Abstract
Weathered coal is a widely used raw material of farm-oriented humic acid in China, while the high heterogeneity impedes its sufficient utilization. In this study, we fractionated the humic acid derived from Chinese weathered coal by ultrafiltration, and three fractions with the molecular range of ≥100 kDa, 10–100 kDa, ≤10 kDa were obtained. Subsequently, the chemical and spectral properties of the fractions were characterized by elemental analysis, potentiometric titration, Fourier transform infrared (FTIR), nuclear magnetic resonance (NMR), and X-ray photoelectron (XPS) and other spectroscopy. The results showed that more than 60% of humic acid by weight was concentrated in the molecular range higher than 100 kDa, while only 3.25% was assigned to that with molecular weight lower than 10 kDa. The humic acid fraction with molecular weight higher than 100 kDa showed more carbon content, lower H/C atomic ratio, while higher E4/E6 ratio, more aromatic structure in FTIR, 13C NMR, and XPS spectra, indicating a higher degree of aromaticity and stronger hydrophobicity. Conversely, there were more carboxyl groups and aliphatic structures, while fewer condensed aromatic rings for the humic acid fraction with molecular weight less than 100 kDa. These differences provide a baseline for the better utilization of weathered coal.
1. Introduction
Weathered coal is a kind of low-rank coal, which is usually exposed to the Earth’s surface or buried at shallow depths. The burial location determines the characteristics of this metamorphic coal. In general, it has a low calorific value, while a high ash and moisture content, leading to a difficulty in the resource utilization of weathered coal in the current industry [1]. Abundant resource reserves of weathered coal are reported to occur in China, estimated at more than 10 billion tons [2]. Due to the low value in industrial production, most of them are unused and thus accumulate, resulting in wasted resources, occupation of space, and pollution of the environment [3]. Therefore, exploring an available way to use weathered coal is an urgent problem, and its solution will own a huge social and environmental value. In addition, the features mentioned above, weathered coal is also characterized with a higher content of humic acid (HA), accounting for 30–80% of its weight [1,4]. The abundant reserve and high HA content contribute weathered coal to an important raw material of HA [5].
HA is an amorphous, colloidal, and polydisperse organic compound with complex compositions and structures [6], and it is composed of various molecular moieties and functional groups [7]. Extensive studies have demonstrated that HA plays an important role in the stimulation of plant growth, improvement of nutrient uptake and use, and complexation with heavy metals and pesticides in soil and water environments [4,8,9,10]. Therefore, it is extensively used as a commercial biostimulant, fertilizer synergist, or soil amendment. During application, HA’s behavior is profoundly influenced by its inherent elemental and structural composition, which is highly dependent on its origin or molecular weight distribution [1,11]. It is reported that HAs with low molecular weight showed a higher bioactivity on the plant root growth and urease activity inhibition than that with higher molecular weight [4,6]. The HAs characterized and applied in previous research are mostly extracted from lignite, peat, compost, and soil [6,11,12,13,14], while there are limited reports on the characteristics of weathered coal derived HA, which hinders its understanding and application. Unlike other raw materials, weathered coal generally contains humic acid with more abundant active functional groups, such as acidic and aromatic structures, which are testified pivotal in HA’s functioning [1,15,16]. It is reported that the HAs with more carboxyl groups showed a high affinity for mineral nutrient, metal, or organic pollutant, indicating its huge potential as fertilizer synergists or soil amendments [1]. In addition, Qian et al. found that HAs with higher O-alkyl and carboxyl groups showed a better performance on plant growth [4], Canellas et al. suggested HAs’ conformational stability affects bioactivity on Arabidopsis and maize seedlings [13]. Thereby, it is important to explore the composition and structure of HA derived from weathered coal by using an appropriate procedure for a clear understanding of its properties, and the exploration has a fundamental significance for the utilization of this resource.
During the HA characterization, researchers usually separate it into different fractions to reduce heterogeneity [6,13,17,18,19,20,21], and this gradually becomes the essential starting point for detailed studies. In a prior study, we fractionated HA derived from weathered coal by adjusting the pH of extraction and found that there were some slight differences among HA fractions, especially in the acidic functional groups and the degree of protonation in aromatic structure [15]. Nonetheless, the limited variation impeded the comprehensive understanding of weathered coal derived HA. Therefore, a more suitable fractionation method should be explored to obtain fractions with large differences. Ultrafiltration is an important and extensively employed method that uses membranes with different pore sizes, and its yielded fractions vary greatly in structure and molecular weight [6,19,22]. Dong et al. ultra-filtrated Chinese lignite HA, and found that the fraction with molecular weight above 50 kDa exhibited a higher C content and aromatic structure, while a lower O and carboxyl structure than the fraction with molecular weight of 10–50 kDa [6]. Lu et al. ultra-filtrated lignite HA into fractions with more fine molecular weight range, and the variation of their elemental and structural composition along with molecular weight was in accordance with that reported by Dong et al. [6,23]. In addition, ultrafiltration is also convenient to batch process for HA fractionation [24]. However, there was no ultrafiltration been used in the fractionation of weathered coal derived HA.
In this context, we employed ultrafiltration to fractionate weathered coal derived HA to obtain corresponding fractions with different nominal molecular weight ranges. Subsequently, the HA fractions were characterized by using techniques, including elemental analysis, potentiometric titration, ultraviolet-visible (UV-Vis) spectroscopy, Fourier transform infrared (FTIR) spectroscopy, solid-state 13 C nuclear magnetic resonance (NMR) spectroscopy, and X-ray photoelectron spectroscopy (XPS). Thus, we achieved the main objective of this work to isolate weathered coal derived HA and to elucidate the features of the corresponding HA fractions. These results will provide a more comprehensive understanding of the structure of weathered coal derived HA and develop a basis for how to effectively use the resources of weathered coal.
2. Materials and Methods
2.1. Materials
2.1.1. Extraction and Purification of HA from Weathered Coal
The Chinese weathered coal used in this study was collected from the Huolinhe Minerals Administration Coalmine (E 119°15′, N 45°23′; Tongliao, Inner Mongolian Autonomous Region, Northeast China). Air-dried weathered coal was pulverized and sieved with a 100-mesh sieve. The characteristics of weathered coal were reported in Zhang et al. [15] The extraction and purification of HA was performed according to the procedure developed by Stevenson [25] and modified by Pramanik and Kim [19]. An aliquot of weathered coal powder was suspended in 0.25 M NaOH with the final solid to liquid ratio maintained at 1:10. Then, the solution was stirred at ambient temperature for 24 h and centrifuged at 8000 rpm min−1 for 10 min to remove impurities. The supernatant was filtered through filter paper (diameter < 20 μm) in a vacuum to remove impurities. Subsequently, the filtrate was acidized to pH = 1.0 with 6.0 M HCl and allowed to stand for 24 h. Then, the precipitate was centrifuged at 8000 rpm min−1 for 10 min to obtain crude HA. The crude HA was purified by shaking in a dilute HCl-HF solution (0.1 mol L−1 HCl 0.3 mol L−1 HF = 1:1, approximately HA:HCl-HF = 1:10, on a w:v basis) overnight to remove inorganic contaminants three times, and a wash with distilled water was also performed to completely remove foreign Cl- ions. Finally, the washed HA was freeze-dried to get a powder of humic acid, which was then weighed and stored in a desiccator until use.
2.1.2. Ultrafiltration and Fractionation of HA
The purified HA was fractionated into three fractions with different molecular weight by ultrafiltration using a procedure similar to that described previously [6,7,24]. Eight liters of HA solution (20 g L−1 in 0.25 mol L−1 NaOH) were divided into 20 parts, and each part was filtered by using a 400mL stirred ultrafiltration cell (8400, Amicon®, Darmstadt, Germany) equipped with a membrane (Ultracel®, Amicon, Darmstadt, Germany) with a large pore size (100 kDa). When the volume of solution retained by the membrane was reduced to about 50 mL, filtration was stopped. The solution, containing the HA fraction with nominal molecular weight greater than 100 kDa (HAH) was removed from the cell and collected in a reservoir. Meanwhile, the filtrate was also put together and the filtration was repeated with membranes of a small pore size (molecular weight cut-offs of 10 kDa) to obtain the HA fraction with nominal molecular weight of 10–100 kDa (HAM). The remaining filtrate was also retained as the HA fraction with nominal molecular weight below 10 kDa (HAL). Each solution containing HA fraction was protonated to pH = 1 with 6 mol L−1 HCl, and let stand for 24 hours. The centrifugation was conducted at 8000 rpm min−1 for 10 min. After freeze-drying, the HA fractions were weighed, then stored in a desiccator until use.
2.2. Methods
2.2.1. Elemental Analysis
Elemental analysis (C, H, and N) was carried out in triplicate by using a Vario Micro Cube Elementar elemental analyzer (Elementar Analysensysteme Gmbh, Langenselbold, Germany). The oxygen content was calculated by subtraction: O% = 100 − (C + H + N)%. On the basis of elemental content, we also calculated the atomic ratios: H/C, O/C, O/H, and N/C.
2.2.2. Acidic Functional Groups
The total acidity and carboxylic group contents of HA fractions were determined by using the methods of potentiometric titration after extraction with Ba(OH)2 and Ca(CH3COO)2, respectively, as described in a previous report [15]. Phenolic hydroxyl group content was calculated based on the difference.
2.2.3. UV-Vis Spectroscopy
Each sample was analyzed in triplicate. The light absorbance of samples in 0.05 mol L−1 NaHCO3 was measured using a range of 250 to 800 nm with an Analytik SPECORD 200 PLUS UV/VIS spectrophotometer (Analytik Jena Inc., Jena, Germany). The E4/E6 ratio (the absorbance at 465 nm divided by that at 665 nm) was calculated as an estimation of degree of aromatic rings condensation [26].
2.2.4. FTIR Spectroscopy
Fourier transform infrared spectra of humic acid fractions were collected in KBr tablets with an FTIR 650 spectrometer (TIANJINGGANGDONG, Tianjin, China). The KBr tablets were prepared by grinding 2 mg of freeze-dried HA samples with 200 mg oven-dried KBr in a vibrating puck mill, and then mixtures were compressed into a translucent pellet using a hydraulic compressor. The spectral range was 4000 to 400 cm−1 with a resolution of 4 cm−1, and an average of 32 scans were taken in the individual spectrum. Subsequently, baseline correction and automatic data smoothing correction of the spectrum were conducted to optimize the spectra.
2.2.5. Solid-State 13C NMR Spectroscopy
The solid-state 13C NMR spectrum was measured by using the cross polarization with cross polarization/total sideband suppression (CP/TOSS) and CP/TOSS with dipolar dephasing (DD), as described by Mao et al. [27], Mao et al. [28], and Zhang et al. [15]. The spectra were collected with a Bruker AVANCE III 400 spectrometer (Bruker Instrumental Inc., Karlsruhe, Switzerland) at a magnetic field of 100 MHz with a 90° pulse width of 4 μs and a contact time of 1 ms. The spin rate was 5 kHz, and the delay between pulses was 0.8 s. The corresponding sub-spectra with signals of non-protonated carbons and mobile groups were obtained by 13C CP/TOSS with 40 μs DD. There were 3000 scans performed for each spectrum. Subsequently, the phase position correction and baseline correction were conducted using MestReNova 9.0.1 software (Mestrelab, Research Inc., Calicia, Spain). The spectra and sub-spectra obtained were assigned to different carbon functional groups according to previous studies [28,29,30], and the following resonance intervals were generally attributed to different carbons: alky C (0–64 ppm), O-alkyl C (64–93 ppm), aromatic C (93–143 ppm), aromatic C-O (143–163 ppm), COO/N-C=O (163–190 ppm), and ketones/aldehydes C (190–220 ppm). The relative proportion of each functional group was calculated by integration and expressed as the percentage of the total area. In addition, the areas relative to these resonance intervals were used to evaluate the degree of aromaticity and hydrophobicity (Hydrophobic/Hydrophilic carbons, HB/HI) of humic acid fractions comprehensively as that calculated by Dobbss et al. [14]:
2.2.6. X-ray Photoelectron Spectroscopy
X-ray photoelectron spectroscopy was employed to determine the number and type of functional groups present on the surface of the HA fractions samples by using a Thermo Scientific ESCALab 250 spectrophotometer (Thermo Fisher Scientific Inc., Massachusetts, America). The finely powdered samples were molded into a disc with a diameter of 10 mm, and were analyzed by using a monochromatic Al Kα X-ray source, at a power of 15 kV at 150 W. The survey scans were collected from the binding energy of 0–1350 eV with 1 eV per step. The XPS survey spectra exhibited three prominent peaks around 285 eV, 400 eV, and 532 eV due to carbon, nitrogen, and oxygen, and their corresponding high-resolution spectra were acquired with a step size of 0.1 eV. For calibration purpose, the peak of C 1 s electron binding energy was corrected at 284.8 eV [31]. A non-linear least squares curve-fitting program (XPSPEAK software, Version 4.1) was used for XPS spectral deconvolution and spectra analysis [32].
3. Results and Discussion
3.1. Mass Distribution of the HA Fractions
After extraction and purification, the obtained HA accounted for 42.13% of weathered coal by weight. Subsequently, the three HA fractions were obtained as the test materials by using the ultrafiltration process. The fraction distribution showed that HAH fraction accounted for 61.88% of the whole HA derived from weathered coal, HAM for 20.95%, and HAL for 3.25%. In addition, about 13.93% of the whole HA derived from weathered coal had not been recovered during the fractionation. According to the results above, the fractions with molecular weight of more than 100 kDa were the dominant components of the whole HA derived from weathered coal. This indicated that HA derived from weathered coal showed a higher potential as urease stabilizer or soil amendments [1,6]. The mass distribution in this study is consistent with several studies of some other materials using ultrafiltration techniques to separate humic acid derived from other sources [5,22,33,34]. In contrast, Benner et al. [35] reported that 75% of marine organic carbon was fractionated as low-molecular weight dissolved organic matter, and the results of Li et al. [5] also showed that most fulvic organic carbon is derived from the fraction with the medium molecular weight of 30–50 kDa. These differences are likely because marine organic carbon and fulvic acid generally have much lower molecular weight and higher polarity than HAs derived from soil and weathered coal [36].
3.2. Elemental Analysis
The elemental analysis results for HAH, HAM, and HAL are shown in Table 1. In these three HA fractions, the content of the four test elements were in descending order as: carbon > oxygen > hydrogen > nitrogen, while their values of different HA fractions varied. Along with the molecular weight cut-offs decreased, carbon content gradually decreased from 60.8% to 57.4%, while oxygen content increased. This is consistent with the variation of HA fractions derived from lignite [6,23]. In addition, the hydrogen content changed slightly in current study, while there was a significant variation for pH-fractionated fractions derived from the same raw weathered coal in our previous document [15]. This phenomenon could be attributed to the discrepancy about the inherence theory of fractionation methods, and the acidification before pH-fractionation to some extent. Atomic ratios can be used to deduce the structural characteristics of humic acid [11,21]. The H/C atomic ratio is related to the degree of aromatic condensation. The H/C atomic ratios of HAM and HAL exceeded that of HAH by 5.48% and 3.52%, respectively. Hence, HA fractions separated with smaller molecular weight cut-offs showed more polarity but less aromaticity [33]. The O/C atomic ratio increased from 0.417 to 0.485 as the molecular weight cut-off decreased (Table 1), reflecting the gradual increase in oxygenated functional groups, such as hydroxyl and carboxyl [34], when molecular weight was lower. The H/O ratio implies the oxidation degree and polarity of the organic matter [37]. In this study, the H/O ratio declined from 1.84 to 1.64 with the decrease in HA molecular weight (Table 1), suggesting a gradual increase in the number of oxygen-containing functional groups and polarity of the HA fractions [37].

Table 1.
Elemental composition (moisture and ash free) and atomic ratio of the HA fractions derived from Chinese weathered coal.
Values are means of three replicates. HAH represents HA fraction with nominal molecular weight higher than 100 kDa, HAM represents HA fraction with nominal molecular weight of 10-100 kDa, and HAL represents HA fraction with nominal molecular weight below 10 kDa.
3.3. UV-Vis Spectra and Acidic Functional Groups
The UV-Vis absorbance of HA fractions decreased as the wavelength increased (Figure S1). This is in accordance with previous literature that the spectra were typically broad and featureless [1,21,33]. The E4/E6 ratio is commonly used to reflect the molecular weight and condensation degree of the humic macromolecule [25,26]. In a previous study, we fractionated the weathered coal derived HA by adjusting the extraction pH, and found that the E4/E6 ratio of HA fractions varied from 3.08 to 4.12 [15]. However, the E4/E6 ratio of HA fractions had a range of 3.41 to 15.1 following ultrafiltration in this study (Table 2). This difference indicated that ultrafiltration could fractionate HA more thoroughly and with larger variation. The E4/E6 ratio of HAM was 7.18, approximately twice the value of HAH, while a half of the value of HAL. Hence, there was more condensed aromatic structure while less aliphatic carbon in the HA fraction with a higher molecular weight [11,26]. This information was consistent with that indicated by H/C atomic ratio in Table 1.

Table 2.
The E4/E6 ratio, acidic functional group contents and distribution of HA fractions derived from weathered coal.
The total acidity of HAM and HAL had the values of 7.21 and 7.08 mmol g−1, respectively, which were apparently higher than that of HAH. This confirmed the results from O/C and H/C atomic ratio in Table 1 that there were more oxygen-containing functional groups in HA fractions with a lower molecular weight. In addition, the presence of acidic functional groups bears the negative charge of HA molecule, which is an efficient feature for HA as an adsorbents with positively charged groups [38]. Hence, HAM, and HAL, the HA fractions with more total acidity, have a more broad application prospect as absorbents than HAH. In addition, HAM and HAL also had a higher carboxyl content than HAH, suggesting that HAM and HAL has a stronger affinity for the complexation of ammonium ion [39]. Moreover, there were more phenolic OH groups for HAH, beneficial for the enhancement of phosphate availability [40].
The carboxyl group accounted for more than 60% of the total acidic functional groups in HA fractions, and the proportion increased from 68.6% to 80.9% with the decrease in HA molecular weight. Therefore, the carboxyl group was the predominant acidic functional groups in HA fractions, especially for that with lower molecular weight. The abundant acidic, especially carboxyl, groups provided a basis for the resource utilization of humic acid derived from weathered coal as chelating agent for fertilizer and pollutant [1,16,37].
3.4. FTIR Spectroscopy
FTIR spectra of the three HA fractions are shown in Figure 1. It is obvious that all samples occupied similar absorption bands at the following wavenumbers: around 3420 cm−1 (H-bonded OH stretching of carboxyl alcohol and phenol), 1720 cm−1 (C=O stretching of COOH and ketones), 1620 cm−1 (C=O asymmetric stretching of COO-, H-bonded C=O, aromatic C=C, alkenes in conjugation with C=O), 1420 cm−1 (aliphatic C-H bending and COO- asymmetric stretching), and 1250 cm−1 (-C-O- stretching and O-H-deformation in COOH, C-O stretching of aryl esters) [25,33]. However, the appreciable differences in relative vibration intensity among samples could be inspected further. The spectra of HAM and HAL had stronger peak intensity at the 3420 and 1420 cm−1 wavenumbers than HAH. In addition, we also observed a pronounced vibration at the region of 3000 to 2800 cm−1 (aliphatic C-H stretching) in the samples of HAM and HAL, while there was no distinct peak at this region in HAH. Thus, HAM and HAL fractions were suggested to have more hydroxyl and aliphatic groups, consistent with the results from UV-Vis spectra and elemental analysis (Table 1 and Table 2). In addition, the adjacent peak at the regions of 1720 and 1620 cm−1 exhibited different relative changes in the HA fractions. In the HAH fraction, the vibration intensity at 1620 cm−1 was stronger than that at 1720 cm−1, while there was a reverse trend in the other two samples. The spectra deconvolution could be used to quantified the difference about vibration intensity [41], and The results showed that the ratio of C=C (1650–1520 cm−1) to C=O (1800−1650 cm−1) was 2.12 for HAH, 1.52 for HAM, and 1.14 for HAL (Figure S2 and Table S1). This indicated that there were more aromatic functional groups, while less carboxyl functional groups in HA fraction with higher molecular [5,6,33], corresponding to its lower E4/E6 value, lower carboxyl groups and O/C atomic ratio (Table 1 and Table 2). This indicates that HAL probably enhances plant growth and urease inhibition more than HAM and HAH fractions, while HAH will showed more stability and exert less bioactivity [4,6,13].
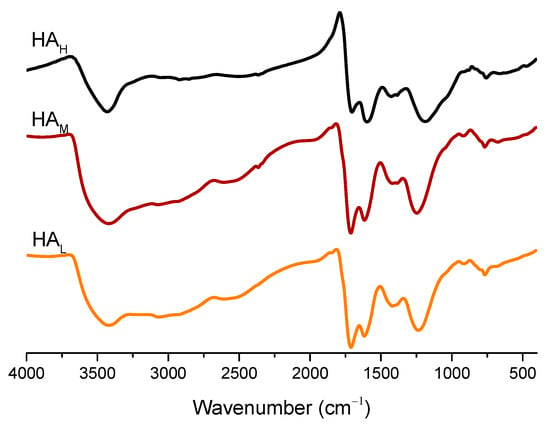
Figure 1.
Fourier transform infrared (FTIR) spectra of HA fractions derived from weathered coal.
3.5. Solid-State 13C NMR Spectra
The CP/TOSS 13C NMR spectra and their corresponding sub-spectra by CP/TOSS with DD techniques for HA fractions are presented in Figure 2. In general, each sample showed a similar major spectral band, and their major peaks on the resonance areas concentrated at the regions of 100 to 150 ppm and 150 to 200 ppm, implying that the HA fractions were dominated by aromatic and carboxyl structures [28]. Such a phenomenon was consistent with the spectra in our previous study [15], while distinct from the common 13C NMR spectra of HA derived from soil and peat [11,17,30,42], providing support that weathered coal was subject to a high degree of oxidization and aromaticity [1]. Additionally, compared with the fractions obtained by pH-fractionation, each HA fraction obtained by ultrafiltration had more non-protonated aromatic carbon, which seems to be attributed to the acidification prior to pH-fractionation [15].
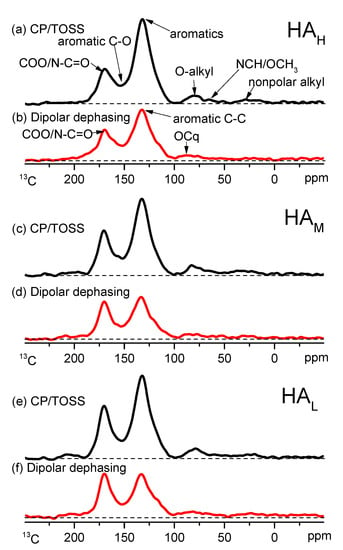
Figure 2.
13C nuclear magnetic resonance (NMR) spectra of HA fractions derived from weathered coal by unselective cross polarization with cross polarization/total sideband suppression (CP/TOSS) (a,c,e) and dipolar dephased techniques (b,d,f).
The relative intensities in spectra of HA fractions varied greatly (Figure 2). The difference of relative resonance intensities at 100–150 ppm and 150–200 ppm was narrowed in HAM and HAL compared to HAH. This trend was more apparent in the spectra of CP/TOSS with DD (Figure 2b,d,f). Specifically, the peak height at 100–150 ppm was significantly higher than that at 150–200 ppm in the HAH fraction. However, the peak heights in these two chemical shifts were similar in HAM and HAL, and HAL even showed a slightly higher resonance intensity at 150–200 ppm. Therefore, the ratio of aromatic C-C and carboxyl C in HAM and HAL was lower than that in HAH, suggesting that more carbon was assigned to aromatic structure in HAH, while HAM and HAL had more carbon belonging to carboxyl groups [28]. These findings should be verified by the assignment of carbon-containing functional groups.
The proportion of carbon-containing functional groups in each sample based on semi-quantitative calculation of 13C NMR spectra is summarized in Table 3 to complement the chemical and structural properties of HA fractions by FTIR. Aromatic structure was the predominant assignment of carbon and accounted for more than 65% of total carbon functional groups, and could be divided into two categories, aromatic C and aromatic C-O. Their proportion declined as the molecular weight of HA fractions decreased, verifying the above conjectural results that there were more aromatic and phenolic OH groups in HAH (Table 2, Figure 1). This feature was conformed to previous research on the HA fractions derived from lignite [6,23]. The second largest functional group, COO/N-C=O, had an ascending change with the lowered molecular weight of HA fractions, in accordance with the results by the potentiometric titration that more carboxyl groups appeared in the HAL (Table 2). In addition, HAM and HAL also exhibited a larger value in the chemical shift regions of alkyl C, O-alkyl C and ketones/aldehydes than that of HAH, a signal of a higher aliphatic structure, leading to a lower index of aromaticity (in Table 3). The maximum hydrophobic index value occurred in the HAH fraction, while the minimum value occurred in HAL, indicating the decrease in hydrophobicity of the HA fraction with the decreased molecular weight [14]. This was consistent with the inference from the change in the H/C atomic ratio in Table 1.

Table 3.
Distribution of 13C in resonance intervals (ppm) of CP/TOSS, CP/TOSS with DD NMR C intensity in different regions of NMR spectra of HA fractions and their reaction derivatives.
3.6. XPS Spectroscopy
There were three chemical states distinguished in the XPS C 1 s spectra of HA fractions (Figure 3). The chemical assignments, binding energy (BE), and relative proportion (RP) of each functional group in the C 1 s spectra of HA fractions were listed in Table S2. The relative proportion of carbon functional groups decreased according to the sequence aromatic carbon (about 70–73%) > carboxyl carbon (about 15–19%) > ketonic carbon (10–13%), which was in consistent with the results from 13C NMR in Figure 2 that there was more carbon assigned to aromatic structure, and carboxyl structure followed. Along with the decrease in molecular weight, the relative content of aromatic carbon decreased, while the carboxyl content increased, in consistent with the results from 13C NMR in Figure 2, as well. However, there was no apparent relationship between the ketonic carbon and molecular weight of HA fractions.
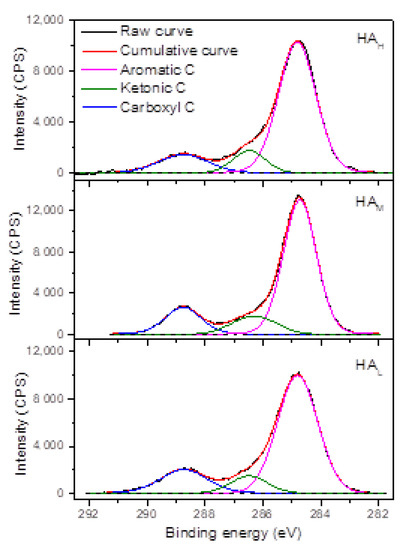
Figure 3.
High-resolution C 1 s X-ray photoelectron spectra of HA fractions. The black dash line means the baseline of each sample. Count per second, the intensity unit, is abbreviated as CPS in the figures.
For the XPS N 1 s signals, two peaks were fitted as shown in Figure 4. The peaks were centered at about 399.7 eV (aromatic N including imine, heterocyclic C=N, and aromatic amine), 400.6 eV (peptide bond N including other amides, pyrrole, secondary and tertiary amines, and imide) or 401.7 eV (primary amine N including other protonated N) [43,44]. The data shown in Table S3 clearly demonstrated that aromatic nitrogen (about 80–87%) was the most abundant form of organic bound nitrogen in weathered coal derived HA, and it was positively correlated with the molecular weight of HA fractions. This is attributed to higher aromatic carbon of the HA fraction with high molecular weight to some extent, in agreement with the results from the H/C atomic ratio in Table 1, E4/E6 ratio in Table 2, and the spectra of FTIR and 13C NMR and XPS C1s (Figure 1, Figure 2 and Figure 3).
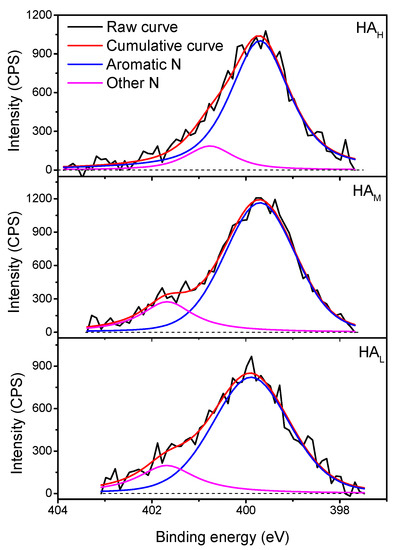
Figure 4.
High-resolution N 1 s X-ray photoelectron spectra of HA fractions. The black dash line means the baseline of each sample. Other N indicates the peptide bond N or primary amine N.
The XPS O 1 s spectra were deconvoluted into two or three Gaussian curves with peak centers at about 531.5 eV, 533.1 eV, 536.2 eV (Figure 5). The peak around 531.1 eV was assigned to carbonyl oxygen atoms, and the peak round 533.1 eV was attributed to the ether oxygen atoms in esters and anhydrides [32]. In addition, there was a peak located at 536.1 eV observed in the sample of HAH, which was the contribution of water [32]. Considering the use of NaOH during HA preparation, this curve is also probably attributed to Na+. The relative distribution of oxygen functionalities in HA fractions was listed in Table S4. There was more oxygen attributed to carbonyl, ranging from 44.46% to 59.65%, than that to ether with the relative distribution less than 48.81%. For individual samples, the relative proportion of carbonyl oxygen increased when the molecular weight decreased, in consistency with the results from the potentiometric titration in Table 2 and the spectra of FTIR, 13C NMR, XPS C1 s (Figure 1, Figure 2 and Figure 3).
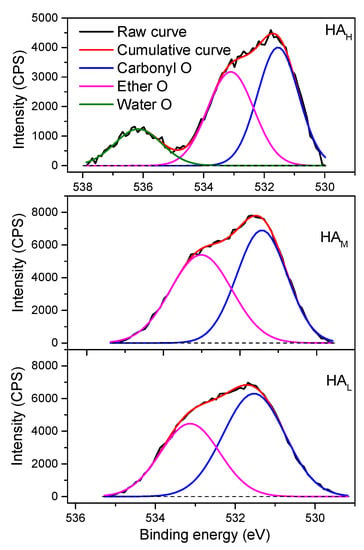
Figure 5.
High-resolution O 1 s X-ray photoelectron spectra of HA fractions. The black dash line means the baseline of each sample.
All above verified that there were more aromatic structures in HA factions with molecular weight higher than 100 kDa, while more carboxyl groups and aliphatic structures in that with molecular weight less than 100 kDa. Therefore, they will have a discrepant performance on biostimulation, fertilizer-efficiency enhancement, or soil amendment [1]. However, the detailed results should be verified with further research.
4. Conclusions
The fraction with the molecular weight higher than 100 kDa was the main component of HA derived from weathered coal, while the fraction with the molecular weight lower than 10 kDa only accounted for 3.25% of its total mass. This indicated that HA derived from weathered coal showed a higher potential as urease stabilizer or soil amendments. Compared with fractionation by adjusting the pH value of the extraction, ultrafiltration was a more feasible way to obtain HA with significant differences in chemical and structural composition. The compositional and spectroscopic investigation of HA fractions showed that their characteristics determined by various methods were basically consistent. HA fractions with molecular weight higher than 100 kDa had higher carbon content, a larger number of condensed aromatic rings and non-protonated carbon. Thus, these fractions also possessed a higher degree of aromaticity and stronger hydrophobicity. In contrast, the oxygen content and oxygen-containing functional groups, especially carboxyl groups, were more prevalent in HA fractions with molecular weight less than 100 kDa. Moreover, their aliphatic structure was also more apparent. The above different composition and functional group properties are expected to provide a basis for the utilization of HA fractions that the HA fractions with low molecular weight as biostimulant or nutrient chelating agent, while that with higher molecular weight as urease stabilizer or soil amendments.
Supplementary Materials
The following are available online at www.mdpi.com/article/10.3390/agronomy11102030/s1, Figure S1: UV-Vis spectra of HA fractions derived from weathered coal. Figure S2: Curve-fitting FTIR spectra of oxygen-containing structure in HA fractions. Table S1: Structural parameters determined from curve-fitting FTIR spectra in HA fractions. Table S2: Relative distribution of carbon functionalities in HA fractions determined by XPS. Table S3: Relative distribution of nitrogen functionalities in HA fractions determined by XPS. Table S4. Relative distribution of oxygen functionalities in HA fractions determined by XPS.
Author Contributions
Conceptualization, B.Z.; methodology, B.Z. and L.Y.; software, S.Z.; validation, S.Z. and L.Y.; formal analysis, S.Z. and L.Y.; investigation, S.Z. and L.Y.; resources, L.Y. and Y.L.; data curation, S.Z.; writing—original draft preparation, S.Z.; writing—review and editing, S.Z., L.Y. and B.Z.; visualization, S.Z. and L.Y.; supervision, B.Z. and Y.L.; project administration, B.Z. and Y.L.; funding acquisition, B.Z. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by National Key Research and Development Program of China, grant number 2016YFD0200402; Fundamental Research Funds for Central Non-profit Scientific Institution, grant number 1610132019019, and National Natural Science Foundation of China, grant number 31601827; China Agriculture Research System, grant number CARS-03.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All discussed and analyzed data are contained within the article or Supplementary Materials.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zheng, P. The Application and Production of Coal Humic Acids; Chemical Industry Press: Beijing, China, 1991. [Google Scholar]
- Yuan, L.; Huang, T.; Wang, G.; Wang, X. Effects of humic acid and humic organic fertilizer on nutrient allocation and fertilizer use efficiency of maize. Chin. Agric. Sci. Bull. 2014, 30, 98–102. [Google Scholar]
- Li, S.; Dou, X. Current situation and forecast for the utilization of weathered coals. Coal Chem. Ind. 1996, 1, 1–4. [Google Scholar]
- Qian, S.; Ding, W.; Li, Y.; Liu, G.; Sun, J.; Ding, Q. Characterization of humic acids derived from Leonardite using a solid-state NMR spectroscopy and effects of humic acids on growth and nutrient uptake of snap bean. Chem. Speciat. Bioavailab. 2015, 27, 156–161. [Google Scholar] [CrossRef]
- Li, H.; Li, Y.; Li, C. Characterization of humic acids and fulvic acids derived from sewage sludge. Asian J. Chem. 2013, 25, 10087–10091. [Google Scholar] [CrossRef]
- Dong, L.; Yang, J.; Yuan, H.; Wang, E.; Chen, W. Chemical characteristics and influences of two fractions of Chinese lignite humic acids on urease. Eur. J. Soil Biol. 2008, 44, 166–171. [Google Scholar] [CrossRef]
- Shin, H.S.; Monsallier, J.M.; Choppin, G.R. Spectroscopic and chemical characterizations of molecular size fractionated humic acid. Talanta 1999, 50, 641–647. [Google Scholar] [CrossRef]
- Senesi, N.; Plaza, C.; Brunetti, G.; Polo, A. A comparative survey of recent results on humic-like fractions in organic amendment and effects on native soil humic substances. Soil Biol. Biochem. 2007, 39, 1244–1262. [Google Scholar] [CrossRef]
- Fu, F.; Wang, Q. Removal of heavy metal ions from wastewaters: A review. J. Environ. Manage. 2011, 92, 407–418. [Google Scholar] [CrossRef]
- Canellas, L.P.; Olivares, F.L.; Aguiar, N.O.; Jones, D.L.; Nebbioso, A.; Mazzei, P.; Piccolo, A. Humic and fulvic acids as biostimulants in horticulture. Sci. Hortic. 2015, 196, 15–27. [Google Scholar] [CrossRef]
- Botero, W.G.; Oliveira, L.C.D.; Rocha, J.C.; Rosa, A.H.; Santos, A.D. Peat humic substances enriched with nutrients for agricultural applications: Competition between nutrients and non-essential metals present in tropical soils. J. Hazard. Mater. 2010, 177, 307–311. [Google Scholar] [CrossRef]
- Mao, J.; Fang, X.; Schmidt-Rohr, K.; Carmo, A.M.; Hundal, L.S.; Thompson, M.L. Molecular-scale heterogeneity of humic acid in particle-size fractions of two Iowa soils. Geoderma 2007, 140, 17–29. [Google Scholar] [CrossRef]
- Canellas, L.P.; Piccolo, A.; Dobbss, L.B.; Spaccini, R.; Olivares, F.L.; Zandonadi, D.B.; Façanha, A.R. Chemical composition and bioactivity properties of size-fractions separated from a vermicompost humic acid. Chemosphere 2010, 78, 457–466. [Google Scholar] [CrossRef]
- Dobbss, L.B.; Canellas, L.P.; Olivares, F.L. Bioactivity of chemically transformed humic matter from vermicompost on plant root growth. J. Agric. Food Chem. 2010, 58, 3681–3688. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Characterization of pH-fractionated humic acids derived from Chinese weathered coal. Chemosphere 2017, 166, 334–342. [Google Scholar] [CrossRef]
- Zhang, S.; Yuan, L.; Li, W.; Lin, Z.; Li, Y.; Hu, S.; Zhao, B. Effects of urea enhanced with different weathered coal-derived humic acid components on maize yield and fate of fertilizer nitrogen. J. Integr. Agric. 2019, 18, 656–666. [Google Scholar] [CrossRef]
- Fujitake, N.; Kusumoto, A.; Tsukamoto, M.; Kawahigashi, M.; Suzuki, T.; Otsuka, H. Properties of soil humic substances in fractions obtained by sequential extraction with pyrophosphate solutions at different pHs. Soil Sci. Plant Nutr. 1999, 44, 253–260. [Google Scholar] [CrossRef]
- Asakawa, D.; Kiyota, T.; Yanagi, Y.; Fujitake, N. Optimization of conditions for high-performance size-exclusion chromatography of different soil humic acids. Anal. Sci. 2008, 24, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Pramanik, P.; Kim, P.J. Fractionation and characterization of humic acids from organic amended rice paddy soils. Sci. Total Environ. 2014, 466, 952–956. [Google Scholar] [CrossRef]
- Klučáková, M.; Kalina, M. Composition, particle size, charge, and colloidal stability of pH-fractionated humic acids. J. Soils Sediments 2015, 15, 1–9. [Google Scholar] [CrossRef]
- Enev, V.; Sedláček, P.; Kubíková, L.; Sovová, Š.; Doskočil, L.; Klučáková, M.; Pekař, M. Polarity-Based sequential extraction as a simple tool to reveal the structural complexity of humic acids. Agronomy 2021, 11, 587. [Google Scholar] [CrossRef]
- Francioso, O.; Sánchez-Cortés, S.; Casarini, D.; Garcia-Ramos, J.V.; Ciavatta, C.; Gessa, C. Spectroscopic study of humic acids fractionated by means of tangential ultrafiltration. J. Mol. Struct. 2002, 609, 137–147. [Google Scholar] [CrossRef]
- Lu, M.; Zhang, Y.; Su, Z.; Jiang, T. The NMR and spectral study on the structure of molecular size-fractionated lignite humic acid. Resour. Environ. Sustain. 2020, 2, 100004. [Google Scholar] [CrossRef]
- Pantano, G.; Tadini, A.M.; Bisinoti, M.C.; Moreira, A.B.; Santos, A.D.; Oliveira, L.C.D.; Martin, C.S. Development of a simple and versatile ultrafiltration system for the fractionation of aquatic humic substances. Org. Geochem. 2012, 43, 156–161. [Google Scholar] [CrossRef]
- Stevenson, F.J. Humus Chemistry; Wiley: Hoboken, NJ, USA, 1994. [Google Scholar]
- Chen, Y.; Senesi, N.; Schnitzer, M. Information provided on humic substances by E4/E6 ratios. Soil Sci. Soc. Am. J. 1977, 41, 352–358. [Google Scholar] [CrossRef]
- Mao, J.; Schmidt-Rohr, K. Accurate quantification of aromaticity and nonprotonated aromatic carbon fraction in natural organic matter by 13C solid-state nuclear magnetic resonance. Environ. Sci. Technol. 2004, 38, 2680–2684. [Google Scholar] [CrossRef]
- Mao, J.; Dan, C.O.; Fang, X.; He, Z.; Schmidt-Rohr, K. Influence of animal manure application on the chemical structures of soil organic matter as investigated by advanced solid-state NMR and FT-IR spectroscopy. Geoderma 2008, 146, 353–362. [Google Scholar] [CrossRef]
- Mao, J.; Hu, W.; Schmidtrohr, K.; Davies, G.; Ghabbour, E.A.; Xing, B. Quantitative characterization of humic substances by solid-state carbon-13 nuclear magnetic resonance. Soil Sci. Soc. Am. J. 2000, 64, 873–884. [Google Scholar] [CrossRef]
- Xu, J.; Zhao, B.; Chu, W.; Mao, J.; Olk, D.C.; Xin, X.; Zhang, J. Altered humin compositions under organic and inorganic fertilization on an intensively cultivated sandy loam soil. Sci. Total Environ. 2017, 601, 356–364. [Google Scholar] [CrossRef] [PubMed]
- Jing, J.; Zhang, S.; Yuan, L.; Li, Y.; Lin, Z.; Xiong, Q.; Zhao, B. Combining humic acid with phosphate fertilizer affects humic acid structure and its stimulating efficacy on the growth and nutrient uptake of maize seedlings. Sci. Rep. 2020, 10, 17502. [Google Scholar] [CrossRef]
- Chiang, Y.; Lee, C.; Lee, H. Surface chemistry of polyacrylonitrile- and rayon-based activated carbon fibers after post-heat treatment. Mater. Chem. Phys. 2007, 101, 199–210. [Google Scholar] [CrossRef]
- Li, L.; Zhao, Z.; Huang, W.; Peng, P.A.; Sheng, G.; Fu, J. Characterization of humic acids fractionated by ultrafiltration. Org. Geochem. 2004, 35, 1025–1037. [Google Scholar] [CrossRef]
- González-Pérez, M.; Torrado, P.V.; Colnago, L.A.; Martin-Neto, L.; Otero, X.L.; Milori, D.M.B.P.; Gomes, F.H. 13C NMR and FTIR spectroscopy characterization of humic acids in spodosols under tropical rain forest in southeastern Brazil. Geoderma 2008, 146, 425–433. [Google Scholar] [CrossRef]
- Benner, R.; Biddanda, B.; Black, B.; Mccarthy, M. Abundance, size distribution, and stable carbon and nitrogen isotopic compositions of marine organic matter isolated by tangential-flow ultrafiltration. Mar. Chem. 1997, 57, 243–263. [Google Scholar] [CrossRef]
- Perminova, I.V.; Kovalevskii, D.V.; Abbt-Braun, G.; Kudryavtsev, A.V.; Hesse, S.; Frimmel, F.H. Development of predictive model for calculation of molecular weight of humic substances. Water Res. 1998, 32, 872–881. [Google Scholar] [CrossRef]
- Paolis, F.D.; Kukkonen, J. Binding of organic pollutants to humic and fulvic acids: Influence of pH and the structure of humic material. Chemosphere 1997, 34, 1693–1704. [Google Scholar] [CrossRef]
- Shuang, C.; Wang, M.; Li, P.; Li, A.; Zhou, Q.; Pan, F.; Zhou, W. Adsorption of humic acid fractions with different molecular weight by magnetic polyacrylic anion exchange resin. J. Soils Sediments 2014, 14, 312–319. [Google Scholar] [CrossRef]
- Liang, Z.; Cheng, S. Study on mechanism of interaction between coal humic acid and urea. J. Fuel Chem. Technol. 1999, 27, 176–181. (In Chinese) [Google Scholar]
- Tan, K.H.; Nopamornbodi, V. Effect of different levels of humic acids on nutrient content and growth of corn (Zea mays L.). Plant Soil 1979, 51, 283–287. [Google Scholar] [CrossRef]
- Li, W.; Zhu, Y. Structural characteristics of coal vitrinite during pyrolysis. Energy Fuels 2014, 28, 3645–3654. [Google Scholar] [CrossRef]
- Kasim, S.; Ahmed, O.H.; Nik Muhamad, A.M.; Yusop, M.K.; Jalloh, M.B. Reduction of ammonia loss by mixing urea with liquid humic and fulvic acids isolated from tropical peat soil. Am. J. Agric. Biol. Sci. 2009, 4, 18–23. [Google Scholar] [CrossRef][Green Version]
- Abe, T.; Maie, N.; Watanabe, A. Investigation of humic acid N with X-ray photoelectron spectroscopy: Effect of acid hydrolysis and comparison with 15N cross polarization/magic angle spinning nuclear magnetic resonance spectroscopy. Org. Geochem. 2005, 36, 1490–1497. [Google Scholar] [CrossRef]
- Abe, T.; Watanabe, A. X-ray photoelectron spectroscopy of nitrogen functional groups in soil humic acids. Soil Sci. 2004, 169, 35–43. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).