A Promising Predator-In-First Strategy to Control Western Corn Rootworm Population in Maize Fields
Abstract
1. Introduction
2. Material and Methods
2.1. Greenhouse Trial
2.1.1. Plant Material
2.1.2. Arthropods Production and Preparation
2.1.3. Experimental Setup
2.1.4. Statistical Analyses
2.2. Field Trial
2.2.1. Plant Material
2.2.2. Arthropods Production and Preparation
2.2.3. Experimental Setup
2.2.4. Statistical Analysis
3. Results
3.1. Greenhouse Trials
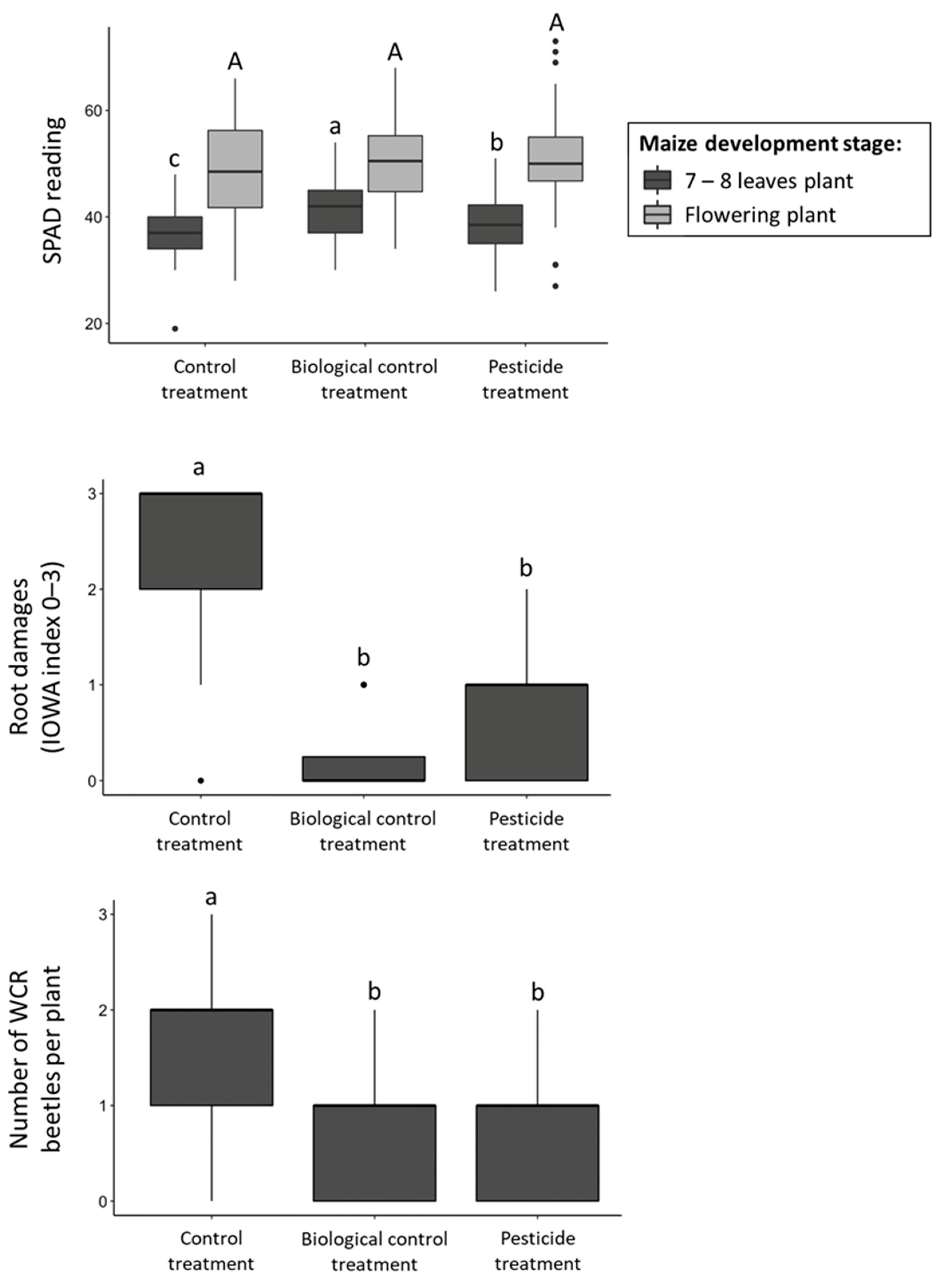
3.2. Field Trials
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Amin, M.R.; Khanjani, M.; Zahiri, B. Preimaginal development and fecundity of Gaeolaelaps aculeifer (Acari: Laelapidae) feeding on Rhizoglyphus echinopus (Acari: Acaridae) at constant temperatures. J. Crop. Prot. 2014, 3, 581–587. [Google Scholar]
- Baatrup, E.; Bayley, M.; Axelsen, J.A. Predation of the mite Hypoaspis aculeifer on the springtail Folsomia fimetaria and the influence of sex, size, starvation, and poisoning. Entomol. Exp. Appl. 2006, 118, 61–70. [Google Scholar] [CrossRef]
- Barbosa, M.F.C.; de Moraes, G.J. Potential of astigmatid mites (Acari: Astigmatina) as prey for rearing edaphic predatory mites of the families Laelapidae and Rhodacaridae (Acari: Mesostigmata). Exp. Appl. Acarol. 2016, 69, 289–296. [Google Scholar] [CrossRef] [PubMed]
- Bažok, R.; Lemić, D.; Chiarini, F.; Furlan, L. Western Corn Rootworm (Diabrotica virgifera virgifera LeConte) in Europe: Current Status and Sustainable Pest Management. Insects 2021, 12, 195. [Google Scholar] [CrossRef] [PubMed]
- Benjamin, E.O.; Grabenweger, G.; Strasser, H.; Wesseler, J. The socioeconomic benefits of biological control of western corn rootworm Diabrotica virgifera virgifera and wireworms Agriotes spp. in maize and potatoes for selected European countries. J. Plant. Dis. Prot. 2018, 125, 273–285. [Google Scholar] [CrossRef]
- Bergman, M.K.; Turpin, F.T. Phenology of Field Populations of Corn Rootworms (Coleoptera: Chrysomelidae) Relative to Calendar Date and Heat Units. Environ. Entomol. 1986, 15, 109–112. [Google Scholar] [CrossRef]
- Branson, T.F.; Sutter, G.R.; Fisher, J.R. Plant Response to Stress Induced by Artificial Infestations of Western Corn Rootworm. Environ. Entomol. 1980, 9, 253–257. [Google Scholar] [CrossRef]
- Cai, H.; You, M.; Lin, C. Effects of intercropping systems on community composition and diversity of predatory arthropods in vegetable fields. Acta Ecol. Sin. 2010, 30, 190–195. [Google Scholar] [CrossRef]
- Chakwizira, E.; Dawson, A.E.; Stafford, A. Yara N-Tester Chlorophyll Meter Calibration: A Prequel. 2020. Available online: https://www.yara.co.nz/crop-nutrition/farmers-toolbox/n-tester/ (accessed on 28 August 2021).
- Ciosi, M.; Miller, N.J.; Kim, K.S.; Giordano, R.; Estoup, A.; Guillemaud, T. Invasion of Europe by the western corn rootworm, Diabrotica virgifera virgifera: Multiple transatlantic introductions with various reductions of genetic diversity. Mol. Ecol. 2008, 17, 3614–3627. [Google Scholar] [CrossRef] [PubMed]
- Cortet, J.; Ronce, D.; Poinsot-Balaguer, N.; Beaufreton, C.; Chabert, A.; Viaux, P.; Cancela de Fonseca, J.P. Impacts of different agricultural practices on the biodiversity of microarthropod communities in arable crop systems. Eur. J. Soil Biol. 2002, 38, 239–244. [Google Scholar] [CrossRef]
- Curzi, M.J.; Zavala, J.A.; Spencer, J.L.; Seufferheld, M.J. Abnormally high digestive enzyme activity and gene expression explain the contemporary evolution of a Diabrotica biotype able to feed on soybeans. Ecol. Evol. 2012, 2, 2005–2017. [Google Scholar] [CrossRef]
- David, P.; Thébault, E.; Anneville, O.; Duyck, P.-F.; Chapuis, E.; Loeuille, N. Impacts of Invasive Species on Food Webs. In Advances in Ecological Research; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–60. [Google Scholar] [CrossRef]
- Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides (Text with EEA Relevance). Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 28 August 2021).
- Dwyer, L.M.; Anderson, A.M.; Stewart, D.W.; Ma, B.L.; Tollenaar, M. Changes in maize hybrid photosynthetic response to leaf nitrogen, from pre-anthesis to grain fill. Agron. J. 1995, 87, 1221–1225. [Google Scholar] [CrossRef]
- Pereira, A.E.; Souza, D.; Zukoff, S.N.; Meinke, L.J.; Siegfried, B.D. Cross-resistance and synergism bioassays suggest multiple mechanisms of pyrethroid resistance in western corn rootworm populations. PLoS ONE 2017, 12, e0179311. [Google Scholar] [CrossRef]
- Elliott, N.C.; Gustin, R.D.; Hanson, S.L. Influence of adult diet on the reproductive biology and survival of the western corn rootworm, Diabrotica virgifera virgifera. Entomol. Exp. Appl. 1990, 56, 15–21. [Google Scholar] [CrossRef]
- Faostat. FAOSTAT (Food and Agriculture Organization of the United Nations). Statistics Database. 2018. Available online: http://www.fao.org/faostat/en/#data (accessed on 2 February 2018).
- Fisher, J.R. Greenhouse Method for Studying Development and Survival of Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1987, 80, 286–289. [Google Scholar] [CrossRef]
- Fountain, M.T.; Medd, N. Integrating pesticides and predatory mites in soft fruit crops. Phytoparasitica 2015, 43, 657–667. [Google Scholar] [CrossRef]
- Furlan, L. The biology of Agriotes sordidus Illiger (Col., Elateridae). J. Appl. Entomol. 2004, 128, 696–706. [Google Scholar] [CrossRef]
- Gaeolaelaps aculeifer (HYSPAC) [Documents]|EPPO Global Database [WWW Document]. Available online: https://gd.eppo.int/taxon/HYSPAC/documents (accessed on 20 August 2021).
- Gerson, U.; Weintraub, P.G. Mites (Acari) as a factor in greenhouse management. Annu. Rev. Entomol. 2012, 57, 229–247. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, D.R.; Quiring, D.M.J. Biological control of fungus gnats, bradysia spp. (diptera: Sciaridae), and western flower thrips, frankliniella occidentals (pergande) (thysanoptera: Thripidae), in greenhouses using a soil-dwelling predatory mite, geolaelaps sp. Nr. Aculeifer (canestrini) (acari: Laelapidae). Can. Entomol. 1990, 122, 975–983. [Google Scholar]
- Godfrey, L.D.; Meinke, L.J.; Wright, R.J. Vegetative and Reproductive Biomass Accumulation in Field Com: Response to Root Injury by Western Com Rootworm and Sustainable Pest Management (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1993, 86, 1557–1573. [Google Scholar] [CrossRef]
- Haegele, J.W.; Cook, K.A.; Nichols, D.M.; Below, F.E. Changes in Nitrogen Use Traits Associated with Genetic Improvement for Grain Yield of Maize Hybrids Released in Different Decades. Crop. Sci. 2013, 53, 1256–1268. [Google Scholar] [CrossRef]
- Hamers, T.; Krogh, P.H. Predator–Prey Relationships in a Two-Species Toxicity Test System. Ecotoxicol. Environ. Saf. 1997, 37, 203–212. [Google Scholar] [CrossRef]
- Inserra, R.N.; Davis, D.W. Hypoaspis nr. aculeifer: A Mite Predacious on Root-knot and Cyst Nematodes. J. Nematol. 1983, 15, 324–325. [Google Scholar]
- Jaffuel, G.; Imperiali, N.; Shelby, K.; Campos-Herrera, R.; Geisert, R.; Maurhofer, M.; Loper, J.; Keel, C.; Turlings, T.C.J.; Hibbard, B.E. Protecting maize from rootworm damage with the combined application of arbuscular mycorrhizal fungi, Pseudomonas bacteria and entomopathogenic nematodes. Sci. Rep. 2019, 9, 3127. [Google Scholar] [CrossRef]
- Jensen, K.; Sørensen, J.G.; Holmstrup, M. Interactive effects of temperature and time on cold tolerance and spring predation in overwintering soil predatory mites (Gaeolaelaps aculeifer Canestrini). Biol. Control. 2019, 132, 169–176. [Google Scholar] [CrossRef]
- Kahler, A.L.; Olness, A.E.; Sutter, G.R.; Dybing, C.D.; Devine, O.J. Root Damage by Western Corn Rootworm and Nutrient Content in Maize1. Agron. J. 1985, 77, 769. [Google Scholar] [CrossRef]
- Kautz, T.; López-Fando, C.; Ellmer, F. Abundance and biodiversity of soil microarthropods as influenced by different types of organic manure in a long-term field experiment in Central Spain. Appl. Soil Ecol. 2006, 33, 278–285. [Google Scholar] [CrossRef]
- Kevan, D.K.M.; Sharma, G.D. Observations on the biology of Hypoaspis aculeifer (Canestrini 1884), apparently new to North America (Acarina: Mesostigmata: Laelaptidae). Acarologia 1964, 6, 647–658. [Google Scholar]
- Kiss, J.; Edwards, C.R.; Berger, H.K.; Cate, P.; Cean, M.; Cheek, S.; Derron, J.; Festic, H.; Furlan, L.; Igrc-Barcic, J. Monitoring of western corn rootworm (Diabrotica virgifera virgifera LeConte) in Europe 1992–2003. West. Corn Rootworm Ecol. Manag. 2005, 29–39. [Google Scholar] [CrossRef]
- Koehler, H.H. Mesostigmata (Gamasina, Uropodina), efficient predators in agroecosystems. Agric. Ecosyst. Environ. 1997, 62, 105–117. [Google Scholar] [CrossRef]
- Kriticos, J.; Reynaud, P.; Baker, R.H.A.; Eyre, D. Estimating the global area of potential establishment for the western corn rootworm (Diabrotica virgifera virgifera) under rain-fed and irrigated agriculture*: The potential distribution of western corn rootworm. EPPO Bull. 2012, 42, 56–64. [Google Scholar] [CrossRef]
- Krysan, J.L.; Jackson, J.J.; Lew, A.C. Field Termination of Egg Diapause in Diabrotica with New Evidence of Extended Diapause in D. barberi (Coleoptera: Chrysomelidae). Environ. Entomol. 1984, 13, 1237–1240. [Google Scholar] [CrossRef]
- Kuhlmann, U.; van der Burgt, A.C.M. Possibilities for biological control of the western corn rootworm, Diabrotica virgifera virgifera LeConte, in Central Europe. Biocontrol News Inf. 1998, 19, 59N–68N. [Google Scholar]
- Kumar, V.; Mehra, L.; McKenzie, C.L.; Osborne, L.S. “Predator-In-First”: A Preemptive Biological Control Strategy for Sustainable Management of Pepper Pests in Florida. Sustainability 2020, 12, 7816. [Google Scholar] [CrossRef]
- Kumar, V.; Xiao, Y.; McKenzie, C.L.; Osborne, L.S. Early establishment of the phytoseiid mite Amblyseius swirskii (Acari: Phytoseiidae) on pepper seedlings in a Predator-in-First approach. Exp. Appl Acarol. 2015, 65, 465–481. [Google Scholar] [CrossRef]
- Kutuk, H.; Yigit, A. Pre-establishment of Amblyseius swirskii (Athias-Henriot) (Acari: Phytoseiidae) using Pinus brutia (Ten.) (Pinales: Pinaceae) pollen for thrips (Thysanoptera: Thripidae) control in greenhouse peppers. Int. J. Acarol. 2011, 37, 95–101. [Google Scholar] [CrossRef]
- Lesna, I.; Conijn, C.G.M.; Sabelis, M.W.; van Straalen, N.M. Biological Control of the Bulb Mite, Rhizoglyphus robini, by the Predatory Mite, Hypoaspis aculeifer, on Lilies: Predator-Prey Dynamics in the Soil, under Greenhouse and Field Conditions. Biocontrol Sci. Technol. 2000, 10, 179–193. [Google Scholar] [CrossRef]
- Machado, R.A.R.; Thönen, L.; Arce, C.C.M.; Theepan, V.; Prada, F.; Wüthrich, D.; Robert, C.A.M.; Vogiatzaki, E.; Shi, Y.-M.; Schaeren, O.P.; et al. Publisher Correction: Engineering bacterial symbionts of nematodes improves their biocontrol potential to counter the western corn rootworm. Nat. Biotechnol. 2020, 38, 649. [Google Scholar] [CrossRef] [PubMed]
- Martin, P.A.W.; Hirose, E.; Aldrich, J.R. Toxicity of Chromobacterium subtsugae to Southern Green Stink Bug (Heteroptera: Pentatomidae) and Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2007, 100, 680–684. [Google Scholar] [CrossRef]
- Mayerhofer, J.; Rauch, H.; Hartmann, M.; Widmer, F.; Gschwend, F.; Strasser, H.; Leuchtmann, A.; Enkerli, J. Response of soil microbial communities to the application of a formulated Metarhizium brunneum biocontrol strain. Biocontrol Sci. Technol. 2019, 29, 547–564. [Google Scholar] [CrossRef]
- McMurtry, J.A.; Sourassou, N.F.; Demite, P.R. The Phytoseiidae (Acari: Mesostigmata) as Biological Control Agents. In Prospects for Biological Control of Plant Feeding Mites and Other Harmful Organisms; Carrillo, D., de Moraes, G.J., Peña, J.E., Eds.; Springer International Publishing: Cham, Switzerland, 2015; pp. 133–149. [Google Scholar]
- Meinke, L.J.; Sappington, T.W.; Onstad, D.W.; Guillemaud, T.; Miller, N.J.; Komáromi, J.; Levay, N.; Furlan, L.; Kiss, J.; Toth, F. Western corn rootworm (Diabrotica virgifera virgifera LeConte) population dynamics. Agric. For. Entomol. 2009, 11, 29–46. [Google Scholar] [CrossRef]
- Nishimatsu, T.; Jackson, J.J. Interaction of Insecticides, Entomopathogenic Nematodes, and Larvae of the Western Corn Root worm (Coleoptera: Chrysomelidae). J. Econ. Entomol. 1998, 91, 410–418. [Google Scholar] [CrossRef]
- Nomikou, M.; Sabelis, M.W.; Janssen, A. Pollen subsidies promote whitefly control through the numerical response of predatory mites. BioControl 2010, 55, 253–260. [Google Scholar] [CrossRef]
- Oleson, J.D.; Park, Y.-L.; Nowatzki, T.M.; Tollefson, J.J. Node-Injury Scale to Evaluate Root Injury by Corn Rootworms (Coleoptera: Chrysomelidae). J. Econ. Entomol. 2005, 98, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.T.; Kommedahl, T. Root-infecting Fusarium species in relation to rootworm infestations in corn. Phytopathology 1969. Available online: https://agris.fao.org/agris-search/search.do?recordID=US201301227104 (accessed on 15 July 2021).
- Pasquier, A.; Andrieux, T.; Martinez-Rodiguez, P.; Vercken, E.; Ferrero, M. Predation capacity of soil-dwelling predatory mites on two major maize pests. Acarologia 2021, 61, 577–580. [Google Scholar] [CrossRef]
- Pérez-Rodríguez, J.; Calvo, J.; Urbaneja, A.; Tena, A. The soil mite Gaeolaelaps (Hypoaspis) aculeifer (Canestrini) (Acari: Laelapidae) as a predator of the invasive citrus mealybug Delottococcus aberiae (De Lotto) (Hemiptera: Pseudococcidae): Implications for biological control. Biol. Control 2018, 127, 64–69. [Google Scholar] [CrossRef]
- Petzold-Maxwell, J.L.; Jaronski, S.T.; Gassmann, A.J. Tritrophic interactions among Bt maize, an insect pest and entomopathogens: Effects on development and survival of western corn rootworm. Ann. Appl. Biol. 2012, 160, 43–55. [Google Scholar] [CrossRef]
- Pilz, C.; Keller, S.; Kuhlmann, U.; Toepfer, S. Comparative efficacy assessment of fungi, nematodes and insecticides to control western corn rootworm larvae in maize. BioControl 2009, 54, 671–684. [Google Scholar] [CrossRef]
- Postma-Blaauw, M.B.; de Goede, R.G.M.; Bloem, J.; Faber, J.H.; Brussaard, L. Soil biota community structure and abundance under agricultural intensification and extensification. Ecology 2010, 91, 460–473. [Google Scholar] [CrossRef]
- Prischmann, D.A.; Knutson, E.M.; Dashiell, K.E.; Lundgren, J.G. Generalist-feeding subterranean mites as potential biological control agents of immature corn rootworms. Exp. Appl. Acarol. 2011, 55, 233–248. [Google Scholar] [CrossRef]
- Prischmann-Voldseth, D.A.; Dashiell, K.E. Effects of releasing a generalist predator (Acari: Gaeolaelaps aculeifer) on a subterranean insect herbivore (Coleoptera: Diabrotica virgifera virgifera). Biol. Control 2013, 65, 190–199. [Google Scholar] [CrossRef]
- Ragusa, S.; Zedan, M.A.; Sciacchitano, M.A. The effects of food from plant and animal sources on the development and egg production of the predaceous mite Hypoaspis aculeifer (Canestrini) (Parasitiformes, Dermanyssidae). Redia 1986, 69, 481–488. [Google Scholar]
- Ramakers, P.M. Manipulation of phytoseiid thrips predators in the absence of thrips. Bull. SROP 1990, 13, 169–172. [Google Scholar]
- Rauch, H.; Steinwender, B.M.; Mayerhofer, J.; Sigsgaard, L.; Eilenberg, J.; Enkerli, J.; Zelger, R.; Strasser, H. Field efficacy of Heterorhabditis bacteriophora (Nematoda: Heterorhabditidae), Metarhizium brunneum (Hypocreales: Clavicipitaceae), and chemical insecticide combinations for Diabrotica virgifera virgifera larval management. Biol. Control 2017, 107, 1–10. [Google Scholar] [CrossRef]
- Riedell, W.E.; Reese, R.N. Maize morphology and shoot CO2 assimilation after root damage by western corn rootworm larvae. Crop. Sci. 1999, 39, 1332–1340. [Google Scholar] [CrossRef]
- Rueda-Ramirez, D.M.; Rios-Malaver, D.M.; Varela-Ramirez, A.; Moraes, G.J. de Colombian population of the mite Gaeolaelaps aculeifer as a predator of the thrips Frankliniella occidentalis and the possible use of an astigmatid mite as its factitious prey. Syst. Appl. Acarol. 2018, 23, 2359. [Google Scholar]
- Schroder, R.F.W.; Athanas, M.M. Biological observations of Centistes gasseni Shaw (Hymenoptera: Braconidae), a parasitoid of Diabrotica spp. (Coleoptera: Chrysomelidae). Proc. Entomol. Soc. Wash. 2002, 104, 554–562. [Google Scholar]
- Shaw, M.W. (Department of A.B., 1996. Simulating Dispersal of Fungal Spores by Wind, and the Resulting Patterns. Aspects of Applied Biology (United Kingdom). Available online: https://www.researchgate.net/publication/46820144_Dispersal_of_fungal_spores_on_a_cooperatively_generated_wind (accessed on 28 August 2021).
- Shrestha, R.B.; Gassmann, A.J. Field and Laboratory Studies of Resistance to Bt Corn by Western Corn Rootworm (Coleoptera: Chrysomelidae). J. Econ. Entomol 2019, 112, 2324–2334. [Google Scholar] [CrossRef] [PubMed]
- Spike, B.P.; Tollefson, J.J. Western Corn Rootworm (Coleoptera: Chrysomelidae) Larval Survival and Damage Potential to Corn Subjected to Nitrogen and Plant Density Treatments. J. Econ. Entomol. 1988, 81, 1450–1455. [Google Scholar] [CrossRef]
- Strnad, S.P.; Bergman, M.K.; Fulton, W.C. First-instar Western Corn Rootworm (Coleoptera: Chrysomelidae) Response to Carbon Dioxide. Env. Entomol. 1986, 15, 839–842. [Google Scholar] [CrossRef]
- Sutter, G.R.; Lance, D.R. New strategies for reducing insecticide use in the corn belt. In Sustainable Agriculture Research and Education in the Field: Proceedings; The National Academies: Washington, DC, USA, 1991; pp. 230–249. [Google Scholar]
- Toepfer, S.; Burger, R.; Ehlers, R.-U.; Peters, A.; Kuhlmann, U. Controlling western corn rootworm larvae with entomopathogenic nematodes: Effect of application techniques on plant-scale efficacy. J. Appl. Entomol. 2009, 134, 467–480. [Google Scholar] [CrossRef]
- Toepfer, S.; Gueldenzoph, C.; Ehlers, R.-U.; Kuhlmann, U. Screening of entomopathogenic nematodes for virulence against the invasive western corn rootworm, Diabrotica virgifera virgifera (Coleoptera: Chrysomelidae) in Europe. Bull. Entomol. Res. 2005, 95, 473–482. [Google Scholar] [CrossRef] [PubMed]
- Toepfer, S.; Hatala-Zseller, I.; Ehlers, R.-U.; Peters, A.; Kuhlmann, U. The effect of application techniques on field-scale efficacy: Can the use of entomopathogenic nematodes reduce damage by western corn rootworm larvae? Agric. For. Entomol. 2010, 12, 389–402. [Google Scholar] [CrossRef]
- Urías-López, M.A.; Meinke, L.J.; Higley, L.G.; Haile, F.J. Influence of western corn rootworm (Coleoptera: Chrysomelidae) larval injury on photosynthetic rate and vegetative growth of different types of maize. Environ. Entomol. 2000, 29, 861–867. [Google Scholar] [CrossRef][Green Version]
- Vidal, S.; Kuhlmann, U.; Edwards, C.R. (Eds.) Western Corn Rootworm: Ecology and Management; CABI Publisher: Wallingford, UK; Cambridge, MA, USA, 2005. [Google Scholar]
- Wiethoff, J.; Poehling, H.-M.; Meyhöfer, R. Combining plant-and soil-dwelling predatory mites to optimise biological control of thrips. Exp. Appl. Acarol. 2004, 34, 239–261. [Google Scholar] [CrossRef]
- Wright, E.M.; Chambers, R.J. The biology of the predatory mite Hypoaspis miles (Acari: Laelapidae), a potential biological control agent of Bradysia paupera (Dipt.: Sciaridae). Entomophaga 1994, 39, 225–235. [Google Scholar] [CrossRef]

| Variables to Explain | Number of Adult Emerged | Chlorophyll Index | Root Damage Index | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Young Plant | Flowering Plant | ||||||||||||
| Explanatory Variables | Deviance | Df | p Value | F Value | Df | p Value | F Value | Df | p Value | Deviance | Df | p Value | |
| Greenhouse Experiment | Maize vs. Maize + WCR vs. Maize + WCR + Mites | 151.0 | 2 | <0.001 | 34.6 | 2.56 | <0.001 | 23 | 2 | <0.001 | |||
| Maize + WCR + 100 Mites vs. Maize+ WCR + 500 Mites vs. Maize + WCR + 1000 Mites | 1.3 | 2 | 0.532 | 0.13 | 2.33 | 0.877 | 2.9 | 2 | 0.235 | ||||
| Field Experiment | Control vs. Biological control vs. pesticide | 67.1 | 2 | <0.001 | 23.9 | 2 | <0.001 | 1.5 | 2 | 0.223 | 56.4 | 2 | <0.001 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pasquier, A.; Monticelli, L.S.; Moreau, A.; Kaltenbach, B.; Chabot, C.; Andrieux, T.; Ferrero, M.; Vercken, E. A Promising Predator-In-First Strategy to Control Western Corn Rootworm Population in Maize Fields. Agronomy 2021, 11, 1984. https://doi.org/10.3390/agronomy11101984
Pasquier A, Monticelli LS, Moreau A, Kaltenbach B, Chabot C, Andrieux T, Ferrero M, Vercken E. A Promising Predator-In-First Strategy to Control Western Corn Rootworm Population in Maize Fields. Agronomy. 2021; 11(10):1984. https://doi.org/10.3390/agronomy11101984
Chicago/Turabian StylePasquier, Antoine, Lucie S. Monticelli, Adeline Moreau, Benjamin Kaltenbach, Candice Chabot, Thibault Andrieux, Maxime Ferrero, and Elodie Vercken. 2021. "A Promising Predator-In-First Strategy to Control Western Corn Rootworm Population in Maize Fields" Agronomy 11, no. 10: 1984. https://doi.org/10.3390/agronomy11101984
APA StylePasquier, A., Monticelli, L. S., Moreau, A., Kaltenbach, B., Chabot, C., Andrieux, T., Ferrero, M., & Vercken, E. (2021). A Promising Predator-In-First Strategy to Control Western Corn Rootworm Population in Maize Fields. Agronomy, 11(10), 1984. https://doi.org/10.3390/agronomy11101984






