Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Plant Material
2.2. Experimental Design and Management
2.3. Data Collection
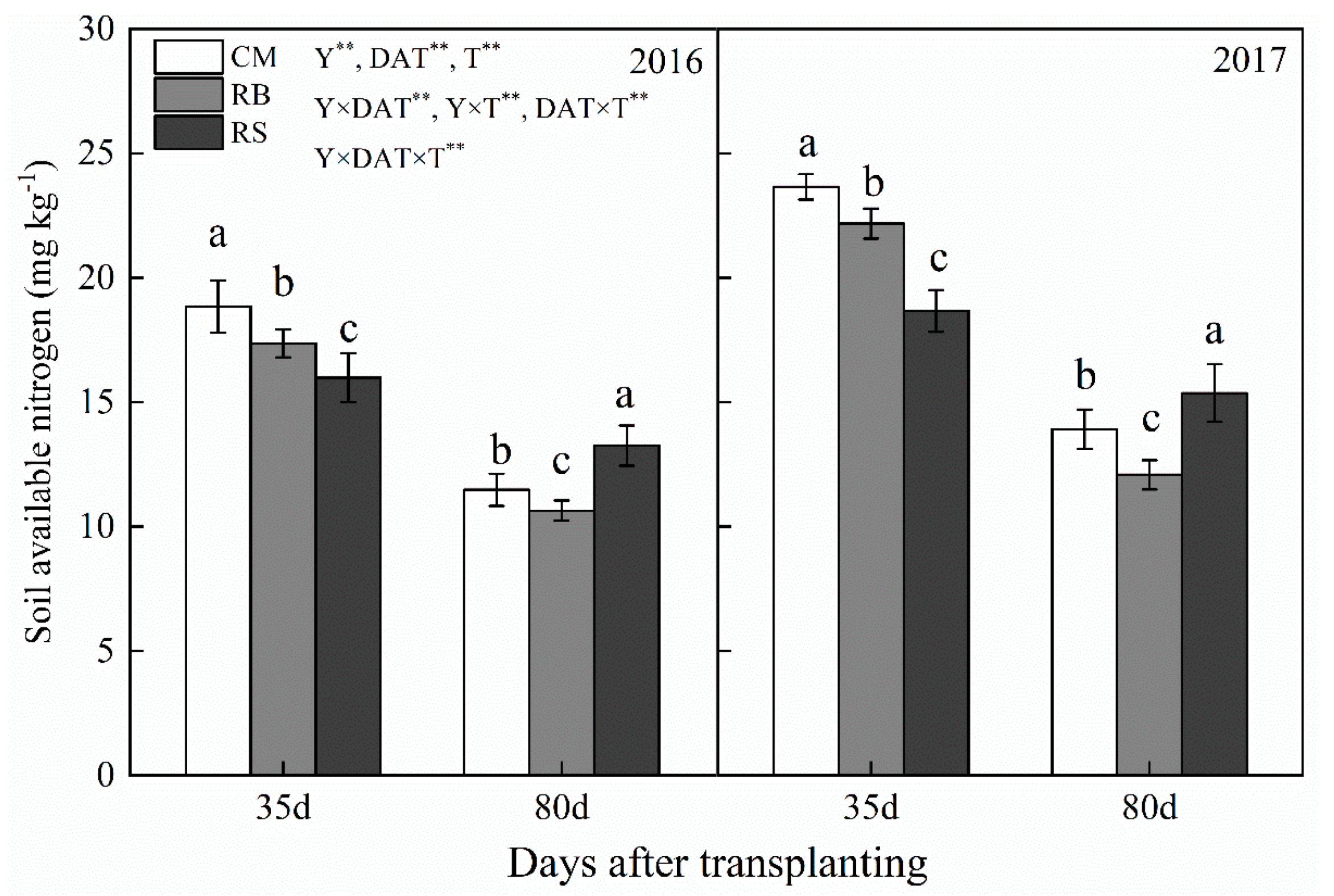
2.3.1. Soil-Available Nitrogen
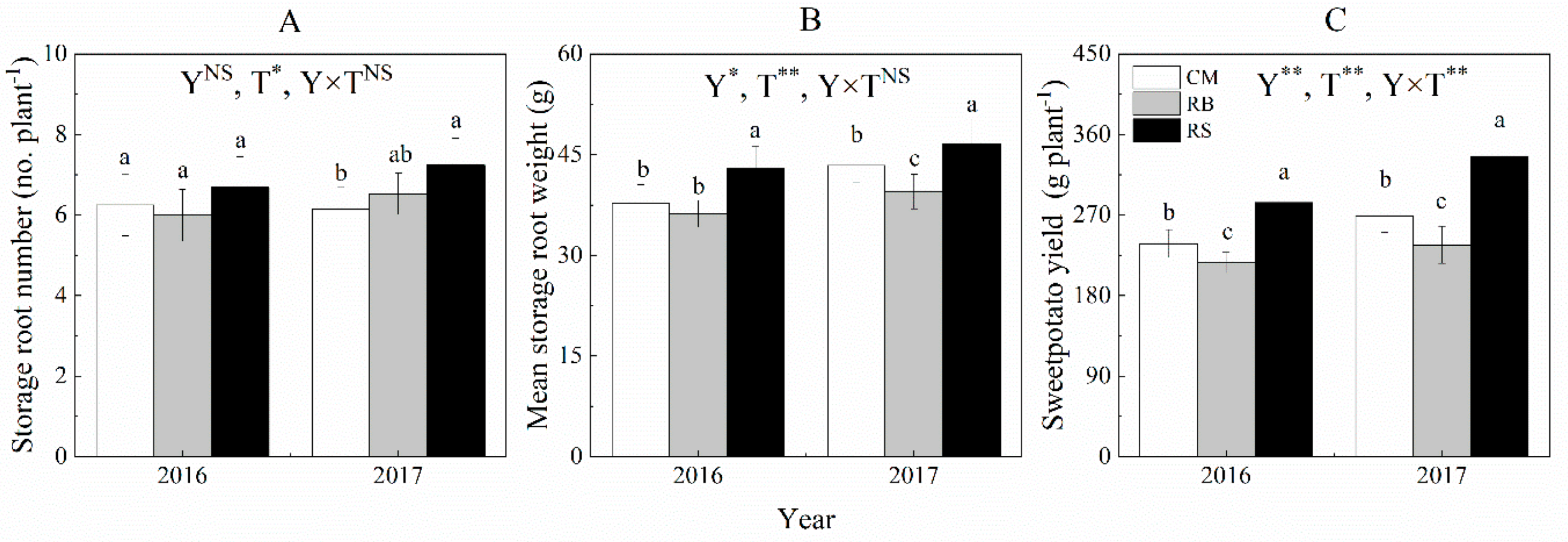
2.3.2. Sweet Potato Yield and Yield Component
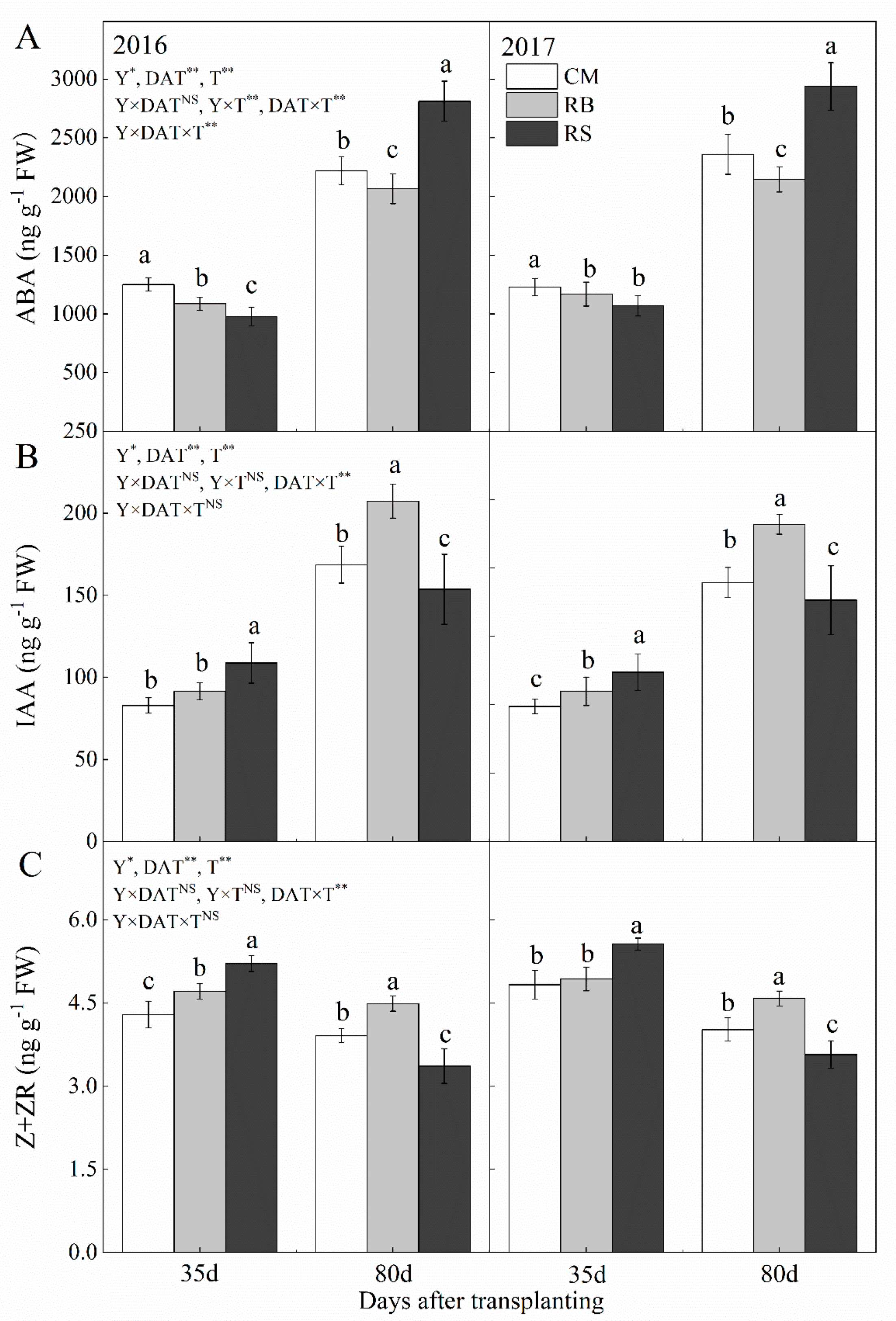
2.3.3. Nitrogen Content, Carbon Content and Endogenous Hormones
2.4. Statistical Analysis
3. Results
3.1. Soil-Available N Content
3.2. Sweet Potato Yield and Yield Components
3.3. N Content, C Content and C/N Ratio in Storage Roots of Sweet Potato
3.4. Endogenous Hormone Levels in Storage Root of Sweet Potato
3.5. Correlation between Endogenous Hormone Levels, N Content, C Content, C/N Ratio, Storage Root Number and Mean Storage Root Weight
4. Discussion
4.1. Enhanced IAA and Z + ZR Contents and Reduced ABA Content, Together with Increased C/N Ratio during the Storage Root Formation Period Promoted Storage Root Formation
4.2. Increased ABA Content Promoted C Allocated in Storage Root during the Storage Root Bulking Period and Increased the Mean Storage Root Weight
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Disclosure Statement
Abbreviations
| ABA | abscisic acid |
| AV-N | Soil-available N |
| CM | conventional N management |
| DAT | days after transplanting |
| IAA | auxin |
| RB | 20.0% reduced N was applied as basal |
| RS | split application of 20.0% reduced N at transplanting and 35 DAT |
| Z + ZR | zeatin and zeatin riboside |
References
- FAO. Production Year Book 1986; Food and Agricultural Organization: Rome, Italy, 2000. [Google Scholar]
- Lowe, S.B.; Wilson, L.A. Yield and yield components of six sweetpotato (Ipomoea batatas) cultivars. II. Variability and possible sources of variation. Exp. Agric. 1975, 11, 49–58. [Google Scholar] [CrossRef]
- Guertal, E.A.; Kemble, J.A. Nitrogen rate and within-row plant spacing effects on sweetpotato yield and grade. J. Plant Nutr. 1997, 20, 355–360. [Google Scholar] [CrossRef]
- Chen, X.; Ding, Y.; Tang, Z.; Wei, M.; Shi, X.; Zhang, A.; Li, H. Suitable nitrogen rate for storage root yield and quality of sweet potato. J. Plant Nutr. Fertil. 2015, 21, 979–986, (In Chinese, with English abstract). [Google Scholar]
- Bourke, R.M. Influence of nitrogen and potassium fertilizer on growth of sweet potato (Ipomoea batatas) in Papua New Guinea. Field Crops Res. 1985, 12, 363–375. [Google Scholar] [CrossRef]
- Hammett, L.; Miller, C.; Swallow, W. Influence of N source, N rate, and K rate on the yield and mineral concentration of sweet potato. J. Am. Soc. Hortic. Sci. 1984, 109, 294–298. [Google Scholar]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.; Drinkwater, L.; Holland, E.; Johnes, P.; Katzenberger, J.; Martinelli, L.; Matson, P. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Mullen, R.W. Nutrient cycling in soils: Nitrogen. In Soil Management: Building a Stable Base for Agriculture; Hatfield, J., Sauer, T., Eds.; American Society of Agronomy and Soil Science Society of America: Madison, WI, USA, 2011; pp. 67–78. [Google Scholar]
- Abdin, M.; Bansal, K.; Abrol, Y. Effect of split nitrogen application on growth and yield of wheat (T. aestivum L.) genotypes with different N-assimilation potential. J. Agron. Crop Sci. 1996, 176, 83–90. [Google Scholar] [CrossRef]
- Forde, B.G. Local and long-range signaling pathways regulating plant responses to nitrate. Annu. Rev. Plant Biol. 2002, 53, 203–224. [Google Scholar] [CrossRef]
- Zhu, C.; Zhu, J.; Zeng, Q.; Liu, G.; Xie, Z.; Tang, H.; Cao, J.; Zhao, X. Elevated CO2 accelerates flag leaf senescence in wheat due to ear photosynthesis which causes greater ear nitrogen sink capacity and ear carbon sink limitation. Funct. Plant Biol. 2009, 36, 291. [Google Scholar] [CrossRef]
- Matsuo, T.; Mitsuzono, H.; Okada, R.; Itoo, S. Variations in the levels of major free cytokinins and free abscisic acid during tuber development of sweet potato. J. Plant Growth Regul. 1988, 7, 249–258. [Google Scholar] [CrossRef]
- Nakatani, M.; Komeichi, M. Changes in the endogenous level of zeatin riboside, abscisic acid and indole acetic acid during formation and thickening of tuberous roots in sweet potato. Jpn. J. Crop Sci. 1991, 60, 91–100. [Google Scholar] [CrossRef]
- Ku, Y.; Wang, Y.; Ma, D.; Yeh, K. IbMADS1 (Ipomoea batatas MADS-box 1 gene) is Involved in Tuberous Root Initiation in Sweet Potato (Ipomoea batatas). Ann. Bot. 2008, 102, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Noh, S.A.; Lee, H.S.; Huh, E.J.; Huh, G.H.; Paek, K.H.; Shin, J.S.; Bae, J.M. SRD1 is involved in the auxin-mediated initial thickening growth of storage root by enhancing proliferation of metaxylem and cambium cells in sweetpotato (Ipomoea batatas). J. Exp. Bot. 2010, 61, 1337–1349. [Google Scholar] [CrossRef] [PubMed]
- Ning, Y.; Ma, H.; Zhang, H.; Xu, J.; Wang, J.; Xu, X.; Zhang, Y. Effects of nitrogen, phosphorus and potassium on root morphology and endogenous hormone contents of sweet potato at early growing stages. Jiangsu J. Agr. Sci. 2013, 29, 1326–1332, (In Chinese, with English abstract). [Google Scholar]
- Duan, W.; Wang, Q.; Zhang, H.; Xie, B.; Li, A.; Hou, F.; Dong, S.; Wang, B.; Qin, Z.; Zhang, L. Comparative study on carbon–nitrogen metabolism and endogenous hormone contents in normal and overgrown sweetpotato. S. Afr. J. Bot. 2018, 115, 199–207. [Google Scholar] [CrossRef]
- Caba, J.M.; Centeno, M.L.; Fernández, B.; Gresshoff, P.M.; Ligero, F. Inoculation and nitrate alter phytohormone levels in soybean roots: Differences between a supernodulating mutant and the wild type. Planta 2000, 211, 98–104. [Google Scholar] [CrossRef]
- Liu, J.; An, X.; Cheng, L.; Chen, F.; Bao, J.; Yuan, L.; Zhang, F.; Mi, G. Auxin transport in maize roots in response to localized nitrate supply. Ann. Bot. 2010, 106, 1019–1026. [Google Scholar] [CrossRef]
- Chen, J.; Cheng, S.; Cao, W.; Zhou, X. Involvement of endogenous plant hormones in the effect of mixed nitrogen source on growth and tillering of wheat. J. Plant Nutr. 1998, 21, 87–97. [Google Scholar] [CrossRef]
- Dahnke, W.; Johnson, G.V. Testing soils for available nitrogen. In Testing Soils for Available Nitrogen; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990; pp. 128–139. [Google Scholar]
- Kim, S.H.; Mizuno, K.; Sawada, S.; Fujimura, T. Regulation of tuber formation and ADP-glucose pyrophosphorylase (AGPase) in sweet potato (Ipomoea batatas (L.) Lam.) by nitrate. Plant Growth Regul. 2002, 37, 207–213. [Google Scholar] [CrossRef]
- Chen, X.; Kou, M.; Tang, Z.; Zhang, A.; Wei, M. Responses of root physiological characteristics and yield of sweet potato to humic acid urea fertilizer. PLoS ONE 2017, 12, e0189715. [Google Scholar] [CrossRef]
- Wang, C.J.; Wang, Y.Z.; Chu, Z.H.; Wang, P.S.; Luan, B.H. Root promoting effect and its regulation mechanisms of endophytic Bacillus amyloliquefaciens YTB1407 in sweet potato. J. Appl. Ecol. 2018, 29, 3819–3828, (In Chinese, with English abstract). [Google Scholar] [CrossRef]
- Ogg, C.L. Determination of nitrogen by the micro-Kjeldahl method. J. Assoc. Off. Agric. Chem. 1960, 689–693. [Google Scholar] [CrossRef]
- Yang, T.; Wei, A.; Zheng, Y.; Yang, H.; Yang, X.; Zhang, R. Simultaneous determination of 8 endogenous hormones in apricot floral bud by high performance liquid chromatography. Chin. J. Anal. Chem. 2007, 35, 1359, (In Chinese, with English abstract). [Google Scholar]
- Yang, W.; Wang, Z.; Yin, Y.; Li, W.; Li, Y.; Chen, X.; Wang, P.; Chen, E.; Guo, J.; Cai, T. Effects of spraying exogenous ABA or GA on the endogenous hormones concentration and filling of wheat grains. Sci. Agric. Sin. 2011, 44, 2673–2682, (In Chinese, with English abstract). [Google Scholar]
- Villordon, A.Q.; Bonte, D.R.L.; Firon, N.; Kfir, Y.; Pressman, E.; Schwartz, A. Characterization of adventitious root development in sweetpotato. Hortscience 2009, 44, 651–655. [Google Scholar] [CrossRef]
- Villordon, A.; LaBonte, D.; Firon, N. Development of a simple thermal time method for describing the onset of morpho-anatomical features related to sweetpotato storage root formation. Sci. Hortic Amst. 2009, 121, 374–377. [Google Scholar] [CrossRef]
- Wilson, L. Stimulation of adventitious bud production in detached sweet potato leaves by high levels of nitrogen supply. Euphytica 1973, 22, 324–326. [Google Scholar] [CrossRef]
- Gifford, M.L.; Alexis, D.; Gutierrez, R.A.; Coruzzi, G.M.; Birnbaum, K.D. Cell-specific nitrogen responses mediate developmental plasticity. Proc. Nat. Acad. Sci. USA 2008, 105, 803–808. [Google Scholar] [CrossRef]
- Hahn, S. Sweet potato. In Ecophysiology of Tropical Crops; Goldsworthy, P., Fisher, N., Eds.; Wiley: New York, NY, USA, 1977; pp. 551–567. [Google Scholar]
- Nieminen, K.; Immanen, J.; Laxell, M.; Kauppinen, L.; Tarkowski, P.; Dolezal, K.; Tähtiharju, S.; Elo, A.; Decourteix, M.; Ljung, K. Cytokinin signaling regulates cambial development in poplar. Proc. Nat. Acad. Sci. USA 2008, 105, 20032–20037. [Google Scholar] [CrossRef]
- McDavid, C.; Alamu, S. The effect of growth regulators on tuber initiation and growth in rooted leaves of two sweet potato cultivars. Ann. Bot. 1980, 45, 363–364. [Google Scholar] [CrossRef]
- Kiba, T.; Kudo, T.; Kojima, M.; Sakakibara, H. Hormonal control of nitrogen acquisition: Roles of auxin, abscisic acid, and cytokinin. J. Exp. Bot. 2011, 62, 1399–1409. [Google Scholar] [CrossRef] [PubMed]
- Gawronska, H.; Deji, A.; Sakakibara, H.; Sugiyama, T. Hormone-mediated nitrogen signaling in plants: Implication of participation of abscisic acid in negative regulation of cytokinin-inducible expression of maize response regulator. Plant Physiol. Biochem 2003, 41, 605–610. [Google Scholar] [CrossRef]
- Xiong, E. Effects of nitrogen fertilizer on grain yield and root morphology physiology in different rice varieties. J. Yangzhou Univ. 2015. (In Chinese, with English abstract). [Google Scholar] [CrossRef]
- Akita, S. Studies on the small-tuber-setting method in sweet potato cultivation. Bull. Chugoku Agric. Exp. Stn. 1962, 8, 75–128. [Google Scholar]
- Nakatani, M. In vitro formation of tuberous roots in sweet potato. Jpn. J. Crop Sci. 1994, 63, 158–159. [Google Scholar] [CrossRef][Green Version]
- Wang, C.; Shi, C.; Wang, Z.; Chai, S.; Liu, H.; Shi, Y. Effects of plastic film mulching cultivation on young roots growth development, tuber formation and tuber yield of sweet potato. Acta Agron. Sin. 2014, 40, 1677–1685, (In Chinese, with English abstract). [Google Scholar] [CrossRef]
- Baker, D.A. Long-distance vascular transport of endogenous hormones in plants and their role in source: Sink regulation. Isr. J. Plant Sci. 2000, 48, 199–203. [Google Scholar] [CrossRef]
- Roitsch, T.; Ehneß, R.; Goetz, M.; Hause, B.; Hofmann, M.; Sinha, A.K. Regulation and function of extracellular invertase from higher plants in relation to assimilate partitioning, stress responses and sugar signalling. Funct. Plant Biol. 2000, 27, 815–825. [Google Scholar] [CrossRef]
- Du, X.; Kong, L.; Xi, M.; Zhang, X. Split application improving sweetpotato yield by enhancing photosynthetic and sink capacity under reduced nitrogen condition. Field Crops Res. 2019, 238, 56–63. [Google Scholar] [CrossRef]
- Nakatani, M.; Tanaka, M.; Yoshinaga, M. Physiological and anatomical characterization of a late-storage root-forming mutant of sweetpotato. J. Am. Soc. Hortic. Sci. 2002, 127, 178–183. [Google Scholar] [CrossRef][Green Version]
- Wang, Z.; Fang, B.; Chen, J.; Zhang, X.; Luo, Z.; Huang, L.; Chen, X.; Li, Y. De novo assembly and characterization of root transcriptome using Illumina paired-end sequencing and development of cSSR markers in sweetpotato (Ipomoea batatas). BMC Genom. 2010, 11, 726. [Google Scholar] [CrossRef] [PubMed]
- Ackerson, R.C. Abscisic Acid and Precocious Germination in Soybeans. J. Exp. Bot. 1984, 35, 414–421. [Google Scholar] [CrossRef]
- Frascaroli, E.; Tuberosa, R. Effect of Abscisic Acid on Pollen Germination and Tube Growth of Maize Genotypes. Plant Breed. 1993, 110, 250–254. [Google Scholar] [CrossRef]

| Correlation with | N Content | C Content | C/N Ratio | IAA | ABA | Z + ZR | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 35 DAT | 80 DAT | 35 DAT | 80 DAT | 35 DAT | 80 DAT | 35 DAT | 80 DAT | 35 DAT | 80 DAT | 35 DAT | 80 DAT | |
| Storage root number | −0.495 * | −0.006 | −0.526 * | 0.445 | 0.559 * | 0.516 * | 0.497 * | −0.175 | −0.537 * | 0.369 | 0.865 ** | 0.448 |
| Storage root weight | −0.554 * | 0.593 ** | −0.711 ** | 0.816 ** | 0.688 ** | 0.810 ** | 0.465 | −0.581 * | −0.668 ** | 0.866 ** | 0.112 | −0.432 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Du, X.; Zhang, X.; Kong, L.; Xi, M. Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato. Agronomy 2020, 10, 1243. https://doi.org/10.3390/agronomy10091243
Du X, Zhang X, Kong L, Xi M. Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato. Agronomy. 2020; 10(9):1243. https://doi.org/10.3390/agronomy10091243
Chicago/Turabian StyleDu, Xiangbei, Xinyue Zhang, Lingcong Kong, and Min Xi. 2020. "Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato" Agronomy 10, no. 9: 1243. https://doi.org/10.3390/agronomy10091243
APA StyleDu, X., Zhang, X., Kong, L., & Xi, M. (2020). Split Application under Reduced Nitrogen Rate Favors High Yield by Altering Endogenous Hormones and C/N Ratio in Sweet Potato. Agronomy, 10(9), 1243. https://doi.org/10.3390/agronomy10091243




