Estimating Soil Properties and Nutrients by Visible and Infrared Diffuse Reflectance Spectroscopy to Characterize Vineyards
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Area and Soil Sampling
2.2. Spectral Reflectance Acquisition
2.2.1. PG Setup Measurements
2.2.2. CP Setup Measurements
2.2.3. Preprocessing
2.2.4. Soil Property Estimation by PLSR
3. Results
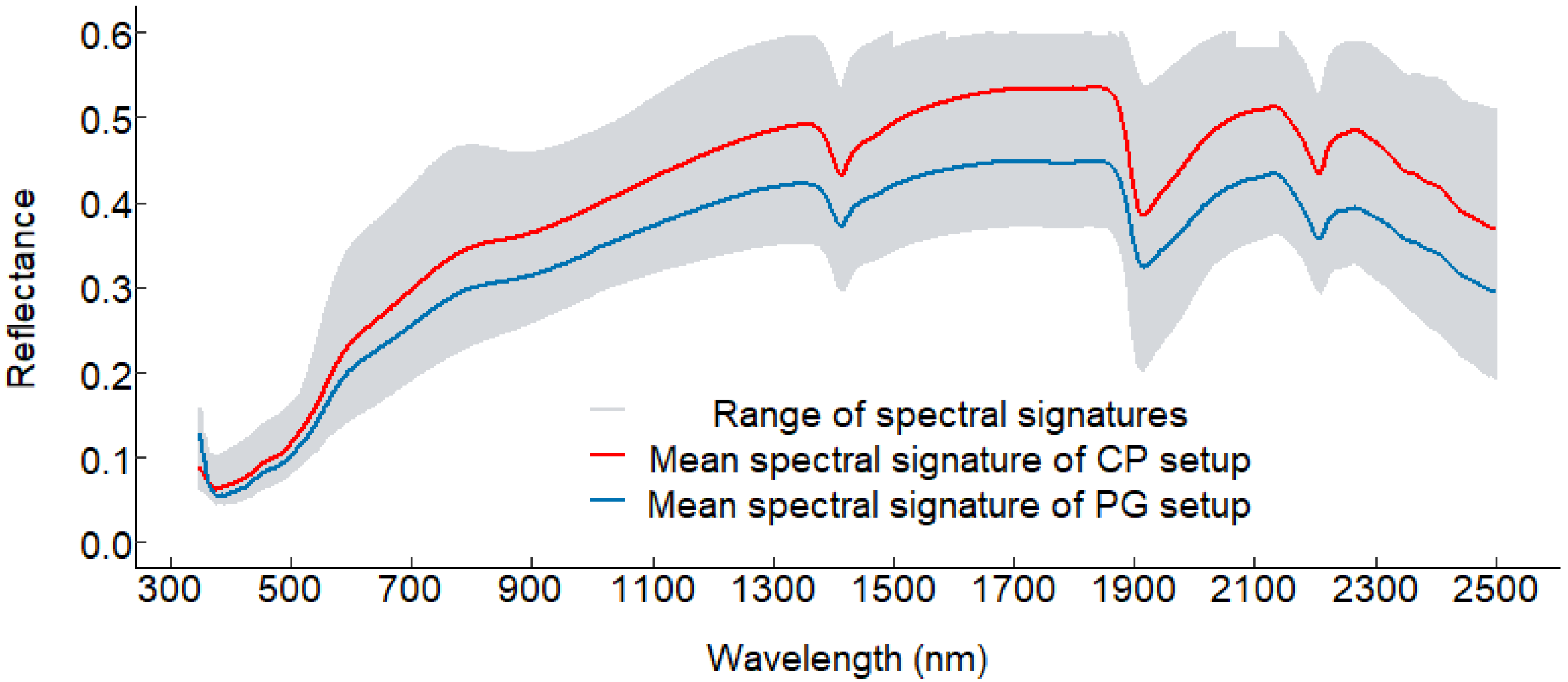
3.1. Soil Reflectance Spectra
3.2. Laboratory Analysis
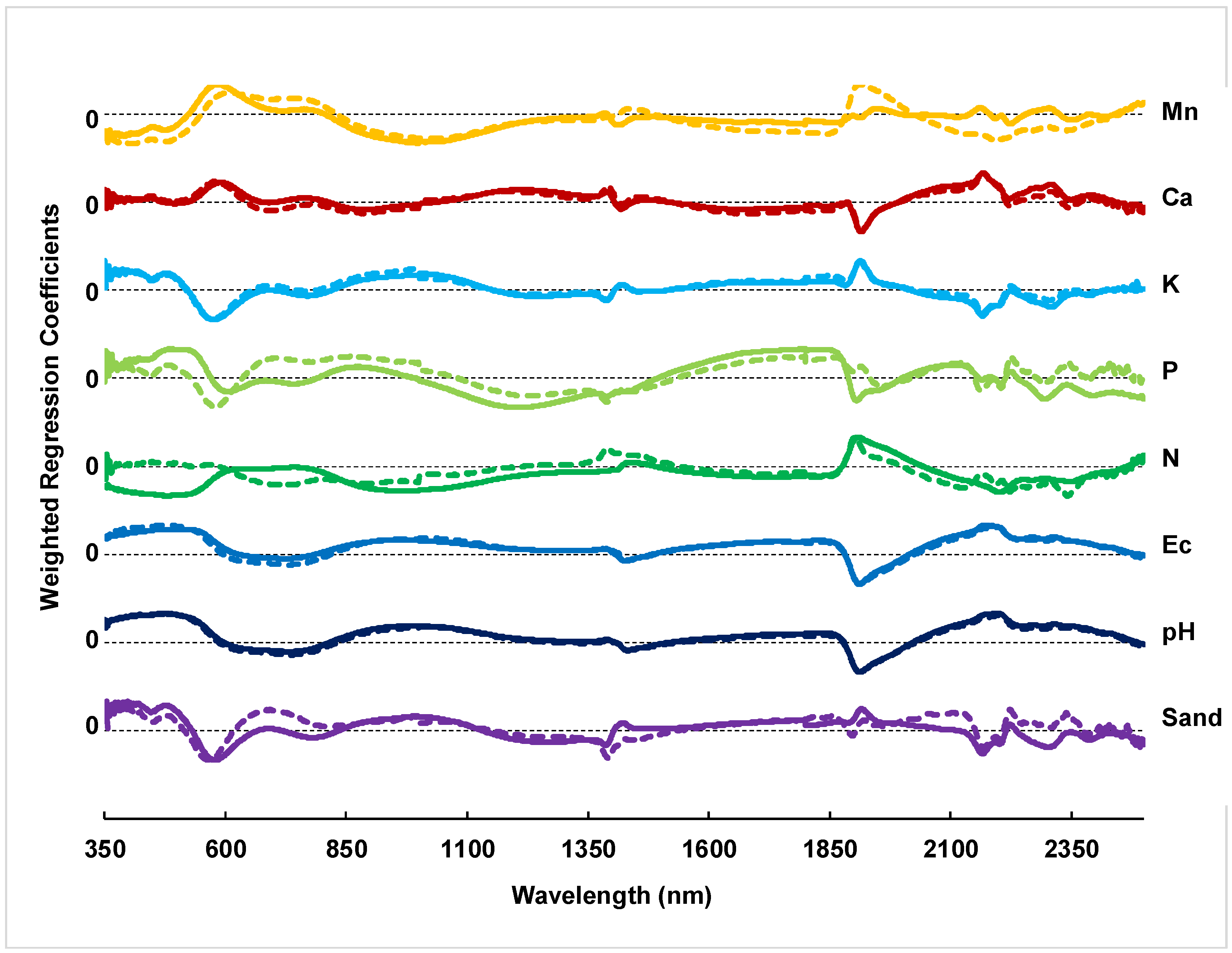
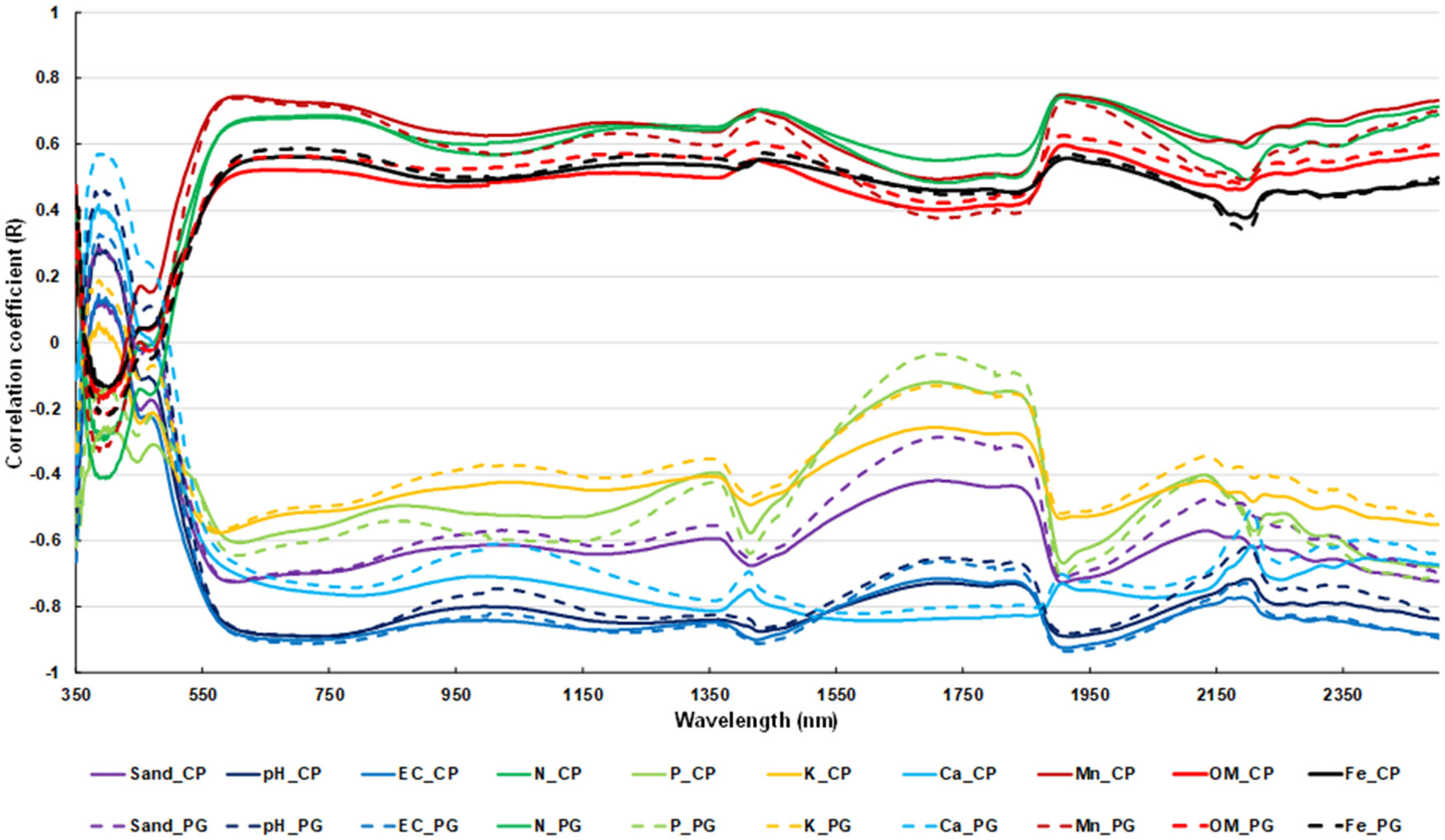
3.3. PLSR Model Predictions
4. Discussion
4.1. Soil Property Predictions
4.2. PLSR Model Performance
4.3. Data Preprocessing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Mashalaba, L.; Galleguillos, M.; Seguel, O.; Poblete-Olivares, J. Predicting Spatial Variability of Selected Soil Properties Using Digital Soil Mapping in a Rainfed Vineyard of Central Chile. Geoderma Reg. 2020, 22, e00289. [Google Scholar] [CrossRef]
- Cozzolino, D.; Morón, A. The Potential of Near-Infrared Reflectance Spectroscopy to Analyse Soil Chemical and Physical Characteristics. J. Agric. Sci. 2003, 140, 65–71. [Google Scholar] [CrossRef]
- Barra, I.; Haefele, S.M.; Sakrabani, R.; Kebede, F. Soil Spectroscopy with the Use of Chemometrics, Machine Learning and Pre-Processing Techniques in Soil Diagnosis: Recent Advances—A Review. TrAC Trends Anal. Chem. 2021, 135, 116166. [Google Scholar] [CrossRef]
- Nocita, M.; Stevens, A.; van Wesemael, B.; Aitkenhead, M.; Bachmann, M.; Barthès, B.; Ben Dor, E.; Brown, D.J.; Clairotte, M.; Csorba, A.; et al. Chapter Four—Soil Spectroscopy: An Alternative to Wet Chemistry for Soil Monitoring. In Advances in Agronomy; Sparks, D.L., Ed.; Academic Press: Cambridge, MA, USA, 2015; Volume 132, pp. 139–159. [Google Scholar]
- Osborne, B.G. Near-Infrared Spectroscopy in Food Analysis. In Encyclopedia of Analytical Chemistry; American Cancer Society: Atlanta, GA, USA, 2006; ISBN 978-0-470-02731-8. [Google Scholar]
- Islam, K.; Singh, B.; McBratney, A. Simultaneous Estimation of Several Soil Properties by Ultra-Violet, Visible, and near-Infrared Reflectance Spectroscopy. Soil Res. 2003, 41, 1101–1114. [Google Scholar] [CrossRef]
- Zumdahl, S.S.; Zumdahl, S.A.; DeCoste, D.J. Molecular spectroscopy. In Chemistry; Cengage Learning: Boston, MA, USA; University of Illinois: Boston, MA, USA, 2017; ISBN 978-1-305-95740-4. [Google Scholar]
- Kharbach, M.; Marmouzi, I.; Kamal, R.; Yu, H.; Barra, I.; Cherrah, Y.; Alaoui, K.; Heyden, Y.V.; Bouklouze, A. Extra Virgin Argan Oils’ Shelf-Life Monitoring and Prediction Based on Chemical Properties or FTIR Fingerprints and Chemometrics. Food Control 2021, 121, 107607. [Google Scholar] [CrossRef]
- Barra, I.; Kharbach, M.; Qannari, E.M.; Hanafi, M.; Cherrah, Y.; Bouklouze, A. Predicting Cetane Number in Diesel Fuels Using FTIR Spectroscopy and PLS Regression. Vib. Spectrosc. 2020, 111, 103157. [Google Scholar] [CrossRef]
- Martínez-España, R.; Bueno-Crespo, A.; Soto, J.; Janik, L.J.; Soriano-Disla, J.M. Developing an Intelligent System for the Prediction of Soil Properties with a Portable Mid-Infrared Instrument. Biosyst. Eng. 2019, 177, 101–108. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Banin, A. Near-Infrared Analysis as a Rapid Method to Simultaneously Evaluate Several Soil Properties. Soil Sci. Soc. Am. J. 1995, 59, 364–372. [Google Scholar] [CrossRef]
- Ben-Dor, E. Quantitative remote sensing of soil properties. In Advances in Agronomy; Academic Press: Cambridge, MA, USA, 2002; Volume 75, pp. 173–243. [Google Scholar]
- Ben-Dor, E.; Irons, J.R.; Epema, G.F.; Rencz, A.N. Soil reflectance. In Remote Sensing for the Earth Sciences: Manual of Remote Sensing 3/3; John Wiley & Sons: Hoboken, NJ, USA, 1999; pp. 111–188. [Google Scholar]
- Stenberg, B.; Viscarra Rossel, R.A.; Mouazen, A.M.; Wetterlind, J. Visible and Near Infrared Spectroscopy in Soil Science. Adv. Agron. 2010, 107, 163–215. [Google Scholar] [CrossRef] [Green Version]
- Kuang, B.; Mahmood, H.S.; Quraishi, Z.; Hoogmoed, W.B.; Mouazen, A.M.; van Henten, E. Sensing Soil Properties in the Laboratory, in Situ, and on-Line: A Review. Adv. Agron. 2012, 114, 155–223. [Google Scholar] [CrossRef]
- Mouazen, A.M.; Kuang, B.; De Baerdemaeker, J.; Ramon, H. Comparison among Principal Component, Partial Least Squares and Back Propagation Neural Network Analyses for Accuracy of Measurement of Selected Soil Properties with Visible and near Infrared Spectroscopy. Geoderma 2010, 158, 23–31. [Google Scholar] [CrossRef]
- Viscarra-Rossel, R.A.; Walvoort, D.J.J.; McBratney, A.B.; Janik, L.J.; Skjemstad, J.O. Visible, near Infrared, Mid Infrared or Combined Diffuse Reflectance Spectroscopy for Simultaneous Assessment of Various Soil Properties. Geoderma 2006, 131, 59–75. [Google Scholar] [CrossRef]
- Bilgili, A.V.; Van Es, H.M.; Akbas, F.; Durak, A.; Hively, W.D. Visible-near Infrared Reflectance Spectroscopy for Assessment of Soil Properties in a Semi-Arid Area of Turkey. J. Arid. Env. 2010, 74, 229–238. [Google Scholar] [CrossRef]
- Marín-González, O.; Kuang, B.; Quraishi, M.Z.; Munóz-García, M.Á.; Mouazen, A.M. On-Line Measurement of Soil Properties without Direct Spectral Response in near Infrared Spectral Range. Soil Tillage Res. 2013, 132, 21–29. [Google Scholar] [CrossRef] [Green Version]
- Pimstein, A.; Notesco, G.; Ben-Dor, E. Performance of Three Identical Spectrometers in Retrieving Soil Reflectance under Laboratory Conditions. Soil Sci. Soc. Am. J. 2011, 75, 746–759. [Google Scholar] [CrossRef]
- Ben-Dor, E.; Ong, C.; Lau, I.C. Reflectance Measurements of Soils in the Laboratory: Standards and Protocols. Geoderma 2015, 245–246, 112–124. [Google Scholar] [CrossRef]
- Silveira, M.L.; Kohmann, M.M. Chapter 3—Maintaining soil fertility and health for sustainable pastures. In Management Strategies for Sustainable Cattle Production in Southern Pastures; Rouquette, M., Aiken, G.E., Eds.; Academic Press: Cambridge, MA, USA, 2020; pp. 35–58. ISBN 978-0-12-814474-9. [Google Scholar]
- Cozzolino, D.; Cynkar, W.U.; Dambergs, R.G.; Shah, N.; Smith, P. In Situ Measurement of Soil Chemical Composition by Near-Infrared Spectroscopy: A Tool Toward Sustainable Vineyard Management. Commun. Soil Sci. Plant Anal. 2013, 44, 1610–1619. [Google Scholar] [CrossRef]
- Angelopoulou, T.; Balafoutis, A.; Zalidis, G.; Bochtis, D. From Laboratory to Proximal Sensing Spectroscopy for Soil Organic Carbon Estimation—A Review. Sustainability 2020, 12, 443. [Google Scholar] [CrossRef] [Green Version]
- Thomas, C.L.; Hernandez-Allica, J.; Dunham, S.J.; McGrath, S.P.; Haefele, S.M. A Comparison of Soil Texture Measurements Using Mid-Infrared Spectroscopy (MIRS) and Laser Diffraction Analysis (LDA) in Diverse Soils. Sci. Rep. 2021, 11, 16. [Google Scholar] [CrossRef]
- Muganu, M.; Paolocci, M.; Gnisci, D.; Barnaba, F.E.; Bellincontro, A.; Mencarelli, F.; Grosu, I. Effect of Different Soil Management Practices on Grapevine Growth and on Berry Quality Assessed by NIR-AOTF Spectroscopy. Acta Hortic 2013, 978, 125. [Google Scholar] [CrossRef]
- Páscoa, R.N.M.J.; Lopo, M.; Teixeira dos Santos, C.A.; Graça, A.R.; Lopes, J.A. Exploratory Study on Vineyards Soil Mapping by Visible/near-Infrared Spectroscopy of Grapevine Leaves. Comput. Electron. Agric. 2016, 127, 15–25. [Google Scholar] [CrossRef]
- Lopo, M.; Teixeira dos Santos, C.A.; Páscoa, R.N.M.J.; Graça, A.R.; Lopes, J.A. Near Infrared Spectroscopy as a Tool for Intensive Mapping of Vineyards Soil. Precis. Agric 2018, 19, 445–462. [Google Scholar] [CrossRef]
- Munnaf, M.A.; Nawar, S.; Mouazen, A.M. Estimation of Secondary Soil Properties by Fusion of Laboratory and On-Line Measured Vis–NIR Spectra. Remote Sens. 2019, 11, 2819. [Google Scholar] [CrossRef] [Green Version]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, Update 2015 International Soil Classification System Fort Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- Ministerio de Agricultura Pesca y Alimentación–MAPA. Métodos Oficiales de Análisis; Secretaría General Técnica del Ministerio de Agricultura, Pesca y Alimentación: Madrid, Spain, 1994; Volume III, ISBN 978-84-491-0003-1.
- ASD Inc. FieldSpect4 User Manual. Available online: https://www.malvernpanalytical.com/en/learn/knowledge-center/user-manuals/fieldspec-4-user-guide (accessed on 25 June 2021).
- Rosero-Vlasova, O.A.; Pérez-Cabello, F.; Montorio Llovería, R.; Vlassova, L. Assessment of Laboratory VIS-NIR-SWIR Setups with Different Spectroscopy Accessories for Characterisation of Soils from Wildfire Burns. Biosyst. Eng. 2016, 152, 51–67. [Google Scholar] [CrossRef]
- Barnes, R.J.; Dhanoa, M.S.; Lister, S.J. Standard Normal Variate Transformation and De-Trending of Near-Infrared Diffuse Reflectance Spectra. Appl. Spectrosc. 1989, 43, 772–777. [Google Scholar] [CrossRef]
- Chang, C.W.; Laird, D.A.; Mausbach, M.J.; Hurburgh, C.R. Near-Infrared Reflectance Spectroscopy–Principal Components Regression Analyses of Soil Properties. Soil Sci. Soc. Am. J. 2001, 65, 480–490. [Google Scholar] [CrossRef] [Green Version]
- Geladi, P.; Kowalski, B.R. Partial Least-Squares Regression: A Tutorial. Anal. Chim. Acta 1986, 185, 1–17. [Google Scholar] [CrossRef]
- Wold, S.; Sjöström, M.; Eriksson, L. PLS-Regression: A Basic Tool of Chemometrics. Chemom. Intell. Lab. Syst. 2001, 58, 109–130. [Google Scholar] [CrossRef]
- Williams, P.; Dardenne, P.; Flinn, P. Tutorial: Items to Be Included in a Report on a near Infrared Spectroscopy Project. J. Near Infrared Spectrosc. JNIRS 2017, 25, 85–90. [Google Scholar] [CrossRef]
- Conforti, M.; Froio, R.; Matteucci, G.; Buttafuoco, G. Visible and near Infrared Spectroscopy for Predicting Texture in Forest Soil: An Application in Southern Italy. Iforest—Biogeosciences For. 2015, 8, 339. [Google Scholar] [CrossRef]
- Wetzel, D.L. Near-Infrared Reflectance Analysis. Anal. Chem. 1983, 55, 1165A–1176A. [Google Scholar] [CrossRef]
- Barthès, B.G.; Brunet, D.; Ferrer, H.; Chotte, J.L.; Feller, C. Determination of Total Carbon and Nitrogen Content in a Range of Tropical Soils Using near Infrared Spectroscopy: Influence of Replication and Sample Grinding and Drying. J. Near Infrared Spectrosc. 2006, 14, 341–348. [Google Scholar] [CrossRef]
- Zornoza, R.; Guerrero, C.; Mataix-Solera, J.; Scow, K.M.; Arcenegui, V.; Mataix-Beneyto, J. Near Infrared Spectroscopy for Determination of Various Physical, Chemical and Biochemical Properties in Mediterranean Soils. Soil Biol. Biochem. 2008, 40, 1923–1930. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.D.; Malley, D.F.; Manning, G.; Fuller, L. Determination of Soil Organic Carbon and Nitrogen at the Field Level Using Near-Infrared Spectroscopy. Can. J. Soil. Sci. 2002, 82, 413–422. [Google Scholar] [CrossRef]
- Curcio, D.; Ciraolo, G.; D’Asaro, F.; Minacapilli, M. Prediction of Soil Texture Distributions Using VNIR-SWIR Reflectance Spectroscopy. Procedia Environ. Sci. 2013, 19, 494–503. [Google Scholar] [CrossRef] [Green Version]
- Stevens, A.; van Wesemael, B.; Bartholomeus, H.; Rosillon, D.; Tychon, B.; Ben-Dor, E. Laboratory, Field and Airborne Spectroscopy for Monitoring Organic Carbon Content in Agricultural Soils. Geoderma 2008, 144, 395–404. [Google Scholar] [CrossRef] [Green Version]
- Du, C.; Zhou, J. Evaluation of Soil Fertility Using Infrared Spectroscopy—A Review. In Climate Change, Intercropping, Pest Control and Beneficial Microorganisms; Lichtfouse, E., Ed.; Sustainable Agriculture Reviews; Springer: Dordrecht, The Netherlands, 2009; pp. 453–483. ISBN 978-90-481-2716-0. [Google Scholar]
- Linker, R. Waveband Selection for Determination of Nitrate in Soil Using Mid-Infrared Attenuated Total Reflectance Spectroscopy. Appl. Spectrosc. 2004, 58, 1277–1281. [Google Scholar] [CrossRef]
- Linker, R.; Shmulevich, I.; Kenny, A.; Shaviv, A. Soil Identification and Chemometrics for Direct Determination of Nitrate in Soils Using FTIR-ATR Mid-Infrared Spectroscopy. Chemosphere 2005, 61, 652–658. [Google Scholar] [CrossRef]
- Borenstein, A.; Linker, R.; Shmulevich, I.; Shaviv, A. Determination of Soil Nitrate and Water Content Using Attenuated Total Reflectance Spectroscopy. Appl. Spectrosc. 2006, 60, 1267–1272. [Google Scholar] [CrossRef]
- Sithole, N.J.; Ncama, K.; Magwaza, L.S. Robust Vis-NIRS Models for Rapid Assessment of Soil Organic Carbon and Nitrogen in Feralsols Haplic Soils from Different Tillage Management Practices. Comput. Electron. Agric. 2018, 153, 295–301. [Google Scholar] [CrossRef]
- Kuang, B.; Tekin, Y.; Mouazen, A.M. Comparison between Artificial Neural Network and Partial Least Squares for On-Line Visible and near Infrared Spectroscopy Measurement of Soil Organic Carbon, PH and Clay Content. Soil Tillage Res. 2015, 146, 243–252. [Google Scholar] [CrossRef]
- Sorenson, P.T.; Small, C.; Tappert, M.C.; Quideau, S.A.; Drozdowski, B.; Underwood, A.; Janz, A. Monitoring Organic Carbon, Total Nitrogen, and pH for Reclaimed Soils Using Field Reflectance Spectroscopy. Can. J. Soil. Sci. 2017, 97, 241–248. [Google Scholar] [CrossRef]
- Soriano-Disla, J.M.; Janik, L.J.; Allen, D.J.; McLaughlin, M.J. Evaluation of the Performance of Portable Visible-Infrared Instruments for the Prediction of Soil Properties. Biosyst. Eng. 2017, 161, 24–36. [Google Scholar] [CrossRef]
- Shepherd, K.D.; Walsh, M.G. Development of Reflectance Spectral Libraries for Characterization of Soil Properties. Soil Sci. Soc. Am. J. 2002, 66, 988–998. [Google Scholar] [CrossRef]
- Lei, S.; Bao, N.; Liu, S.; Liu, X. Modelling and Predicting of Soil Electrical Conductivity and pH from Semi-Arid Grassland Using VIS-NIR Spectroscopy Technology. In Proceedings of the Computer and Computing Technologies in Agriculture; Li, D., Zhao, C., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 442–453. [Google Scholar]
- Farifteh, J.; Van der Meer, F.; Atzberger, C.; Carranza, E.J.M. Quantitative Analysis of Salt-Affected Soil Reflectance Spectra: A Comparison of Two Adaptive Methods (PLSR and ANN). Remote Sens. Environ. 2007, 110, 59–78. [Google Scholar] [CrossRef]
- Mashimbye, Z.E.; Cho, M.A.; Nell, J.P.; De Clercq, W.P.; Van niekerk, A.; Turner, D.P. Model-Based Integrated Methods for Quantitative Estimation of Soil Salinity from Hyperspectral Remote Sensing Data: A Case Study of Selected South African Soils. Pedosphere 2012, 22, 640–649. [Google Scholar] [CrossRef]
- Sherman, D.M.; Waite, T.D. Electronic Spectra of Fe3 + Oxides and Oxide Hydroxides in the near IR to near UV. Am. Mineral. 1985, 70, 8. [Google Scholar]
- Vašát, R.; Kodešová, R.; Klement, A.; Borůvka, L. Simple but Efficient Signal Pre-Processing in Soil Organic Carbon Spectroscopic Estimation. Geoderma 2017, 298, 46–53. [Google Scholar] [CrossRef]
- Buddenbaum, H.; Steffens, M. The Effects of Spectral Pretreatments on Chemometric Analyses of Soil Profiles Using Laboratory Imaging Spectroscopy. Appl. Environ. Soil Sci. 2012, 2012, e274903. [Google Scholar] [CrossRef] [Green Version]
- Engel, J.; Gerretzen, J.; Szymańska, E.; Jansen, J.J.; Downey, G.; Blanchet, L.; Buydens, L.M.C. Breaking with Trends in Pre-Processing? TrAC Trends Anal. Chem. 2013, 50, 96–106. [Google Scholar] [CrossRef]
- Hill, J.; Udelhoven, T.; Vohland, M.; Stevens, A. The Use of Laboratory Spectroscopy and Optical Remote Sensing for Estimating Soil Properties. In Precision Crop Protection—The Challenge and Use of Heterogeneity; Oerke, E.-C., Gerhards, R., Menz, G., Sikora, R.A., Eds.; Springer: Dordrecht, The Netherlands, 2010; pp. 67–85. ISBN 978-90-481-9277-9. [Google Scholar]
- Miller, C.E.; Williams, P.C.; Norris, K.H. Chemical principles of near infrared technology. In Near Infrared Technology in the Agricultural and Food Industries; American Association of Cereal Chemist: St. Paul, MN, USA, 2001; pp. 19–39. [Google Scholar]
- Martínez-Carreras, N.; Krein, A.; Udelhoven, T.; Gallart, F.; Iffly, J.F.; Hoffmann, L.; Pfister, L.; Walling, D.E. A Rapid Spectral-Reflectance-Based Fingerprinting Approach for Documenting Suspended Sediment Sources during Storm Runoff Events. J. Soils Sediments 2010, 10, 400–413. [Google Scholar] [CrossRef]
- Wu, Y.; Chen, J.; Ji, J.; Gong, P.; Liao, Q.; Tian, Q.; Ma, H. A Mechanism Study of Reflectance Spectroscopy for Investigating Heavy Metals in Soils. Soil Sci. Soc. Am. J. 2007, 71, 918–926. [Google Scholar] [CrossRef]

| Municipality | Designation of Origin | Grape Cultivar | Longitude | Latitude | Soil Classification |
|---|---|---|---|---|---|
| Cacabelos | Bierzo | Mencía | 6.754 W | 42.626 N | Dystric Cambisol |
| Camponaraya | Bierzo | Godello | 6.692 W | 42.606 N | Chromic Cambisol |
| Valbuena de Duero | Ribera de Duero | Tempranillo | 4.391 W | 41.631 N | Lithic Leptosol |
| Matapozuelos | Rueda | Verdejo | 4.765 W | 41.364 N | Albic Arenosol |
| Soil Property | N | Min | Max | Range | Median | Mean | SD | CoV |
|---|---|---|---|---|---|---|---|---|
| Silt (%) | 48 | 14 | 56 | 42 | 36 | 34.58 | 11.00 | 31.81 |
| Clay (%) | 48 | 10 | 32 | 22 | 19 | 18.46 | 5.91 | 32.02 |
| Sand (%) | 48 | 24 | 76 | 52 | 46 | 46.96 | 15.10 | 32.15 |
| pH | 48 | 5.33 | 8.47 | 3.14 | 7.63 | 7.24 | 1.16 | 16.07 |
| Ec (dS m−1) | 48 | 0.02 | 0.12 | 0.10 | 0.08 | 0.07 | 0.03 | 49.27 |
| Organic matter (%) | 48 | 0.37 | 2.40 | 2.03 | 0.81 | 1.02 | 0.52 | 51.04 |
| Total N (%) | 48 | 0.05 | 0.16 | 0.11 | 0.08 | 0.09 | 0.03 | 33.76 |
| P (mg kg−1) | 35 | 5.65 | 58.38 | 52.73 | 16.39 | 26.12 | 20.67 | 79.14 |
| K (cmol kg−1) | 48 | 0.13 | 0.80 | 0.67 | 0.40 | 0.38 | 0.14 | 37.55 |
| Ca (cmol kg−1) | 48 | 1.56 | 20.90 | 19.34 | 9.80 | 10.49 | 6.44 | 61.37 |
| Mn (mg kg−1) | 48 | 1.90 | 28.40 | 26.50 | 10.65 | 11.02 | 7.09 | 64.35 |
| Fe (mg kg−1) | 48 | 2.56 | 212.41 | 209.85 | 8.00 | 28.60 | 41.49 | 145.11 |
| PG Setup | CP Setup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil Property | R2 | RMSE | SE | RPD | Factors | R2 | RMSE | SE | RPD | Factors |
| Sand | 0.75 | 7.678 | 7.759 | 1.95 | 7 | 0.70 | 8.327 | 8.415 | 1.79 | 6 |
| pH | 0.95 | 0.340 | 0.343 | 3.38 | 4 | 0.92 | 0.329 | 0.334 | 3.47 | 4 |
| Ec | 0.89 | 0.011 | 0.011 | 2.73 | 3 | 0.90 | 0.011 | 0.011 | 2.73 | 4 |
| N | 0.68 | 0.017 | 0.017 | 1.76 | 6 | 0.62 | 0.018 | 0.018 | 1.67 | 3 |
| P | 0.90 | 6.530 | 6.619 | 3.09 | 7 | 0.90 | 6.647 | 6.741 | 3.04 | 4 |
| K | 0.65 | 0.086 | 0.087 | 1.61 | 6 | 0.64 | 0.087 | 0.088 | 1.59 | 6 |
| Ca | 0.87 | 2.332 | 2.357 | 2.73 | 6 | 0.89 | 2.141 | 2.163 | 2.98 | 6 |
| Mn | 0.62 | 4.399 | 4.446 | 1.59 | 3 | 0.66 | 4.195 | 4.239 | 1.67 | 5 |
| PG Setup | CP Setup | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Soil Property | R2 | RMSE | SE | RPD | Factors | R2 | RMSE | SE | RPD | Factors |
| SVN | ||||||||||
| Sand | 0.76 | 7.556 | 7.635 | 1.98 | 6 | 0.72 | 8.028 | 8.112 | 1.86 | 5 |
| pH | 0.96 | 0.317 | 0.320 | 3.63 | 4 | 0.92 | 0.336 | 0.339 | 3.42 | 3 |
| Ec | 0.89 | 0.011 | 0.011 | 2.73 | 2 | 0.91 | 0.010 | 0.011 | 2.73 | 3 |
| N | 0.70 | 0.016 | 0.016 | 1.88 | 5 | 0.65 | 0.018 | 0.018 | 1.67 | 4 |
| P | 0.92 | 5.891 | 5.977 | 3.43 | 5 | 0.93 | 5.701 | 5.784 | 3.54 | 5 |
| K | 0.66 | 0.084 | 0.085 | 1.65 | 5 | 0.66 | 0.084 | 0.085 | 1.65 | 5 |
| Ca | 0.89 | 2.119 | 2.141 | 3.01 | 5 | 0.91 | 1.960 | 1.981 | 3.25 | 5 |
| Mn | 0.65 | 4.115 | 4.154 | 1.71 | 5 | 0.69 | 3.992 | 4.032 | 1.76 | 5 |
| DT | ||||||||||
| Sand | 0.74 | 7.725 | 7.805 | 1.93 | 4 | 0.73 | 7.902 | 7.985 | 1.89 | 7 |
| pH | 0.91 | 0.356 | 0.359 | 3.23 | 3 | 0.92 | 0.343 | 0.346 | 3.35 | 3 |
| Ec | 0.90 | 0.011 | 0.011 | 2.73 | 3 | 0.90 | 0.007 | 0.011 | 2.73 | 3 |
| N | 0.68 | 0.017 | 0.017 | 1.76 | 4 | 0.67 | 0.017 | 0.017 | 1.76 | 3 |
| P | 0.91 | 6.307 | 6.398 | 3.20 | 4 | 0.93 | 5.719 | 5.795 | 3.53 | 4 |
| K | 0.68 | 0.082 | 0.083 | 1.69 | 4 | 0.64 | 0.087 | 0.087 | 1.44 | 4 |
| Ca | 0.86 | 2.412 | 2.437 | 2.64 | 4 | 0.90 | 2.043 | 2.064 | 3.12 | 5 |
| Mn | 0.68 | 4.075 | 4.118 | 1.72 | 4 | 0.64 | 4.315 | 4.360 | 1.63 | 1 |
| SVN + DT | ||||||||||
| Sand | 0.77 | 7.280 | 7.74 | 1.95 | 6 | 0.76 | 7.550 | 7.62 | 1.98 | 6 |
| pH | 0.93 | 0.315 | 0.32 | 3.64 | 4 | 0.92 | 0.342 | 0.35 | 3.35 | 3 |
| Ec | 0.90 | 0.011 | 0.01 | 2.73 | 2 | 0.91 | 0.011 | 0.01 | 2.73 | 3 |
| N | 0.71 | 0.016 | 0.02 | 1.88 | 7 | 0.70 | 0.016 | 0.02 | 1.88 | 7 |
| P | 0.92 | 6.083 | 6.17 | 3.32 | 4 | 0.92 | 5.900 | 5.99 | 3.42 | 4 |
| K | 0.68 | 0.083 | 0.08 | 1.69 | 6 | 0.63 | 0.088 | 0.09 | 1.57 | 4 |
| Ca | 0.89 | 2.198 | 2.22 | 2.90 | 4 | 0.91 | 2.005 | 2.03 | 3.18 | 4 |
| Mn | 0.71 | 3.887 | 3.93 | 1.80 | 5 | 0.69 | 3.983 | 4.03 | 1.76 | 5 |
| PG Setup | CP Setup | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Soil Property | Transformation | Spectral Subset | R2 | RMSE | SE | RPD | Factors | R2 | RMSE | SE | RPD | Factors |
| Sand | SVN + DT | SWIR | 0.77 | 7.276 | 7.353 | 2.05 | 6 | 0.77 | 7.326 | 7.396 | 2.04 | 6 |
| pH | None | SWIR | 0.94 | 0.284 | 0.287 | 4.04 | 3 | 0.94 | 0.287 | 0.29 | 4.00 | 5 |
| Ec | SVN + DT | SWIR | 0.92 | 0.010 | 0.01 | 3.00 | 3 | 0.92 | 0.010 | 0.01 | 3.00 | 3 |
| N | SVN | SWIR | 0.77 | 0.014 | 0.014 | 2.14 | 7 | 0.84 | 0.012 | 0.016 | 1.88 | 7 |
| P | SVN | SWIR | 0.92 | 5.816 | 5.891 | 3.48 | 5 | 0.93 | 5.571 | 5.653 | 3.62 | 5 |
| K | SVN + DT | SWIR | 0.72 | 0.076 | 0.077 | 1.82 | 4 | 0.71 | 0.078 | 0.079 | 1.77 | 4 |
| Ca | SVN + DT | VIS | 0.94 | 1.603 | 1.983 | 3.25 | 5 | 0.92 | 1.859 | 2.277 | 2.83 | 5 |
| Mn | SVN + DT | VIS + NIR + SWIR | 0.71 | 3.887 | 3.928 | 1.80 | 5 | 0.69 | 3.983 | 4.025 | 1.76 | 5 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rodríguez-Pérez, J.R.; Marcelo, V.; Pereira-Obaya, D.; García-Fernández, M.; Sanz-Ablanedo, E. Estimating Soil Properties and Nutrients by Visible and Infrared Diffuse Reflectance Spectroscopy to Characterize Vineyards. Agronomy 2021, 11, 1895. https://doi.org/10.3390/agronomy11101895
Rodríguez-Pérez JR, Marcelo V, Pereira-Obaya D, García-Fernández M, Sanz-Ablanedo E. Estimating Soil Properties and Nutrients by Visible and Infrared Diffuse Reflectance Spectroscopy to Characterize Vineyards. Agronomy. 2021; 11(10):1895. https://doi.org/10.3390/agronomy11101895
Chicago/Turabian StyleRodríguez-Pérez, José Ramón, Víctor Marcelo, Dimas Pereira-Obaya, Marta García-Fernández, and Enoc Sanz-Ablanedo. 2021. "Estimating Soil Properties and Nutrients by Visible and Infrared Diffuse Reflectance Spectroscopy to Characterize Vineyards" Agronomy 11, no. 10: 1895. https://doi.org/10.3390/agronomy11101895
APA StyleRodríguez-Pérez, J. R., Marcelo, V., Pereira-Obaya, D., García-Fernández, M., & Sanz-Ablanedo, E. (2021). Estimating Soil Properties and Nutrients by Visible and Infrared Diffuse Reflectance Spectroscopy to Characterize Vineyards. Agronomy, 11(10), 1895. https://doi.org/10.3390/agronomy11101895






