Metabolomics Analysis Reveals the Mechanism of Hydrogen Cyanamide in Promoting Flower Bud Break in Blueberry
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Chemicals
2.2. HC Treatment and Sampling
2.3. Metabolite Extractions
2.4. GC–TOFMS Analysis
3. Results
3.1. Bud Break Rate after HC Treatment
3.2. Global Metabolic Response of Blueberry Buds after HC Treatment
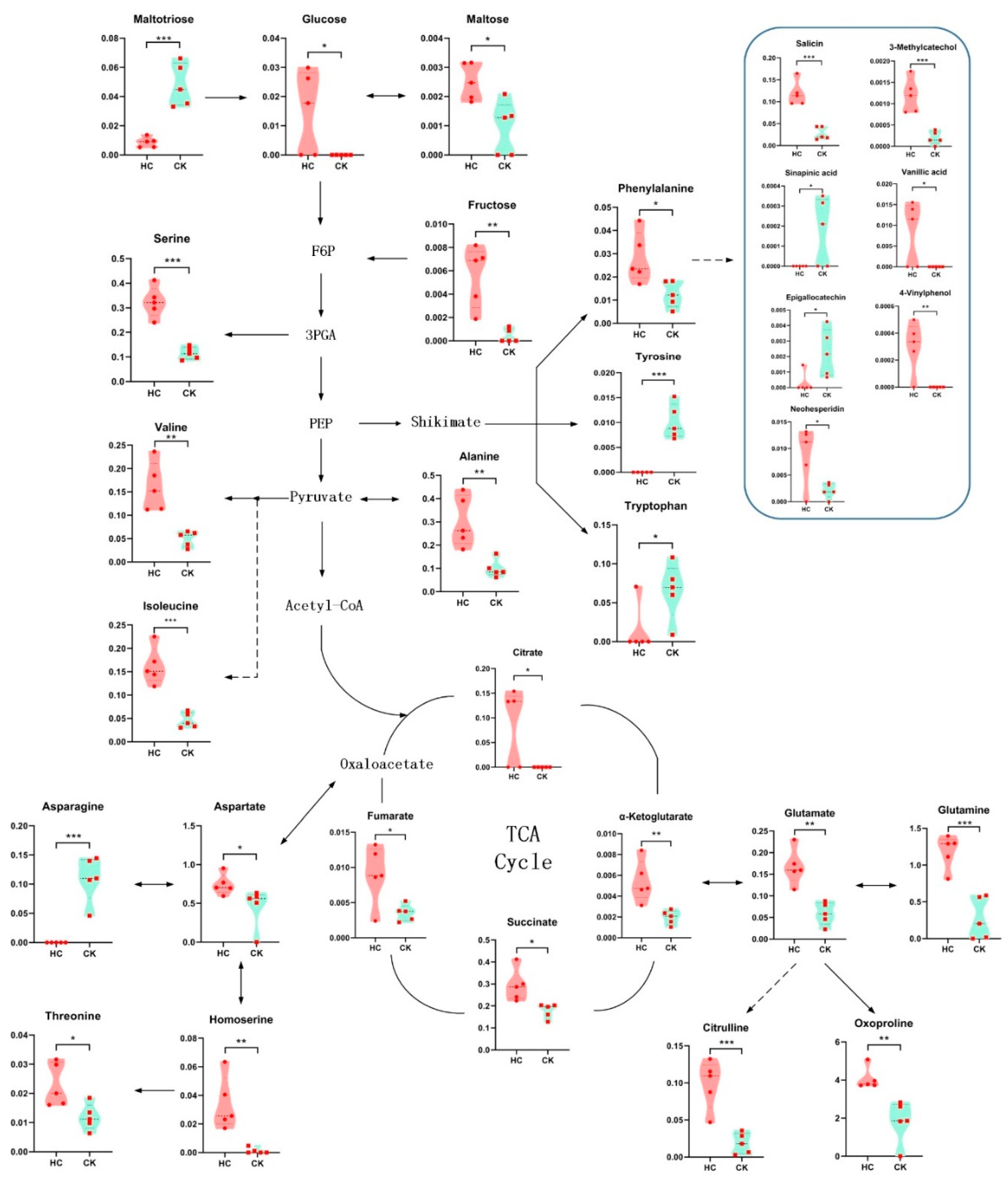
3.3. Changes in Metabolite Levels after HC Treatment
3.4. Effects of HC Treatment on Carbohydrate Metabolism and TCA Cycle of Blueberry Buds
3.5. Effects of HC Treatment on Phenylpropanoid Metabolites of Blueberry Buds
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Singh, R.K.; Svystun, T.; AlDahmash, B.; Jonsson, A.M.; Bhalerao, R.P. Photoperiod- and temperature-mediated control of phenology in trees—A molecular perspective. New Phytol. 2017, 213, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, C.J.; Brennan, R.M.; Jones, H.G. Declining chilling and its impact on temperate perennial crops. Environ. Exp. Bot. 2013, 91, 48–62. [Google Scholar] [CrossRef]
- Liang, D.; Huang, X.; Shen, Y.; Shen, T.; Zhang, H.; Lin, L.; Wang, J.; Deng, Q.; Lyu, X.; Xia, H. Hydrogen cyanamide induces grape bud endodormancy release through carbohydrate metabolism and plant hormone signaling. BMC Genom. 2019, 20, 1034. [Google Scholar] [CrossRef] [PubMed]
- Ionescu, I.A.; Lopez-Ortega, G.; Burow, M.; Bayo-Canha, A.; Junge, A.; Gericke, O.; Moller, B.L.; Sanchez-Perez, R. Transcriptome and metabolite changes during hydrogen cyanamide-induced floral bud break in sweet cherry. Front. Plant Sci. 2017, 8, 1233. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Beckman, T.G. Effect of a late spring application of hydrogen cyanamide on high-chill peaches. Agronomy 2019, 9, 726. [Google Scholar] [CrossRef]
- Williamson, J.G.; Maust, B.E.; Nesmith, D.S. Timing and concentration of hydrogen cyanamide affect blueberry bud development and flower mortality. HortScience 2001, 36, 922–924. [Google Scholar] [CrossRef]
- Shangguan, L.; Chen, M.; Fang, X.; Xie, Z.; Gong, P.; Huang, Y.; Wang, Z.; Fang, J. Comparative transcriptome analysis provides insight into regulation pathways and temporal and spatial expression characteristics of grapevine (Vitis vinifera) dormant buds in different nodes. BMC Plant Biol. 2020, 20, 390. [Google Scholar] [CrossRef] [PubMed]
- Sudawan, B.; Chang, C.S.; Chao, H.F.; Ku, M.S.; Yen, Y.F. Hydrogen cyanamide breaks grapevine bud dormancy in the summer through transient activation of gene expression and accumulation of reactive oxygen and nitrogen species. BMC Plant Biol. 2016, 16, 202. [Google Scholar] [CrossRef]
- Oracz, K.; El-Maarouf-Bouteau, H.; Bogatek, R.; Corbineau, F.; Bailly, C. Release of sunflower seed dormancy by cyanide: Cross-talk with ethylene signalling pathway. J. Exp. Bot. 2008, 59, 2241–2251. [Google Scholar] [CrossRef]
- Shi, Z.; Halaly-Basha, T.; Zheng, C.; Weissberg, M.; Ophir, R.; Galbraith, D.W.; Pang, X.; Or, E. Transient induction of a subset of ethylene biosynthesis genes is potentially involved in regulation of grapevine bud dormancy release. Plant Mol. Biol. 2018, 98, 507–523. [Google Scholar] [CrossRef]
- Khalil-Ur-Rehman, M.; Wang, W.; Dong, Y.; Faheem, M.; Xu, Y.; Gao, Z.; Shen, Z.G.; Tao, J. Comparative transcriptomic and proteomic analysis to deeply investigate the role of hydrogen cyanamide in grape bud dormancy. Int. J. Mol. Sci. 2019, 20, 3528. [Google Scholar] [CrossRef] [PubMed]
- Pasamontes, A.; Cheung, W.H.K.; Simmons, J.; Aksenov, A.A.; Peirano, D.J.; Grafton-Cardwell, E.E.; Kapaun, T.; Dandekar, A.M.; Fiehn, O.; Davis, C.E. Citrus tristeza virus infection in sweet orange trees and a mandarin × tangor cross alters low molecular weight metabolites assessed using gas chromatography mass spectrometry (GC/MS). Metabolomics 2016, 12, 41. [Google Scholar] [CrossRef]
- Luo, Q.; Wang, S.; Sun, L.-n.; Wang, H. Metabolic profiling of root exudates from two ecotypes of Sedum alfredii treated with Pb based on GC-MS. Sci. Rep. 2017, 7, 39878. [Google Scholar] [CrossRef] [PubMed]
- Fettke, J.; Fernie, A.R. Intracellular and cell-to-apoplast compartmentation of carbohydrate metabolism. Trends Plant Sci. 2015, 20, 490–497. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Zeng, L.; Zhao, H.; Ye, Q. Proteomic analysis of the early development of the phalaenopsis amabilis flower bud under low temperature induction using the iTRAQ/MRM approach. Molecules 2020, 25, 1244. [Google Scholar] [CrossRef]
- Farokhzad, A.; Nobakht, S.; Alahveran, A.; Sarkhosh, A.; Mohseniazar, M. Biochemical changes in terminal buds of three different walnut (Juglans regia L.) genotypes during dormancy break. Biochem. Syst. Ecol. 2018, 76, 52–57. [Google Scholar] [CrossRef]
- Sayyari, M.; Babalar, M.; Kalantari, S.; Martínez-Romero, D.; Guillén, F.; Serrano, M.; Valero, D. Vapour treatments with methyl salicylate or methyl jasmonate alleviated chilling injury and enhanced antioxidant potential during postharvest storage of pomegranates. Food Chem. 2011, 124, 964–970. [Google Scholar] [CrossRef]
- Khalil-Ur-Rehman, M.; Wang, W.; Xu, Y.S.; Haider, M.S.; Li, C.X.; Tao, J.M. Comparative study on reagents involved in grape bud break and their effects on different metabolites and related gene expression during winter. Front. Plant Sci. 2017, 8, 1340. [Google Scholar] [CrossRef]
- Fernandez, E.; Cuneo, I.F.; Luedeling, E.; Alvarado, L.; Farias, D.; Saa, S. Starch and hexoses concentrations as physiological markers in dormancy progression of sweet cherry twigs. Trees 2019, 33, 1187–1201. [Google Scholar] [CrossRef]
- Ben Mohamed, H.; Vadel, A.M.; Geuns, J.M.C.; Khemira, H. Carbohydrate changes during dormancy release in Superior Seedless grapevine cuttings following hydrogen cyanamide treatment. Sci. Hortic. 2012, 140, 19–25. [Google Scholar] [CrossRef]
- Matsoukas, I.G. Interplay between sugar and hormone signaling pathways modulate floral signal transduction. Front. Genet. 2014, 5, 218. [Google Scholar] [CrossRef] [PubMed]
- Tang, L.; Chhajed, S.; Vashisth, T.; Olmstead, M.A.; Olmstead, J.W.; Colquhoun, T.A. Transcriptomic study of early responses to the bud dormancy-breaking agent hydrogen cyanamide in ‘TropicBeauty’ peach. J. Am. Soc. Hortic. Sci. 2019, 144, 244–256. [Google Scholar] [CrossRef]
- Zhang, T.; Yuan, Y.; Zhan, Y.; Cao, X.; Liu, C.; Zhang, Y.; Gai, S. Metabolomics analysis reveals Embden Meyerhof Parnas pathway activation and flavonoids accumulation during dormancy transition in tree peony. BMC Plant Biol. 2020, 20, 484. [Google Scholar] [CrossRef] [PubMed]
- Michailidis, M.; Karagiannis, E.; Tanou, G.; Sarrou, E.; Adamakis, I.-D.; Karamanoli, K.; Martens, S.; Molassiotis, A. Metabolic mechanisms underpinning vegetative bud dormancy release and shoot development in sweet cherry. Environ. Exp. Bot. 2018, 155, 1–11. [Google Scholar] [CrossRef]
- Horikoshi, H.M.; Sekozawa, Y.; Kobayashi, M.; Saito, K.; Kusano, M.; Sugaya, S. Metabolomics analysis of ’Housui’ Japanese pear flower buds during endodormancy reveals metabolic suppression by thermal fluctuation. Plant Physiol. Biochem. 2018, 126, 134–141. [Google Scholar] [CrossRef] [PubMed]
- Hildebrandt, T.M.; Nunes Nesi, A.; Araujo, W.L.; Braun, H.P. Amino acid catabolism in plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Zhao, D.; Liu, Q. Connections between amino acid metabolisms in plants: Lysine as an example. Front. Plant Sci. 2020, 11, 928. [Google Scholar] [CrossRef]
- Götz, K.-P.; Chmielewski, F.-M.; Gödeke, K.; Wolf, K.; Jander, E.; Sievers, S.; Homann, T.; Huschek, G.; Rawel, H.M. Assessment of amino acids during winter rest and ontogenetic development in sweet cherry buds (Prunus avium L.). Sci. Hortic. 2017, 222, 102–110. [Google Scholar] [CrossRef]
- Galili, G.; Amir, R.; Fernie, A.R. The regulation of essential amino acid synthesis and accumulation in plants. Annu. Rev. Plant Biol. 2016, 67, 153–178. [Google Scholar] [CrossRef]
- Qiu, X.M.; Sun, Y.Y.; Ye, X.Y.; Li, Z.G. Signaling role of glutamate in plants. Front. Plant Sci. 2019, 10, 1743. [Google Scholar] [CrossRef]
- Kirma, M.; Araujo, W.L.; Fernie, A.R.; Galili, G. The multifaceted role of aspartate-family amino acids in plant metabolism. J. Exp. Bot. 2012, 63, 4995–5001. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Curtis, T.Y.; Powers, S.J.; Raffan, S.; Gao, R.; Huang, J.; Heiner, M.; Gilbert, D.R.; Halford, N.G. Genomic, biochemical, and modeling analyses of asparagine synthetases from wheat. Front. Plant Sci. 2017, 8, 2237. [Google Scholar] [CrossRef] [PubMed]
- Xing, A.; Last, R.L. A regulatory hierarchy of the arabidopsis branched-chain amino acid metabolic network. Plant Cell 2017, 29, 1480–1499. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Uygun, S.; Shiu, S.H.; Last, R.L. The impact of the branched-chain ketoacid dehydrogenase complex on amino acid homeostasis in Arabidopsis. Plant Physiol. 2015, 169, 1807–1820. [Google Scholar] [CrossRef] [PubMed]
- Ros, R.; Munoz-Bertomeu, J.; Krueger, S. Serine in plants: Biosynthesis, metabolism, and functions. Trends Plant Sci. 2014, 19, 564–569. [Google Scholar] [CrossRef]
- Timm, S.; Florian, A.; Wittmiss, M.; Jahnke, K.; Hagemann, M.; Fernie, A.R.; Bauwe, H. Serine acts as a metabolic signal for the transcriptional control of photorespiration-related genes in Arabidopsis. Plant Physiol. 2013, 162, 379–389. [Google Scholar] [CrossRef]
- Maeda, H.; Dudareva, N. The shikimate pathway and aromatic amino acid biosynthesis in plants. Annu. Rev. Plant Biol. 2012, 63, 73–105. [Google Scholar] [CrossRef]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araujo, W.L.; Braun, H.P.; Hildebrandt, T.M. The role of amino acid metabolism during abiotic stress release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef]
- Savoi, S.; Wong, D.C.; Arapitsas, P.; Miculan, M.; Bucchetti, B.; Peterlunger, E.; Fait, A.; Mattivi, F.; Castellarin, S.D. Transcriptome and metabolite profiling reveals that prolonged drought modulates the phenylpropanoid and terpenoid pathway in white grapes (Vitis vinifera L.). BMC Plant Biol. 2016, 16, 67. [Google Scholar] [CrossRef]
- Orrantia-Araujo, M.A.; Martínez-Téllez, M.Á.; Rivera-Domínguez, M.; Hernández-Oñate, M.Á.; Vargas-Arispuro, I. Changes in the endogenous content and gene expression of salicylic acid correlate with grapevine bud dormancy release. Plant Growth Regul. 2020. [Google Scholar] [CrossRef]
- Beauvieux, R.; Wenden, B.; Dirlewanger, E. Bud dormancy in perennial fruit tree species: A pivotal role for oxidative cues. Front. Plant Sci. 2018, 9, 657. [Google Scholar] [CrossRef] [PubMed]
- Mazzitelli, L.; Hancock, R.D.; Haupt, S.; Walker, P.G.; Pont, S.D.; McNicol, J.; Cardle, L.; Morris, J.; Viola, R.; Brennan, R.; et al. Co-ordinated gene expression during phases of dormancy release in raspberry (Rubus idaeus L.) buds. J. Exp. Bot. 2007, 58, 1035–1045. [Google Scholar] [CrossRef] [PubMed]
- Khalil-Ur-Rehman, M.; Wang, W.; Zheng, H.; Faheem, M.; Iqbal, S.; Shen, Z.G.; Tao, J. Role of hydrogen cyanamide (HC) in grape bud dormancy release: Proteomic approach. 3 Biotech 2020, 10, 229. [Google Scholar] [CrossRef] [PubMed]
- Pérez, F.J.; Vergara, R.; Or, E. On the mechanism of dormancy release in grapevine buds: A comparative study between hydrogen cyanamide and sodium azide. Plant Growth Regul. 2009, 59, 145–152. [Google Scholar] [CrossRef]

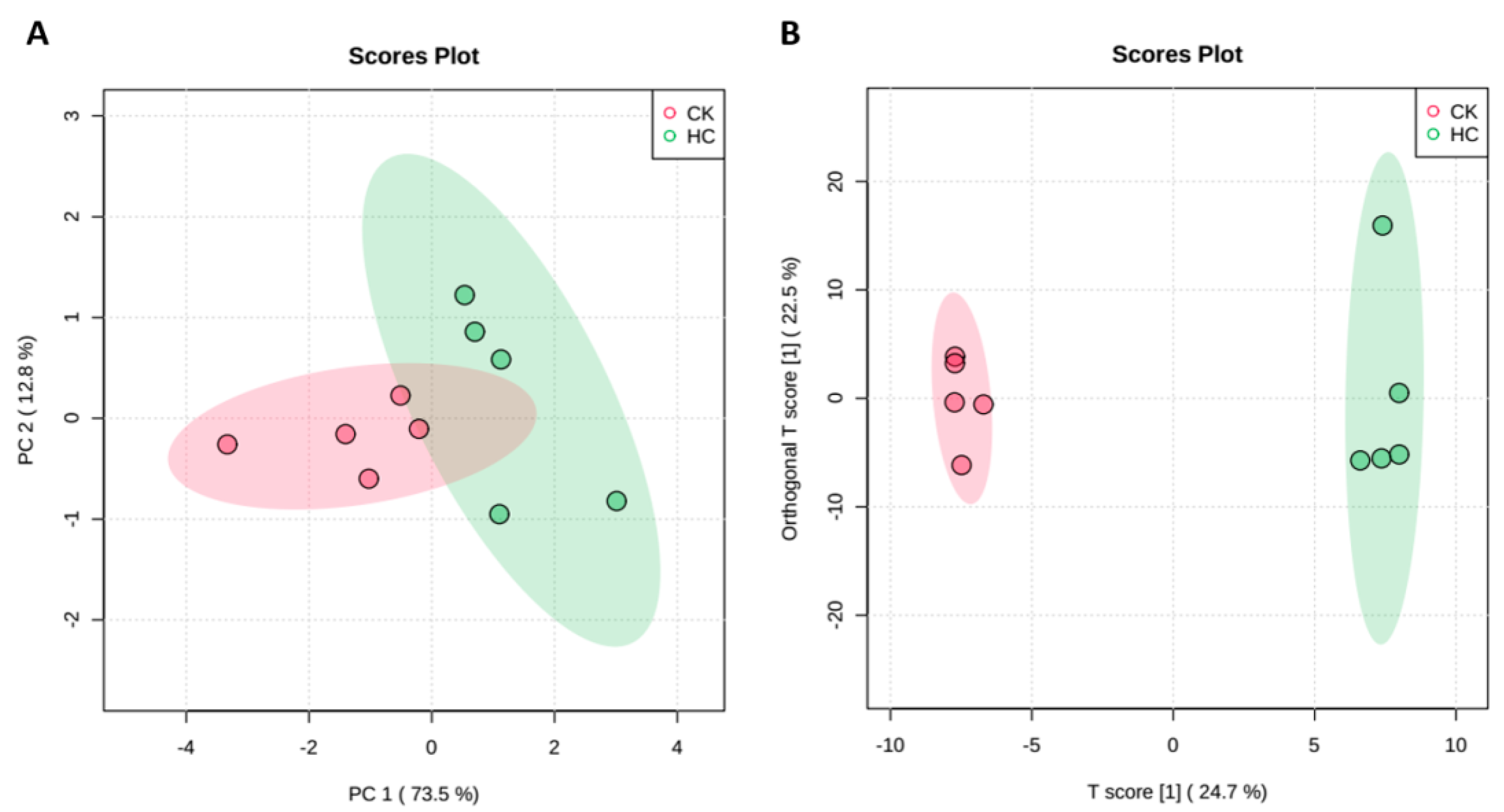

| Metabolites | HC vs. CK | ||
|---|---|---|---|
| VIP | P | FC | |
| Oxoproline | 7.93 | 0.004 | 1.15 |
| Glutamine | 5.14 | <0.001 | 2.11 |
| Serine | 2.47 | <0.001 | 1.49 |
| Alanine | 2.30 | 0.004 | 1.61 |
| Isoleucine | 1.84 | <0.001 | 1.83 |
| Asparagine | 1.77 | <0.001 | −29.39 |
| Glutamic acid | 1.75 | 0.001 | 1.50 |
| Citrulline | 1.52 | 0.001 | 2.43 |
| Allothreonine | 1.35 | <0.001 | 1.52 |
| Galactinol | 2.76 | 0.035 | −1.72 |
| Myo-inositol | 1.33 | 0.010 | 0.69 |
| Succinic acid | 1.79 | 0.013 | 0.72 |
| Citric acid | 1.41 | 0.041 | 28.58 |
| Phosphate | 1.71 | 0.005 | 0.72 |
| Salicin | 1.63 | <0.001 | 2.09 |
| Maltotriose | 1.04 | <0.001 | −2.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, H.; Xia, X.; An, L. Metabolomics Analysis Reveals the Mechanism of Hydrogen Cyanamide in Promoting Flower Bud Break in Blueberry. Agronomy 2021, 11, 102. https://doi.org/10.3390/agronomy11010102
Wang H, Xia X, An L. Metabolomics Analysis Reveals the Mechanism of Hydrogen Cyanamide in Promoting Flower Bud Break in Blueberry. Agronomy. 2021; 11(1):102. https://doi.org/10.3390/agronomy11010102
Chicago/Turabian StyleWang, Hao, Xiuying Xia, and Lijia An. 2021. "Metabolomics Analysis Reveals the Mechanism of Hydrogen Cyanamide in Promoting Flower Bud Break in Blueberry" Agronomy 11, no. 1: 102. https://doi.org/10.3390/agronomy11010102
APA StyleWang, H., Xia, X., & An, L. (2021). Metabolomics Analysis Reveals the Mechanism of Hydrogen Cyanamide in Promoting Flower Bud Break in Blueberry. Agronomy, 11(1), 102. https://doi.org/10.3390/agronomy11010102





