Organic Matter Composition of Digestates Has a Stronger Influence on N2O Emissions than the Supply of Ammoniacal Nitrogen
Abstract
:1. Introduction
2. Materials and Methods
2.1. Digestate Collection and Composition
2.2. Experimental Site
2.3. Field Experiment
2.4. Incubation Experiment
2.5. Trace Gas Analysis and Flux Rate Calculation
2.6. Statistical Analysis
3. Results
3.1. Field Experiment
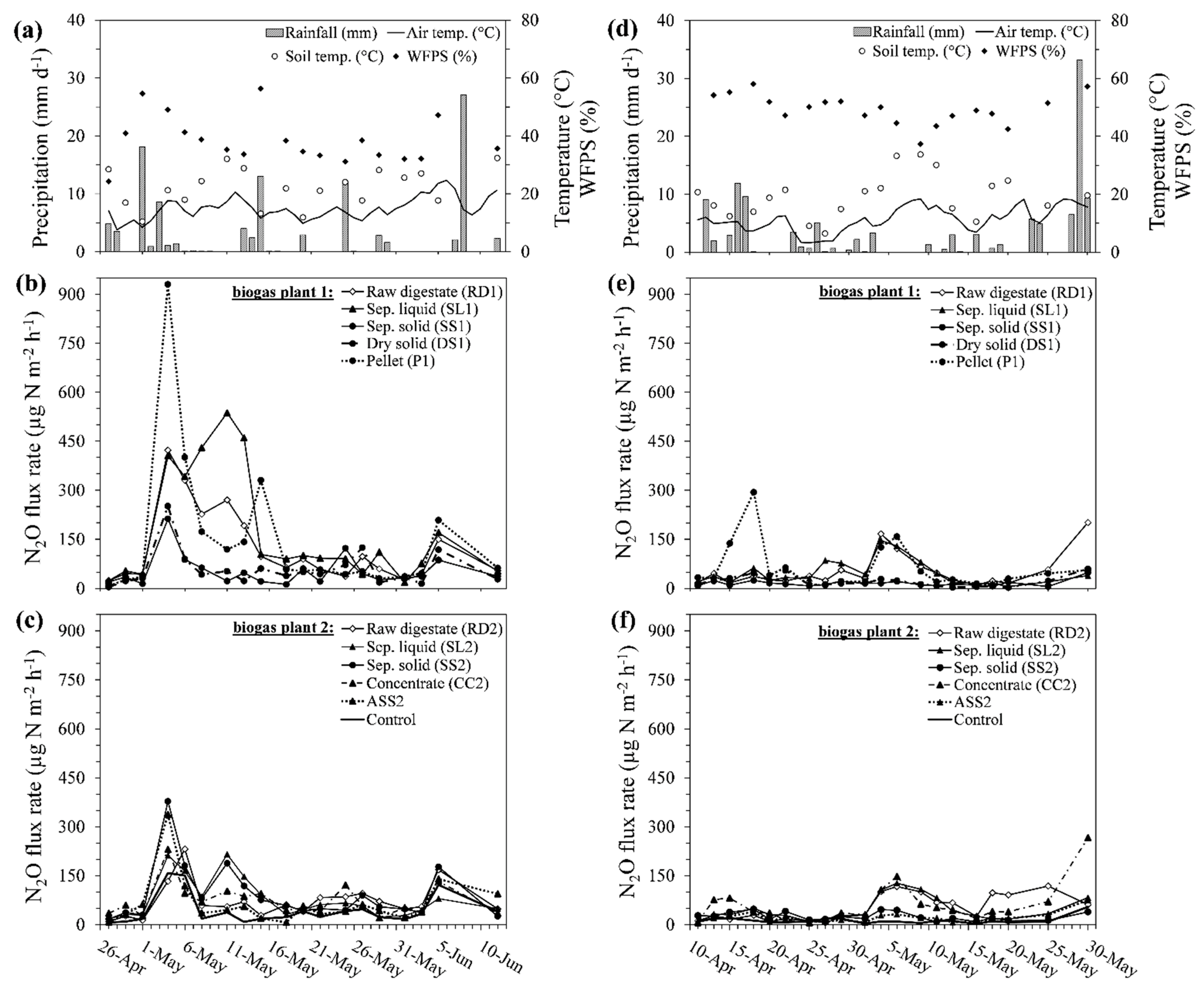
3.1.1. Weather Conditions in 2015
3.1.2. Daily N2O Fluxes in 2015
3.1.3. Weather Conditions in 2016
3.1.4. Daily N2O Fluxes in 2016
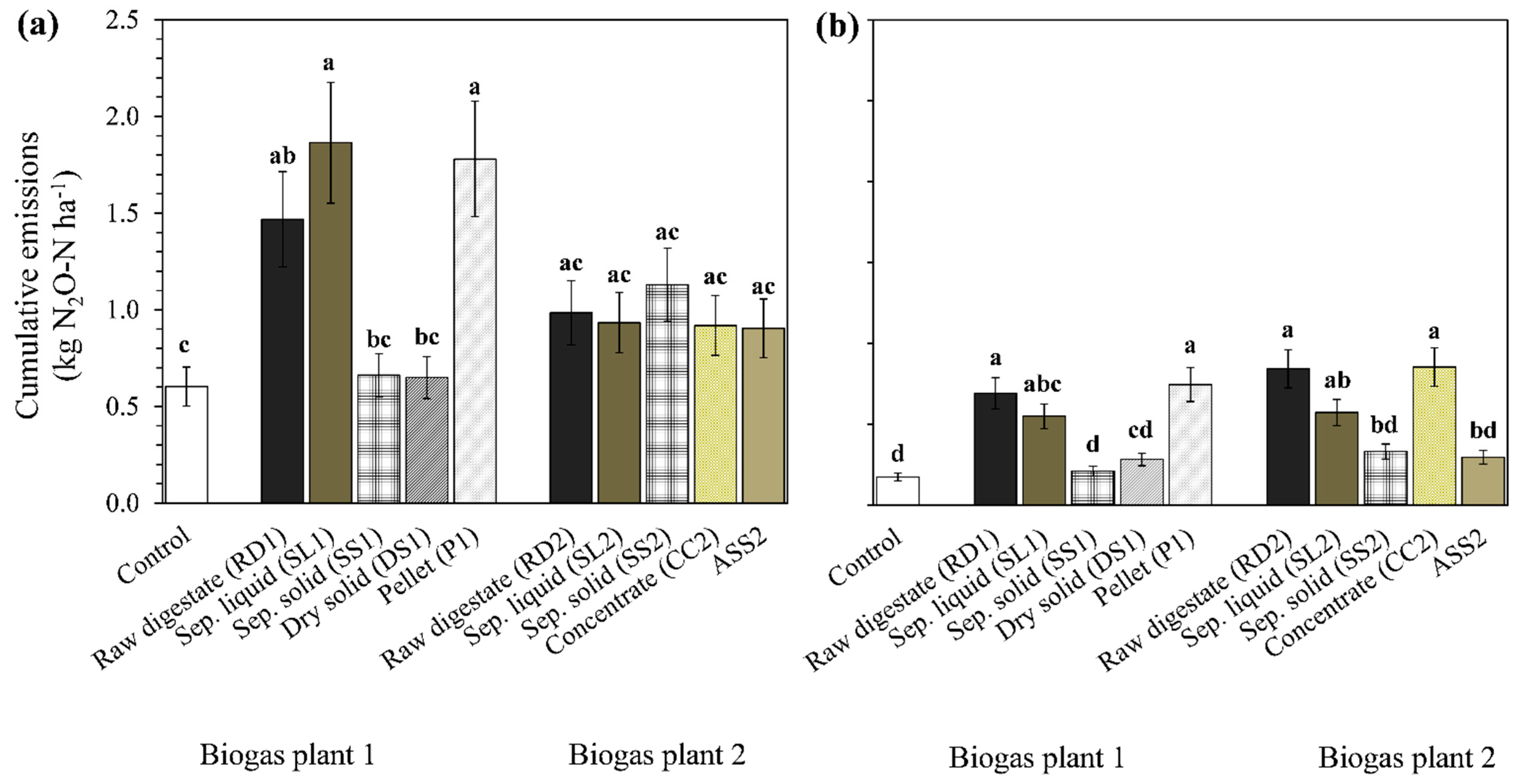
3.1.5. Cumulative N2O Emissions
3.2. Incubation Experiment
3.2.1. Daily N2O Fluxes
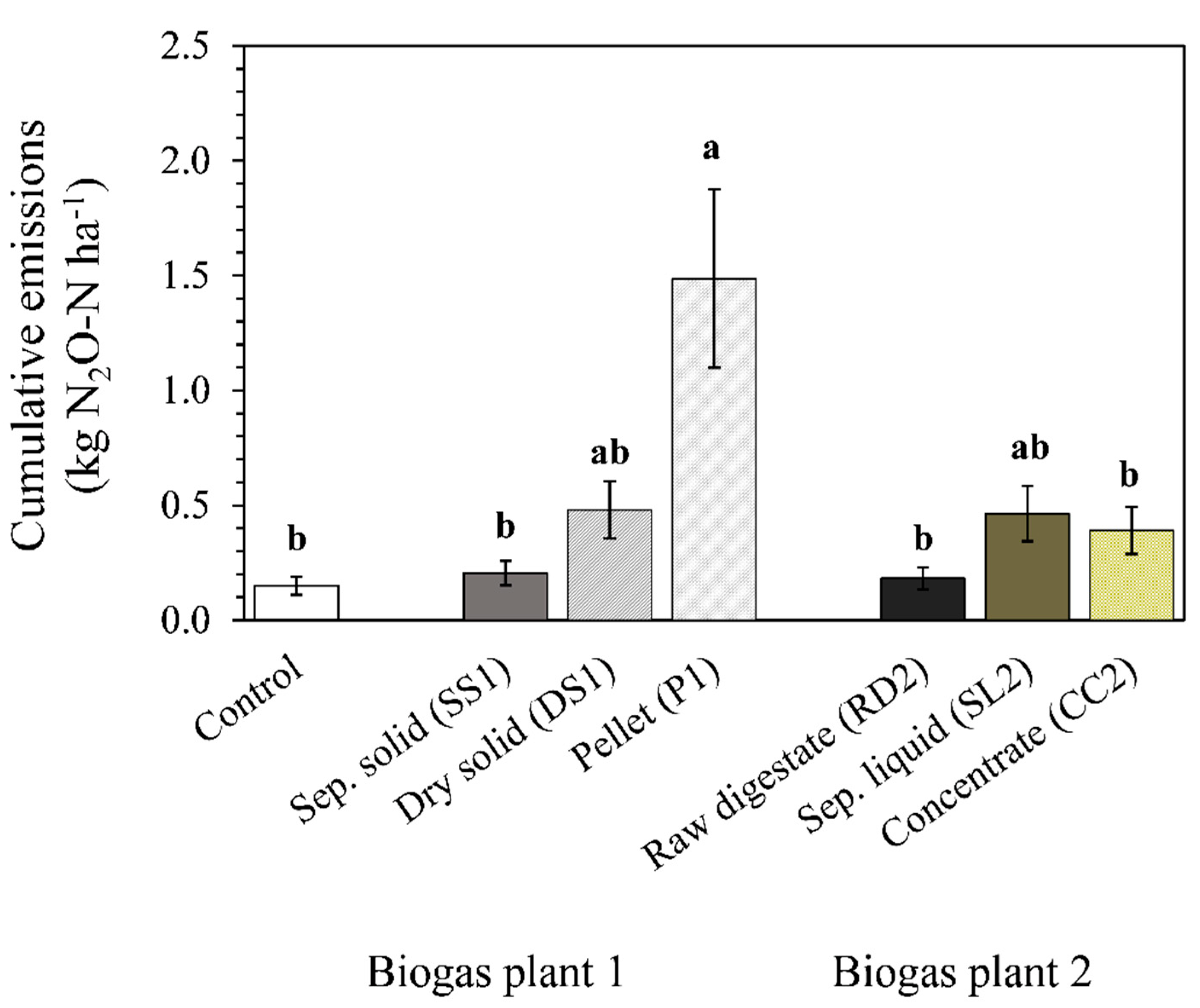
3.2.2. Cumulative N2O Emissions
4. Discussion
4.1. General Factors Influencing the N2O Release
4.2. N2O Emissions Affected by Changes in Digestate Composition Due to Processing
4.2.1. Solid–Liquid Separation
4.2.2. Subsequent Processing of Separated Liquid
4.2.3. Further Processing of Separated Solid
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Bäurle, H.; Tamásy, C. Regionale Konzentrationen der Nutztierhaltung in Deutschland. Mitteilungen/Institut für Strukturforschung und Planung in Agrarischen Intensivgebieten-Universität Vechta 2012, 79, 1–89. [Google Scholar]
- Tamásy, C. Intensivierung der Landwirtschaft im Oldenburger Münsterland. Standort 2014, 38, 203–207. [Google Scholar] [CrossRef]
- Häußermann, U.; Bach, M.; Klement, L.; Breuer, L. Stickstoff-Flächenbilanzen für Deutschland mit Regionalgliederung Bundesländer und Kreise—Jahre 1995 bis 2017: Methodik, Ergebnisse und Minderungsmaßnahmen No. 131; Umweltbundesamt: Dessau-Roßlau, Germany, 2019. [Google Scholar]
- Häußermann, U.; Klement, L.; Breuer, L.; Ullrich, A.; Wechsung, G.; Bach, M. Nitrogen soil surface budgets for districts in Germany 1995 to 2017. Environ. Sci. Eur. 2020, 32, 109. [Google Scholar] [CrossRef]
- Council of the European Union. Council Directive of 12 December 1991 concerning the protection of waters against pollution caused by nitrates from agricultural sources (91/676/EEC). Off. J. Eur. Commun. 1991, 1–8. [Google Scholar]
- DüV. Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen. Bundesgesetzblatt 2017, 1, 1305. [Google Scholar]
- Amon, B.; Kryvoruchko, V.; Amon, T.; Zechmeister-Boltenstern, S. Methane, nitrous oxide and ammonia emissions during storage and after application of dairy cattle slurry and influence of slurry treatment. Agric. Ecosyst. Environ. 2006, 112, 153–162. [Google Scholar] [CrossRef]
- Chantigny, M.H.; MacDonald, J.D.; Beaupré, C.; Rochette, P.; Angers, D.A.; Massé, D.; Parent, L.-É. Ammonia volatilization following surface application of raw and treated liquid swine manure. Nutr. Cycl. Agroecosyst. 2009, 85, 275–286. [Google Scholar] [CrossRef]
- Chantigny, M.H.; Rochette, P.; Angers, D.A.; Bittman, S.; Buckley, K.; Massé, D.; Bélanger, G.; Eriksen-Hamel, N.; Gasser, M.-O. Soil nitrous oxide emissions following band-incorporation of fertiliser nitrogen and swine manure. J. Environ. Qual. 2010, 39, 1545–1553. [Google Scholar] [CrossRef]
- Hansen, M.N.; Henriksen, K.; Sommer, S.G. Observations of production and emission of greenhouse gases and ammonia during storage of solids separated from pig slurry: Effects of covering. Atmos. Environ. 2006, 40, 4172–4181. [Google Scholar] [CrossRef] [Green Version]
- Møller, H.; Lund, I.; Sommer, S.G. Solid–liquid separation of livestock slurry: Efficiency and cost. Bioresour. Technol. 2000, 74, 223–229. [Google Scholar] [CrossRef]
- Möller, K.; Müller, T. Effects of anaerobic digestion on digestate nutrient availability and crop growth: A review. Eng. Life Sci. 2012, 12, 242–257. [Google Scholar] [CrossRef]
- Askri, A.; Laville, P.; Trémier, A.; Houot, S. Influence of origin and post-treatment on greenhouse gas emissions after anaerobic digestate application to soil. Waste Biomass Valor. 2016, 7, 293–306. [Google Scholar] [CrossRef]
- Singla, A.; Inubushi, K. Effect of biogas digested liquid on CH4 and N2O flux in paddy ecosystem. J. Integr. Agric. 2014, 13, 635–640. [Google Scholar] [CrossRef]
- Thomas, B.W.; Hao, X. Nitrous oxide emitted from soil receiving anaerobically digested solid cattle manure. J. Environ. Qual. 2017, 46, 741–750. [Google Scholar] [CrossRef] [Green Version]
- Dietrich, M.; Fongen, M.; Foereid, B. Greenhouse gas emissions from digestate in soil. Int. J. Recycl. Org. Waste Agricult. 2020, 9, 1–19. [Google Scholar] [CrossRef]
- Gómez-Muñoz, B.; Case, S.D.C.; Jensen, L.S. Pig slurry acidification and separation techniques affect soil N and C turnover and N2O emissions from solid, liquid and biochar fractions. J. Environ. Manag. 2016, 168, 236–244. [Google Scholar] [CrossRef] [PubMed]
- Holly, M.A.; Larson, R.A.; Powell, J.M. Gas reduction benefits from storage to application of anaerobic digestion and solid-liquid separation of dairy manure. In Proceedings of the 2015 ASABE Annual International Meeting, New Orleans, LA, USA, 26–29 July 2015; American Society of Agricultural and Biological Engineers: St. Joseph, MI, USA, 2015. [Google Scholar]
- Singla, A.; Iwasa, H.; Inubushi, K. Effect of biogas digested slurry based-biochar and digested liquid on N2O, CO2 flux and crop yield for three continuous cropping cycles of komatsuna (Brassica rapa var. perviridis). Biol. Fertil. Soils 2014, 50, 1201–1209. [Google Scholar] [CrossRef]
- Verdi, L.; Mancini, M.; Ljubojevic, M.; Orlandini, S.; Dalla Marta, A. Greenhouse gas and ammonia emissions from soil: The effect of organic matter and fertilisation method. Ital. J. Agron. 2018, 13, 260–266. [Google Scholar] [CrossRef] [Green Version]
- Akiyama, H.; McTaggart, I.P.; Ball, B.C.; Scott, A. N2O, NO, and NH3 emissions from soil after the application of organic fertilisers, urea and water. Water Air Soil Pollut. 2004, 156, 113–129. [Google Scholar] [CrossRef]
- Hayakawa, A.; Akiyama, H.; Sudo, S.; Yagi, K. N2O and NO emissions from an Andisol field as influenced by pelleted poultry manure. Soil Biol. Biochem. 2009, 41, 521–529. [Google Scholar] [CrossRef]
- Pampuro, N.; Busato, P.; Cavallo, E. Gaseous emissions after soil application of pellet made from composted pig slurry solid fraction: Effect of application method and pellet diameter. Agriculture 2018, 8, 119. [Google Scholar] [CrossRef] [Green Version]
- Velthof, G.L.; Rietra, R.P.J.J. Nitrogen use efficiency and gaseous nitrogen losses from the concentrated liquid fraction of pig slurries. Int. J. Agron. 2019, 2019, 9283106. [Google Scholar] [CrossRef]
- Die chemische Untersuchung von Futtermitteln: The Chemical Analysis of Feedstuffs. VDLUFA; Bassler, R. (Eds.) 3.Aufl.; VDLUFA-Verl.: Darmstadt, Germany, 1976; ISBN 978-3-941273-14-6. [Google Scholar]
- van Soest, P.J.; Wine, R.H. Determination of lignin and cellulose in acid-detergent fiber with permanganate. J. Assoc. Off. Anal. Chem. 1968, 51, 780–785. [Google Scholar] [CrossRef]
- DüV. Verordnung über die Anwendung von Düngemitteln, Bodenhilfsstoffen, Kultursubstraten und Pflanzenhilfsmitteln nach den Grundsätzen der guten fachlichen Praxis beim Düngen. Bundesgesetzblatt 2006, 1, 20–29. [Google Scholar]
- Hutchinson, G.L.; Mosier, A.R. Improved soil cover method for field measurement of nitrous oxide fluxes. Soil Sci. Soc. Am. J. 1981, 45, 311–316. [Google Scholar] [CrossRef]
- Laville, P.; Lehuger, S.; Loubet, B.; Chaumartin, F.; Cellier, P. Effect of management, climate and soil conditions on N2O and NO emissions from an arable crop rotation using high temporal resolution measurements. Agric. For. Meteorol. 2011, 151, 228–240. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Schilling, R.; Steindl, H.; Beese, F. Soil compaction and fertilisation effects on nitrous oxide and methane fluxes in potato fields. Soil Sci. Soc. Am. J. 1998, 62, 1587–1595. [Google Scholar] [CrossRef]
- Guzman-Bustamante, I.; Winkler, T.; Schulz, R.; Müller, T.; Mannheim, T.; Laso Bayas, J.C.; Ruser, R. N2O emissions from a loamy soil cropped with winter wheat as affected by N-fertilizer amount and nitrification inhibitor. Nutr. Cycl. Agroecosyst. 2019, 114, 173–191. [Google Scholar] [CrossRef]
- Petrova, I.P.; Ruser, R.; Guzman-Bustamante, I. Pellets from biogas digestates: A substantial source of N2O emissions. Waste Biomass Valor. 2021, 12, 2433–2444. [Google Scholar] [CrossRef]
- Fuß, R.; Greenhouse Gas Flux Calculation from Chamber Measurements. R-Package version 0.2-1. Available online: https://cran.r-project.org/web/packages/gasfluxes/gasfluxes.pdf (accessed on 24 April 2018).
- Leiber-Sauheitl, K.; Fuß, R.; Voigt, C.; Freibauer, A. High CO2 fluxes from grassland on histic Gleysol along soil carbon and drainage gradients. Biogeosciences 2014, 11, 749–761. [Google Scholar] [CrossRef] [Green Version]
- Piepho, H.-P. A SAS macro for generating letter displays of pairwise mean comparisons. Commun. Biometry Crop. Sci. 2012, 7, 4–13. [Google Scholar]
- Bertora, C.; Alluvione, F.; Zavattaro, L.; Van Groenigen, J.W.; Velthof, G.; Grignani, C. Pig slurry treatment modifies slurry composition, N2O, and CO2 emissions after soil incorporation. Soil Biol. Biochem. 2008, 40, 1999–2006. [Google Scholar] [CrossRef]
- Jones, S.K.; Rees, R.M.; Skiba, U.M.; Ball, B.C. Influence of organic and mineral N fertiliser on N2O fluxes from a temperate grassland. Agric. Ecosyst. Environ. 2007, 121, 74–83. [Google Scholar] [CrossRef]
- Kusa, K.; Hu, R.; Sawamoto, T.; Hatano, R. Three years of nitrous oxide and nitric oxide emissions from silandic andosols cultivated with maize in Hokkaido, Japan. Soil Sci. Plant. Nutr. 2006, 52, 103–113. [Google Scholar] [CrossRef]
- Köster, J.R.; Cárdenas, L.; Senbayram, M.; Bol, R.; Well, R.; Butler, M.; Mühling, K.H.; Dittert, K. Rapid shift from denitrification to nitrification in soil after biogas residue application as indicated by nitrous oxide isotopomers. Soil Biol. Biochem. 2011, 43, 1671–1677. [Google Scholar] [CrossRef]
- Häfner, F.; Ruser, R.; Claß-Mahler, I.; Möller, K. Field application of organic fertilisers triggers N2O emissions from the soil N pool as indicated by 15N-labeled digestates. Front. Sustain. Food Syst. 2021, 4. [Google Scholar] [CrossRef]
- Granli, T.; Bøckman, O. Nitrous oxide from agriculture. Nor. J. Agric. Sci. 1994, 12, 7–128. [Google Scholar]
- Flessa, H.; Beese, F. Laboratory estimates of trace gas emissions following surface application and injection of cattle slurry. J. Environ. Qual. 2000, 29, 262–268. [Google Scholar] [CrossRef]
- Petersen, S.O.; Sommer, S.G. Ammonia and nitrous oxide interactions: Roles of manure organic matter management. Anim. Feed Sci. Tech. 2011, 166–167, 503–513. [Google Scholar] [CrossRef]
- Smith, K.A. The potential for feedback effects induced by global warming on emissions of nitrous oxide by soils. Glob. Chang. Biol. 1997, 3, 327–338. [Google Scholar] [CrossRef]
- Pfab, H.; Palmer, I.; Buegger, F.; Fiedler, S.; Müller, T.; Ruser, R. N2O fluxes from a Haplic Luvisol under intensive production of lettuce and cauliflower as affected by different N-fertilisation strategies. J. Plant. Nutr. Soil Sci. 2011, 174, 545–553. [Google Scholar] [CrossRef]
- Ruser, R.; Fuß, R.; Andres, M.; Hegewald, H.; Kesenheimer, K.; Köbke, S.; Räbiger, T.; Quinones, T.S.; Augustin, J.; Christen, O.; et al. Nitrous oxide emissions from winter oilseed rape cultivation. Agric. Ecosyst. Environ. 2017, 249, 57–69. [Google Scholar] [CrossRef]
- Senbayram, M.; Chen, R.; Wienforth, B.; Herrmann, A.; Kage, H.; Mühling, K.H.; Dittert, K. Emission of N2O from biogas crop production systems in Northern Germany. Bioenergy Res. 2014, 7, 1223–1236. [Google Scholar] [CrossRef]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Meijide, A.; García-Torres, L.; Arce, A.; Vallejo, A. Nitrogen oxide emissions affected by organic fertilisation in a non-irrigated Mediterranean barley field. Agric. Ecosyst. Environ. 2009, 132, 106–115. [Google Scholar] [CrossRef]
- Davidson, E.A. Fluxes of nitrous oxide and nitric oxide from terrestrial ecosystems: In Microbial Production and Consumption of Greenhouse Gases: Methane, Nitrogen Oxides and Halomethanes; American Society for Microbiology: Washington, DC, USA, 1991; pp. 219–235. [Google Scholar]
- Fechter, M. Technischer Vergleich von Aufbereitungsverfahren für Gärreste in der Biogastechnik. Doctoral dissertation, Technische Universität Berlin, Berlin, Germany, 2019. [Google Scholar]
- Petersen, J.; Sørensen, P. Loss of nitrogen and carbon during storage of the fibrous fraction of separated pig slurry and influence on nitrogen availability. J. Agric. Sci. 2008, 146, 403–413. [Google Scholar] [CrossRef]
- Marcato, C.E.; Pinelli, E.; Pouech, P.; Winterton, P.; Guiresse, M. Particle size and metal distributions in anaerobically digested pig slurry. Bioresour. Technol. 2008, 99, 2340–2348. [Google Scholar] [CrossRef] [Green Version]
- Møller, H.B.; Sommer, S.G.; Ahring, B.K. Separation efficiency and particle size distribution in relation to manure type and storage conditions. Bioresour. Technol. 2002, 85, 189–196. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; de la Fuente, Carlos; Bernal, M. P. Chemical properties of anaerobic digestates affecting C and N dynamics in amended soils. Agric. Ecosyst. Environ. 2012, 160, 15–22. [Google Scholar] [CrossRef]
- Collins, H.P.; Alva, A.K.; Streubel, J.D.; Fransen, S.F.; Frear, C.; Chen, S.; Kruger, C.; Granatstein, D. Greenhouse gas emissions from an irrigated silt loam soil amended with anaerobically digested dairy manure. Soil Sci. Soc. Am. J. 2011, 75, 2206–2216. [Google Scholar] [CrossRef]
- Ingold, M.; Khanal, G.; Dyckmans, J.; Wachendorf, C.; Buerkert, A. NH3 volatilization, N2O emission and microbial biomass turnover from 15N-labeled manure under laboratory conditions. Commun. Soil Sci. Plant. Anal. 2018, 49, 537–551. [Google Scholar] [CrossRef]
- Roman-Perez, C.C.; Hernandez-Ramirez, G. Sources and priming of nitrous oxide production across a range of moisture contents in a soil with high organic matter. J. Environ. Qual. 2021, 50, 94–109. [Google Scholar] [CrossRef]
- Manfredini, A.; Negri, M.; Cavalli, D.; Bechini, L.; Marino, P. Carbon and nitrogen mineralization of raw and separated, digested animal manures. In Treatment and Use of organic Residues in Agriculture: Challenges and Opportunities towards Sustainable Management, 14th RAMIRAN International Conference, Lisbon, Portugal, 13–15 September; Marques dos Santos Cordovil, Cláudia S.C., Ferreira, L., Eds.; ISA Press: Lisbon, Portugal, 2010; ISBN 978-972-8669-47-8. [Google Scholar]
- Ellington, A. Ammonia volatilization losses from fertilisers applied to acid soil in the field. Fertil. Res. 1986, 8, 283–296. [Google Scholar] [CrossRef]
- He, Z.L.; Alva, A.K.; Calvert, D.V.; Banks, D.J. Ammonia volatilization from different fertiliser sources and effects of temperature and soil pH. Soil Sci. 1999, 164, 750–758. [Google Scholar] [CrossRef]
- Powlson, D.S.; Dawson, C.J. Use of ammonium sulphate as a sulphur fertiliser: Implications for ammonia volatilization. Soil Use Manag. 2021. [Google Scholar] [CrossRef]
- Sommer, S.G.; Jensen, C. Ammonia volatilization from urea and ammoniacal fertilisers surface applied to winter wheat and grassland. Fertil. Res. 1994, 37, 85–92. [Google Scholar] [CrossRef]
- Turner, D.A.; Edis, R.E.; Chen, D.; Freney, J.R.; Denmead, O.T. Ammonia volatilization from nitrogen fertilisers applied to cereals in two cropping areas of southern Australia. Nutr. Cycl. Agroecosyst. 2012, 93, 113–126. [Google Scholar] [CrossRef]
- Fangueiro, D.; Coutinho, J.; Cabral, F.; Fidalgo, P.; Bol, R.; Trindade, H. Nitric oxide and greenhouse gases emissions following the application of different cattle slurry particle size fractions to soil. Atmos. Environ. 2012, 47, 373–380. [Google Scholar] [CrossRef]
- Hadas, A.; Bar-Yosef, B.; Davidov, S.; Sofer, M. Effect of pelleting, temperature, and soil type on mineral nitrogen release from poultry and dairy manures. Soil Sci. Soc. Am. J. 1983, 47, 1129–1133. [Google Scholar] [CrossRef]
- Aguirre-Villegas, H.A.; Larson, R.A.; Sharara, M.A. Anaerobic digestion, solid-liquid separation, and drying of dairy manure: Measuring constituents and modeling emission. Sci. Total Environ. 2019, 696, 134059. [Google Scholar] [CrossRef]
- Maurer, C.; Müller, J. Ammonia (NH3) emissions during drying of untreated and dewatered biogas digestate in a hybrid waste-heat/solar dryer. Eng. Life Sci. 2012, 12, 321–326. [Google Scholar] [CrossRef]
- Ingold, M.; Dietz, H.; Schlecht, E.; Buerkert, A. Gaseous N and C losses during sun-drying of goat manure—Effects of drying conditions and feed additives. In Proceedings of the Conference on International Research on Food Security, Natural Resource Management and Rural Development, Virtual Conference, 9–11 September 2020. [Google Scholar]
- Prenafeta-Boldú, F.X.; Fernández, B.; Viñas, M.; Noguerol, J.; Soler, J.; Illa, J. Combined acidification and solar drying of pig slurries for nutrient recovery and controlled atmospheric emissions. Agronomy 2021, 11, 222. [Google Scholar] [CrossRef]
- Hartley, I.D.; Wood, L.J. Hygroscopic properties of densified softwood pellets. Biomass Bioenergy 2008, 32, 90–93. [Google Scholar] [CrossRef]
- Nurk, L.; Bühle, L.; Wachendorf, M. Degradation of fibre and non-fibre fractions during anaerobic digestion in silages of maize, sunflower and Sorghum-Sudangrass of different maturities. Bioenergy Res. 2016, 9, 720–730. [Google Scholar] [CrossRef]
- Suppadit, T.; Pongpiachan, S.; Panomsri, S. Effects of moisture content in quail litter on the physical characteristics after pelleting using a Siriwan Model machine. Anim. Sci. J. 2012, 83, 350–357. [Google Scholar] [CrossRef]
- Oberberger, I.; Thek, G. The Pellet Handbook: The Production and Thermal Utilisation of Biomass Pellets; Earthscan: London, UK, 2010; ISBN 978-84407-631-4. [Google Scholar]
- Dosch, P.; Gutser, R. Reducing N losses (NH3, N2O, N2) and immobilization from slurry through optimized application techniques. Fertil. Res. 1995, 43, 165–171. [Google Scholar] [CrossRef]
- Velthof, G.L.; Kuikman, P.J.; Oenema, O. Nitrous oxide emission from animal manures applied to soil under controlled conditions. Biol. Fertil. Soils 2003, 37, 221–230. [Google Scholar] [CrossRef]

| Biogas Plant | Product | Year | DM 4 | Ct 6 | Corg 7 | Nt 8 | NH4–N 9 | NH4–N:Nt | C:N | pH |
|---|---|---|---|---|---|---|---|---|---|---|
| (g·kg−1 FM 5) | (g·kg−1 DM) | |||||||||
| 1 | Raw digestate (RD1) | 2015 | 79 | 348 | 345 | 64.1 | 30.2 | 0.47 | 5.4 | 8.1 |
| Sep. liquid (SL1) 1 | 56 | 358 | 354 | 86.5 | 43.1 | 0.50 | 4.1 | 8.0 | ||
| Sep. solid (SS1) 1 | 311 | 431 | 429 | 25.2 | 6.4 | 0.25 | 17.1 | 8.2 | ||
| Dry solid (DS1) | 902 | 416 | 413 | 25.6 | 1.7 | 0.07 | 16.2 | 8.2 | ||
| Pellet (P1) | 889 | 402 | 398 | 35.1 | 4.3 | 0.12 | 11.5 | 7.7 | ||
| Raw digestate (RD1) | 2016 | 108 | 435 | 432 | 47.6 | 18.2 | 0.38 | 9.1 | 7.3 | |
| Sep. liquid (SL1) 1 | 65 | 398 | 394 | 78.4 | 36.0 | 0.46 | 5.1 | 7.8 | ||
| Sep. solid (SS1) 1 | 299 | 456 | 454 | 18.6 | 3.9 | 0.21 | 24.6 | 8.6 | ||
| Dry solid (DS1) | 546 | 461 | 459 | 21.4 | 1.0 | 0.05 | 21.5 | 7.8 | ||
| Pellet (P1) | 851 | 447 | 445 | 34.9 | 2.4 | 0.07 | 12.8 | 7.9 | ||
| 2 | Raw digestate (RD2) | 2015 | 54 | 381 | 376 | 96.1 | 62.2 | 0.65 | 4.0 | 7.8 |
| Sep. liquid (SL2) 1 | 50 | 368 | 362 | 103.4 | 68.6 | 0.66 | 3.6 | 7.8 | ||
| Sep. solid (SS2) 1 | 225 | 398 | 396 | 28.1 | 15.0 | 0.53 | 14.1 | 8.3 | ||
| Concentrate (CC2) 2 | 85 | 375 | 373 | 48.8 | 19.7 | 0.40 | 7.7 | 9.0 | ||
| ASS2 3 | 322 | <2 | <2 | 185.8 | 181.9 | 0.98 | 0.01 | 1.3 | ||
| Raw digestate (RD2) | 2016 | 77 | 406 | 403 | 68.6 | 41.8 | 0.61 | 5.9 | 7.7 | |
| Sep. liquid (SL2) 1 | 47 | 393 | 388 | 106.4 | 60.8 | 0.57 | 3.7 | 7.9 | ||
| Sep. solid (SS2) 1 | 174 | 434 | 432 | 32.1 | 14.4 | 0.45 | 13.5 | 8.9 | ||
| Concentrate (CC2) 2 | 136 | 399 | 395 | 38.7 | 6.5 | 0.17 | 10.3 | 8.9 | ||
| ASS2 3 | 278 | 1 | 1 | 202.9 | 193.7 | 0.95 | 0.007 | 2.4 | ||
| Biogas Plant | Product | aNDF 4 (g·kg−1 DM) | ADF 5 (g·kg−1 DM) | ADL 6 (g·kg−1 DM) | |||
|---|---|---|---|---|---|---|---|
| 2015 | 2016 | 2015 | 2016 | 2015 | 2016 | ||
| 1 | Raw digestate (RD1) | 411 | 477 | 377 | 438 | 170 | 223 |
| Sep. liquid (SL1) 1 | 205 | 379 | 204 | 382 | 109 | 244 | |
| Sep. solid (SS1) 1 | 683 | 785 | 518 | 649 | 216 | 426 | |
| Dry solid (DS1) | 533 | 653 | 493 | 607 | 227 | 393 | |
| Pellet (P1) | 517 | 583 | 463 | 520 | 225 | 316 | |
| 2 | Raw digestate (RD2) | 96 | 455 | 228 | 411 | 134 | 187 |
| Sep. liquid (SL2) 1 | 21 | 189 | 254 | 298 | 174 | 193 | |
| Sep. solid (SS2) 1 | 727 | 693 | 563 | 524 | 207 | 179 | |
| Concentrate (CC2) 2 | 89 | 169 | 260 | 259 | 112 | 141 | |
| ASS2 3 | <10 7,8 | <10 7,8 | <5 7,8 | <5 7,8 | <5 7,8 | <5 7,8 | |
| Year | Sand | Silt | Clay | Bulk Density | pH | Corg | Nt | Nmin 1 |
|---|---|---|---|---|---|---|---|---|
| (%) | (%) | (%) | (Mg·m−³) | (CaCl2) | (%) | (%) | (kg·ha−1) | |
| 2015 | 2 | 68 | 30 | 1.29 | 6.5 | 1.8 | 0.16 | 19.9 |
| 2016 | 9 | 69 | 22 | 1.24 | 7.0 | 1.1 | 0.14 | 7.3 |
| N2O Release 1 | Model R2 | Air Temp | Rainfall | Soil Temp | WFPS 2 | Final Equation of Stepwise Forward Regression | |||
|---|---|---|---|---|---|---|---|---|---|
| (g N2O-N ha−1 d−1) | (°C) | (mm) | (°C) | (%) | |||||
| Partial R2 | p-Value | Partial R2 | p-Value | Partial R2 | p-Value | ||||
| RD1 | 0.55 | 0.29 | 0.002 | n.s. | n.s. | 0.26 | 0.008 | y = −250.1 + 10.6x1 + 4.4x2 | |
| SL1 | 0.27 | 0.27 | 0.020 | n.s. | n.s. | n.s. | y = −78.7 + 12.4x1 | ||
| SS1 | 0.61 | 0.33 | <0.001 | n.s. | 0.28 | 0.004 | n.s. | y = −11.7 + 8.6x1 + 3.7x3 | |
| DS1 | 0.68 | 0.31 | <0.001 | n.s. | 0.15 | <0.001 | 0.22 | n.s. | y = 8.3 + 11.2x1 − 6.1x3 |
| P1 | 0.65 | 0.28 | 0.003 | n.s. | n.s. | 0.37 | <0.001 | y = −553.9 + 16x1 + 10.7x2 | |
| RD2 | 0.67 | 0.34 | <0.001 | n.s. | 0.33 | 0.001 | n.s. | y = −26.2 + 15.5x1 − 6.9x3 | |
| SL2 | 0.60 | 0.36 | <0.001 | n.s. | n.s. | 0.24 | 0.007 | y = −126.3 + 6.1x1 + 2.2x2 | |
| SS2 | 0.65 | 0.32 | <0.001 | n.s. | 0.33 | 0.001 | n.s. | y = −23.9 + 17.3x1 − 7.8x3 | |
| CC2 | 0.68 | 0.32 | <0.001 | n.s. | 0.36 | <0.001 | n.s. | y = −5.5 + 12.1x1 − 5.6x3 | |
| ASS2 | 0.64 | 0.37 | <0.001 | n.s. | 0.27 | 0.003 | n.s. | y = −43.2 + 15.7x1 − 6.5x3 | |
| Control | 0.75 | 0.35 | <0.001 | n.s. | 0.40 | <0.001 | n.s. | y = −24.4 + 12.5x1 − 5.9x3 | |
| N2O Release 1 | Model R2 | Air Temp | Rainfall | Soil Temp | WFPS 2 | Final Equation of Stepwise Forward Regression | ||||
|---|---|---|---|---|---|---|---|---|---|---|
| (g N2O-N ha−1 d−1) | (°C) | (mm) | (°C) | (%) | ||||||
| Partial R2 | p-Value | Partial R2 | p-Value | Partial R2 | p-Value | Partial R2 | p-Value | |||
| RD1 | 0.54 | n.s. | 0.40 | <0.001 | 0.14 | 0.037 | n.s. | y = −22.1+ 14x2 + 2.2x4 | ||
| SL1 | n.s. | n.s. | n.s. | n.s. | y = Intercept | |||||
| SS1 | 0.50 | n.s. | 0.50 | <0.001 | n.s. | n.s. | y = 7.4 + 3.1x2 | |||
| DS1 | 0.31 | n.s. | 0.31 | 0.010 | n.s. | n.s. | y = 10.6 + 3.5x2 | |||
| P1 | n.s. | n.s. | n.s. | n.s. | y = Intercept | |||||
| RD2 | 0.49 | 0.20 | 0.018 | 0.29 | 0.016 | n.s. | n.s. | y = −35.8+ 6.2x1 + 11.1x2 | ||
| SL2 | 0.36 | 0.36 | 0.005 | n.s. | n.s. | n.s. | y = −11.6 + 3.7x1 | |||
| SS2 | n.s. | n.s | n.s. | n.s. | y = Intercept | |||||
| CC2 | 0.77 | n.s. | 0.57 | <0.001 | 0.13 | 0.002 | 0.06 | 0.051 | y = −215.8 + 18.6x2 + 3.2x3 + 4.3x4 | |
| ASS2 | 0.45 | n.s. | 0.45 | 0.001 | n.s. | n.s. | y = 10.2 + 4.8x2 | |||
| Control | 0.70 | n.s. | 0.70 | <0.001 | n.s. | n.s. | y = 4.9 + 4x2 | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Petrova, I.P.; Pekrun, C.; Möller, K. Organic Matter Composition of Digestates Has a Stronger Influence on N2O Emissions than the Supply of Ammoniacal Nitrogen. Agronomy 2021, 11, 2215. https://doi.org/10.3390/agronomy11112215
Petrova IP, Pekrun C, Möller K. Organic Matter Composition of Digestates Has a Stronger Influence on N2O Emissions than the Supply of Ammoniacal Nitrogen. Agronomy. 2021; 11(11):2215. https://doi.org/10.3390/agronomy11112215
Chicago/Turabian StylePetrova, Ioana Petrova, Carola Pekrun, and Kurt Möller. 2021. "Organic Matter Composition of Digestates Has a Stronger Influence on N2O Emissions than the Supply of Ammoniacal Nitrogen" Agronomy 11, no. 11: 2215. https://doi.org/10.3390/agronomy11112215
APA StylePetrova, I. P., Pekrun, C., & Möller, K. (2021). Organic Matter Composition of Digestates Has a Stronger Influence on N2O Emissions than the Supply of Ammoniacal Nitrogen. Agronomy, 11(11), 2215. https://doi.org/10.3390/agronomy11112215






