric. 2013, 3, 163–171. [Google Scholar]
- Aase, J.K.; Bjorneborg, D.L.; Westermann, D.T. Phosphorus runoff from two water sources on a calcareous soil. J. Environ. Qual. 2001, 30, 1315–1323. [Google Scholar] [CrossRef] [Green Version]
- Jarvis, N.J. A review of non-equilibrium water flow and solute transport in soil macropores: Principles, controlling factors and consequences for water quality. Eur. J. Soil Sci. 2007, 58, 523–546. [Google Scholar] [CrossRef]
- McDowell, R.W.; Gray, C.W.; Cameron, K.C.; Di, H.J.; Pellow, R. The efficacy of good practice to prevent long-term leaching losses of phosphorus from an irrigated dairy farm. Agric. Ecosyst. Environ. 2019, 273, 86–94. [Google Scholar] [CrossRef]
- Ulen, B.; Larsbo, M.; Koestel, J.; Hellner, Q.; Blomberg, M.; Geranmayeh, P. Assessing strategies to mitigate phosphorus leaching from drained clay soils. Ambio 2018, 47, 114–123. [Google Scholar] [CrossRef] [Green Version]
- Coelho, B.B.; Murray, R.; Lapen, D.; Topp, E.; Bruin, A. Phosphorus and sediment loading to surface waters from liquid swine manure application under different drainage and tillage practices. Agric. Water Manag. 2012, 104, 51–61. [Google Scholar] [CrossRef]
- Sharpley, A.; Smith, S.; Stewart, B.; Mathers, A. Forms of phosphorus in soil receiving cattle feedlot waste. J. Environ. Qual. 1984, 13, 211–215. [Google Scholar] [CrossRef] [Green Version]
- Ch’ng, H.Y.; Ahmed, O.H.; Majid, N.M.A. Improving phosphorus availability, nutrient uptake and dry matter production of Zea mays L. on a tropical acid soil using poultry manure biochar and pineapple leaves compost. Exp. Agric. 2016, 52, 447–465. [Google Scholar] [CrossRef]
- Asap, A.; Haruna, A.O.; Ab Majid, N.M.; Ali, M. Amending triple superphosphate with chicken litter biochar improves phosphorus availability. Eurasian J. Soil Sci. 2018, 7, 121–132. [Google Scholar] [CrossRef]
- Bot, A.; Benites, J. The Importance of Soil Organic Matter: Key to Drought-Resistant Soil and Sustained Food Production; FAO Soils Bulletin 80; FAO: Rome, Italy, 2005. [Google Scholar]
- Nadi, M.; Sedaghati, E.; Fuleky, G. Evaluation of humus quality of forest soils with two extraction methods. Int. J. For. Soil Eros. 2012, 2, 124–127. [Google Scholar]
- Dixon, R.K.; Wisniewski, J. Global forest systems: An uncertain response to atmospheric pollutants and global climate change. Water Air Soil Pollut. 1995, 85, 101–110. [Google Scholar] [CrossRef]
- Bouyoucos, G.J. Hydrometer method improved for making particle size analysis of soils. Agron. J. 1962, 54, 464–465. [Google Scholar] [CrossRef]
- Peech, H.M. Hydrogen-ion Activity. In Methods of Soil Analysis Chemical and Microbiological Properties; Black, C.A., Evans, D.D., White, J.L., Ensminger, L.E., Clark, F.E., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 914–926. [Google Scholar]
- Chefetz, B.; Hatcher, P.H.; Hadar, Y.; Chen, Y. Chemical and biological characterization of organic matter during composting of municipal solid waste. J. Environ. Qual. 1996, 25, 776–785. [Google Scholar] [CrossRef]
- Bremner, J.M. Total Nitrogen. In Methods of Soil Analysis: Part 2; Black, C.A., Evans, D.D., Ensminger, L.E., White, J.L., Clark, F.F., Dinauer, R.C., Eds.; American Society of Agronomy: Madison, WI, USA, 1965; pp. 1149–1178. [Google Scholar]
- Cottenie, A. Soil Testing and Plant Testing as a Basis of Fertilizer Recommendation; FAO Soils Bulletin 38/2; FAO: Rome, Italy, 1980; pp. 1–119. [Google Scholar]
- Rowell, D.L. Soil Science: Methods and Application; Longman Publishers Ltd.: London, UK, 1994; p. 169. [Google Scholar]
- Bernas, B. New method for decomposition and comprehensive analysis of silicates by atomic absorption spectrometry. Anal. Chem. 1968, 40, 1682–1686. [Google Scholar] [CrossRef]
- Mehlich, A. Determination of P, Ca, Mg, K, Na and NH4. Short Test Methods Used in Soil Testing Division; Department of Agriculture: Raleigh, NC, USA, 1953.
- Murphy, J.; Riley, J.P. A modified single solution method for the determination of phosphate in natural waters. Anal. Chim. Acta 1962, 27, 31–36. [Google Scholar] [CrossRef]
- Paramananthan, S. Soil of Malaysia: Their Characteristics and Identification; Academy of Sciences Malaysia: Kuala Lumpur, Malaysia, 2000; Volume 1, pp. 11–125. [Google Scholar]
- Free, H.F.; McGill, C.R.; Rowarth, J.S.; Hedley, M.J. The effect of biochars on maize (Zea mays) germination. N. Z. J. Agric. Res. 2010, 53, 1–4. [Google Scholar] [CrossRef]
- Ndor, E.; Dauda, S.N.; Azagaku, E.D. Response of maize varieties (Zea mays) to biochar amended soil in Lafia, Nigeria. J. Exp. Agric. Int. 2015, 5, 525–531. [Google Scholar] [CrossRef]
- Mandre, M.; Pärn, H.; Ots, K. Short-term effects of wood ash on the soil and the lignin concentration and growth of Pinus sylvestris L. For. Ecol. Manag. 2006, 223, 349–357. [Google Scholar] [CrossRef]
- Ozolincius, R.; Buozyte, R.; Varnagiryte, K.I. Wood ash and nitrogen influence on ground vegetation cover and chemical composition. Biomass Bioenergy 2007, 31, 710–716. [Google Scholar] [CrossRef]
- Perucci, P.; Monaci, E.; Onofri, A.; Vischetti, C.; Casucci, C. Changes in physico-chemical and biochemical parameters of soil following addition of wood ash: A field experiment. Eur. J. Agron. 2008, 28, 155–161. [Google Scholar] [CrossRef]
- Costello, R.C.; Sullivan, D.M. Determining the pH buffering capacity of compost via titration with dilute sulfuric acid. Waste Biomass Valorization 2014, 5, 505–513. [Google Scholar] [CrossRef]
- Malaysian Agricultural Research and Development Institute (MARDI). Jagung Manis Baru, Masmadu [New Sweet Corn, Masmadu]; Malaysian Agricultural Research and Development: Serdang, Malaysia, 1993; pp. 3–5.
- Nelson, P.N.; Su, N. Soil pH buffering capacity: A descriptive function and its application to some acidic tropical soils. Soil Res. 2010, 48, 201–207. [Google Scholar] [CrossRef]
- Wei, H.; Liu, Y.; Xiang, H.; Zhang, J.; Li, S.; Yang, J. Soil pH responses to simulated acid rain leaching in three agricultural soils. Sustainability 2020, 12, 280. [Google Scholar] [CrossRef] [Green Version]
- Xu, R.K.; Zhao, A.Z.; Yuan, J.H.; Jiang, J. pH buffering capacity of acid soils from tropical and subtropical regions of China as influenced by incorporation of crop straw biochars. J. Soils Sediments 2012, 12, 494–502. [Google Scholar] [CrossRef]
- Jusop, S.; Ishak, C.F. Weathered Tropical Soils the Ultisols and Oxisols; Universiti Putra Malaysia Press: Serdang, Malaysia, 2010. [Google Scholar]
- Shi, R.Y.; Li, J.Y.; Ni, N.I.; Xu, R.K. Understanding the biochar’s role in ameliorating soil acidity. J. Integr. Agric. 2019, 18, 1508–1517. [Google Scholar] [CrossRef]
- Wiklund, J. Effects of Wood Ash on Soil Fertility and Plant Performance in Southwestern Kenya. Master’s Thesis, Swedish University of Agricultural Sciences, Uppsala, Sweden, 2017. [Google Scholar]
- Bang-Andreasen, T.; Nielsen, J.T.; Voriskova, J.; Heise, J.; Rønn, R.; Kjøller, R.; Hansen, H.C.; Jacobsen, C.S. Wood ash induced pH changes strongly affect soil bacterial numbers and community composition. Front. Microbiol. 2017, 8, 1400. [Google Scholar] [CrossRef]
- Demeyer, A.; Voundi Nkana, J.; Verloo, M. Characteristics of wood ash influence on soil properties and nutrient uptake: An overview. Bioresour. Technol. 2001, 77, 287–295. [Google Scholar] [CrossRef]
- Arvidsson, H.; Lundkvist, H. Effects of crushed wood ash on soil chemistry in young Norway spruce stands. For. Ecol. Manag. 2003, 176, 121–132. [Google Scholar] [CrossRef]
- Lim, S.S.; Choi, W.J.; Lee, K.S.; Ro, H.M. Reduction in CO2 emission from normal and saline soils amended with coal fly ash. J. Soils Sediments 2012, 12, 1299–1308. [Google Scholar] [CrossRef]
- Basso, A.S.; Miguez, F.E.; Laird, D.A.; Horton, R.; Westgate, M. Assessing potential of biochar for increasing water-holding capacity of sandy soils. GCB Bioenergy 2013, 5, 132–143. [Google Scholar] [CrossRef] [Green Version]
- Batista, E.M.; Shultz, J.; Matos, T.T.; Fornari, M.R.; Ferreira, T.M.; Szpoganicz, B.; de Freitas, R.A.; Mangrich, A.S. Effect of surface and porosity of biochar on water holding capacity aiming indirectly at preservation of the Amazon biome. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef] [Green Version]
- Eduah, J.O.; Nartey, E.K.; Abekoe, M.K.; Breuning-Madsen, H.; Andersen, M.N. Phosphorus retention and availability in three contrasting soils amended with rice husk and corn cob biochar at varying pyrolysis temperatures. Geoderma 2019, 341, 10–17. [Google Scholar] [CrossRef]
- Matin, N.H.; Jalali, M.; Antoniadis, V.; Shaheen, S.M.; Wang, J.; Zhang, T.; Wang, H.; Rinklebe, J. Almond and walnut shell-derived biochars affect sorption-desorption, fractionation, and release of phosphorus in two different soils. Chemosphere 2020, 241, 124888. [Google Scholar] [CrossRef]
- Monaghan, R.M.; De Klein, C.A.; Muirhead, R.W. Prioritisation of farm scale remediation efforts for reducing losses of nutrients and faecal indicator organisms to waterways: A case study of New Zealand dairy farming. J. Environ. Manag. 2008, 87, 609–622. [Google Scholar] [CrossRef]
- Hasbullah, N.A. Use of Clinoptilolite Zeolite to Improve Efficiency of Phosphorus Use in Acid Soils. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2016. [Google Scholar]
- Palanivell, P. Organic and Mineral Amendments on Rice (Oryza sativa L.) Yield and Nutrients Recovery Efficiency. Ph.D. Thesis, Universiti Putra Malaysia, Serdang, Malaysia, 2016. [Google Scholar]
- Xu, G.; Sun, J.; Shao, H.; Chang, S.X. Biochar had effects on phosphorus sorption and desorption in three soils with differing acidity. Ecol. Eng. 2014, 62, 54–60. [Google Scholar] [CrossRef]
- Masulili, A.; Utomo, W.H.; Syechfani, M.S. Rice husk biochar for rice based cropping system in acid soil 1. The characteristics of rice husk biochar and its influence on the properties of acid sulfate soils and rice growth in West Kalimantan, Indonesia. J. Agric. Sci. 2010, 2, 39–47. [Google Scholar] [CrossRef] [Green Version]
- Etiegni, L.; Campbell, A.G. Physical and chemical characteristics of wood ash. Bioresour. Technol. 1991, 37, 173–178. [Google Scholar] [CrossRef]
- Ohno, T. Neutralization of soil acidity and release of phosphorus and potassium by wood ash. J. Environ. Qual. 1992, 21, 433–438. [Google Scholar] [CrossRef]
- Cai, Z.; Wang, B.; Xu, M.; Zhang, H.; He, X.; Zhang, L.; Gao, S. Intensified soil acidification from chemical N fertilization and prevention by manure in an 18-year field experiment in the red soil of southern China. J. Soils Sediments 2015, 15, 260–270. [Google Scholar] [CrossRef]
- Gondek, M.; Weindorf, D.C.; Thiel, C.; Kleinheinz, G. Soluble salts in compost and their effects on soil and plants: A review. Compost. Sci. Util. 2020, 28, 59–75. [Google Scholar] [CrossRef]
- Jones, D.L.; Quilliam, R.S. Metal contaminated biochar and wood ash negatively affect plant growth and soil quality after land application. J. Hazard. Mater. 2014, 276, 362–370. [Google Scholar] [CrossRef] [PubMed]
- Lucchini, P.; Quilliam, R.S.; DeLuca, T.H.; Vamerali, T.; Jones, D.L. Increased bioavailability of metals in two contrasting agricultural soils treated with waste wood-derived biochar and ash. Environ. Sci. Pollut. Res. 2014, 21, 3230–3240. [Google Scholar] [CrossRef]
- Bauder, J.W.; Brock, T.A. Irrigation water quality, soil amendment, and crop effects on sodium leaching. Arid Land Res. Manag. 2001, 15, 101–113. [Google Scholar] [CrossRef]
- Assis, M.R.; Brancheriau, L.; Napoli, A.; Trugilho, P.F. Factors affecting the mechanics of carbonized wood: Literature review. Wood Sci. Technol. 2016, 50, 519–536. [Google Scholar] [CrossRef]
- Troeh, F.R.; Thompson, L.M. Soils and Soil Fertility; Blackwell: New York, NY, USA, 2005. [Google Scholar]
- Asmare, M.; Heluf, G.; Markku, Y.; Birru, Y. Phosphorus Status, Inorganic Phosphorus Forms, and Other Physicochemical Properties of Acid Soils of Farta District, Northwestern Highlands of Ethiopia. Appl. Environ. Soil Sci. 2015, 2015, 748390. [Google Scholar] [CrossRef] [Green Version]
- Xu, G.; Zhang, Y.; Sun, J.; Shao, H. Negative interactive effects between biochar and phosphorus fertilization on phosphorus availability and plant yield in saline sodic soil. Sci. Total Environ. 2016, 568, 910–915. [Google Scholar] [CrossRef]
- Oburger, E.; Kirk, G.J.; Wenzel, W.W.; Puschenreiter, M.; Jones, D.L. Interactive effects of organic acids in the rhizosphere. Soil Biol. Biochem. 2009, 41, 449–457. [Google Scholar] [CrossRef]




| Property | Value Obtained | Standard Range |
|---|---|---|
| pH (H2O) | 4.61 ± 0.05 | 4.6–4.9 |
| pH (KCl) | 3.95 ± 0.01 | 3.8–4.0 |
| EC (µS cm−1) | 35.10 ± 0.15 | NA |
| Bulk density (g cm−1) | 1.25 ± 0.04 | NA |
| Total carbon (%) | 2.16 ± 0.05 | 0.57–2.51 |
| Total N (%) | 0.08 ± 0.01 | 0.04–0.17 |
| Total P (mg kg−1) | 23.65 ± 1.09 | NA |
| Available P (mg kg−1) | 1.13 ± 0.02 | NA |
| CEC (cmol kg−1) | 4.67 ± 0.29 | 3.86–8.46 |
| Exchangeable acidity (cmol kg−1) | 1.15 ± 0.03 | NA |
| Exchangeable Al3+ (cmol kg−1) | 1.02 ± 0.03 | NA |
| Exchangeable H+ (cmol kg−1) | 0.13 ± 0.02 | NA |
| Exchangeable K+ (cmol kg−1) | 0.060 ± 0.002 | 0.05–0.19 |
| Exchangeable Ca2+ (cmol kg−1) | 0.020 ± 0.001 | 0.01 |
| Exchangeable Mg2+ (cmol kg−1) | 0.22 ± 0.003 | 0.07–0.21 |
| Exchangeable Na+ (cmol kg−1) | 0.030 ± 0.001 | 0.01 |
| Exchangeable Mn2+ (cmol kg−1) | 0.010 ± 0.001 | NA |
| Exchangeable Fe2+ (cmol kg−1) | 1.09 ± 0.02 | NA |
| Soil texture | Sand (%): 71.9 | Sand (%): 72–76 |
| Silt (%): 13.5 | Silt (%): 8–9 | |
| Clay (%): 14.6 | Clay (%): 16–19 | |
| Sandy loam | Sandy clay loam |
| Property | Charcoal | Sago Bark Ash |
|---|---|---|
| pH (H2O) | 7.74 ± 0.02 | 9.99 ± 0.03 |
| pH (KCl) | 7.31 ± 0.05 | 9.66 ± 0.02 |
| EC (dS m−1) | 0.270 ± 0.006 | 5.75 ± 0.02 |
| Available P (mg kg−1) | 31.25 ± 1.15 | 55.83 ± 1.32 |
| Exchangeable K+ (cmol kg−1) | 3.67 ± 0.06 | 23.33 ± 0.25 |
| Exchangeable Ca2+ (cmol kg−1) | 11.71 ± 0.32 | 16.77 ± 0.48 |
| Exchangeable Mg2+ (cmol kg−1) | 3.37 ± 0.03 | 3.57 ± 0.03 |
| Exchangeable Na+ (cmol kg−1) | 0.43 ± 0.01 | 1.51 ± 0.03 |
| Exchangeable Fe2+ (cmol kg−1) | 0.150 ± 0.003 | 0.030 ± 0.001 |
| Treatment | Soil (kg) | ERP (g) | Charcoal (g) | Sago Bark Ash (g) | Charcoal:Ash Ratio |
|---|---|---|---|---|---|
| S1 | 1 | − | − | − | Soil only |
| E1 | 1 | 7.7 | − | − | ERP only |
| C1A0 | 1 | 7.7 | 51.4 | − | Charcoal only |
| C0A1 | 1 | 7.7 | − | 25.7 | Ash only |
| C1A1 | 1 | 7.7 | 51.4 | 25.7 | 100:100 |
| C7A7 | 1 | 7.7 | 38.6 | 19.3 | 75:75 |
| C5A7 | 1 | 7.7 | 25.7 | 19.3 | 50:75 |
| C2A7 | 1 | 7.7 | 12.9 | 19.3 | 25:75 |
| C7A5 | 1 | 7.7 | 38.6 | 12.9 | 75:50 |
| C5A5 | 1 | 7.7 | 25.7 | 12.9 | 50:50 |
| C2A5 | 1 | 7.7 | 12.9 | 12.9 | 25:50 |
| C7A2 | 1 | 7.7 | 38.6 | 6.4 | 75:25 |
| C5A2 | 1 | 7.7 | 25.7 | 6.4 | 50:25 |
| C2A2 | 1 | 7.7 | 12.9 | 6.4 | 25:25 |
| Treatment Code | Initial pH | pH Buffering Capacity (mol H+ kg−1 Sample) | Regression Coefficient, R2 |
|---|---|---|---|
| T1 | 5.31 ± 0.05 | 0.25 | 0.92 * |
| T2 | 7.76 ± 0.06 | 0.29 | 0.90 * |
| T3 | 9.78 ± 0.00 | 0.34 | 0.92 * |
| T4 | 6.51 ± 0.03 | 0.26 | 0.97 * |
| T5 | 6.41 ± 0.02 | 0.28 | 0.93 * |
| T6 | 6.65 ± 0.03 | 0.29 | 0.92 * |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johan, P.D.; Ahmed, O.H.; Omar, L.; Hasbullah, N.A. Charcoal and Sago Bark Ash on pH Buffering Capacity and Phosphorus Leaching. Agronomy 2021, 11, 2223. https://doi.org/10.3390/agronomy11112223
Johan PD, Ahmed OH, Omar L, Hasbullah NA. Charcoal and Sago Bark Ash on pH Buffering Capacity and Phosphorus Leaching. Agronomy. 2021; 11(11):2223. https://doi.org/10.3390/agronomy11112223
Chicago/Turabian StyleJohan, Prisca Divra, Osumanu Haruna Ahmed, Latifah Omar, and Nur Aainaa Hasbullah. 2021. "Charcoal and Sago Bark Ash on pH Buffering Capacity and Phosphorus Leaching" Agronomy 11, no. 11: 2223. https://doi.org/10.3390/agronomy11112223
APA StyleJohan, P. D., Ahmed, O. H., Omar, L., & Hasbullah, N. A. (2021). Charcoal and Sago Bark Ash on pH Buffering Capacity and Phosphorus Leaching. Agronomy, 11(11), 2223. https://doi.org/10.3390/agronomy11112223






