Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set †
Abstract
1. Introduction
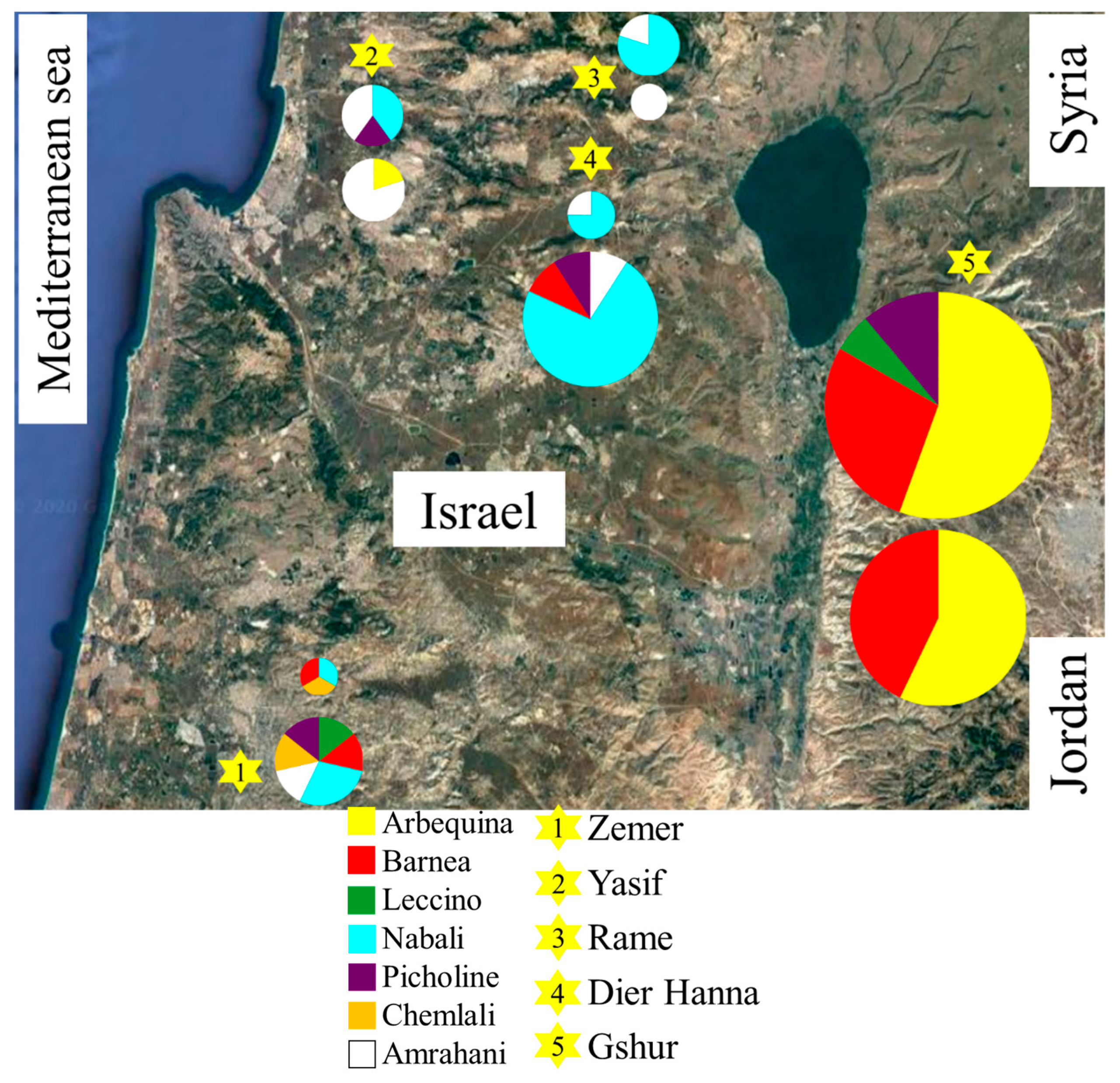
1.1. Souri Cultivar in Israel
1.2. Compatibility between Olive Cultivars
2. Material and Methods
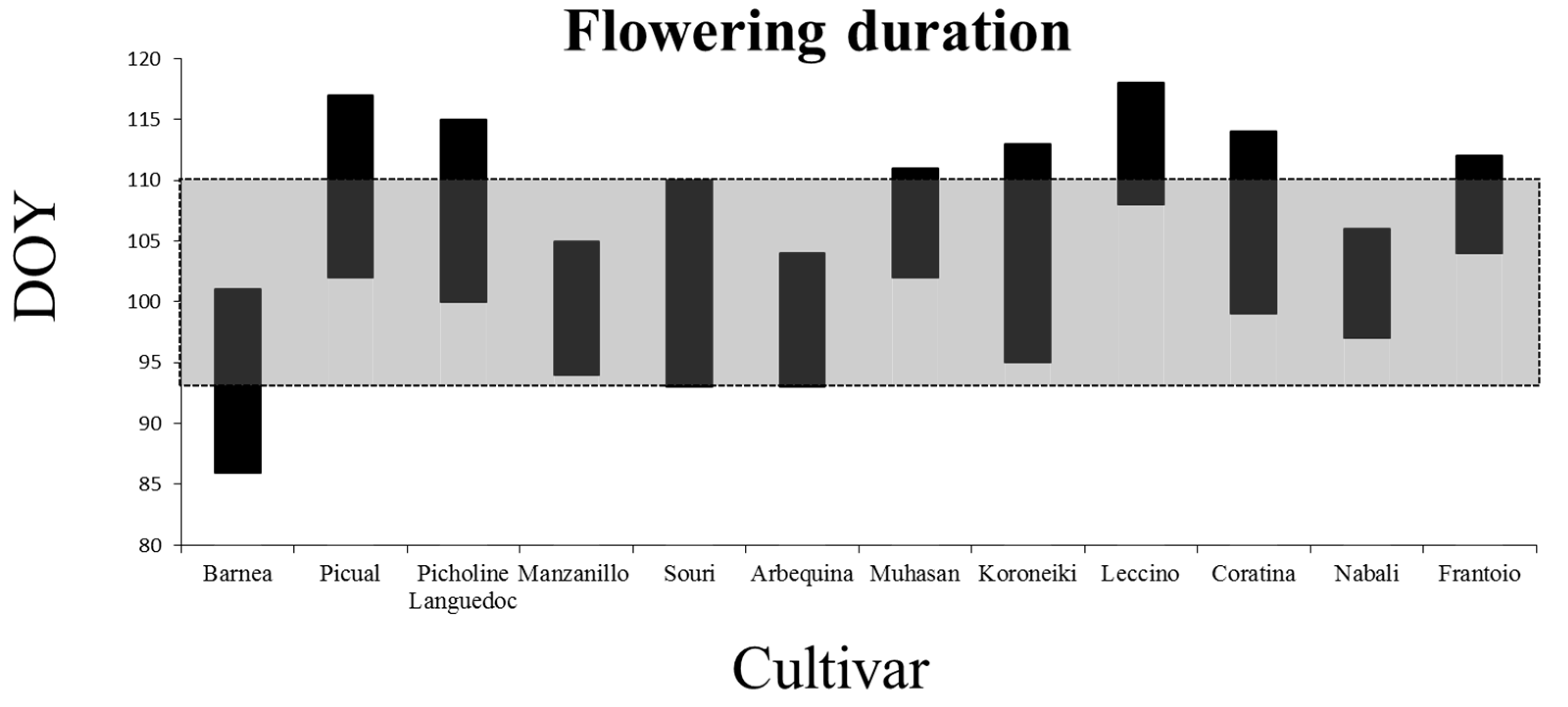
2.1. Characterization of the Flowering Period
2.2. Artificial Pollination
2.2.1. Self-Compatibility Examination
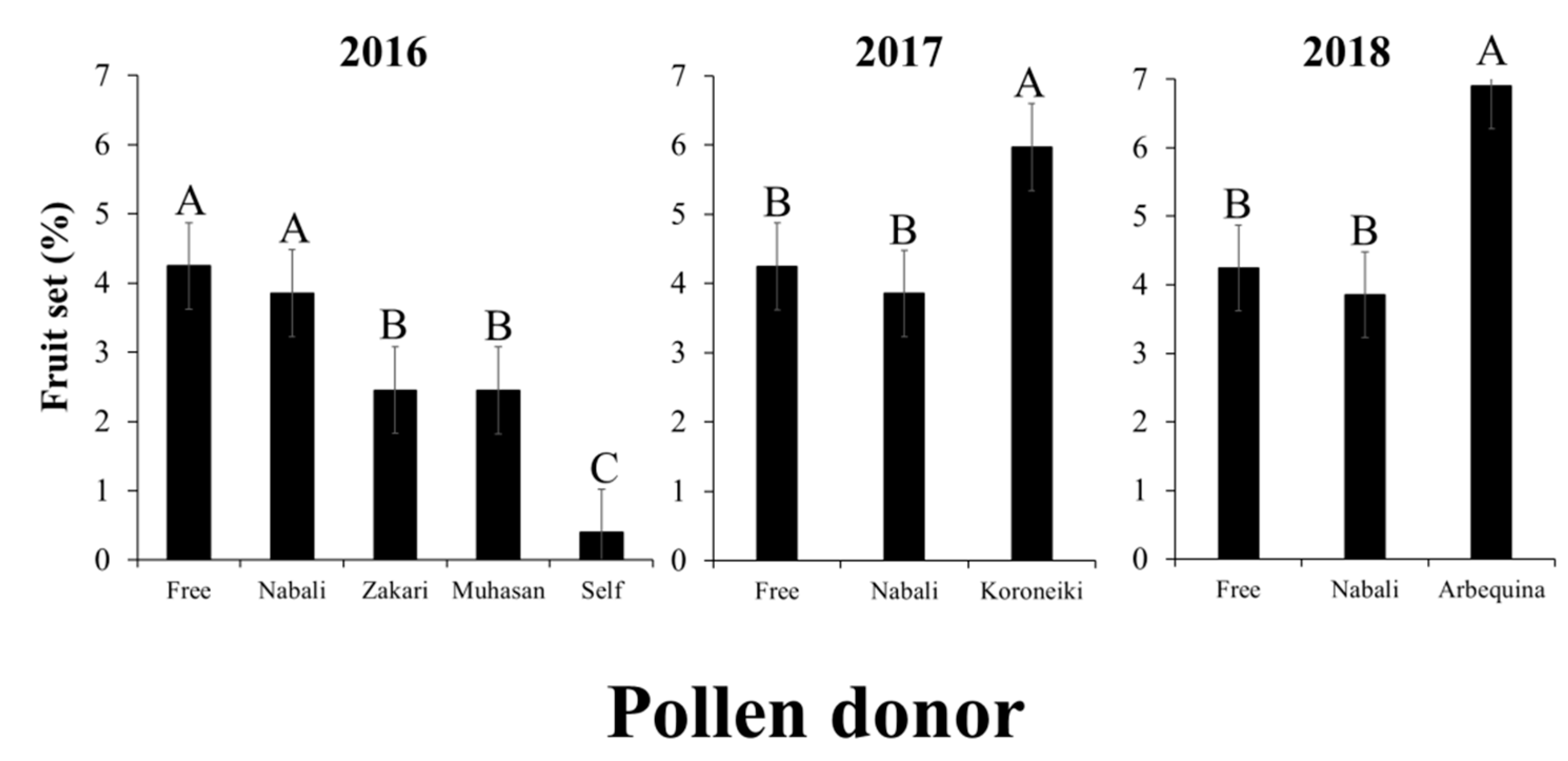
2.2.2. Artificial Cross-Pollination
2.3. Paternity Analysis
DNA Extraction and Genotyping
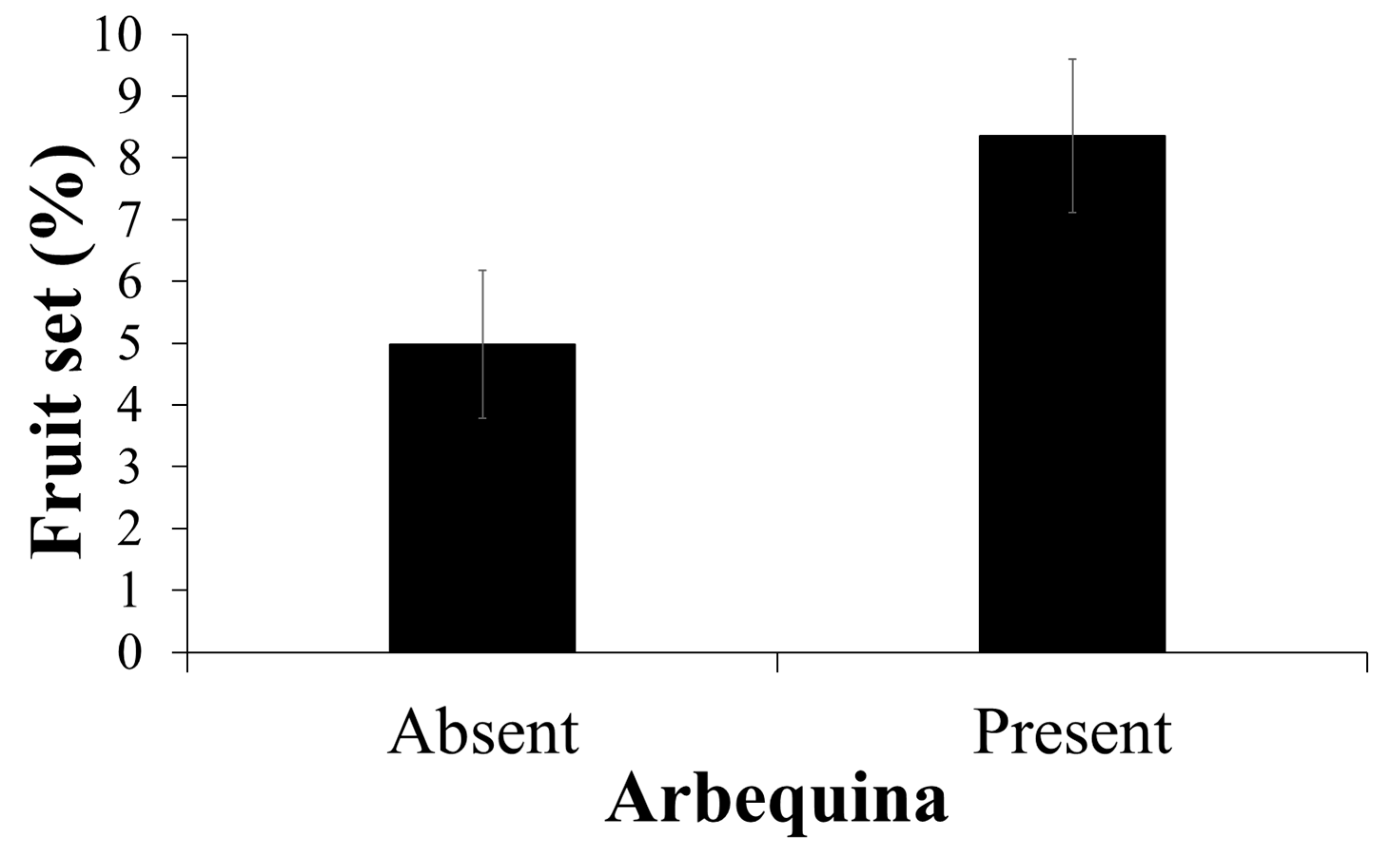
2.4. Increasing Fruit Set by Cross Pollination with Arbequina
2.5. Statistical Analysis
3. Results
3.1. Flowering Period
3.2. Artificial Pollination
3.3. Paternity Analysis
3.4. Increasing Fruit Set by a Compatible Cultivar
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgley, M. Flowering and fruit set in olive: A review. Iran. J. Plant Physiol. 2015, 5, 1263–1272. [Google Scholar]
- Benlloch-González, M.; Sanchez-Lucas, R.; Benlloch, M.; Ricardo, F.-E. An approach to global warming effects on flowering and fruit set of olive trees growing under field conditions. Sci. Hortic. 2018, 240, 405–410. [Google Scholar] [CrossRef]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Billiard, S.; Gallina, S.; Essalouh, L.; Mhaïs, A.; Moukhli, A.; El Bakkali, A.; Barcaccia, G.; et al. Elucidation of the genetic architecture of self-incompatibility in olive: Evolutionary consequences and perspectives for orchard management. Evol. Appl. 2017, 10, 867–880. [Google Scholar] [CrossRef] [PubMed]
- Shemer, A.; Biton, I.; Many, Y.; Vaknin, Y.; Lavee, S.; Avidan, B.; Ben-Ari, G. The olive cultivar ‘Picual’ is an optimal pollen donor for ‘Barnea’. Sci. Hortic. 2014, 172, 278–284. [Google Scholar] [CrossRef]
- Vuletin Selak, G.; Cuevas, J.; Goreta Ban, S.; Perica, S. Determination of compatibility relationships between olive cultivars: An overview of available methods. Acta Hortic. 2018, 1199, 115–120. [Google Scholar] [CrossRef]
- Ben-Ari, G.; Biton, I.; Mani, Y.; Avidan, B.; Lavee, S. The diversity in performance of commercial olive clones selected from the autochthonous cv. Souri population for intensive irrigated cultivation. HortScience 2014, 49, 425–429. [Google Scholar] [CrossRef]
- Jbara, G.; Jawhar, A.; Bido, Z.; Cardone, G.; Dragotta, A.; Famiani, F. Fruit and oil characteristics of the main Syrian olive cultivars. Ital. J. Food Sci. 2010, 22, 395–400. [Google Scholar]
- Omar, R.J. Morphological and Genetical Characterisation of the Main Palestinian Olive (Olea europaea L.) Cultivars. Master’s Thesis, An-Najah National University, Nablus, Pakistan, 2012. [Google Scholar]
- Goor, A. Olive Cultivars in Israel; Hassade Press: Tel-Aviv, Israel, 1948. (In Hebrew) [Google Scholar]
- Grattan, S.R.; Berenquer, M.J.; Connell, J.H.; Polito, V.S.; Vossen, P.M. Olive oil production as influenced by different quantities of applied water. Agric. Water Manag. 2006, 85, 133–140. [Google Scholar] [CrossRef]
- Greven, M.; Neal, S.; Green, S.; Dichio, B.; Clothier, B. The effects of drought on the water use, fruit development and oil yield from young olive trees. Agric. Water Manag. 2009, 96, 1525–1531. [Google Scholar] [CrossRef]
- Cockerell, T.D.A. The Pollination of the Olive. Nature 1908, 78, 31. [Google Scholar] [CrossRef]
- González-Minero, F.J.; Candau, P. Olea europaea airborne pollen in southern Spain. Ann. Allergy Asthma Immunol. 1997, 78, 278–284. [Google Scholar] [CrossRef]
- Pinillos, V.; Cuevas, J. Open-pollination provides sufficient levels of cross-pollen in spanish monovarietal olive orchards. HortScience 2009, 44, 499–502. [Google Scholar] [CrossRef]
- Alagna, F.; Caceres, M.E.; Pandolfi, S.; Collani, S.; Mousavi, S.; Mariotti, R.; Cultrera, N.G.M.; Baldoni, L.; Barcaccia, G. The paradox of self-fertile varieties in the context of self-incompatible genotypes in olive. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [PubMed]
- Cruden, R.W. Pollen grains: Why so many? Plant Syst. Evol. 2000, 222, 143–165. [Google Scholar] [CrossRef]
- García-Mozo, H.; Comtois, P.; Kuehne, E. Aerobiological clines: The role of topography as a barrier for establishing dispersal corridors. Aerobiologia 2004, 20, 161–172. [Google Scholar] [CrossRef]
- Rojo, J.; Orlandi, F.; Pérez-Badia, R.; Aguilera, F.; Ben Dhiab, A.; Bouziane, H.; Díaz de la Guardia, C.; Galán, C.; Gutiérrez-Bustillo, A.M.; Moreno-Grau, S.; et al. Modeling olive pollen intensity in the Mediterranean region through analysis of emission sources. Sci. Total Environ. 2016, 55–1552, 73–82. [Google Scholar] [CrossRef]
- Breton, C.; Bervillé, A. From the olive flower to the drupe: Flower types, pollination, self and inter-compatibility and fruit set. In The Mediterranean Genetic Code: Grapevine and Olive; Sladonja, B., Poljuha, D., Eds.; IntechOpen: Rijeka, Croatia, 2013. [Google Scholar] [CrossRef]
- Mookerjee, S.; Guerin, J.; Collins, G.; Ford, C.; Sedgley, M. Paternity analysis using microsatellite markers to identify pollen donors in an olive grove. Theor. Appl. Genet. 2005, 111, 1174–1182. [Google Scholar] [CrossRef]
- Seifi, E.; Guerin, J.; Kaiser, B.; Sedgeley, M. Sexual compatibility of the olive cultivar ‘Kalamata’ assessed by paternity analysis. Span. J. Agric. Res. 2012, 10, 731–740. [Google Scholar] [CrossRef]
- Diaz, A.; Martín, A.; Rallo, P.; Diego, B.; de la Rosa, R. Self-incompatibility of ‘Arbequina’ and ‘Picual’ Olive Assessed by SSR Markers. J. Am. Soc. Hortic. Sci. 2006, 131, 250–255. [Google Scholar] [CrossRef]
- Belaj, A.; de la Rosa, R.; Lorite, I.J.; Mariotti, R.; Cultrera, N.G.M.; Beuzón, C.R.; González-Plaza, J.J.; Muñoz-Mérida, A.; Trelles, O.; Baldoni, L. Usefulness of a new large set of high throughput EST-SNP markers as a tool for olive germplasm collection management. Front. Plant Sci. 2018, 9. [Google Scholar] [CrossRef]
- Biton, I.; Doron-Faigenboim, A.; Jamwal, M.; Mani, Y.; Eshed, R.; Rosen, A.; Sherman, A.; Ophir, R.; Lavee, S.; Avidan, B.; et al. Development of a large set of SNP markers for assessing phylogenetic relationships between the olive cultivars composing the Israeli olive germplasm collection. Mol. Breed. 2015, 35, 107. [Google Scholar] [CrossRef]
- İpek, A.; Yılmaz, K.; Sıkıcı, P.; Tangu, N.A.; Öz, A.T.; Bayraktar, M.; İpek, M.; Gülen, H. SNP discovery by GBS in olive and the construction of a high-density genetic linkage map. Biochem. Genet. 2016, 54, 313–325. [Google Scholar] [CrossRef] [PubMed]
- Ben-Ari, G.; Lavi, U. Marker-assisted selection in plant breeding. In Plant Biotechnology and Agriculture: Prospects for the 21st Century; Altman, A., Hasegawa, P.M., Eds.; Elsevier: London, UK, 2012; pp. 163–184. [Google Scholar] [CrossRef]
- Biton, I.; Shevtsov, S.; Ostersetzer, O.; Mani, Y.; Lavee, S.; Avidan, B.; Ben-Ari, G. Genetic relationships and hybrid vigour in olive (Olea europaea L.) by microsatellites. Plant Breed. 2012, 131, 767–774. [Google Scholar] [CrossRef]
- Goldental-Cohen, S.; Biton, I.; Many, Y.; Tavrizov, K.; Dourou, A.M.; Zemach, H.; Tonutti, P.; Kerem, Z.; Avidan, B.; Sperling, O.; et al. Removal of flowers or inflorescences affects ‘Barnea’ olive fruitlet post-anthesis abscission. J. Hortic. Sci. Biotechnol. 2019, 94, 488–498. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Rep. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Xin, Z.; Velten, J.P.; Oliver, M.J.; Burke, J.J. High-throughput DNA extraction method suitable for PCR. BioTechniques 2003, 34, 820–826. [Google Scholar] [CrossRef] [PubMed]
- SAS Institute. JMP® Version 7 User’s Guide; SAS Institute Inc.: Cary, NC, USA, 2007. [Google Scholar]
- Cuevas, J.; Pinillos, V.; Polito, V.S. Effective pollination period for ‘Manzanillo’ and ‘Picual’ olive trees. J. Hortic. Sci. Biotechnol. 2009, 84, 370–374. [Google Scholar] [CrossRef]
- Wenden, B.; Campoy, J.A.; Lecourt, J.; López Ortega, G.; Blanke, M.; Radičević, S.; Schüller, E.; Spornberger, A.; Christen, D.; Magein, H.; et al. A collection of European sweet cherry phenology data for assessing climate change. Sci. Data 2016, 3, 160108. [Google Scholar] [CrossRef]
- Mariotti, R.; Fornasiero, A.; Mousavi, S.; Cultrera, N.G.M.; Brizioli, F.; Pandolfi, S.; Passeri, V.; Rossi, M.; Magris, G.; Scalabrin, S.; et al. Genetic mapping of the incompatibility locus in olive and development of a linked Sequence-Tagged Site marker. Front. Plant Sci. 2020, 10. [Google Scholar] [CrossRef]
- Saumitou-Laprade, P.; Vernet, P.; Vekemans, X.; Castric, V.; Barcaccia, G.; Khadari, B.; Baldoni, L. Controlling for genetic identity of varieties, pollen contamination and stigma receptivity is essential to characterize the self-incompatibility system of Olea europaea L. Evol. Appl. 2017, 10, 860–866. [Google Scholar] [CrossRef]
- Marchese, A.; Marra, F.; Costa, F.; Quartararo, A.; Fretto, S.; Caruso, T. An investigation of the self-and inter-incompatibility of the olive cultivars ‘Arbequina’ and ‘Koroneiki’ in the Mediterranean climate of Sicily. Aust. J. Crop Sci. 2016, 88, 88–93. [Google Scholar]
- Ayerza, R.; Coates, W. Supplemental pollination—Increasing olive (olea europaea) yields in hot, arid environments. Exp. Agric. 2004, 40, 481–491. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Biton, I.; Many, Y.; Mazen, A.; Ben-Ari, G. Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set. Agronomy 2020, 10, 910. https://doi.org/10.3390/agronomy10060910
Biton I, Many Y, Mazen A, Ben-Ari G. Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set. Agronomy. 2020; 10(6):910. https://doi.org/10.3390/agronomy10060910
Chicago/Turabian StyleBiton, Iris, Yair Many, Ali Mazen, and Giora Ben-Ari. 2020. "Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set" Agronomy 10, no. 6: 910. https://doi.org/10.3390/agronomy10060910
APA StyleBiton, I., Many, Y., Mazen, A., & Ben-Ari, G. (2020). Compatibility between “Arbequina” and “Souri” Olive Cultivars May Increase Souri Fruit Set. Agronomy, 10(6), 910. https://doi.org/10.3390/agronomy10060910




