Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions
Abstract
1. Introduction
2. Materials and Methods
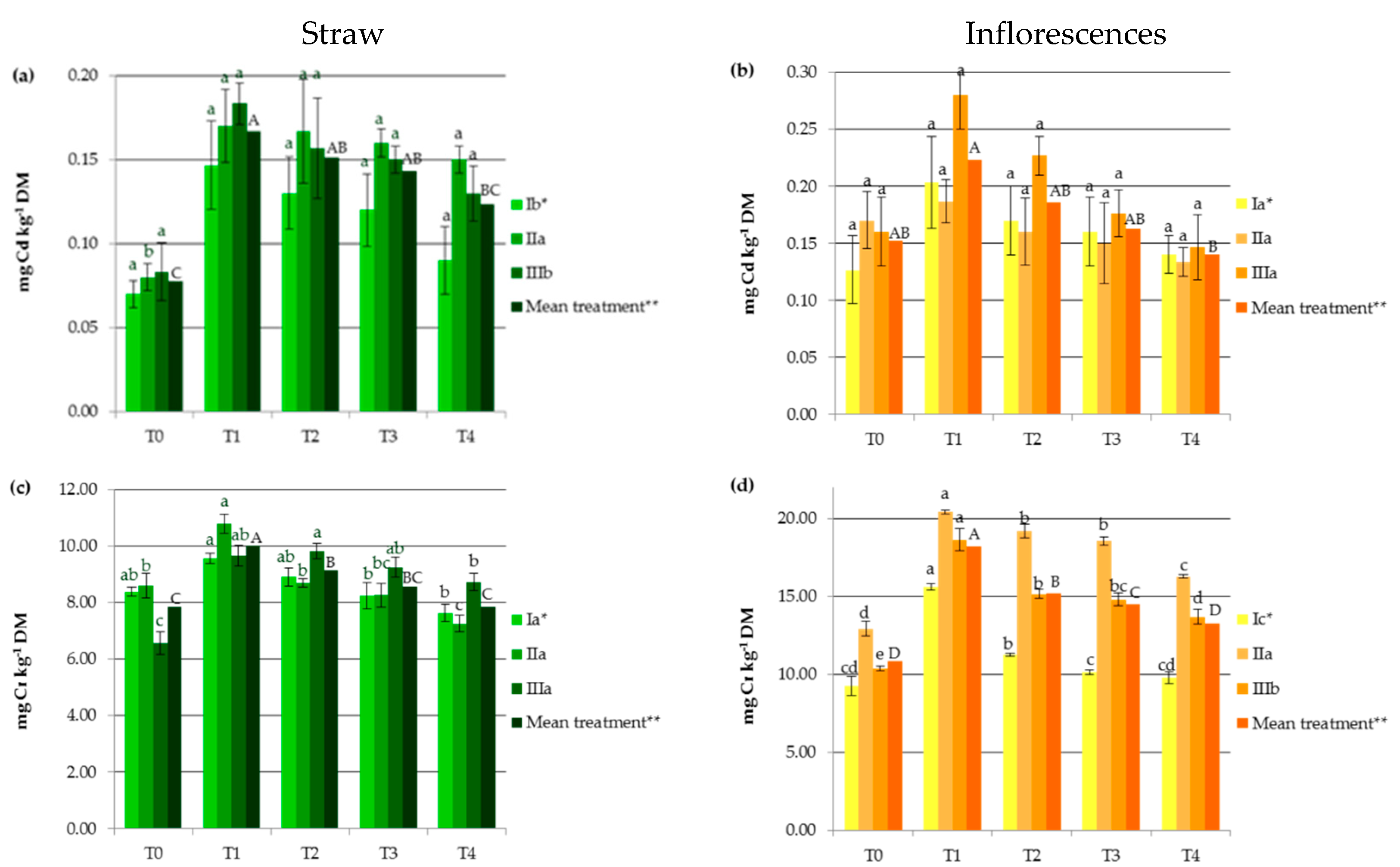
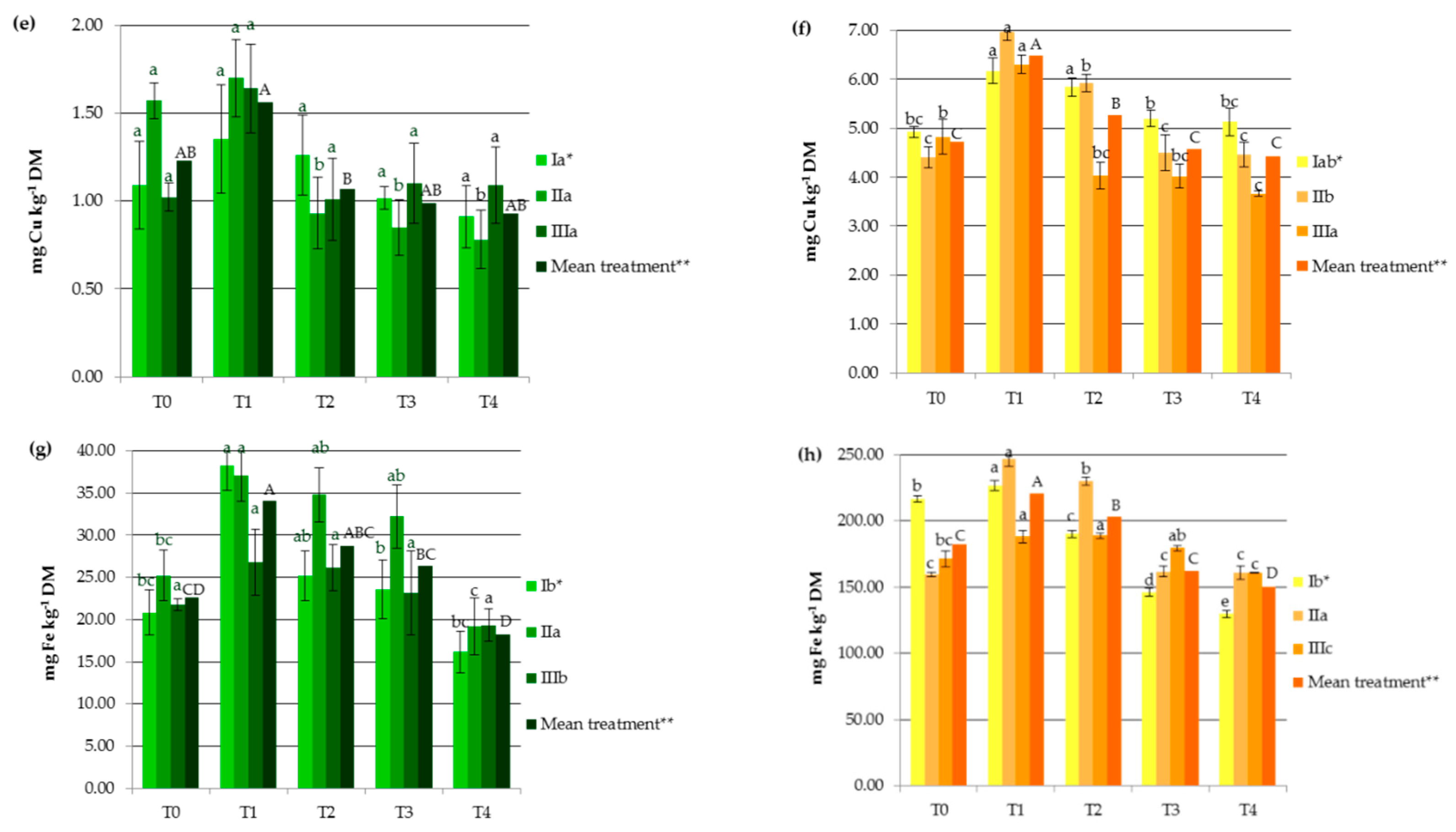
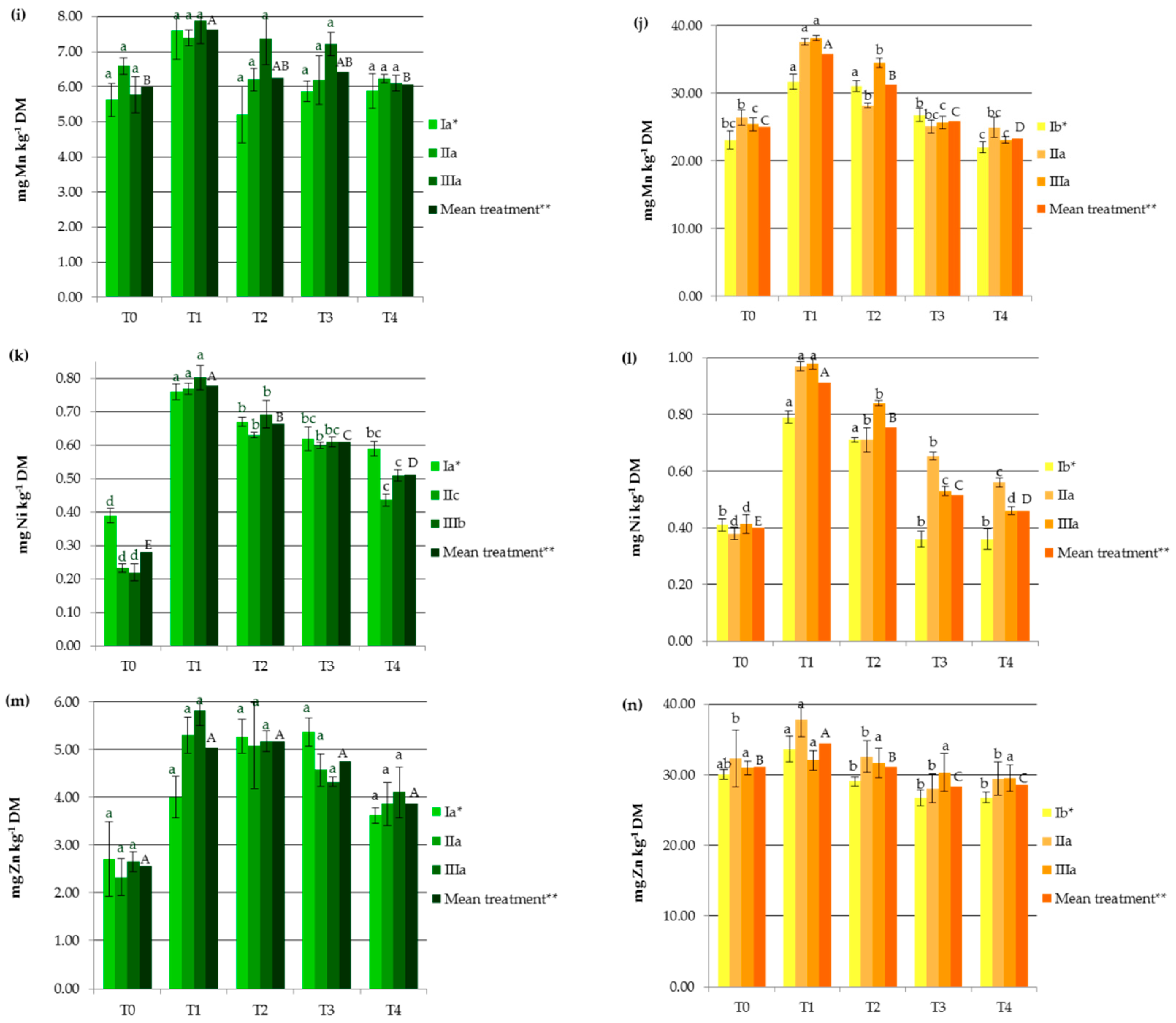
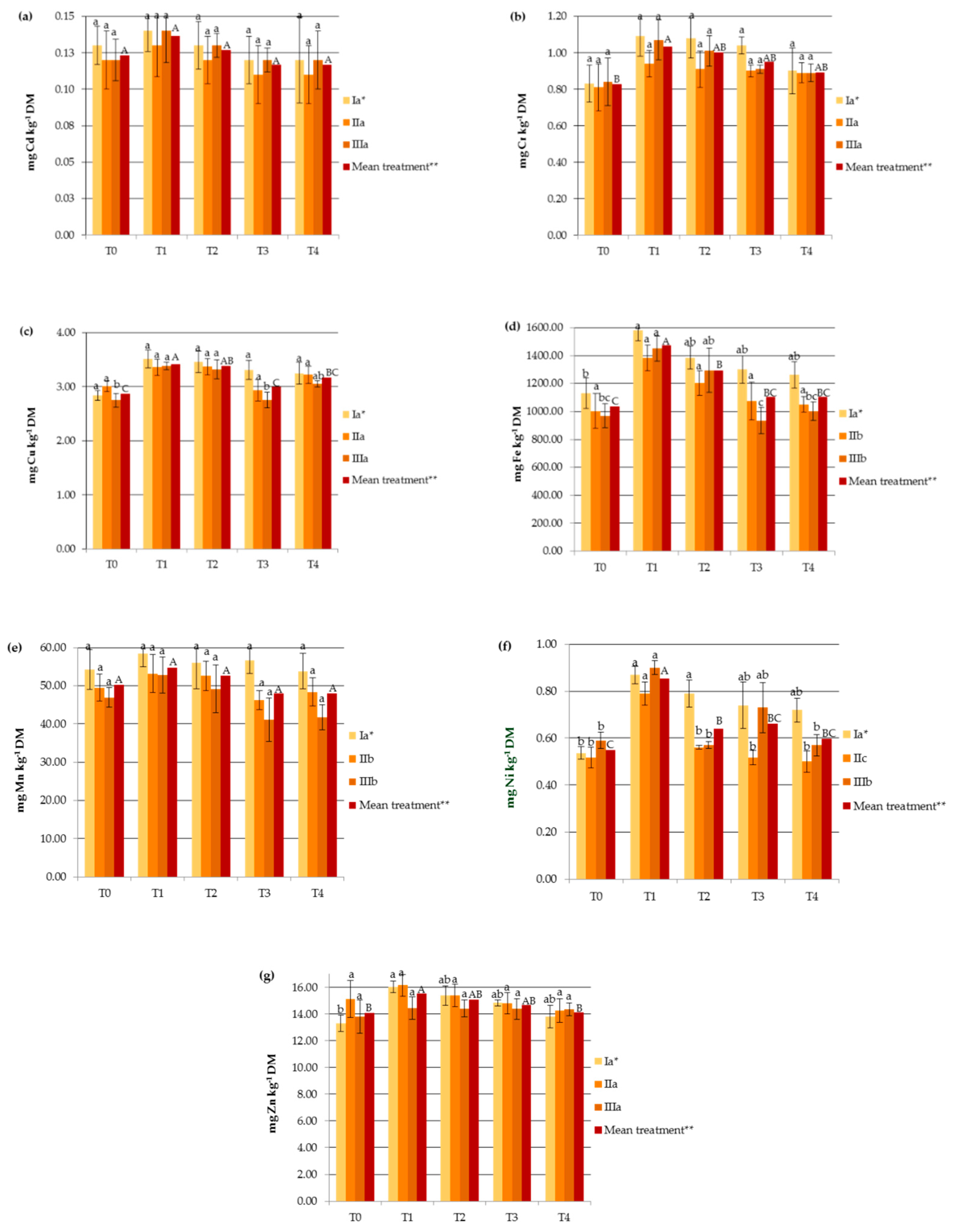
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Ahmad, R.; Tehsin, Z.; Tanvir, S.; Saeed, M.; Asad, A.; Shahzad, M.; Bilal, M.; Shah, M.M.; Khan, S.A. Phytoremediation potential of hemp (Cannabis sativa L.): Identification and characterization of heavy metals responsive genes. Clean Soil Air Water 2016, 44, 195–201. [Google Scholar] [CrossRef]
- Park, J.H.; Lamb, D.; Paneerselvam, P.; Choppala, G.; Bolan, N.; Chung, J.W. Role of organic amendments on enhanced bioremediation of heavy metal(loid) contaminated soils. J. Hazard. Mater. 2011, 185, 549–574. [Google Scholar] [CrossRef]
- Vardhan, K.H.; Kumar, P.S.; Panda, R.C. A review on heavy metal pollution, toxicity and remedial measures: Current trends and future perspectives. J. Mol. Liq. 2019, 290, 111197. [Google Scholar] [CrossRef]
- Vareda, J.P.; Valente, A.J.M.; Durãesa, L. Assessment of heavy metal pollution from anthropogenic activities and remediation strategies: A review. J. Environ. Manage. 2019, 246, 101–118. [Google Scholar] [CrossRef] [PubMed]
- Collivignarelli, M.C.; Abbà, A.; Frattarola, A.; Miino, M.C.; Padovani, S.; Katsoyiannis, I.; Torretta, V. Legislation for the reuse of biosolids on agricultural land in Europe: Overview. Sustainability 2019, 11, 6015. [Google Scholar] [CrossRef]
- Nissim, W.G.; Cincinelli, A.; Martellini, T.; Alvisi, L.; Palm, E.; Mancuso, S.; Azzarello, E. Phytoremediation of sewage sludge contaminated by trace elements and organic compounds. Environ. Res. 2018, 164, 356–366. [Google Scholar] [CrossRef]
- Seleiman, M.F.; Santanen, A.; Stoddard, F.L.; Mäkelä, P. Feedstock quality and growth of bioenergy crops fertilized with sewage sludge. Chemosphere 2012, 89, 1211–1217. [Google Scholar] [CrossRef] [PubMed]
- Laidlaw, W.S.; Arndt, S.K.; Huynh, T.T.; Gregory, D.; Baker, A.J.M. Phytoextraction of heavy metals by willows growing in biosolids under field conditions. J. Environ. Qual. 2012, 41, 134–143. [Google Scholar] [CrossRef]
- Narasimha, M.; Prasad, V.; De Campos Favas, P.J.; Vithanage, M.; Mohan, S.V. (Eds.) Industrial and Municipal Sludge: Emerging Concerns and Scope for Resource, 1st ed.; Butterworth-Heinemann, Elsevier Science: Oxford, UK, 2019; p. 856. [Google Scholar]
- Skowrońska, M.; Bielińska, E.J.; Szymański, K.; Futa, B.; Antonkiewicz, J.; Kolodziej, B. An integrated assessment of the long-term impact of municipal sewage sludge on the chemical and biological properties of soil. Catena 2020, 189, 104484. [Google Scholar] [CrossRef]
- Urra, J.; Alkorta, I.; Garbisu, C. Potential benefits and risks for soil health derived from the use of organic amendments in agriculture. Agronomy 2019, 9, 542. [Google Scholar] [CrossRef]
- Yang, G.-H.; Zhu, G.-Y.; Li, H.L.; Han, X.-M.; Li, J.-M.; Ma, Y.B. Accumulation and bioavailability of heavy metals in a soil-wheat/maize system with long-term sewage sludge amendments. J. Integr. Agric. 2018, 17, 1861–1870. [Google Scholar] [CrossRef]
- Chernysh, Y.; Balintova, M.; Plyatsuk, L.; Holub, M.; Demcak, S. The influence of phosphogypsum addition on phosphorus release in biochemical treatment of sewage sludge. Int. J. Environ. Res. Public Health 2018, 15, 1269. [Google Scholar] [CrossRef] [PubMed]
- Layr, K.; Hartlieb, P. Market analysis for urban mining of phosphogypsum. Berg Huettenmaenn Mon. 2019, 164, 245–249. [Google Scholar] [CrossRef]
- Saadaoui, E.; Ghazel, N.; Romdhane, C.B.; Massoudi, N. Phosphogypsum: Potential uses and problems—A review. J. Environ. Sci. 2017, 74, 558–567. [Google Scholar] [CrossRef]
- Hentati, O.; Abrantes, N.; Caetano, A.L.; Bouguerra, S.; Gonçalves, F.; Römbke, J.; Pereira, R. Phosphogypsum as a soil fertilizer: Ecotoxicity of amended soil and elutriates to bacteria, invertebrates, algae and plants. J. Hazard. Mater. 2015, 294, 80–89. [Google Scholar] [CrossRef]
- Antonkiewicz, J.; Kołodziej, B.; Bielińska, E.J. Phytoextraction of heavy metals from municipal sewage sludge by Rosa multiflora and Sida hermaphrodita. Int. J. Phytoremediat. 2017, 19, 309–318. [Google Scholar] [CrossRef]
- Bauddh, K.; Singh, B.; Korstad, J. (Eds.) Phytoremediation Potential of Bioenergy Plants; Springer: Singapore, 2017; p. 472. [Google Scholar]
- Chandra, R.; Kumar, V. Phytoextraction of heavy metals by potential native plants and their microscopic observation of root growing on stabilized distillery sludge as a prospective tool for in situ phytoremediation of industrial waste. Environ. Sci. Pollut. Res. 2017, 24, 2605–2619. [Google Scholar] [CrossRef]
- Vaverková, M.D.; Zloch, J.; Adamcová, D.; Radziemska, M.; Vyhnánek, T.; Elbl, J.; Trojan, V.; Winkler, J.; Dorđević, B.; Elbl, J.; et al. Landfill Leachate Effects on Germination and Seedling Growth of Hemp Cultivars (Cannabis sativa L.). Waste Biomass Valor. 2019, 10, 369–376. [Google Scholar]
- Adesina, I.; Bhowmik, A.; Sharma, H.; Shahbazi, A. A review on the current state of knowledge of growing conditions, agronomic soil health practices and utilities of hemp in the United States. Agriculture 2020, 10, 129. [Google Scholar] [CrossRef]
- Griga, M.; Bjelková, M. Flax (Linum usitatissimum L.) and hemp (Cannabis sativa L.) as fibre crops for phytoextraction of heavy metals. Biological, agro-technological and economical point of view. In Plant-Based Remediation Process; Gupta, D.K., Ed.; Springer: Heidelberg/Berlin, Germany, 2013; pp. 199–237. [Google Scholar]
- Galić, M.; Perčin, A.; Zgorelec, Ž.; Kisić, I. Evaluation of heavy metals accumulation potential of hemp (Cannabis sativa L.). J. Cent. Eur 2019, 20, 711. [Google Scholar]
- Girdhar, M.; Sharma, N.R.; Rehman, H.; Kumar, A.; Mohan, A. Comparative assessment for hyperaccumulatory and phytoremediation capability of three wild weeds. Biotech 2014, 4, 579–589. [Google Scholar] [CrossRef] [PubMed]
- Praspaliauskas, M.; Žaltauskaitė, J.; Pedišius, N.; Striūgas, N. Comprehensive evaluation of sewage sludge and sewage sludge char soil amendment impact on the industrial hemp growth performance and heavy metal accumulation. Ind. Crops Prod. 2020, 150, 112396. [Google Scholar] [CrossRef]
- Bioremediation and Bioeconomy; Prasad, M.N.V., Ed.; Elsevier: Waltham, MA, USA, 2016; p. 730. [Google Scholar]
- Angelova, V.; Ivanova, R.; Delibaltova, V.; Ivanov, K. Bio-accumulation and distribution of heavy metals in fibre crops (flax, cotton and hemp). Ind. Crops Prod. 2004, 19, 197–205. [Google Scholar] [CrossRef]
- Regulation of the Minister of the Natural Environment on Municipal Sewage Sludge Dated 6 February 2015. J. Laws Pol. 2015, 257.
- European Council. Official Journal of the European Union 56; C 378; Publications Office of the EU: Brussels, Belgium, 2014; pp. 1–44.
- Jones, J.B., Jr.; Case, V.V. Sampling, Handling, and Analyzing Plant Tissue Samples, SSSA Book Series 3. In Soil Testing and Plant Analysis, 3rd ed.; Westerman, R.L., Ed.; Soil Science Society of America: Madison, WI, USA, 1990. [Google Scholar]
- Rinkis, G.Y. Methods of Accelerated Colorimetric Analysis of Trace Elements in Biological Objects; Academy of Sciences of Latvian SSR: Riga, Latvia, 1963. (in Russian) [Google Scholar]
- De Souza, S.C.R.; De Andrade, S.A.L.; De Souza, L.A.; Schiavinato, M.A. Lead tolerance and phytoremediation potential of Brazilian leguminous tree species at the seedling stage. J. Environ. Manag. 2012, 110, 299–307. [Google Scholar] [CrossRef]
- Mahmoud, E.; Abd El-Kader, N. Heavy metal immobilization in contaminated soils using phosphogypsum and rice straw compost. Land Degrad. Dev. 2015, 26, 819–824. [Google Scholar] [CrossRef]
- Petrová, Š.; Benešová, D.; Soudek, P.; Vanĕk, T. Enhancement of metal(loid)s phytoextraction by Cannabis sativa L. J. Food Agric. Environ. 2012, 10, 631–641. [Google Scholar]
- Caporale, A.G.; Violante, A. Chemical processes affecting the mobility of heavy metals and metalloids in soil environments. Curr. Pollut. Rep. 2016, 2, 15–27. [Google Scholar] [CrossRef]
- Fageria, V.D. Nutrient interactions in crop plants. J. Plant Nutr. 2001, 24, 1269–1290. [Google Scholar] [CrossRef]
| Treatment | cv. (cultivar) Białobrzeskie | cv. Tygra | cv. Beniko | |||
|---|---|---|---|---|---|---|
| Heavy Metal Balance g ha−1 | Bioconcentration Factor Straw/Inflorescences | Heavy Metal Balance g ha−1 | Bioconcentration Factor Straw/Inflorescences | Heavy Metal Balance g ha−1 | Bioconcentration Factor Straw/Inflorescences | |
| Cd | ||||||
| T0 | −0.94 | 0.54/0.97 | −1.19 | 0.67/1.42 | −0.93 | 0.69/1.33 |
| T1 | −0.65 | 1.05/1.45 | −0.54 | 1.31/1.44 | −1.19 | 1.31/2.00 |
| T2 | 0.24 | 1.00/1.31 | −0.71 | 1.39/1.33 | 0.12 | 1.21/1.74 |
| T3 | 2.17 | 1.00/1.33 | 0.95 | 1.45/1.36 | 1.40 | 1.25/1.47 |
| T4 | 4.85 | 0.75/1.17 | 3.96 | 1.36/1.21 | 4.63 | 1.08/1.22 |
| Cr | ||||||
| T0 | −105.07 | 10.09/11.16 | −120.02 | 10.60/15.94 | −71.66 | 7.82/12.33 |
| T1 | −112.79 | 8.79/14.30 | −111.38 | 11.47/21.72 | −108.41 | 9.03/17.42 |
| T2 | −84.98 | 8.25/10.42 | −121.90 | 9.54/21.10 | −81.27 | 9.72/15.01 |
| T3 | −69.07 | 7.92/9.75 | −124.41 | 9.18/20.62 | −110.12 | 10.16/16.27 |
| T4 | −55.82 | 8.48/10.89 | −63.81 | 8.16/18.30 | −50.66 | 9.80/15.39 |
| Cu | ||||||
| T0 | −18.46 | 0.38/1.73 | -25.06 | 0.52/1.47 | −13.97 | 0.37/1.75 |
| T1 | 341.23 | 0.39/1.76 | 339.25 | 0.51/2.08 | 339.61 | 0.49/1.86 |
| T2 | 345.37 | 0.36/1.69 | 345.44 | 0.28/1.76 | 355.95 | 0.30/1.21 |
| T3 | 352.05 | 0.31/1.57 | 349.92 | 0.29/1.54 | 351.02 | 0.40/1.46 |
| T4 | 355.98 | 0.28/1.58 | 358.27 | 0.24/1.39 | 360.42 | 0.36/1.20 |
| Fe | ||||||
| T0 | −510.62 | 0.02/0.19 | −537.73 | 0.03/0.16 | −358.54 | 0.02/0.18 |
| T1 | −171.61 | 0.02/0.14 | −70.93 | 0.03/0.18 | 165.62 | 0.02/0.13 |
| T2 | 355.44 | 0.02/0.14 | −37.33 | 0.03/0.19 | 480.70 | 0.02/0.15 |
| T3 | 1311.72 | 0.02/0.11 | 938.90 | 0.03/0.15 | 1235.20 | 0.02/0.19 |
| T4 | 2515.89 | 0.01/0.10 | 2386.74 | 0.02/0.15 | 2549.62 | 0.02/0.16 |
| Mn | ||||||
| T0 | −92.33 | 0.10/0.43 | −117.19 | 0.13/0.53 | −77.48 | 0.12/0.54 |
| T1 | 767.16 | 0.13/0.54 | 782.28 | 0.14/0.71 | 769.41 | 0.15/0.72 |
| T2 | 813.78 | 0.09/0.55 | 778.02 | 0.12/0.54 | 805.05 | 0.15/0.70 |
| T3 | 812.61 | 0.10/0.47 | 772.89 | 0.13/0.54 | 782.48 | 0.17/0.62 |
| T4 | 821.09 | 0.11/0.41 | 806.57 | 0.13/0.52 | 843.08 | 0.15/0.55 |
| Ni | ||||||
| T0 | −4.87 | 0.73/0.76 | −3.30 | 0.45/0.74 | −2.46 | 0.37/0.70 |
| T1 | 24.36 | 0.87/0.91 | 25.92 | 0.97/1.23 | 24.04 | 0.89/1.09 |
| T2 | 27.37 | 0.85/0.90 | 26.37 | 1.13/1.27 | 28.38 | 1.22/1.47 |
| T3 | 32.02 | 0.84/0.49 | 29.33 | 1.16/1.26 | 30.63 | 0.84/0.73 |
| T4 | 36.50 | 0.82/0.50 | 38.38 | 0.87/1.12 | 38.97 | 0.89/0.81 |
| Zn | ||||||
| T0 | −68.78 | 0.20/2.26 | −76.31 | 0.15/2.14 | −52.72 | 0.19/2.25 |
| T1 | 1320.84 | 0.25/2.10 | 1309.75 | 0.33/2.34 | 1310.11 | 0.40/2.22 |
| T2 | 1318.04 | 0.34/1.89 | 1288.99 | 0.33/2.12 | 1339.41 | 0.36/2.20 |
| T3 | 1328.20 | 0.36/1.80 | 1303.42 | 0.31/1.90 | 1332.21 | 0.30/2.11 |
| T4 | 1362.22 | 0.26/1.94 | 1348.57 | 0.27/2.07 | 1376.34 | 0.29/2.06 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zielonka, D.; Szulc, W.; Skowrońska, M.; Rutkowska, B.; Russel, S. Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions. Agronomy 2020, 10, 907. https://doi.org/10.3390/agronomy10060907
Zielonka D, Szulc W, Skowrońska M, Rutkowska B, Russel S. Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions. Agronomy. 2020; 10(6):907. https://doi.org/10.3390/agronomy10060907
Chicago/Turabian StyleZielonka, Dariusz, Wiesław Szulc, Monika Skowrońska, Beata Rutkowska, and Stefan Russel. 2020. "Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions" Agronomy 10, no. 6: 907. https://doi.org/10.3390/agronomy10060907
APA StyleZielonka, D., Szulc, W., Skowrońska, M., Rutkowska, B., & Russel, S. (2020). Hemp-Based Phytoaccumulation of Heavy Metals from Municipal Sewage Sludge and Phosphogypsum Under Field Conditions. Agronomy, 10(6), 907. https://doi.org/10.3390/agronomy10060907






