Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description and Soil Sampling
2.2. Characterization of Vermicompost and Soil
2.3. Cultivation of Moringa in the Greenhouse
2.4. Sample Preparation for Metabolomics Analysis
2.5. Analysis of Metabolites by UPLC-ESI-MS/MS
2.6. Statistical Analysis
3. Results
3.1. Characteristics of Vermicompost and Soil
3.2. Evaluation of Growth
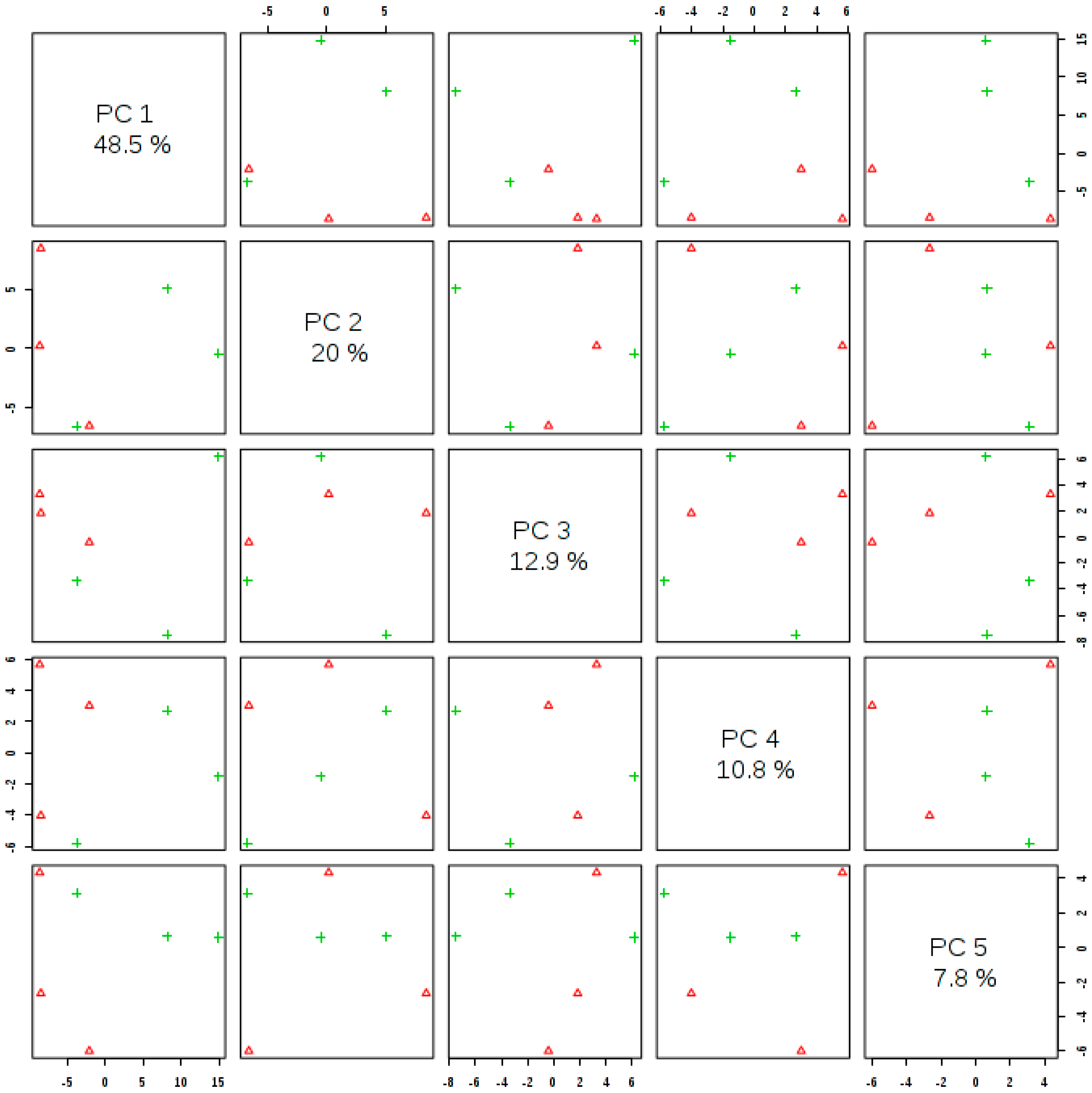
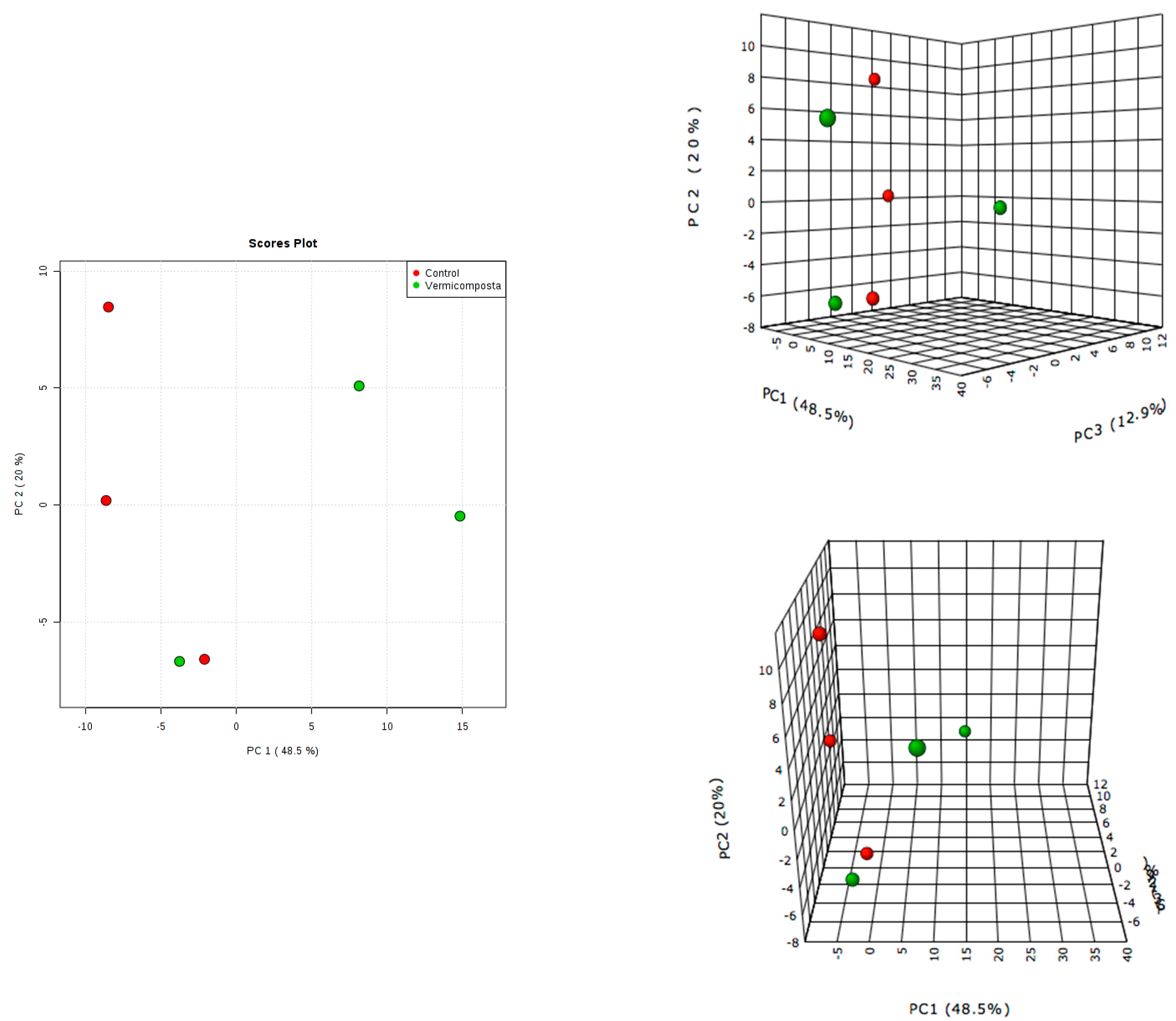
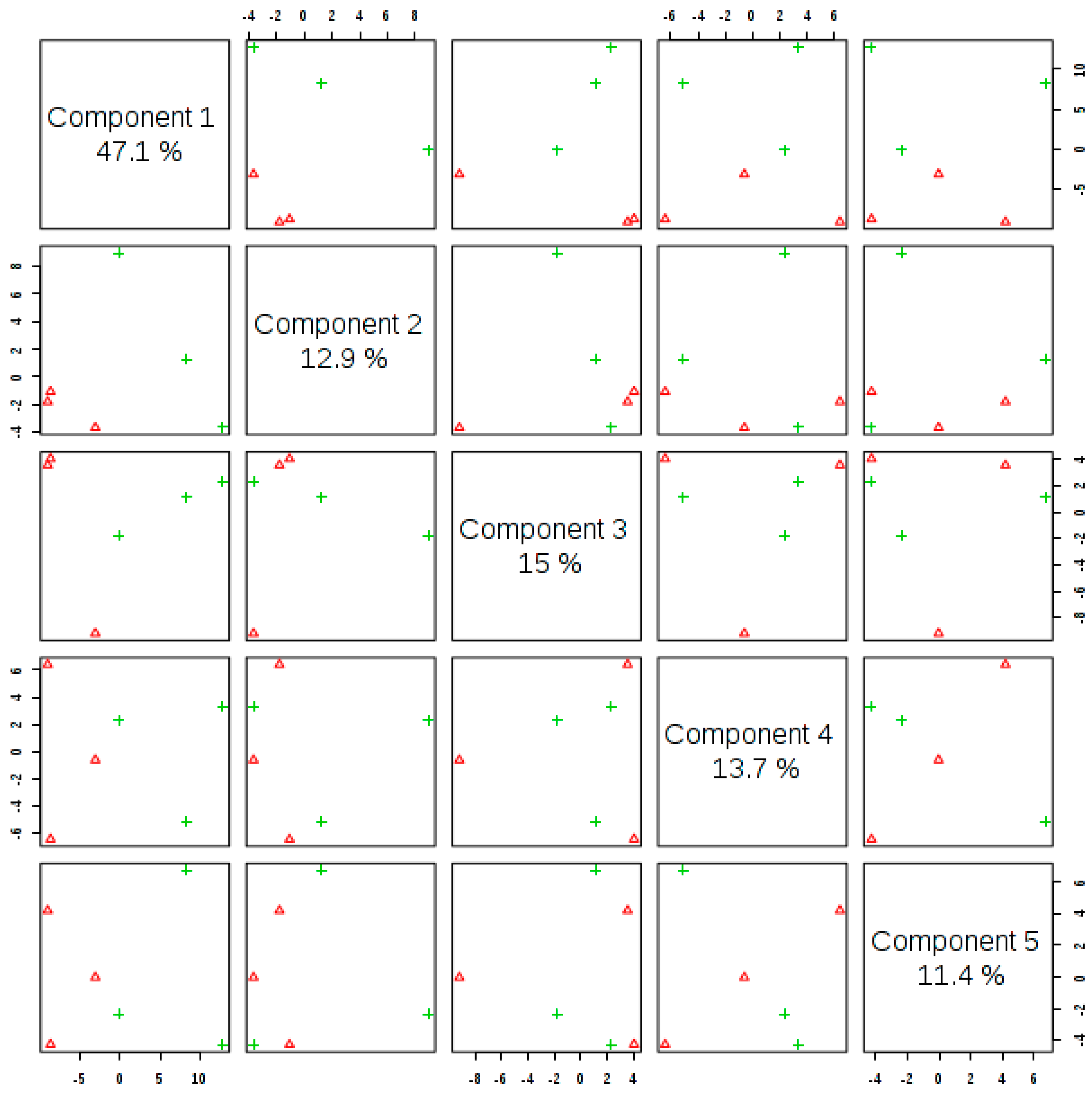
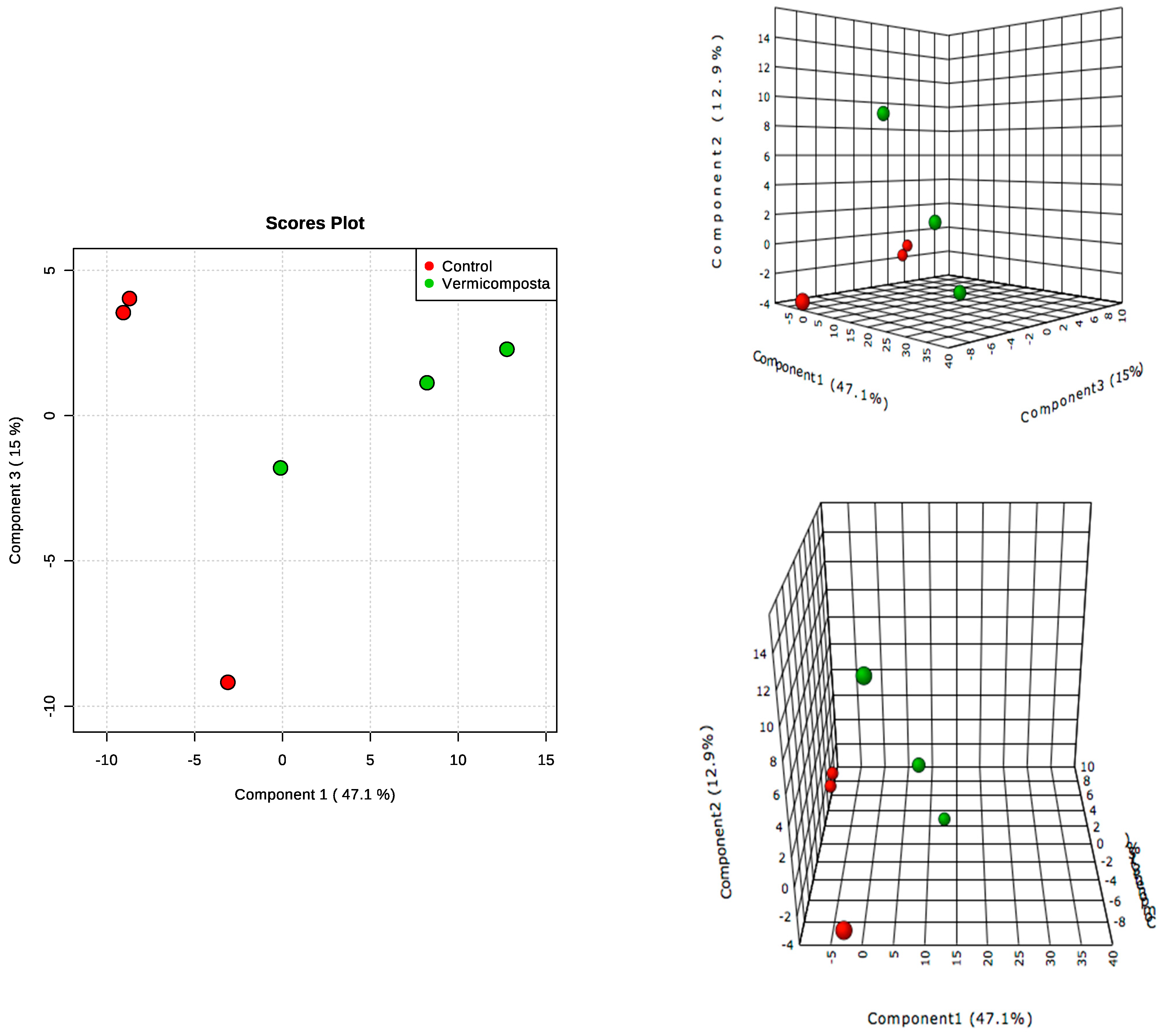
3.3. Metabolomic Profile Changes in Moringa Leaves
3.4. Metabolite Identification
4. Discussion
4.1. Evaluation of Growth
4.2. Metabolite Identification
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050. 2018. Available online: http://www.fao.org/global-perspectives-studies/resources/detail/en/c/1157074 (accessed on 20 August 2021).
- Page, K.L.; Dang, Y.P.; Dalal, R.C. The ability of conservation agriculture to conserve soil organic carbon and the subsequent impact on soil physical, chemical, and biological properties and yield. Front. Sustain. Food Syst. 2020, 4, 31. [Google Scholar] [CrossRef]
- Kuhwald, M.; Blaschek, M.; Brunotte, J.; Duttmann, R. Comparing soil physical properties from continuous conventional tillage with long-term reduced tillage affected by one-time inversion. Soil Use Manag. 2017, 33, 611–619. [Google Scholar] [CrossRef]
- Gutiérrez-Miceli, F.A.; Santiago-Borraz, J.; Montes-Molina, J.A.; Nafate, C.C.; Abud-Archila, M.; Oliva-Llaven, M.A.; Rincón-Rosales, R.; Dendooven, L. Vermicompost as a soil supplement to improve growth, yield and fruit quality of tomato (Lycopersicum esculentum). Bioresour. Technol. 2007, 98, 2781–2786. [Google Scholar] [CrossRef]
- Khan, K.; Pankaj, U.; Verma, S.K.; Gupta, A.K.; Singh, R.P.; Verma, R.K. Bio-inoculants and vermicompost influence on yield, quality of Andrographis paniculata and soil properties. Ind. Crops Prod. 2015, 70, 404–409. [Google Scholar] [CrossRef]
- Arancon, N.Q.; Edwards, C.A.; Lee, S.; Byrne, R. Effects of humic acids from vermicomposts on plant growth. Eur. J. Soil Biol. 2006, 42, S65–S69. [Google Scholar] [CrossRef]
- Xu, L.; Yan, D.; Ren, X.Y.; Wei, Y.Y.; Zhou, J.; Zhao, H.Y.; Liang, M.X. Vermicompost improves the physiological and biochemical responses of blessed thistle (Silybum marianum Gaertn.) and peppermint (Mentha haplocalyx Briq) to salinity stress. Ind. Crops Prod. 2016, 94, 574–585. [Google Scholar] [CrossRef]
- Nardi, S.; Schiavon, M.; Francioso, O. Chemical Structure and Biological Activity of Humic Substances Define Their Role as Plant Growth Promoters. Molecules 2021, 26, 2256. [Google Scholar] [CrossRef] [PubMed]
- Dzuvor, C.K.; Pan, S.; Amanze, C.; Amuzu, P.; Asakiya, C.; Kubi, F. Bioactive components from Moringa oleifera seeds: Production, functionalities and applications–a critical review. Crit. Rev. Biotechnol. 2021, 1–23. [Google Scholar] [CrossRef]
- Milla, P.G.; Peñalver, R.; Nieto, G. Health Benefits of Uses and Applications of Moringa oleifera in Bakery Products. Plants 2021, 10, 318. [Google Scholar] [CrossRef]
- Helene, F.-M.; du Toit, E.S.; Robbertse, P.S.J. Effect of storage conditions on Moringa oleifera Lam. seed oil: Biodiesel feedstock quality. Ind. Crops Prod. 2016, 84, 80–86. [Google Scholar] [CrossRef][Green Version]
- Olson, M.E.; Fahey, J.W. Moringa oleifera: A multipurpose tree for the dry tropics. Rev. Mex. Biodivers. 2011, 82, 1071–1082. [Google Scholar]
- Sreeja, M.; Jayasri, P.; Keerthi, N.; Yeshashwini, J.; Praveen, J. Moringa oleifera: A review on nutritive importance and its potential use as nutraceutical plant. J. Med. Plant Stud. 2021, 9, 15–17. [Google Scholar]
- Chitiyo, S.T.; Ncube, B.; Ndhlala, A.R.; Tsvuura, Z. Biochemical responses of Moringa oleifera Lam. plants to graded moisture deficit. S. Afr. J. Bot. 2021, 138, 41–49. [Google Scholar] [CrossRef]
- Christophe, H.L.; Albert, N.; Martin, Y.; Mbaiguinam, M. Effect of organic fertilizers rate on plant survival and mineral properties of Moringa oleifera under greenhouse conditions. Int. J. Recycl. Org. Waste Agricult. 2019, 8, 123–130. [Google Scholar] [CrossRef]
- Kusano, M.; Fukushima, A.; Redestig, H.; Saito, K. Metabolomic approaches toward understanding nitrogen metabolism in plants. J. Exp. Bot. 2011, 62, 1439–1453. [Google Scholar] [CrossRef]
- Amiour, N.; Imbaud, S.; Clément, G.; Agier, N.; Zivy, V.; Valot, B.; Balliau, T.; Armengaud, P.; Quilleré, I.; Cañas, R.; et al. The use of metabolomics integrated with transcriptomic and proteomic studies for identifying key steps involved in the control of nitrogen metabolism in crops such as maize. J. Exp. Bot. 2012, 63, 5017–5033. [Google Scholar] [CrossRef]
- Moyo, B.; Masika, P.J.; Hugo, A.; Muchenje, V. Nutritional characterization of Moringa (Moringa oleifera Lam.) leaves. Afr. J. Biotechnol. 2011, 10, 12925–12933. [Google Scholar]
- Brusamarello-Santos, L.C.; Gilard, F.; Brulé, L.; Quilleré, I.; Gourion, B.; Ratet, P. Metabolic profiling of two maize (Zea mays L.) inbred lines inoculated with the nitrogen fixing plant-interacting bacteria Herbaspirillum seropedicae and Azospirillum brasilense. PLoS ONE 2017, 12, e0174576. [Google Scholar] [CrossRef]
- James, A.; Zikankuba, V. Moringa oleifera a potential tree for nutrition security in sub-Sahara Africa. Am. J. Res. Commun. 2017, 5, 1–14. [Google Scholar]
- Meireles, D.; Gomes, J.; Lopes, L.; Hinzmann, M.; Machado, J. A review of properties, nutritional and pharmaceutical applications of Moringa oleifera: Integrative approach on conventional and traditional Asian medicine. Adv. Tradit. Med. 2020, 20, 495–515. [Google Scholar] [CrossRef]
- Ruíz-Valdiviezo, V.M.; Luna-Guido, M.; Galzy, A.; Gutiérrez-Miceli, F.A.; Dendooven, L. Greenhouse gas emissions and C and N mineralization in soils of Chiapas (México) amended with leaves of Jatropha curcas L. Appl. Soil Ecol. 2010, 46, 17–25. [Google Scholar] [CrossRef]
- Thomas, G.W. Soil pH and soil acidity. In Methods of Soil Analysis: Chemical Methods; Sparks, D.L., Ed.; American Society of Agronomy: Madison, WI, USA, 1996; pp. 475–490. [Google Scholar]
- Rhoades, J.D.; Mantghi, N.A.; Shause, P.J.; Alves, W. Estimating soil salinity from saturate soil paste electrical conductivity. Soil Sci. Soc. Am. J. 1989, 53, 428–433. [Google Scholar] [CrossRef]
- Bremner, J.M. Nitrogen-Total. In Methods of Soil Analysis: Chemical Methods Part 3, 2nd ed.; Sparks, D.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1996; pp. 1085–1122. [Google Scholar]
- Gee, G.W.; Bauder, J.W. Particle size analysis, hydrometer methods. In Methods of Soil Analysis: Part 1, Physical and Mineralogical Methods; Klute, A., Ed.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 1986; pp. 383–411. [Google Scholar]
- Kim, H.K.; Verpoorte, R. Sample preparation for plant metabolomics. Phytochem. Anal. 2010, 21, 4–13. [Google Scholar] [CrossRef]
- Rhea, J.M.; Snyder, M.L.; Winkler, A.M.; Abou-Diwan, C.; Fantz, C.R.; Ritchie, J.C.; Szlam, F.; Tanaka, K.A.; Molinaro, R.J. Development of a fast and simple liquid chromatography-tandem mass spectrometry method for the quantitation of argatroban in patient plasma samples. J. Chromatogr. B 2012, 893–894, 168–172. [Google Scholar] [CrossRef]
- Pluskal, T.; Castillo, S.; Villar-Briones, A.; Oresic, M. MZmine 2: Modular framework for processing, visualizing, and analyzing mass spectrometry-based molecular profile data. BMC Bioinform. 2010, 11, 395. [Google Scholar] [CrossRef]
- Xia, J.G.; Sinelnikov, I.V.; Han, B.; Wishart, D.S. MetaboAnalyst 3.0-making metabolomics more meaningful. Nucleic Acids Res. 2015, 43, W251–W257. [Google Scholar] [CrossRef] [PubMed]
- Ernest, B.; Gooding, J.R.; Campagna, S.R.; Saxton, A.M.; Voy, B.H. MetabR: An R script for linear model analysis of quantitative metabolomic data. BMC Res. Notes 2012, 5, 596. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Wishart, D.S. Chapter 14. Metabolomic data processing, analysis, and interpretation using MetaboAnalyst. In Current Protocols in Bioinformatics; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 14.10.1–14.10.48. [Google Scholar] [CrossRef]
- Chae, L.; Lee, I.; Shin, J.; Rhee, S.Y. Towards understanding how molecular networks evolve in plants. Curr. Opin. Plant Biol. 2012, 15, 177–184. [Google Scholar] [CrossRef] [PubMed]
- Caspi, R.; Altman, T.; Dreher, K.; Fulcher, C.A.; Subhraveti, P.; Keseler, I.M.; Kothari, A.; Krummenacker, M.; Latendresse, M.; Mueller, L.A.; et al. The MetaCyc database of metabolic pathways and enzymes and the BioCyc collection of Pathway/Genome Databases. Nucleic Acids Res. 2012, 42, D459–D471. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Thiessen, P.A.; Bolton, E.E.; Chen, J.; Fu, G.; Gindulyte, A.; Han, L.; He, J.; He, S.; Shoemaker, B.A.; et al. PubChem Substance and Compound databases. Nucleic Acids Res. 2015, 44, D1202–D1213. [Google Scholar] [CrossRef]
- Horai, H.; Arita, M.; Kanaya, S.; Nihei, Y.; Ikeda, T.; Suwa, K.; Ojima, Y.; Tanaka, K.; Tanaka, S.; Aoshima, K.; et al. MassBank: A public repository for sharing mass spectral data for life sciences. J. Mass Spectrom. 2010, 45, 703–714. [Google Scholar] [CrossRef]
- Wang, L.S.; Li, W.; Liu, Y.H. Hypotensive effect and toxicology of total alkaloids and veratramine from roots and rhizomes of Veratrum nigrum L. in spontaneously hypertensive rats. Pharmazie 2008, 63, 606–610. [Google Scholar] [CrossRef] [PubMed]
- Vanguru, S.; Jilla, L.; Sajja, Y.; Bantu, R.; Nagarapu, L.; Nanubolu, J.B.; Bhaskar, B.; Jain, N.; Sivan, S.; Manga, V. A novel piperazine linked β-amino alcohols bearing a benzosuberone scaffolds as anti-proliferative agents. Bioorg. Med. Chem. Lett. 2017, 27, 792–796. [Google Scholar] [CrossRef] [PubMed]
- Chauhan, P.; Kumar, R.R.; Mendiratta, S.K.; Talukder, S.; Gangwar, M.; Sakunde, D.T.; Meshram, S.K. In-vitro functional efficacy of extracts from Phyllanthus emblica, Eucalyptus globulus, Tinospora cordifolia as pancreatic lipase inhibitor and source of anti-oxidant in goat meat nuggets. Food Chem. 2021, 348, 129087. [Google Scholar] [CrossRef] [PubMed]
- Pratheeba, T.; Taranath, V.; Gopal, D.S.; Natarajan, D. Antidengue potential of leaf extracts of Pavetta tomentosa and Tarenna asiatica (Rubiaceae) against dengue virus and its vector Aedes aegypti (Diptera: Culicidae). Heliyon 2019, 5, e02732. [Google Scholar] [CrossRef]
- Ferreira, P.M.P.; Farias, D.F.; Oliveira, J.T.D.; Carvalho, A.D.U. Moringa oleifera: Bioactive compounds and nutritional potential. Braz. J. Nutrit. 2008, 21, 431–437. [Google Scholar] [CrossRef]
- Sun, M.; Peng, F.; Xiao, Y.; Yu, W.; Zhang, Y.; Gao, H. Exogenous phosphatidylcholine treatment alleviates drought stress and maintains the integrity of root cell membranes in peach. Sci. Hortic. 2020, 259, 108821. [Google Scholar] [CrossRef]
- Guglielmone, H.A.; Agnese, A.M.; Nuñez-Montoya, S.C.; Cabrera, J.L.; Cuadra, G.R. Antithrombotic “in vivo” effects of quercetin 3, 7, 3′, 4′-tetrasulfate isolated from Flaveria bidentis in an experimental thrombosis model in mice. Thromb. Res. 2020, 195, 190–192. [Google Scholar] [CrossRef]
- Yang, Y.; Han, X.; Ma, L.; Wu, Y.; Liu, X.; Fu, H.; Liu, G.; Guo, Y. Dynamic changes of phosphatidylinositol and phosphatidylinositol 4-phosphate levels modulate H+-ATPase and Na+/H+ antiporter activities to maintain ion homeostasis in Arabidopsis under salt stress. Mol. Plant 2021, in press. [Google Scholar] [CrossRef]
- Mansour, A.N.; Thompson, C.; Theil, E.C.; Chasteen, N.D.; Sayers, D.E. Fe (III). ATP complexes. Models for ferritin and other polynuclear iron complexes with phosphate. J. Biol. Chem. 1985, 260, 7975–7979. [Google Scholar] [CrossRef]
- Hachisu, M.; Konishi, K.; Hosoi, M.; Tani, M.; Tomioka, H.; Kitajima, Y.; Inamoto, A.; Hirata, A.; Koganemaru, T.; Tomita, A.; et al. Serum anticholinergic activity as an index of anticholinergic activity load in Alzheimer’s disease. Neurodegener. Dis. 2015, 15, 134–139. [Google Scholar] [CrossRef]
- Barhoi, D.; Upadhaya, P.; Barbhuiya, S.N.; Giri, A.; Giri, S. Aqueous extract of Moringa oleifera exhibit potential anticancer activity and can be used as a possible cancer therapeutic agent: A study involving in vitro and in vivo approach. J. Am. Coll. Nutr. 2021, 40, 70–85. [Google Scholar] [CrossRef] [PubMed]
- Anzai, R.; Adachi, M.; Sho, N.; Muroya, K.; Asakura, Y.; Onigata, K. Long-term 3,5,3’-triiodothyroacetic acid therapy in a child with hyperthyroidism caused by thyroid hormone resistance: Pharmacological study and therapeutic recommendations. Thyroid 2012, 10, 1069–1075. [Google Scholar] [CrossRef] [PubMed]
- Rhetso, T.; Shubharani, R.; Roopa, M.S.; Sivaram, V. Chemical constituents, antioxidant, and antimicrobial activity of Allium chinense G. Don. Future J. Pharm. Sci. 2020, 1, 1–9. [Google Scholar]
- Li, Y.; Yang, X.; Ren, B.; Shen, Q.; Guo, S. Why nitrogen use efficiency decreases under high nitrogen supply in rice (Oryza sativa L.) seedlings. J. Plant Growth Regul. 2012, 31, 47–52. [Google Scholar] [CrossRef]
- Lal, R. Restoring soil quality to mitigate soil degradation. Sustainability 2015, 7, 5875–5895. [Google Scholar] [CrossRef]
- Ostrowska, A.; Porebska, G. Assessment of the C/N ratio as an indicator of the decomposability of organic matter in forest soils. Ecol. Indic. 2015, 49, 104–109. [Google Scholar] [CrossRef]
- Nave, L.E.; Vance, E.D.; Swanston, C.W.; Curtis, P.S. Impacts of elevated N inputs on north temperate forest soil C storage, C/N, and net N-mineralization. Geoderma 2009, 153, 231–240. [Google Scholar] [CrossRef]
- Alok, A.; Tripathi, A.; Soni, P. Vermicomposting: A better option for organic solid waste management. J. Hum. Ecol. 2008, 24, 59–64. [Google Scholar] [CrossRef]
- Mendieta-Araica, B.; Sporndly, E.; Reyes-Sanchez, N.; Salmeron-Miranda, F.; Halling, M. Biomass production and chemical composition of Moringa oleifera under different planting densities and levels of nitrogen fertilization. Agrofor. Syst. 2013, 87, 81–92. [Google Scholar] [CrossRef]
- Reina, L.; Bennadji, Z.; Vinciguerra, V.; Ferreira, F.; Moyna, G.; Menendez, P. Isolation and structural characterization of new piperidine alkaloids from Prosopis affinis. Phytochem. Lett. 2015, 14, 265–269. [Google Scholar] [CrossRef]
- Kim, K.J.; Lee, M.S.; Jo, K.; Hwang, J.K. Piperidine alkaloids from Piper retrofractum Vahl. protect against high-fat diet-induced obesity by regulating lipid metabolism and activating AMP-activated protein kinase. Biochem. Biophys. Res. Commun. 2011, 411, 219–225. [Google Scholar] [CrossRef]
- Anwar, F.; Latif, S.; Ashraf, V.; Gilani, V. Moringa oleifera: A food plant with multiple medicinal uses. Phytother. Res. 2007, 21, 17–25. [Google Scholar] [CrossRef]
- Panda, S.; Kar, A.; Sharma, P.; Sharma, A. Cardioprotective potential of N, α-l-rhamnopyranosyl vincosamide, an indole alkaloid, isolated from the leaves of Moringa oleifera in isoproterenol induced cardiotoxic rats: In vivo and in vitro studies. Bioorg. Med. Chem. Lett. 2013, 23, 959–962. [Google Scholar] [CrossRef] [PubMed]
- Mathur, M.; Kamal, R. Studies on trigonelline from Moringa oleifera and its in vitro regulation by feeding precursor in cell cultures. Rev. Bras. Farmacogn 2012, 22, 994–1001. [Google Scholar] [CrossRef]
- Sahakitpichan, P.; Mahidol, C.; Disadee, W.; Ruchirawat, S.; Kanchanapoom, T. Unusual glycosides of pyrrole alkaloid and 4′-hydroxyphenylethanamide from leaves of Moringa oleifera. Phytochemistry 2011, 72, 791–795. [Google Scholar] [CrossRef]
- Dangi, S.Y.; Jolly, C.I.; Narayanan, S. Antihypertensive activity of the total alkaloids from the leaves of Moringa oleífera. Pharm. Biol. 2002, 40, 144–148. [Google Scholar] [CrossRef]
- Abdulkarim, S.M.; Long, K.; Lai, O.M.; Muhammad, S.K.S.; Ghazali, H.M. Some physico-chemical properties of Moringa oleifera seed oil extracted using solvent and aqueous enzymatic methods. Food Chem. 2005, 93, 253–263. [Google Scholar] [CrossRef]


| Treatment | pH | WHC (g g−1 Soil) | Humidity% | EC (dS m−1) | Organic C (g kg−1 Soil) | Total N (g kg−1 Soil) | C/N | Textural Classification |
|---|---|---|---|---|---|---|---|---|
| SN | 6.77 b | 0.41 b | 5.99 b | 4.31 b | 9.65 c | 1.77 b | 5.5 c | Sandy Clay |
| SA | 5.45 c | 0.42 b | 1.18 c | 3.69 b | 14.12 b | 1.49 c | 9.5 b | Sandy Clay |
| * V | 7.41 a | 0.92 a | 49.25 a | 8.00 a | 233 a | 11.8 a | 19.7 a | - |
| LSD (p < 0.05) | 0.024 | 0.019 | 0.021 | 1.153 | 1.154 | 0.116 | 0.199 |
| Treatment | Plant Height (cm) | Root Length (cm) | Dry Weight Leaves (g) | |||
|---|---|---|---|---|---|---|
| Day | Day | Day | ||||
| 45 | 90 | 45 | 90 | 45 | 90 | |
| Vermicompost | 45.83 a | 66.33 a | 12.33 a | 31.16 a | 1.44 a | 2.11 a |
| Urea | 43.83 a | 66.50 a | 13.00 a | 28.50 a | 0.83 a | 2.52 a |
| Soil + Plant | 43.33 a | 52.83 a | 16.33 a | 26.50 a | 1.39 a | 1.96 a |
| LSD (p < 0.05) | 6.30 | 12.05 | 6.17 | 10.51 | 0.66 | 0.66 |
| Type of Soil | Plant Height (cm/Plant) | Root Length (cm/Plant) | Dry Weight Leaves (g/Plant) | |||
|---|---|---|---|---|---|---|
| Day | Day | Day | ||||
| 45 | 90 | 45 | 90 | 45 | 90 | |
| Native | 54.33 a | 66.00 a | 17.77 a | 29.22 a | 1.93 a | 2.47 a |
| Agricultural | 34.33 b | 57.77 a | 10.00 b | 28.22 a | 0.51 b | 1.93 a |
| LSD (p < 0.05) | 5.14 | 9.84 | 5.04 | 8.58 | 0.54 | 0.54 |
| Treatment | Metabolites | m/z | rt | Activity | Reference |
|---|---|---|---|---|---|
| Agricultural soil vermicompost | Germerine | 694.3914 | 18 | Hypotensive | [37] |
| piperazine-1,4-diyldiethane-2 | 931.671 | 26.3 | Anticancer | [38] | |
| hexanedioic acid | 651.6301 | 20.4 | Antibacterial | [39] | |
| Quercetine 3,3′,4′,7-tetrasulfate | 622.857 | 22.7 | Antioxidant | [40] | |
| Triolein | 885.7467 | 26.7 | Antioxidant | [41] | |
| Agricultural soil control | Phosphatidylcholine | 770.607 | 22.5 | Drought tolerance | [42] |
| Hexabromodiphenyl oxide | 638.528 | 21.7 | Anticoagulant, antiplatelet and profibrinolytic activities | [43] | |
| Phosphatidylinositol | 889.601 | 27 | Ion homeostasis | [44] | |
| ferric-adenosine triphosphate complex | 563.9034 | 23.5 | Intracellular iron transport | [45] | |
| Native soil vermicompost | Atropine | 677.3182 | 23.3 | Anticholinergic activity | [46] |
| palmitate | 257.274 | 20.1 | Anticancer | [47] | |
| 3,3′,5-triiodothyroacetic acid | 622.7689 | 22.8 | Treatment of hyperthyroidism | [48] | |
| Native soil control | palmitate | 257.274 | 20.1 | Anticancer | [47] |
| n-tetratetracontan | 619.7199 | 21.8 | Antibacterial | [49] | |
| 3,3′,5-triiodothyroacetic acid | 622.7689 | 22.8 | Treatment of hyperthyroidism | [48] | |
| Germerine | 694.3914 | 18 | Hypotensive activity | [37] | |
| Atropine | 677.3182 | 23.3 | Anticholinergic activity | [46] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Manzano-Gómez, L.A.; Guzmán-Albores, J.M.; Rincón-Rosales, R.; Winkler, R.; Rincón-Molina, C.I.; Castañón-González, J.H.; Ruiz-Lau, N.; Gutiérrez-Miceli, F.A.; Rincón-Molina, F.A.; Ruíz-Valdiviezo, V.M. Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types. Agronomy 2021, 11, 2061. https://doi.org/10.3390/agronomy11102061
Manzano-Gómez LA, Guzmán-Albores JM, Rincón-Rosales R, Winkler R, Rincón-Molina CI, Castañón-González JH, Ruiz-Lau N, Gutiérrez-Miceli FA, Rincón-Molina FA, Ruíz-Valdiviezo VM. Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types. Agronomy. 2021; 11(10):2061. https://doi.org/10.3390/agronomy11102061
Chicago/Turabian StyleManzano-Gómez, Luis Alberto, Jorge Martín Guzmán-Albores, Reiner Rincón-Rosales, Robert Winkler, Clara Ivette Rincón-Molina, José Humberto Castañón-González, Nancy Ruiz-Lau, Federico Antonio Gutiérrez-Miceli, Francisco Alexander Rincón-Molina, and Víctor Manuel Ruíz-Valdiviezo. 2021. "Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types" Agronomy 11, no. 10: 2061. https://doi.org/10.3390/agronomy11102061
APA StyleManzano-Gómez, L. A., Guzmán-Albores, J. M., Rincón-Rosales, R., Winkler, R., Rincón-Molina, C. I., Castañón-González, J. H., Ruiz-Lau, N., Gutiérrez-Miceli, F. A., Rincón-Molina, F. A., & Ruíz-Valdiviezo, V. M. (2021). Evaluation of Metabolomic Profile and Growth of Moringa oleifera L. Cultivated with Vermicompost under Different Soil Types. Agronomy, 11(10), 2061. https://doi.org/10.3390/agronomy11102061










