Development and Characterization of Synthetic Hexaploid Wheat for Improving the Resistance of Common Wheat to Leaf Rust and Heat Stress
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Materials
2.2. Confirmation of D Genome in SynDT
2.3. LR Infection
2.4. HS Treatment
2.5. Microscopy and Rust Biomass Assay
2.6. Protein Extraction and Enzyme Assays: Chitinase, β-1,3-Glucanase, Peroxidase Activity Assays
2.7. Real Time RT-PCR Analysis
2.8. Data Analysis
3. Results and Discussion
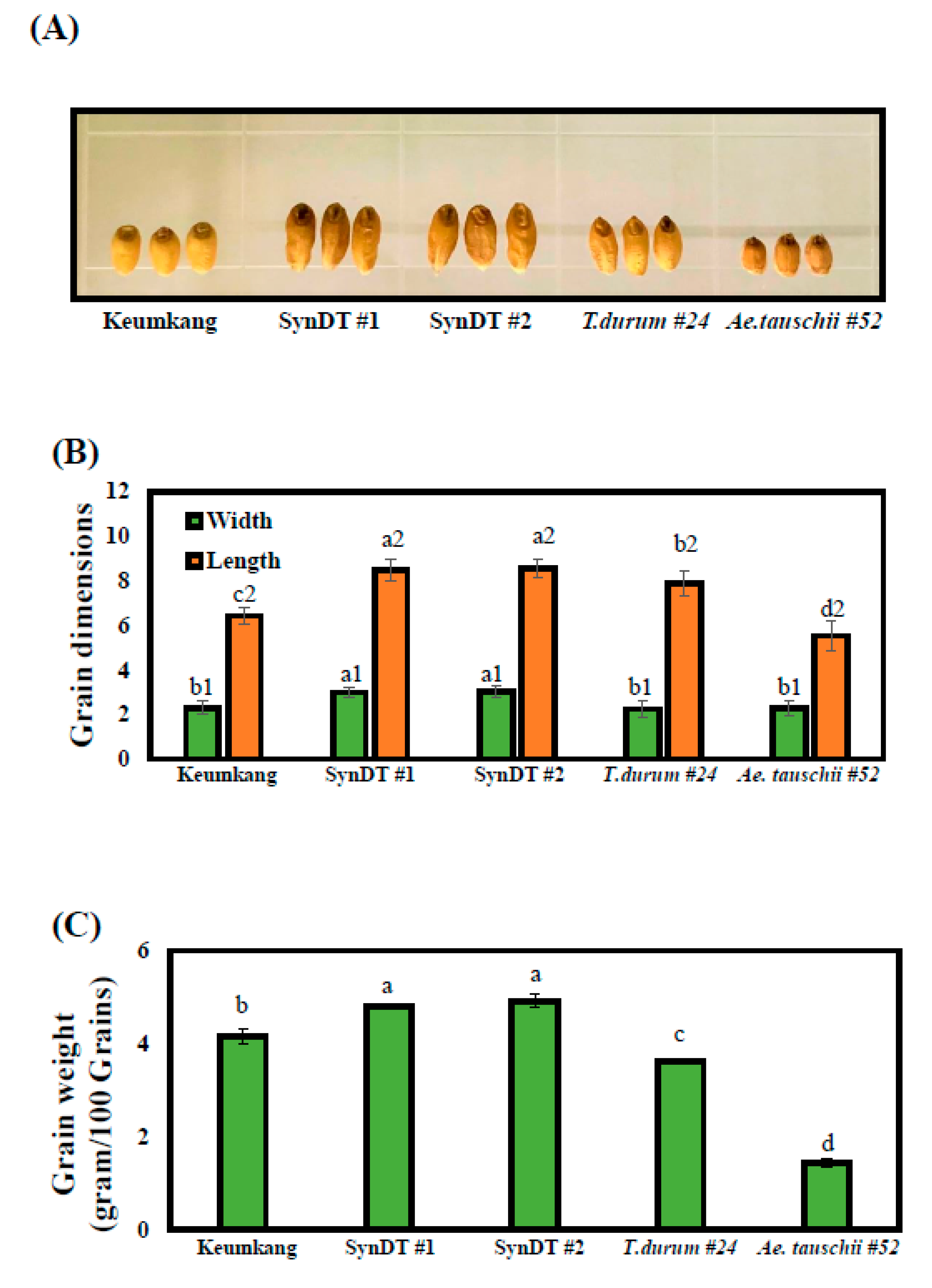
3.1. Process of Generating Synthetic Wheats and Their Morphology
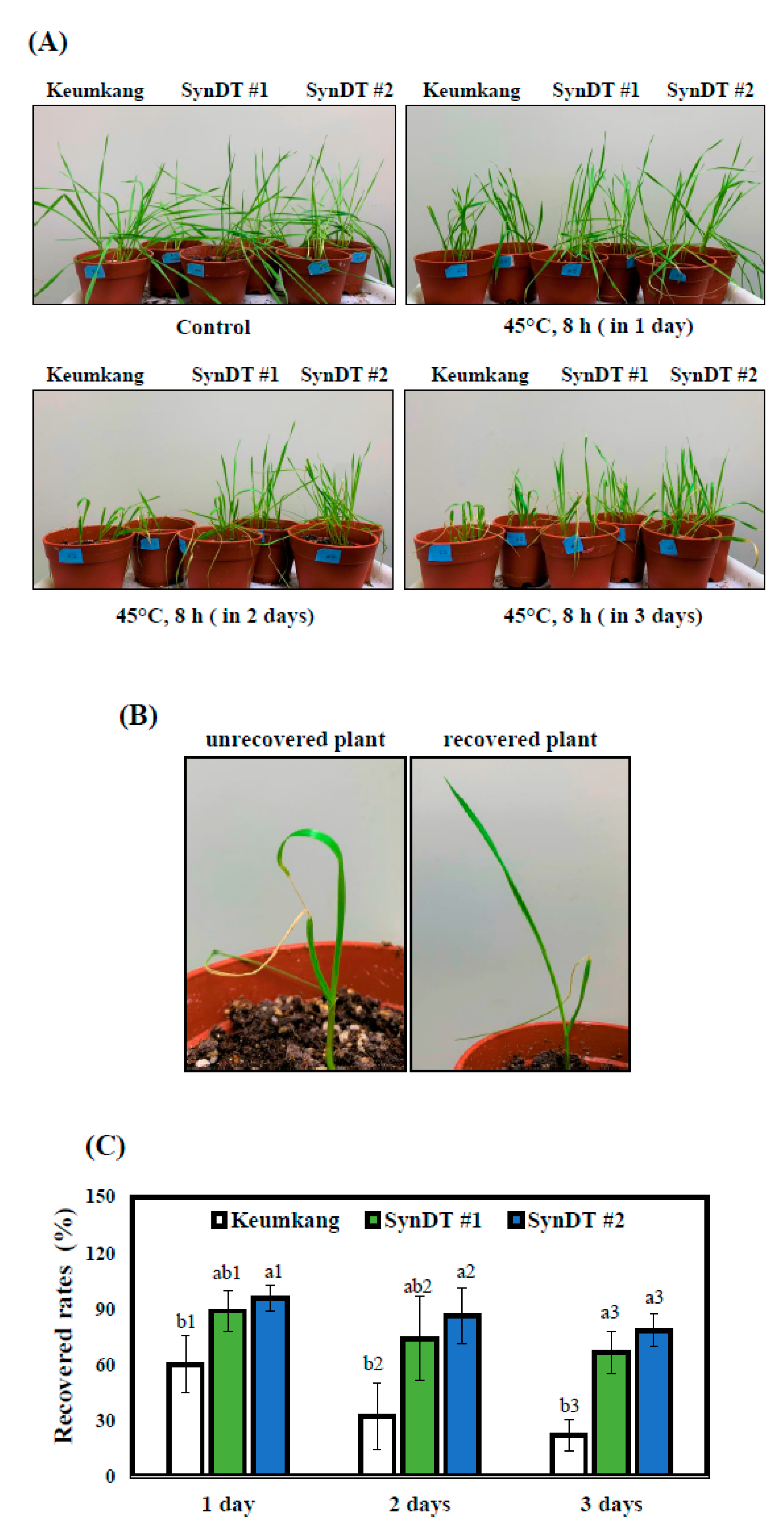
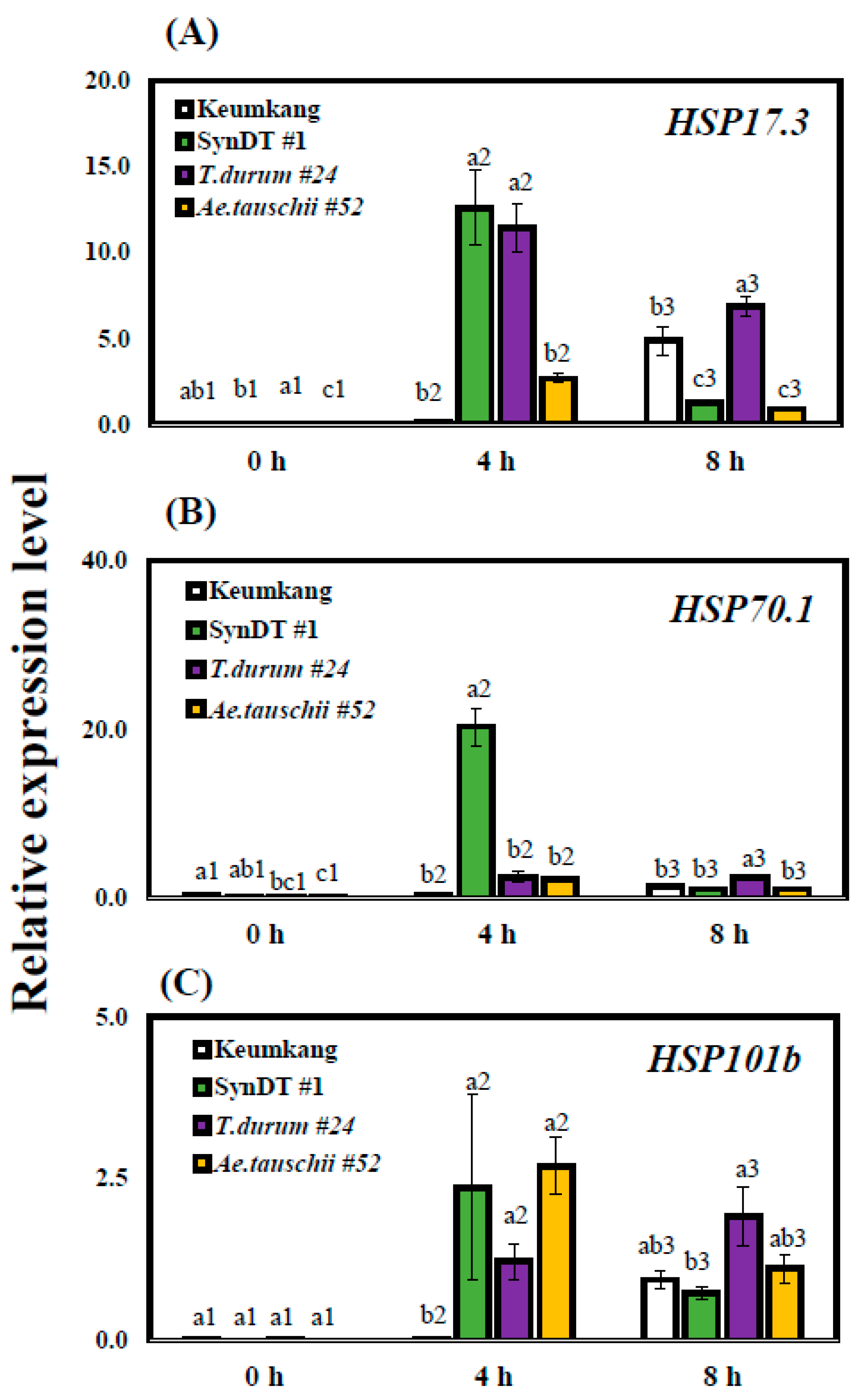
3.2. Tolerance of SynDT to HS Due to the Induction of HSPs
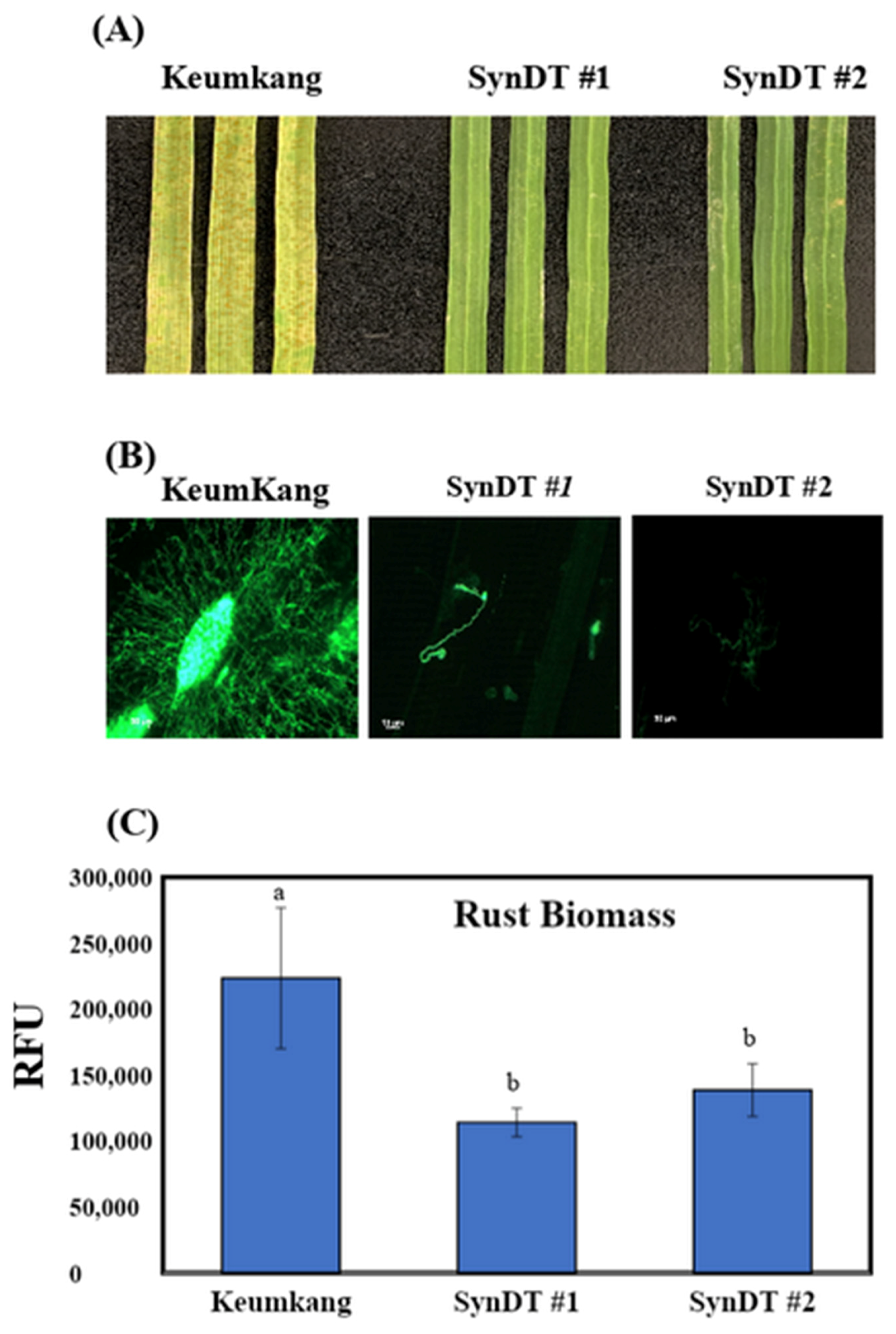
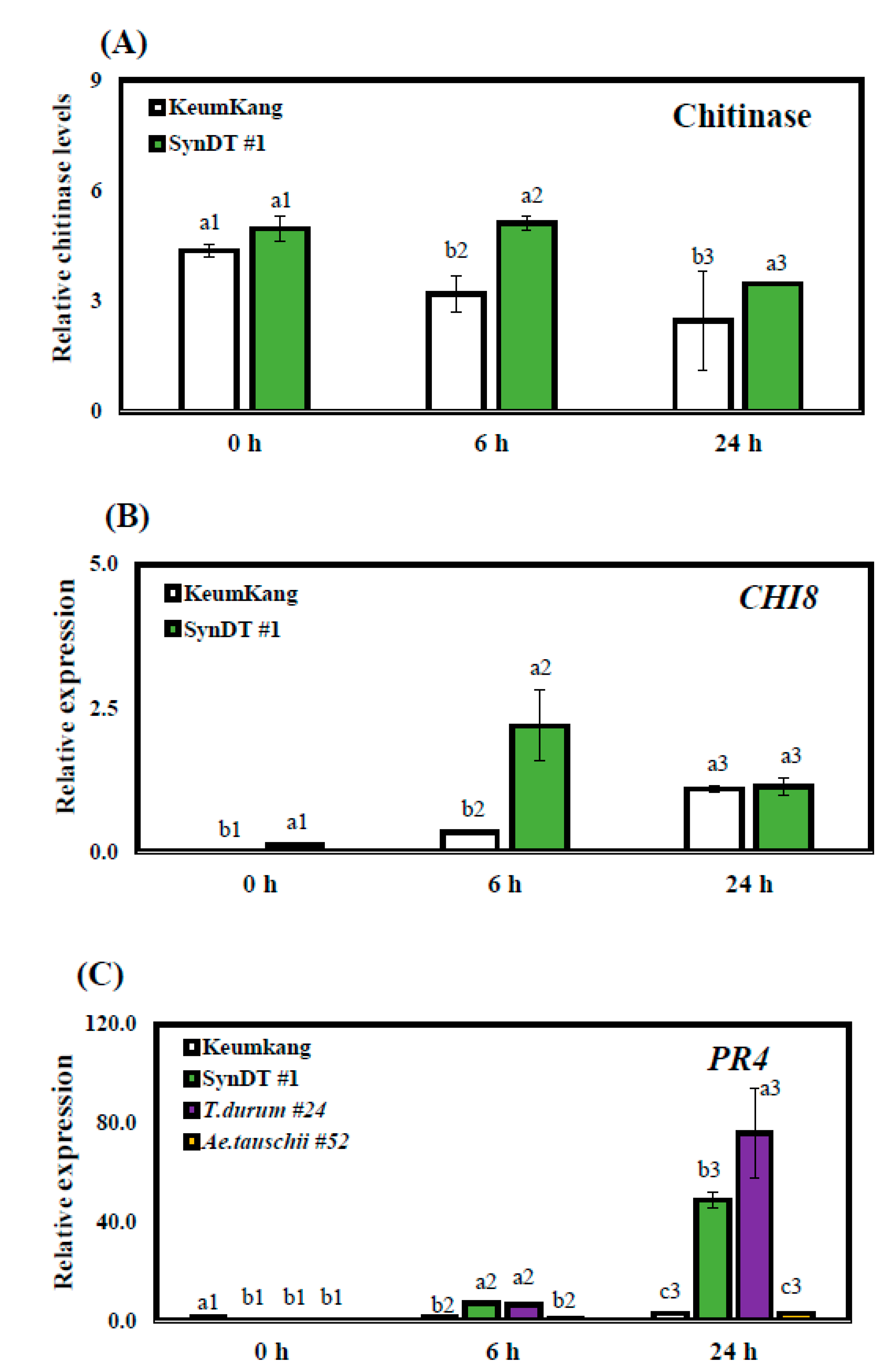
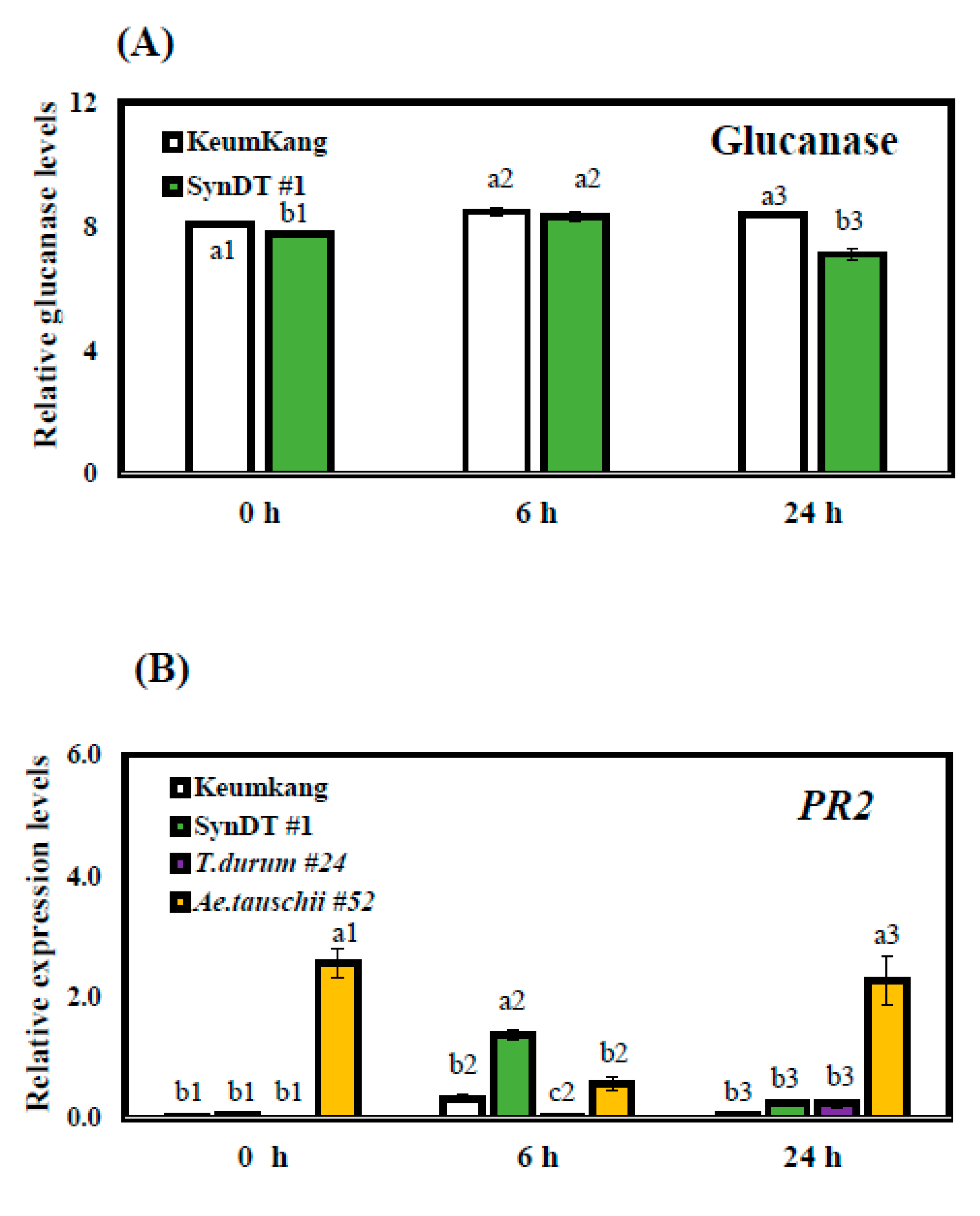
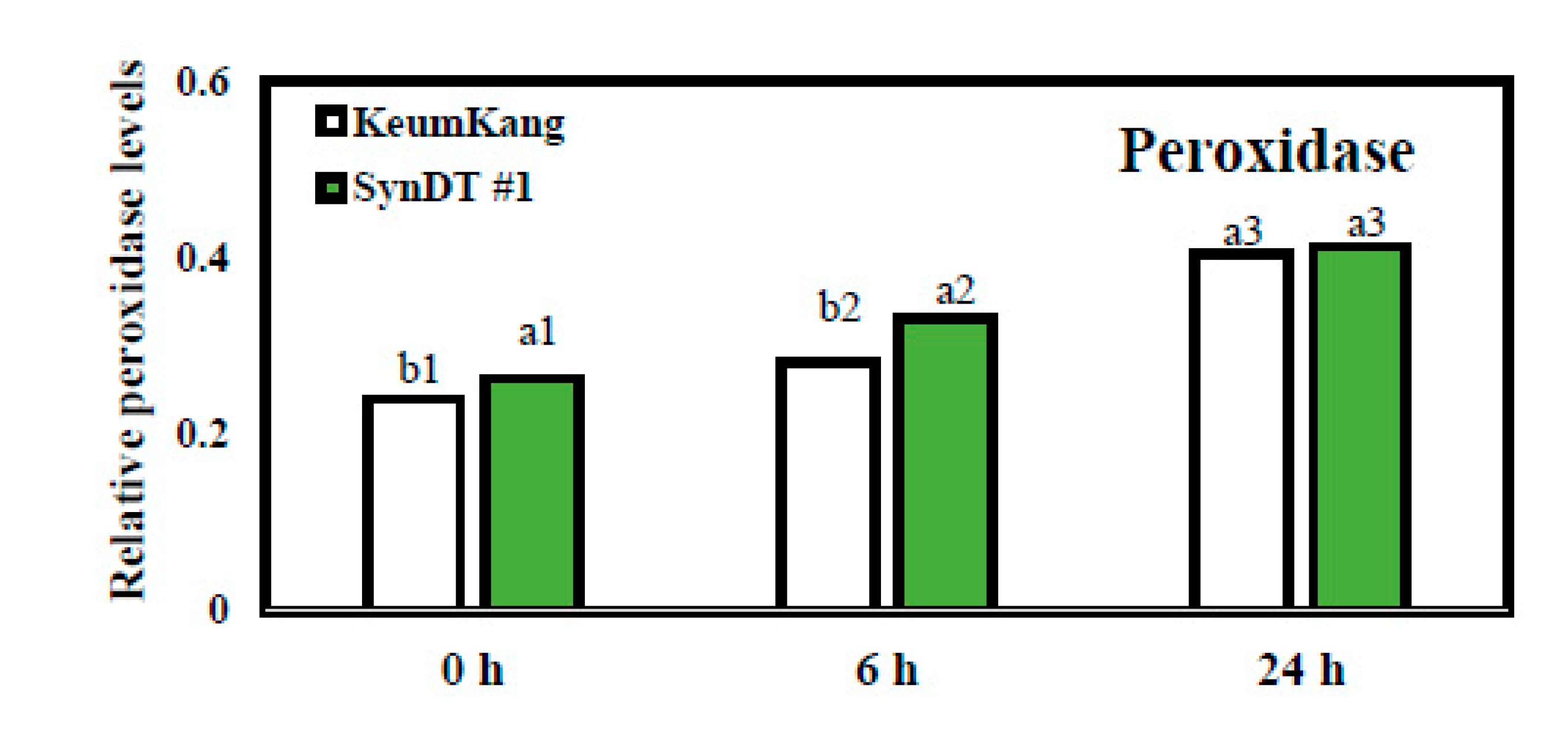
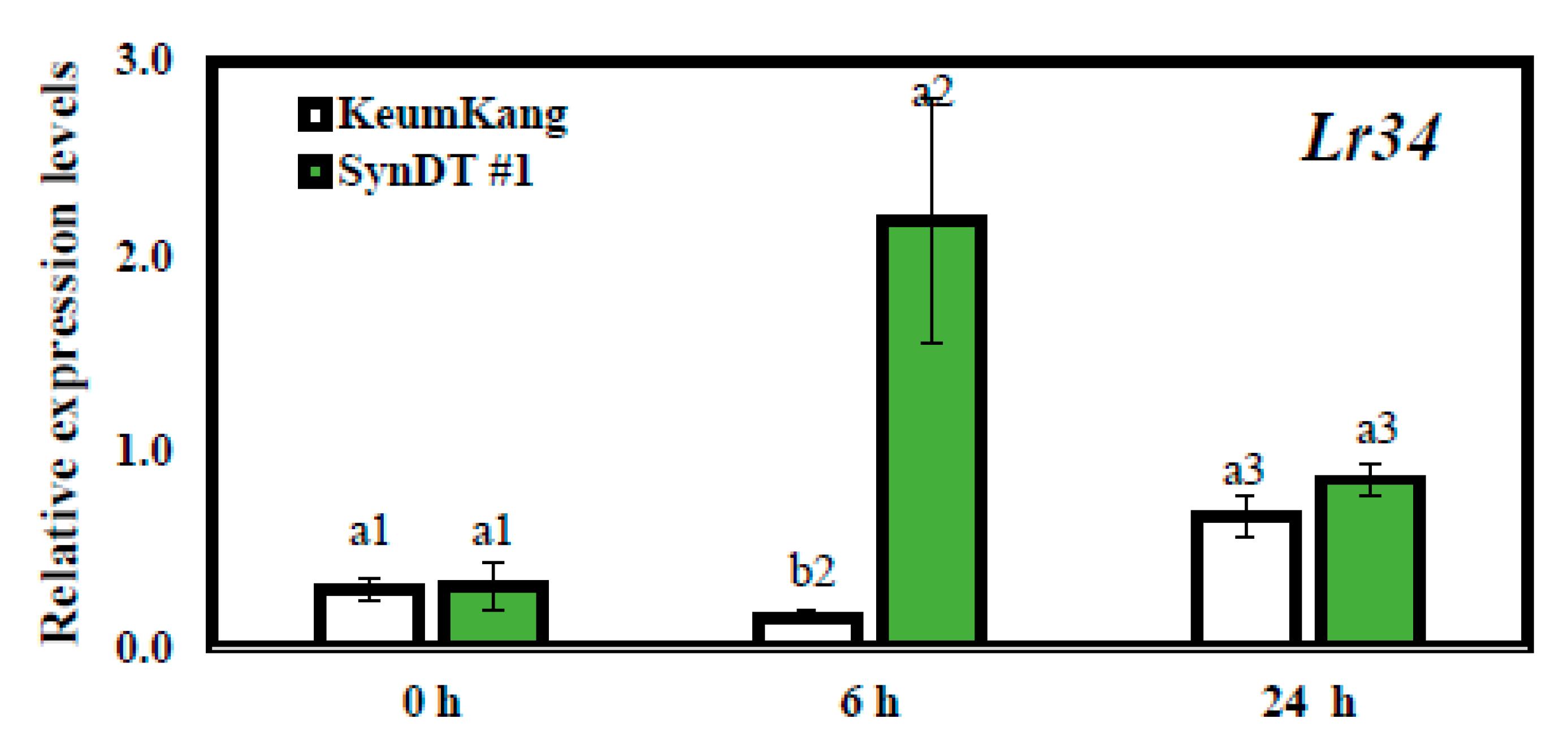
3.3. Resistance of SynDT Wheat Cultivar to LR by the Induction of Chitinase and PR Genes
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Rana, R.M.; Bilal, M.; Rehman, S.U.; Iqbal, F.; Shah, M.K.N. Synthetic Wheat; a New Hope for the Hungry World. Asian J. Agric. Biol. 2013, 1, 91–94. [Google Scholar]
- Li, J.; Wan, H.-S.; Yang, W.-Y. Synthetic hexaploid wheat enhances variation and adaptive evolution of bread wheat in breeding processes. J. Syst. Evol. 2014, 52, 735–742. [Google Scholar] [CrossRef]
- Greveniotis, V.; Zotis, S.; Sioki, E.; Ipsilandis, C.; Breeding, A. Concept to improve the performance of locally cultivated bread wheat (Triticum aestivum L.) cultivars. Czech J. Genet. Plant Breed. 2019, 56, 1–8. [Google Scholar] [CrossRef]
- Das, M.K.; Bai, G.; Mujeeb-Kazi, A.; Rajaram, S. Genetic diversity among synthetic hexaploid wheat accessions (Triticum aestivum) with resistance to several fungal diseases. Genet. Resour. Crop Evol. 2016, 63, 1285–1296. [Google Scholar] [CrossRef]
- Li, A.; Liu, D.; Yang, W.; Kishii, M.; Mao, L. Synthetic hexaploid wheat: Yesterday, today, and tomorrow. Engineering 2018, 4, 552–558. [Google Scholar] [CrossRef]
- Duplessis, S.; Bakkeren, G.; Hamelin, R. Chapter Six-Advancing Knowledge on Biology of Rust Fungi Through Genomics. In Advances in Botanical Research; Martin, F.M., Ed.; Academic Press: Cambridge, MA, USA, 2014; Volume 70, pp. 173–209. [Google Scholar]
- Draz, I.S.; Abou-Elseoud, M.S.; Kamara, A.-E.M.; Alaa-Eldein, O.A.-E.; El-Bebany, A.F. Screening of wheat genotypes for leaf rust resistance along with grain yield. Ann. Agric. Sci. 2015, 60, 29–39. [Google Scholar] [CrossRef] [Green Version]
- Mohler, V.; Schmolke, M.; Zeller, F.J.; Hsam, S.L.K. Genetic analysis of Aegilops tauschii-derived seedling resistance to leaf rust in synthetic hexaploid wheat. J. Appl. Genet. 2020, 61, 163–168. [Google Scholar] [CrossRef] [Green Version]
- Gyani, P.C.; Sharma, J.B.; Vinod; Mallik, N.; Jha, S.K. Inheritance and molecular mapping of leaf rust resistance gene in hexaploid wheat synthetic 45. Indian J. Genet. Plant Breed. 2017, 77, 214. [Google Scholar] [CrossRef]
- Zaidi, N.W.; Dar, M.H.; Singh, S.; Singh, U.S. Chapter 38 Trichoderma species as Abiotic Stress Relievers in Plants. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; pp. 515–525. [Google Scholar]
- Pareek, A.; Dhankher, O.P.; Foyer, C.H. Mitigating the impact of climate change on plant productivity and ecosystem sustainability. J. Exp. Bot. 2020, 71, 451–456. [Google Scholar] [CrossRef]
- Akter, N.; Rafiqul Islam, M. Heat stress effects and management in wheat. A Review. Agron. Sustain. Dev. 2017, 37. [Google Scholar] [CrossRef]
- Cossani, C.M.; Reynolds, M.P. Heat Stress Adaptation in elite lines derived from synthetic hexaploid wheat. Crop. Sci. 2015, 55, 2719–2735. [Google Scholar] [CrossRef]
- Elbashir, A.A.E.; Gorafi, Y.S.A.; Tahir, I.S.A.; Kim, J.S.; Tsujimoto, H. Wheat multiple synthetic derivatives: A new source for heat stress tolerance adaptive traits. Breed. Sci. 2017, 67, 248–256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huseynova, I.M.; Guliyeva, F.B.; Rustamova, S.M.; Aliyev, J.A. PCR-Based Molecular Markers Linked to the Leaf Rust Resistance Gene Lr19 in Different Bread Wheat Cultivars. Adv. Biol. Chem. 2013, 3, 153–158. [Google Scholar] [CrossRef] [Green Version]
- Truong, H.A.; Lee, W.J.; Kishii, M.; Hong, S.-W.; Kang, C.-S.; Lee, B.C.; Lee, H. Assessment of synthetic hexaploid wheats in response to heat stress and leaf rust infection for the improvement of wheat production. Crop. Pasture Sci. 2019, 70, 837–848. [Google Scholar] [CrossRef]
- Jeong, C.Y.; Lee, W.J.; Truong, H.A.; Truon, C.S.; Hong, S.-W.; Lee, H. AtMybL-O modulates abscisic acid biosynthesis to optimize plant growth and aba signaling in response to drought stress. Appl. Biol. Chem. 2018, 61, 473–477. [Google Scholar] [CrossRef]
- Rasheed, A.; Xia, X.; Ogbonnaya, F.; Mahmood, T.; Zhang, Z.; Mujeeb-Kazi, A.; He, Z. Genome-wide association for grain morphology in synthetic hexaploid wheats using digital imaging analysis. BMC Plant Biol. 2014, 14, 128. [Google Scholar] [CrossRef] [Green Version]
- Gan, Y.; Stobbe, E.H. Seedling vigor and grain yield of ‘Roblin’ wheat affected by seed size. Agron.J. 1996, 88, 456–460. [Google Scholar] [CrossRef]
- Mujeeb-Kazi, A.; Gul, A.; Ahmad, I.; Farooq, M.; Rizwan, S.; Bux, H.; Iftikhar, S.; Asad, S.; Delgado, R. Aegilops tauschii, as a spot blotch (Cochliobolus sativus) resistance source for bread wheat improvement. Pak. J. Bot. 2007, 39, 1207–1216. [Google Scholar]
- Reynolds, M.; Dreccer, F.; Trethowan, R. Drought-adaptive traits derived from wheat wild relatives and landraces. J. Exp. Bot. 2007, 58, 177–186. [Google Scholar] [CrossRef] [Green Version]
- Waters, E.R.; Lee, G.J.; Vierling, E. Evolution, structure, and function of the small heat shock proteins in plants. J. Exp. Bot. 1996, 47, 325–338. [Google Scholar] [CrossRef]
- Park, C.J.; Seo, Y.S. Heat shock proteins: A review of the molecular chaperones for plant immunity. Plant Pathol. J. 2015, 31, 323–333. [Google Scholar] [CrossRef] [Green Version]
- Kumar, R.R.; Singh, G.P.; Sharma, S.K.; Singh, K.; Goswami, S.; Rai, R.D. Molecular cloning of HSP17 gene (SHSP) and their differential expression under exogenous putrescine and heat shock in wheat (Triticum aestivum). Afr. J. Biotechnol. 2012, 11, 16800–16808. [Google Scholar]
- Duan, Y.H.; Guo, J.; Ding, K.; Wang, S.J.; Zhang, H.; Dai, X.W.; Chen, Y.Y.; Govers, F.; Huang, L.L.; Kang, Z.S. Characterization of a wheat HSP70 Gene and its expression in response to stripe rust infection and abiotic stresses. Mol. Biol. Rep. 2011, 38, 301–307. [Google Scholar] [CrossRef] [PubMed]
- Parsell, D.A.; Lindquist, S. The function of heat-shock proteins in stress tolerance: Degradation and reactivation of damaged proteins. Annu. Rev. Genet. 1993, 27, 437–496. [Google Scholar] [CrossRef] [PubMed]
- Young, T.E.; Ling, J.; Geisler-Lee, C.J.; Tanguay, R.L.; Caldwell, C.; Gallie, D.R. Developmental and thermal regulation of the maize heat shock protein, HSP101. Plant Physiol. 2001, 127, 777–791. [Google Scholar] [CrossRef] [PubMed]
- Mohammadi, M.; Roohparvar, R.; Torabi, M. Induced chitinase activity in resistant wheat leaves inoculated with an incompatible race of Puccinia striiformis f. sp. tritici, the causal agent of yellow rust disease. Mycopathologia 2002, 154, 119–126. [Google Scholar] [CrossRef] [PubMed]
- Toufiq, N.; Tabassum, B.; Bhatti, M.U.; Khan, A.; Tariq, M.; Shahid, N.; Nasir, I.A.; Husnain, T. Improved antifungal activity of barley derived chitinase I gene that overexpress a 32 KDa recombinant chitinase in Escherichia coli host. Braz. J. Microbiol. 2018, 49, 414–421. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Du, X.; Wang, Q.; Chen, X.; Lv, D.; Xu, K.; Qu, S.; Zhang, Z. Expression of pathogenesis related genes in response to salicylic acid, methyl jasmonate and 1-aminocyclopropane-1-carboxylic acid in Malus hupehensis (Pamp.) Rehd. BMC Res. Notes 2010, 3, 208. [Google Scholar] [CrossRef] [Green Version]
- Selitrennikoff, C.P. Antifungal proteins. Appl. Environ. Microbiol. 2001, 67, 2883–2894. [Google Scholar] [CrossRef] [Green Version]
- Balasubramanian, V.; Vashisht, D.; Cletus, J.; Sakthivel, N. Plant β-1,3-glucanases: Their biological functions and transgenic expression against phytopathogenic fungi. Biotechnol. Lett. 2012, 34, 1983–1990. [Google Scholar] [CrossRef]
- Gupta, P.; Ravi, I.; Sharma, V. Induction of β-1,3-glucanase and chitinase activity in the defense response of Eruca sativa plants against the fungal pathogen Alternaria brassicicola. J. Plant Interact. 2013, 8, 155–161. [Google Scholar] [CrossRef]
- Ali, S.; Mir, Z.A.; Tyagi, A.; Bhat, J.A.; Chandrashekar, N.; Papolu, P.K.; Rawat, S.; Grover, A. identification and comparative analysis of Brassica juncea pathogenesis-related genes in response to hormonal, biotic and abiotic stresses. Acta Physiol. Plant. 2017, 39, 268. [Google Scholar] [CrossRef]
- Zhang, H.; Qiu, Y.; Yuan, C.; Chen, X.; Huang, L. Fine-Tuning of PR genes in wheat responding to different Puccinia rust species. J. Plant Physiol. Pathol. 2018, 6. [Google Scholar] [CrossRef]
- Li, X.-Y.; Gao, L.; Zhang, W.-H.; Liu, J.-K.; Zhang, Y.-J.; Wang, H.-Y.; Liu, D.-Q. Characteristic expression of wheat PR5 gene in response to infection by the leaf rust pathogen, Puccinia triticina. J. Plant Interact. 2015, 10, 132–141. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Boller, T. Antimicrobial Functions of the Plant Hydrolases, Chitinase and ß-1,3-Glucanase. In Mechanisms of Plant Defense Responses; Fritig, B., Legrand, M., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 391–400. [Google Scholar]
- Yusupova, Z.R.; Khairullin, R.M.; Maksimov, I.V. The activity of peroxidase in various cell fractions of wheat plants infected with Septoria nodorum Berk. Russ. J. Plant Physiol. 2006, 53, 807–813. [Google Scholar] [CrossRef]
- German, S.E.; Kolmer, J.A. Effect of gene Lr34 in the enhancement of resistance to leaf rust of wheat. Theoret. J. Appl. Genet. 1992, 84, 97–105. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Truong, H.A.; Lee, H.; Kishii, M.; Hong, S.W.; Lee, H. Development and Characterization of Synthetic Hexaploid Wheat for Improving the Resistance of Common Wheat to Leaf Rust and Heat Stress. Agronomy 2021, 11, 18. https://doi.org/10.3390/agronomy11010018
Truong HA, Lee H, Kishii M, Hong SW, Lee H. Development and Characterization of Synthetic Hexaploid Wheat for Improving the Resistance of Common Wheat to Leaf Rust and Heat Stress. Agronomy. 2021; 11(1):18. https://doi.org/10.3390/agronomy11010018
Chicago/Turabian StyleTruong, Hai An, Hyeri Lee, Masahiro Kishii, Suk Whan Hong, and Hojoung Lee. 2021. "Development and Characterization of Synthetic Hexaploid Wheat for Improving the Resistance of Common Wheat to Leaf Rust and Heat Stress" Agronomy 11, no. 1: 18. https://doi.org/10.3390/agronomy11010018
APA StyleTruong, H. A., Lee, H., Kishii, M., Hong, S. W., & Lee, H. (2021). Development and Characterization of Synthetic Hexaploid Wheat for Improving the Resistance of Common Wheat to Leaf Rust and Heat Stress. Agronomy, 11(1), 18. https://doi.org/10.3390/agronomy11010018




