Effect of Salt-Tolerant Bacterial Inoculations on Rice Seedlings Differing in Salt-Tolerance under Saline Soil Conditions
Abstract
:1. Introduction
2. Materials and Methods
2.1. Determination of Plant Growth-Promoting Properties of Selected Bacterial Isolates
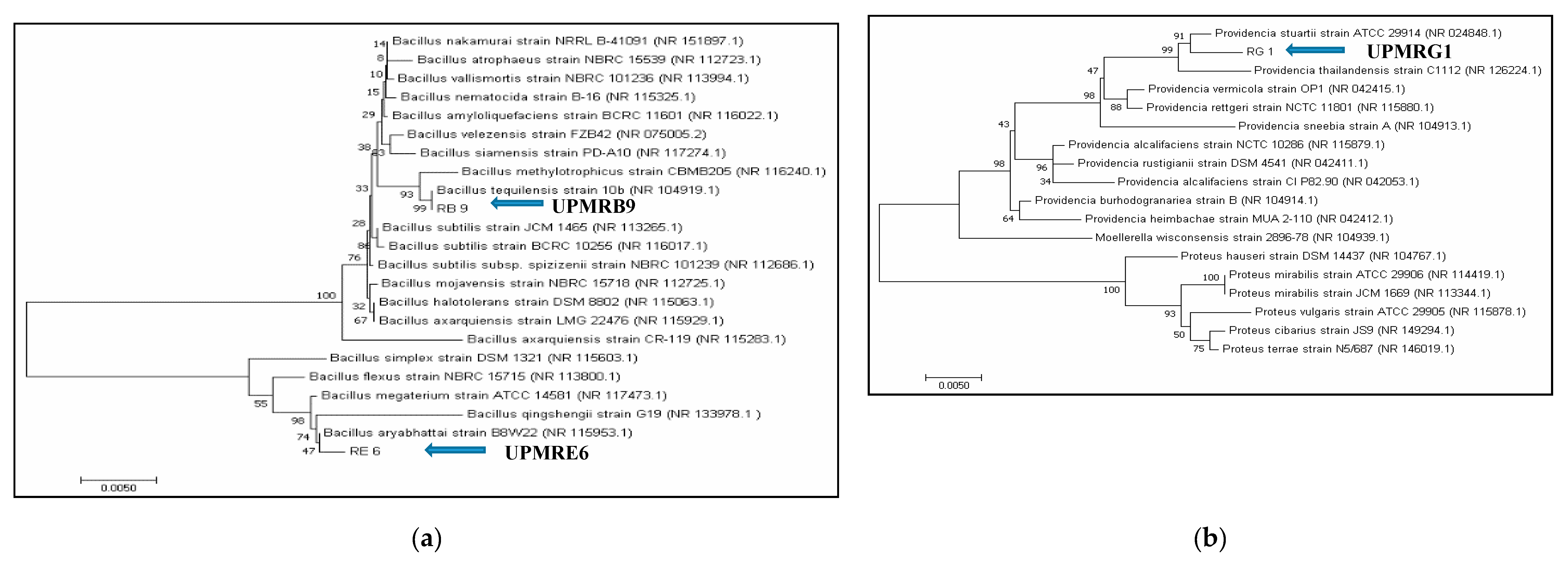
2.2. Identification of Selected Isolates Using a 16S rRNA Gene Sequence
2.3. Glasshouse Trial
2.3.1. Soil Preparation and Fertilizer Application
2.3.2. Inoculum Preparation and Application to Rice Seedlings
2.3.3. Soil Salinity Adjustment
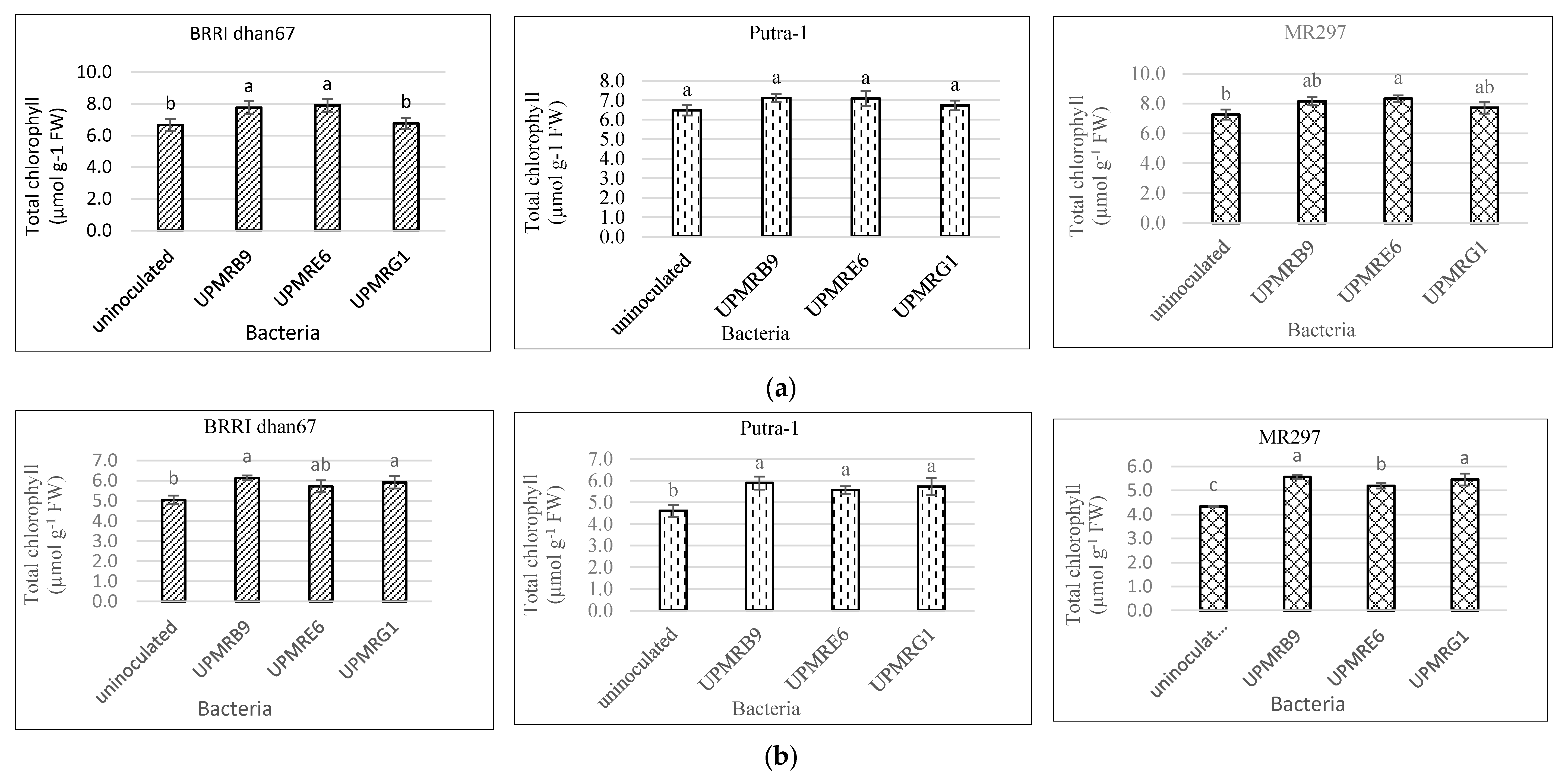
2.3.4. Determination of Total Chlorophyll Content
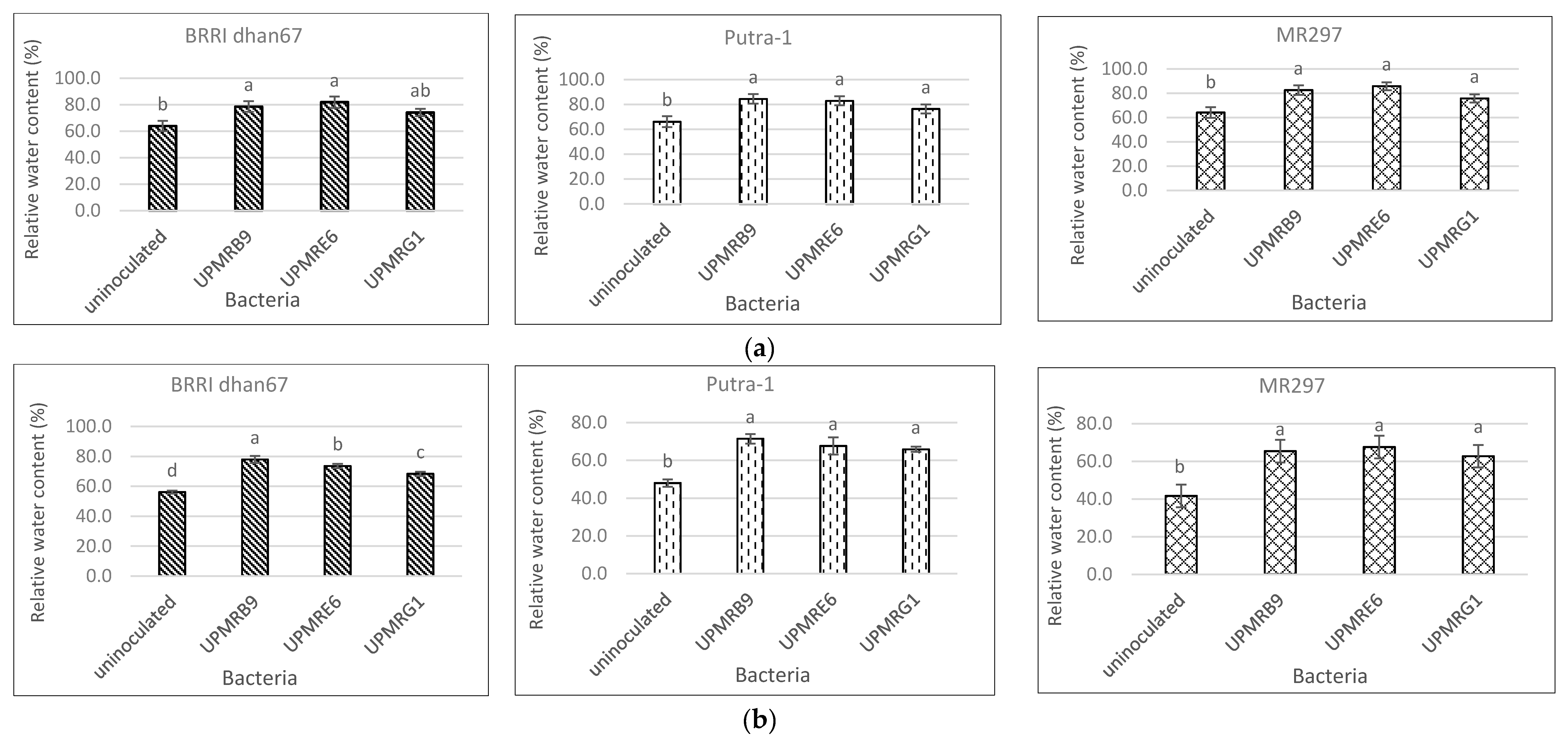
2.3.5. Relative Water Content
2.3.6. Electrolyte Leakage
2.3.7. Measurement of Seedling Dry Matter
2.3.8. Plant and Soil Analyses
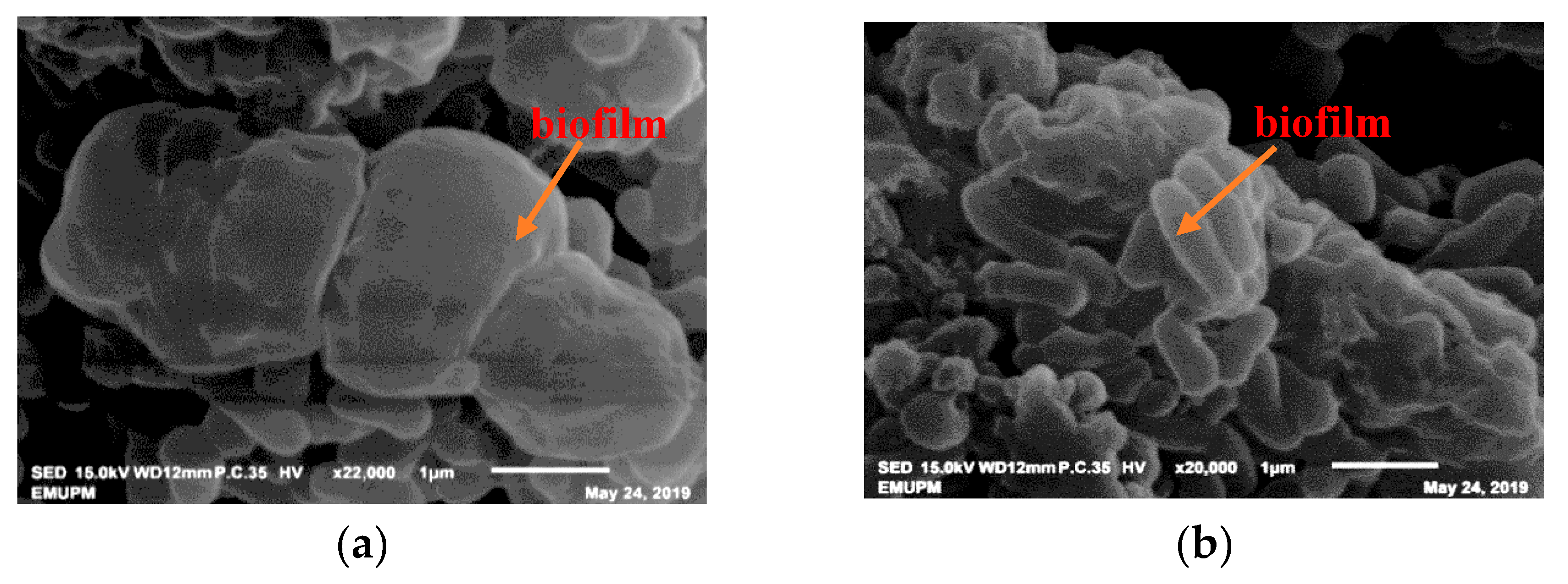
2.4. Observation of Root Colonization by Salt-Tolerant PGPR Using SEM and TEM
2.5. Statistical Analysis
3. Results
3.1. Salt-Tolerance and Plant Growth-Promoting Properties of Selected Bacterial Isolates
3.2. Identification of PGPR Isolates Based on Partial 16S rRNA Gene Sequences
3.3. Effect of Bacterial Inoculation on Total Chlorophyll Content of Three Rice Varieties
3.4. Effect of Bacterial Inoculation on the Relative Water Content of Three Rice Varieties
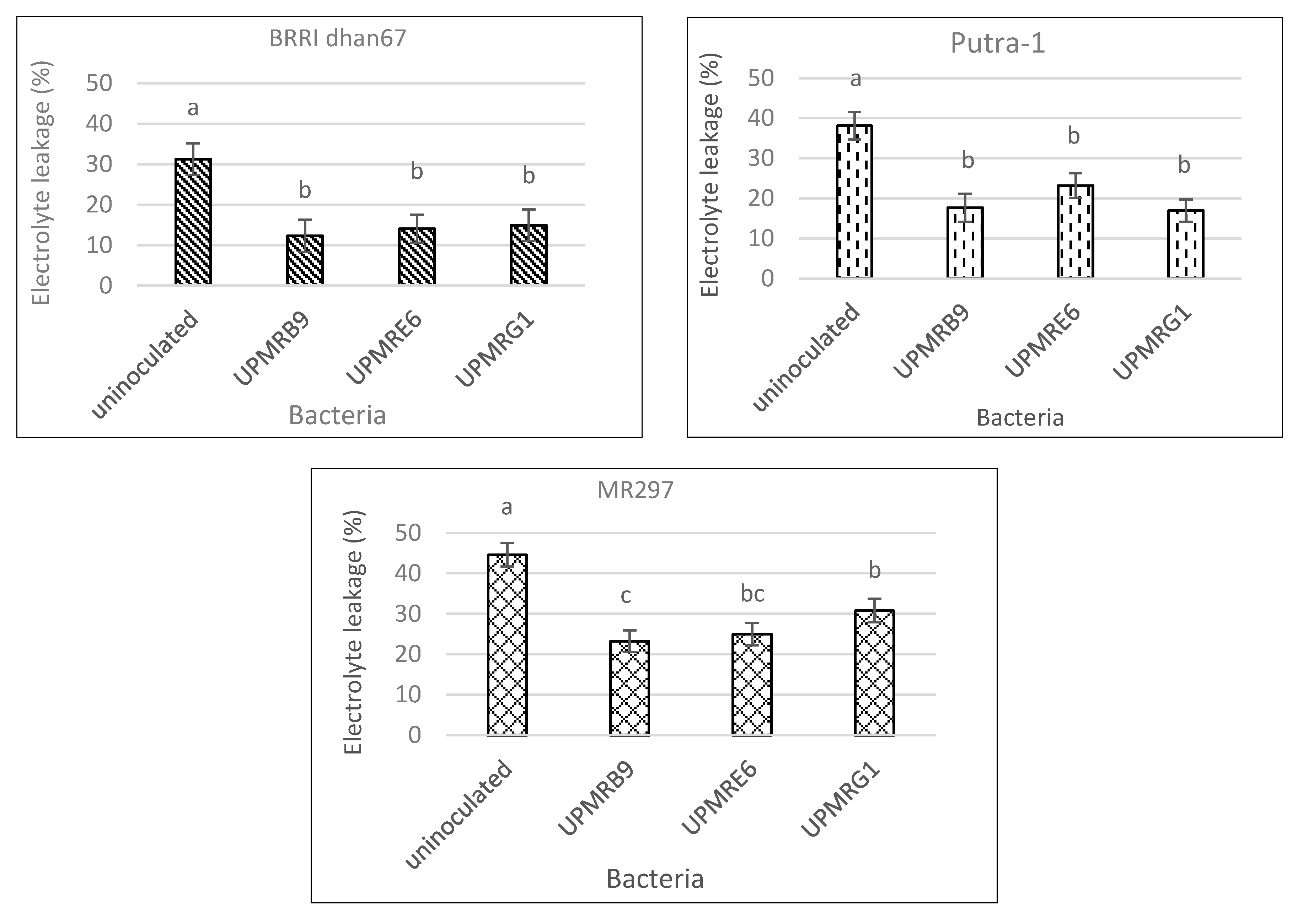
3.5. Effect of Bacterial Inoculation on Electrolyte Leakage of Three Rice Varieties
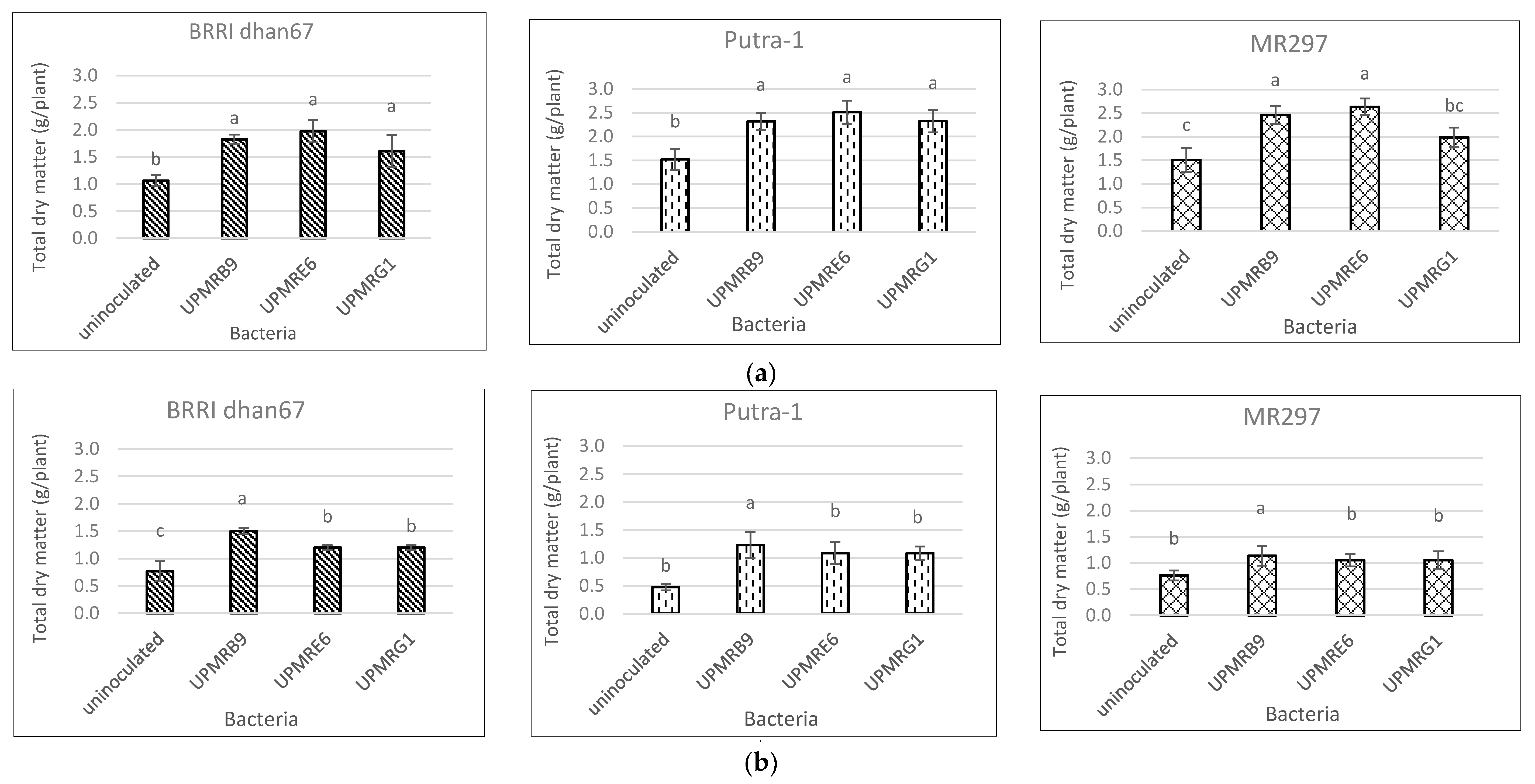
3.6. Effect of Bacterial Inoculations on Total Dry Matter Production of Three Rice Varieties
3.7. Effect of Salinity, PGPR and Rice Varieties on Na/K Ratio in the Above-Ground (Shoot) and Below Ground (Root and Soil) Parts of Three Rice Varieties
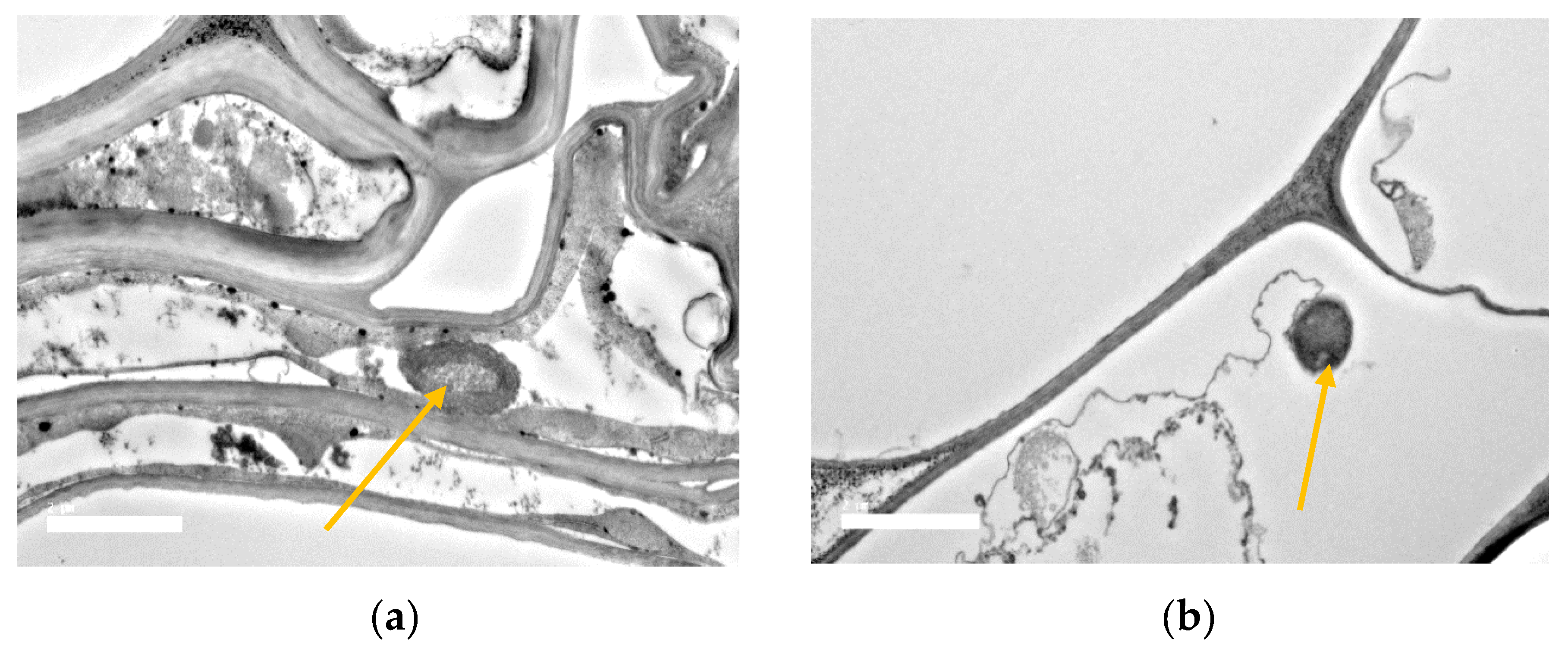
3.8. SEM and TEM Observations of Bacterial Root Colonization
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nemati, I.; Moradi, F.; Gholizadeh, S.; Esmaeili, M.A.; Bihamta, M.R. The effect of salinity stress on ions and soluble sugars distribution in leaves, leaf sheaths and roots of rice (Oryza sativa L.) seedlings. Plant Soil Environ. 2011, 57, 26–33. [Google Scholar] [CrossRef] [Green Version]
- Samant, A.; Jawali, N. Early seedling stage salt tolerance evaluation of genetically diverse rice genotypes. Ann. Biol. Res. 2016, 7, 46–54. [Google Scholar]
- Ghosh, B.; Ali, M.N.; Gantait, S. Response of rice under salinity stress: A review update. Rice Res. Open Access 2016, 4, 1–8. [Google Scholar] [CrossRef]
- Viscardi, S.; Ventorino, V.; Duran, P.; Maggio, A.; De Pascale, S.; Mora, M.L.; Pepe, O. Assessment of plant growth promoting activities and abiotic stress tolerance of Azotobacter chroococcum strains for a potential use in sustainable agriculture. J. Soil Sci. Plant Nutr. 2016, 16, 848–863. [Google Scholar] [CrossRef] [Green Version]
- Jha, B.; Gontia, I.; Hartmann, A. The roots of the halophyte Salicornia brachiata are a source of new halotolerant diazotrophic bacteria with plant growth-promoting potential. Plant Soil 2012, 356, 265–277. [Google Scholar] [CrossRef]
- Timmusk, S.; Kim, S.B.; Nevo, E.; Abd El Daim, I.; Ek, B.; Bergquist, J. Sfp-type PPTase inactivation promotes bacterial biofilm formation and the ability to enhance wheat drought tolerance. Front. Microbiol. 2015, 6, 387. [Google Scholar] [CrossRef]
- Zahid, M. Isolation and identification of indigenous plant growth-promoting rhizobacteria from the Himalayan region of Kashmir and their effect on improving growth and nutrient contents of maize (Zea mays L.). Front. Microbiol. 2015, 6, 207. [Google Scholar] [CrossRef]
- Majeed, A.; Abbasi, M.K.; Hameed, S.; Imran, A.; Rahim, N. Isolation and characterization of plant growth-promoting rhizobacteria from wheat rhizosphere and their effect on plant growth promotion. Front. Microbiol. 2015, 6, 198. [Google Scholar] [CrossRef] [Green Version]
- Whipps, J.M. Microbial interactions and biocontrol in the rhizosphere. J. Exp. Bot. 2001, 52, 487–511. [Google Scholar] [CrossRef]
- Mayak, S.; Tirosh, T.; Glick, B.R. Plant growth-promoting bacteria confer resistance in tomato plants to salt stress. Plant Physiol. Biochem. 2004, 42, 565–572. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Samiyappan, R. ACC-deaminase from Pseudomonas fluorescens mediated saline resistance in groundnut (Arachis hypogaea) plants. J. Appl. Microbiol. 2007, 102, 1283–1292. [Google Scholar] [CrossRef]
- Shukla, P.S.; Agarwal, P.K.; Jha, B. Improved salinity tolerance of Arachis hypogaea (L.) by the interaction of halotolerant plant-growth-promoting rhizobacteria. J. Plant Growth Regul. 2012, 31, 95–206. [Google Scholar] [CrossRef]
- Bal, H.B.; Nayak, L.; Das, S.; Adhya, T.K. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth-promoting activity under salt stress. Plant Soil 2013, 366, 93–105. [Google Scholar] [CrossRef]
- Upadhyay, S.K.; Singh, D.P. Effect of salt-tolerant plant growth-promoting rhizobacteria on wheat plants and soil health in a saline environment. Plant Biol. 2015, 17, 288–293. [Google Scholar] [CrossRef] [PubMed]
- Han, H.S.; Lee, K.D. Plant growth-promoting rhizobacteria effect on antioxidant status, photosynthesis, mineral uptake and growth of lettuce under soil salinity. Res. J. Agric. Biol. Sci. 2005, 1, 210–215. [Google Scholar]
- Rajkumar, M.; Nagendran, R.; Lee, K.J.; Lee, W.H.; Kim, S.Z. Influence of plant growth-promoting bacteria and Cr6+ on the growth of Indian mustard. Chemosphere 2006, 62, 741–748. [Google Scholar] [CrossRef]
- Khan, M.S.; Zaidi, A.; Wani, P.A. Role of phosphate-solubilizing microorganisms in sustainable agriculture—A review. Agron. Sustain. Dev. 2007, 27, 29–43. [Google Scholar] [CrossRef]
- Liu, H.; He, Y.; Jiang, H.; Peng, H.; Huang, X.; Zhang, X.; Thomashow, L.S.; Xu, Y. Characterization of a phenazine-producing strain Pseudomonas chlororaphis GP72 with broad-spectrum antifungal activity from green pepper rhizosphere. Curr. Microbiol. 2007, 54, 302–306. [Google Scholar] [CrossRef]
- Saravanakumar, D.; Vijayakumar, C.; Kumar, N.; Samiyappan, R. PGPR-induced defense responses in the tea plant against blister blight disease. J. Crop Prot. 2007, 26, 556–565. [Google Scholar] [CrossRef]
- Wani, P.A.; Khan, M.S.; Zaidi, A. Effect of metal tolerant plant growth promoting Bradyrhizobium sp. (vigna) on growth, symbiosis, seed yield and metal uptake by green gram plants. Chemosphere 2007, 70, 36–45. [Google Scholar] [CrossRef]
- Ashraf, M.P.J.C.; Harris, P.J.C. Potential biochemical indicators of salinity tolerance in plants. Plant Sci. 2004, 166, 3–16. [Google Scholar] [CrossRef]
- Qureshi, A.W.; Sabri, A.N. Biofilm formation in moderately halophilic bacteria is influenced by varying salinity levels. J. Basic Microbiol. 2012, 52, 566–572. [Google Scholar] [CrossRef]
- Chen, Y.; Yan, F.; Chai, Y.; Liu, H.; Kolter, R.; Losick, R.; Guo, J.H. Biocontrol of tomato wilt disease by Bacillus subtilis isolates from natural environments depends on conserved genes mediating biofilm formation. Environ. Microbiol. 2013, 15, 848–864. [Google Scholar] [CrossRef]
- Gholami, A.; Shahsavani, S.; Nezarat, S. The effect of plant growth-promoting rhizobacteria (PGPR) on germination, seedling growth and yield of maize. Int. J. Biol. Sci. 2009, 1, 35–40. [Google Scholar]
- Yoshida, S. Fundamentals of Rice Crop Production; IRRI: Los Banos, Philippines, 1981. [Google Scholar]
- Hong, B.H.; Joe, M.M.; Selvakumar, G.; Kim, K.Y.; Choi, J.H.; Sa, T.M. Influence of salinity variations on exocellular polysaccharide production, biofilm formation and flocculation in halotolerant bacteria. J. Environ. Biol. 2017, 38, 657. [Google Scholar] [CrossRef]
- Gordon, S.A.; Weber, R.P. Colorimetric estimation of indole acetic acid. Plant Physiol. 1951, 26, 192. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, K.Z.; Radziah, O.; Halimi, M.S.; Khairuddin, A.R.; Habib, S.H.; Shamsuddin, Z.H. Isolation and characterization of rhizobia and plant growth-promoting rhizobacteria and their effects on the growth of rice seedlings. Am. J. Agric. Biol. Sci. 2014, 9, 342–360. [Google Scholar] [CrossRef] [Green Version]
- Lichtenthaler, H.K. Chlorophylls and carotenoids: Pigments of photosynthetic biomembranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Teulat, B.; Zoumarou-Wallis, N.; Rotter, B.; Salem, M.B.; Bahri, H.; This, D. QTL for relative water content in field-grown barley and their stability across Mediterranean environments. Theor. Appl. Genet. 2003, 108, 181–188. [Google Scholar] [CrossRef]
- Yang, G.; Rhodes, D.; Joly, R.J. Effects of high temperature on membrane stability and chlorophyll fluorescence in glycine betaine-deficient and glycine betaine-containing maize lines. Funct. Plant Biol. 1996, 23, 437–443. [Google Scholar] [CrossRef]
- Awang, Y.; Shaharom, A.S.; Mohamad, R.B.; Selamat, A. Chemical and physical characteristics of cocopeat-based media mixtures and their effects on the growth and development of Celosia cristata. Am. J. Agric. Biol. Sci. 2009, 4, 63–71. [Google Scholar] [CrossRef]
- McGrath, S.P.; Cunliffe, C.H.A. Simplified method for the extraction of the metals Fe, Zn, Cu, Ni, Cd, Pb, Cr, Co and Mn from soils and sewage sludges. J. Sci. Food Agric. 1985, 36, 794–798. [Google Scholar] [CrossRef]
- Ghosh, D.; Gupta, A.; Mohapatra, S. A comparative analysis of exopolysaccharide and phytohormone secretions by four drought-tolerant rhizobacterial strains and their impact on osmotic-stress mitigation in Arabidopsis thaliana. World J. Microbiol. Biotechnol. 2019, 35, 90. [Google Scholar] [CrossRef] [PubMed]
- Kasim, W.A.; Gaafar, R.M.; Abou-Ali, R.M.; Omar, M.N.; Hewait, H.M. Effect of biofilm forming plant growth promoting rhizobacteria on salinity tolerance in barley. Ann. Agric. Sci. 2016, 61, 217–227. [Google Scholar] [CrossRef] [Green Version]
- Arora, M.; Kaushik, A.; Rani, N.; Kaushik, C.P. Effect of cyanobacterial exopolysaccharides on salt stress alleviation and seed germination. J. Environ. Biol. 2010, 31, 701–704. [Google Scholar] [PubMed]
- Kang, S.M.; Khan, A.L.; Waqas, M.; Asaf, S.; Lee, K.E.; Park, Y.G.; Kim, A.Y.; Khan, M.A.; You, Y.H.; Lee, I.J. Integrated phytohormone production by the plant growth-promoting rhizobacterium Bacillus tequilensis SSB07 induced thermotolerance in soybean. J. Plant Interact. 2019, 4, 416–423. [Google Scholar] [CrossRef] [Green Version]
- Bhattacharyya, C.; Bakshi, U.; Mallick, I.; Mukherji, S.; Bera, B.; Ghosh, A. Genome-Guided Insights into the Plant Growth Promotion Capabilities of the Physiologically Versatile Bacillus aryabhattai Strain AB211. Front. Microbiol. 2017, 8, 411. [Google Scholar] [CrossRef]
- Chookietwattana, K.; Maneewan, K. Screening of efficient halotolerant phosphate-solubilizing bacterium and its effect on promoting plant growth under saline conditions. World Appl. Sci. J. 2012, 16, 1110–1117. [Google Scholar]
- Singh, G.; Biswas, D.R.; Marwaha, T.S. Mobilization of potassium from waste mica by plant growth promoting rhizobacteria and its assimilation by maize (Zea mays) and wheat (Triticum aestivum L.): A hydroponics study under phytotron growth chamber. J. Plant Nutr. 2010, 33, 1236–1251. [Google Scholar] [CrossRef]
- Yan, N.; Marschner, P.; Cao, W.; Zuo, C.; Qin, W. Influence of salinity and water content on soil microorganisms. Int. Soil Water Conserv. Res. 2015, 3, 316–323. [Google Scholar] [CrossRef]
- Sapre, S.; Gontia-Mishra, I.; Tiwari, S. Klebsiella sp. confers enhanced tolerance to salinity and plant growth promotion in oat seedlings (Avena sativa). Microbiol. Res. 2018, 206, 25–32. [Google Scholar] [CrossRef] [PubMed]
- Habib, S.H.; Kausar, H.; Saud, H.M. Plant growth-promoting rhizobacteria enhance salinity stress tolerance in okra through ROS-scavenging enzymes. BioMed Res. Int. 2016, 2016, 6284547. [Google Scholar] [CrossRef] [Green Version]
- Kang, S.M.; Khan, A.L.; Waqas, M.; You, Y.H.; Kim, J.H.; Kim, J.G.; Hamayun, M.; Lee, I.J. Plant growth-promoting rhizobacteria reduce adverse effects of salinity and osmotic stress by regulating phytohormones and antioxidants in Cucumis sativus. J. Plant Interact. 2014, 9, 673–682. [Google Scholar] [CrossRef] [Green Version]
- Ansari, F.A.; Ahmad, I. Plant growth promoting attributes and alleviation of salinity stress to wheat by biofilm forming Brevibacterium sp. FAB3 isolated from rhizospheric soil. Saudi J. Biol. Sci. 2018. [Google Scholar] [CrossRef]
- Ahamd, M.; Hussain, A.; Akhtar, M.F.U.Z.; Zafar-Ul-Hye, M.; Iqbal, Z.; Naz, T.; Iqbal, M.M. Effectiveness of multi-strain biofertilizer in combination with organic sources for improving the productivity of Chickpea in Drought Ecology. Asian J. Agric. Biol. 2017, 5, 228–237. [Google Scholar]
- Ahmadi, G.; Jaafarinia, M. Evaluating the Effects of Biofertilizers on Growth and Emergence of Barley (Hordeum vulgare). Asian J. Agric. Biol. 2015, 3, 125–131. [Google Scholar]
- Gou, W.; Tian, L.; Ruan, Z.; Zheng, P.E.; Chen, F.U.; Zhang, L.; Cui, Z.; Zheng, P.; Li, Z.; Gao, M.; et al. Accumulation of choline and glycine betaine and drought stress tolerance induced in maize (Zea mays) by three plant growth promoting rhizobacteria (PGPR) strains. Pak. J. Bot. 2015, 47, 581–586. [Google Scholar]
- Katerji, N.; Van Hoorn, J.W.; Hamdy, A.; Mastrorilli, M.; Karzel, E.M. Osmotic adjustment of sugar beets in response to soil salinity and its influence on stomatal conductance, growth and yield. Agric. Water. Manag. 1997, 34, 57–69. [Google Scholar] [CrossRef]
- Baldan, E.; Nigris, S.; Romualdi, C.; D’Alessandro, S.; Clocchiatti, A.; Zottini, M.; Stevanato, P.; Squartini, A.; Baldan, B. Beneficial bacteria isolated from grapevine inner tissues shape Arabidopsis thaliana roots. PLoS ONE 2015, 10, e0140252. [Google Scholar] [CrossRef]
- Ouhaddach, M.H.; Yacoubi, E.l.; Douaik, A.; Rochdi, A. Morpho-Physiological and Biochemical Responses to Salt Stress in Wheat (Triticum aestivum L.) at the Heading Stage. J. Mater. Environ. Sci. 2018, 9, 1899–1907. [Google Scholar]
- Nunkaew, T.; Kantachote, D.; Nitoda, T.; Kanzaki, H.; Ritchie, R.J. Characterization of exopolymeric substances from selected Rhodopseudomonas palustris strains and their ability to adsorb sodium ions. Carbohydr. Polym. 2015, 115, 334–341. [Google Scholar] [CrossRef] [PubMed]
- Nadeem, S.M.; Zahir, Z.A.; Naveed, M.; Asghar, H.N.; Arshad, M. Rhizobacteria capable of producing ACC-deaminase may mitigate salt stress in wheat. Soil Sci. Soc. Am. J. 2010, 74, 533–542. [Google Scholar] [CrossRef]
- Ahmad, M.; Zahir, Z.A.; Nazli, F.; Akram, F.; Arshad, M.; Khalid, M. Effectiveness of halo-tolerant, auxin producing Pseudomonas and Rhizobium strains to improve osmotic stress tolerance in mung bean (Vigna radiata L.). Braz. J. Microbiol. 2013, 44, 1341–1348. [Google Scholar] [CrossRef] [PubMed]
- Ghorai, S.; Pal, K.K.; Dey, R. Alleviation of salinity stress in groundnut by application of PGPR. Int. Res. J. Eng. Tech. 2015, 2, 742–750. [Google Scholar]
- Banerjee, A.; Bareh, D.A.; Joshi, S.R. Native microorganisms as potent bio inoculants for plant growth promotion in shifting agriculture (Jhum) systems. J. Soil Sci. Plant Nutr. 2017, 17, 127–140. [Google Scholar]
- Mahmood, S.; Daur, I.; Al-Solaimani, S.G.; Ahmad, S.; Madkour, M.H.; Yasir, M.; Hirt, H.; Ali, S.; Ali, Z. Plant growth-promoting rhizobacteria and silicon synergistically enhances salinity tolerance of mung bean. Front. Plant Sci. 2016, 7, 876. [Google Scholar] [CrossRef] [Green Version]
- Karlidag, H.; Yildirim, E.; Turan, M.; Pehluvan, M.; Donmez, F. Plant growth-promoting rhizobacteria mitigate deleterious effects of salt stress on strawberry plants (Fragaria× ananassa). Hortscience 2013, 48, 563–567. [Google Scholar] [CrossRef] [Green Version]
- Chandra, D.; Srivastava, R.; Glick, B.R.; Sharma, A.K. Drought-tolerant Pseudomonas spp. improve the growth performance of finger millet (Eleusine coracana (L.) Gaertn.) under non-stressed and drought-stressed conditions. Pedosphere 2018, 28, 227–240. [Google Scholar] [CrossRef]
- Maathuis, F.J. Sodium in plants: Perception, signalling, and regulation of sodium fluxes. J. Exp. Bot. 2013, 65, 849–858. [Google Scholar] [CrossRef]
- Galvan-Ampudia, C.S.; Testerink, C. Salt stress signals shape the plant root. Curr. Opin. Plant Biol. 2011, 14, 296–302. [Google Scholar] [CrossRef]
- Rojas-Tapias, D.; Moreno-Galván, A.; Pardo-Díaz, S.; Obando, M.; Rivera, D.; Bonilla, R. Effect of inoculation with plant growth-promoting bacteria (PGPB) on amelioration of saline stress in maize (Zea mays). Appl. Soil Ecol. 2012, 61, 264–272. [Google Scholar] [CrossRef]
- Niu, S.Q.; Li, H.R.; Paré, P.W.; Aziz, M.; Wang, S.M.; Shi, H.; Li, J.; Han, Q.; Guo, S.; Li, J.; et al. Induced growth promotion and higher salt tolerance in the halophyte grass Puccinellia tenuiflora by beneficial rhizobacteria. Plant Soil 2016, 407, 217–230. [Google Scholar] [CrossRef]
- Kushwaha, P.; Kashyap, P.L.; Kuppusamy, P.; Srivastava, A.K.; Tiwari, R.K. Functional characterization of endophytic bacilli from pearl millet (Pennisetum glaucum) and their possible role in multiple stress tolerance. Plant Biosyst. 2019, 1–12. [Google Scholar] [CrossRef]
- Li, H.; Guan, Y.; Dong, Y.; Zhao, L.; Rong, S.; Chen, W.; Li, L. Isolation and evaluation of endophytic Bacillus tequilensis GYLH001 with potential application for biological control of Magnaporthe oryzae. PLoS ONE 2018, 13, e0203505. [Google Scholar] [CrossRef]

| Bacterial Isolates | Salt-Tolerance Characteristics | Plant Growth-Promoting Characteristics | ||||||
|---|---|---|---|---|---|---|---|---|
| EPS (g L−1) | Floc Yield (g L−1) | Biofilm (590nm) | Sodium Uptake (mg L−1) | IAA (µg mL−1) | P Solubilization (µg mL−1) | K Solubilization (mg L−1) | Nitrogen Fixation | |
| UPMRA4 | 7.36c | 17.83a | 0.44d | 7.55cd | 6.71b | 9.21b | 2.08ab | − |
| UPMRB9 | 30.50a | 19.28a | 1.14a | 23.8a | 8.25a | 15.18a | 2.17ab | + |
| UPMRE3 | 12.24c | 9.73c | 0.31e | 6.40d | 3.50c | 9.34b | 0.70c | − |
| UPMRE6 | 26.18ab | 19.67a | 0.95b | 12.49bc | 6.70b | 15.30a | 2.97a | + |
| UPMRG1 | 21.79b | 12.90b | 0.77c | 13.51b | 6.99ab | 9.24b | 1.72bc | + |
| Variety | Shoot | |||
|---|---|---|---|---|
| Uninoculated | UPMRB9 | UPMRE6 | UPMRG1 | |
| BRRI dhan67 | 0.73a | 0.14c (81%) | 0.22b (70%) | 0.26b (64%) |
| Putra-1 | 1.13a | 0.39c (66%) | 0.57b (50%) | 0.60b (47%) |
| MR297 | 1.37a | 0.81c (41%) | 1.04b (24%) | 1.09b (20%) |
| Variety | Root | Soil | ||||||
|---|---|---|---|---|---|---|---|---|
| CONTROL | UPMRB9 | UPMRE6 | UPMRG1 | CONTROL | UPMRB9 | UPMRE6 | UPMRG1 | |
| BRRI dhan67 | 0.61a | 0.29c (53%) | 0.50b (18%) | 0.53b (13%) | 0.50b | 0.81a (62%) | 0.67ab (34%) | 0.63b (26%) |
| Putra-1 | 0.79a | 0.90a (14%) | 0.42b (47%) | 0.45b (43%) | 0.46b | 0.64a (39%) | 0.48b (4%) | 0.46b (0%) |
| MR297 | 1.11a | 1.04a (6%) | 0.60b (46%) | 0.56b (50%) | 0.27b | 0.59a (119%) | 0.30b (11%) | 0.37b (37%) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shultana, R.; Tan Kee Zuan, A.; Yusop, M.R.; Mohd Saud, H.; Ayanda, A.F. Effect of Salt-Tolerant Bacterial Inoculations on Rice Seedlings Differing in Salt-Tolerance under Saline Soil Conditions. Agronomy 2020, 10, 1030. https://doi.org/10.3390/agronomy10071030
Shultana R, Tan Kee Zuan A, Yusop MR, Mohd Saud H, Ayanda AF. Effect of Salt-Tolerant Bacterial Inoculations on Rice Seedlings Differing in Salt-Tolerance under Saline Soil Conditions. Agronomy. 2020; 10(7):1030. https://doi.org/10.3390/agronomy10071030
Chicago/Turabian StyleShultana, Rakiba, Ali Tan Kee Zuan, Mohd Rafii Yusop, Halimi Mohd Saud, and Arolu Fatai Ayanda. 2020. "Effect of Salt-Tolerant Bacterial Inoculations on Rice Seedlings Differing in Salt-Tolerance under Saline Soil Conditions" Agronomy 10, no. 7: 1030. https://doi.org/10.3390/agronomy10071030
APA StyleShultana, R., Tan Kee Zuan, A., Yusop, M. R., Mohd Saud, H., & Ayanda, A. F. (2020). Effect of Salt-Tolerant Bacterial Inoculations on Rice Seedlings Differing in Salt-Tolerance under Saline Soil Conditions. Agronomy, 10(7), 1030. https://doi.org/10.3390/agronomy10071030





