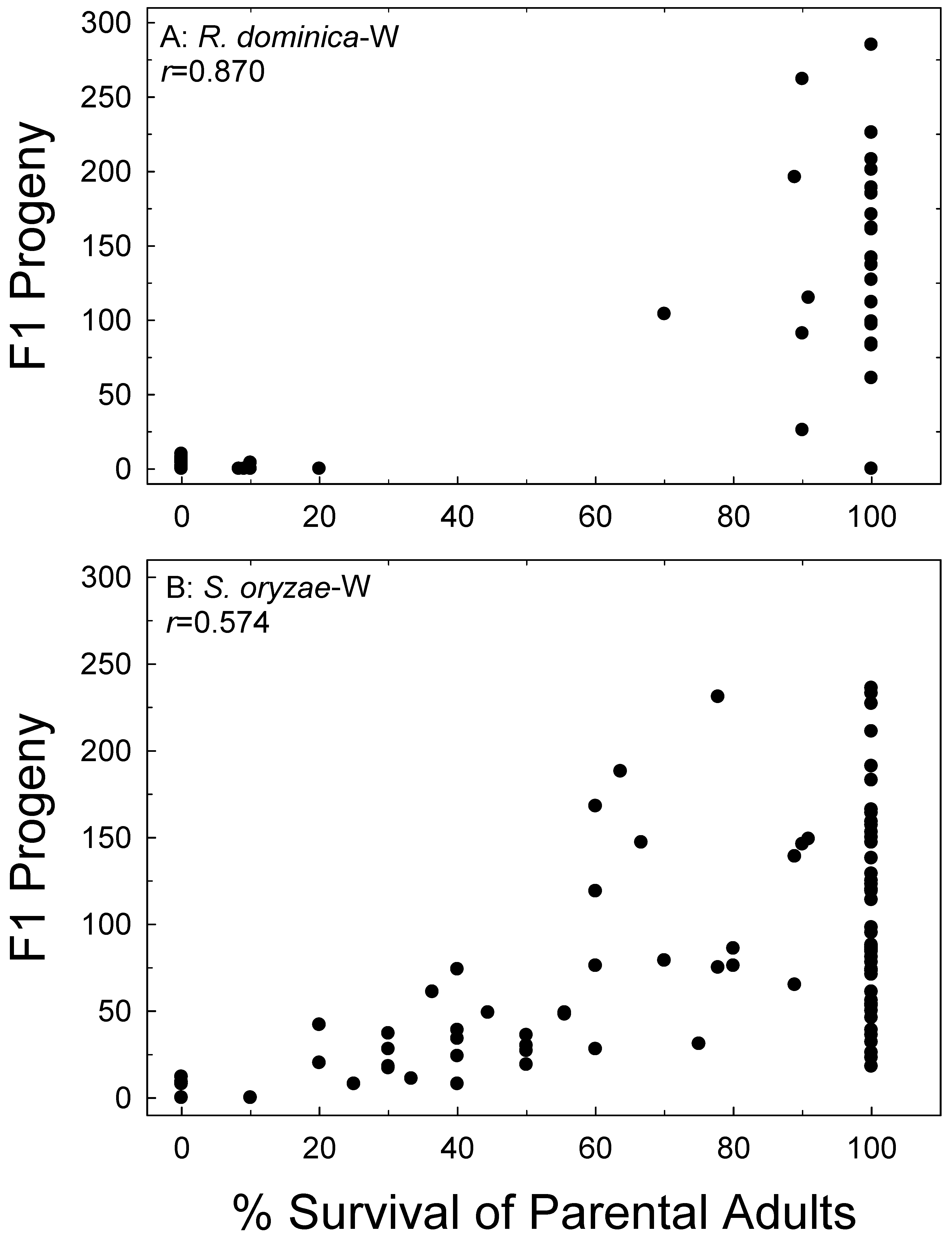
Figure 1.
Correlation of parental survival with F1 progeny production of R. dominica (A) and S. oryzae (B) on wheat (W). Data combined for all treatments and all bioassay months.
Figure 1.
Correlation of parental survival with F1 progeny production of R. dominica (A) and S. oryzae (B) on wheat (W). Data combined for all treatments and all bioassay months.
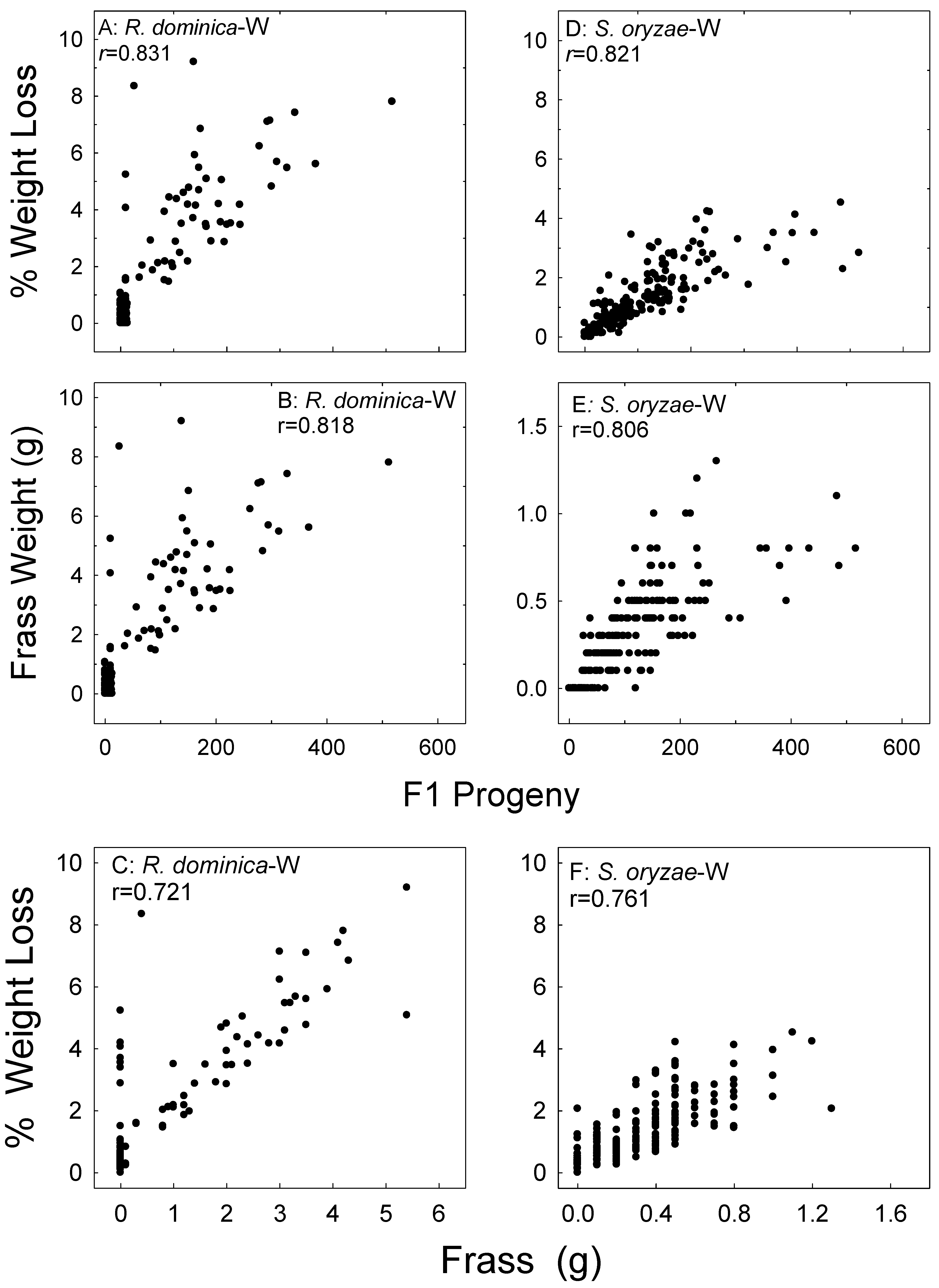
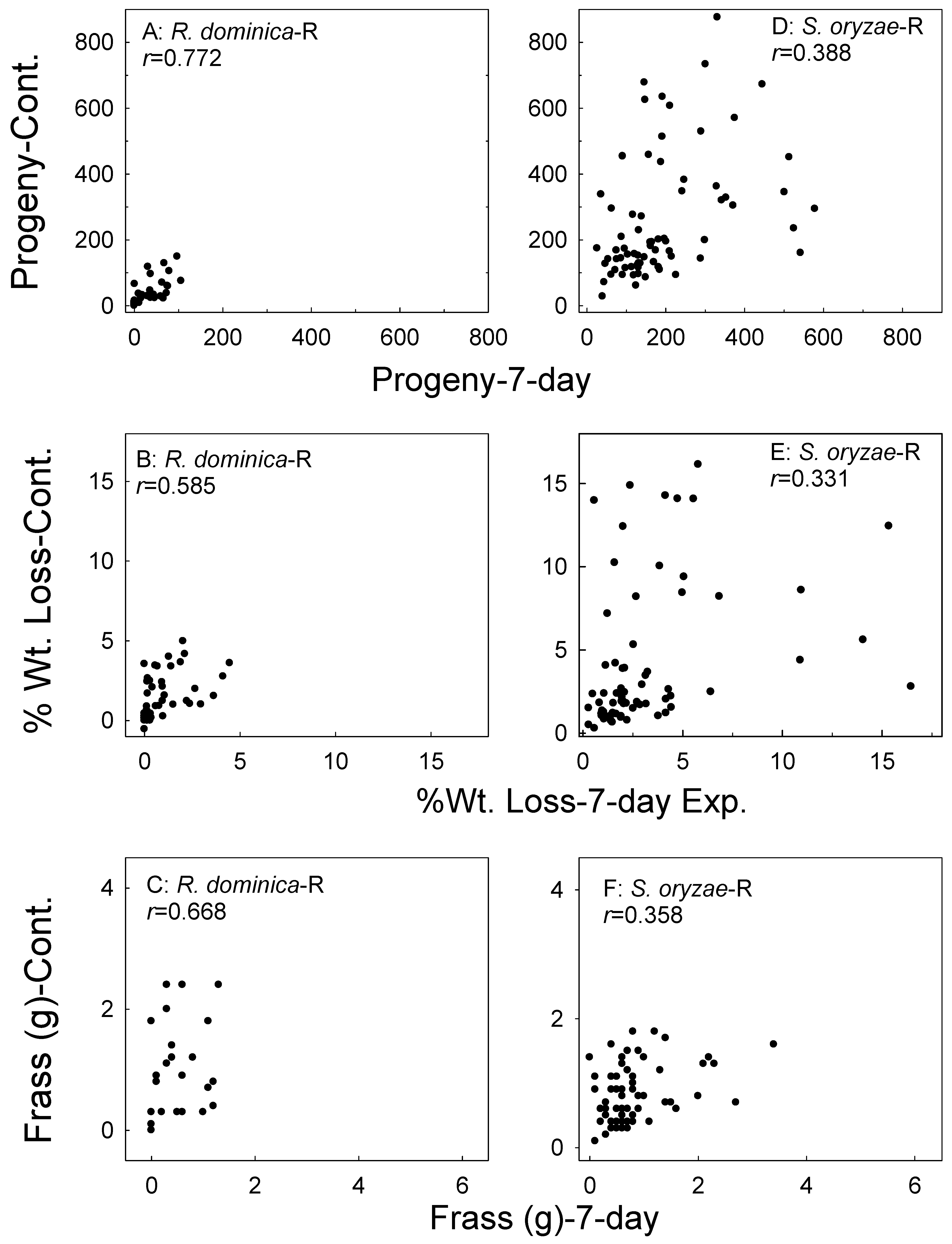
Figure 2.
Correlation of F1 progeny, sample weight loss, and feeding damage (frass weight) in the wheat (W) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for R. dominica (A–C) and S. oryzae (D–F). Data combined for all treatments and all bioassay months.
Figure 2.
Correlation of F1 progeny, sample weight loss, and feeding damage (frass weight) in the wheat (W) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for R. dominica (A–C) and S. oryzae (D–F). Data combined for all treatments and all bioassay months.
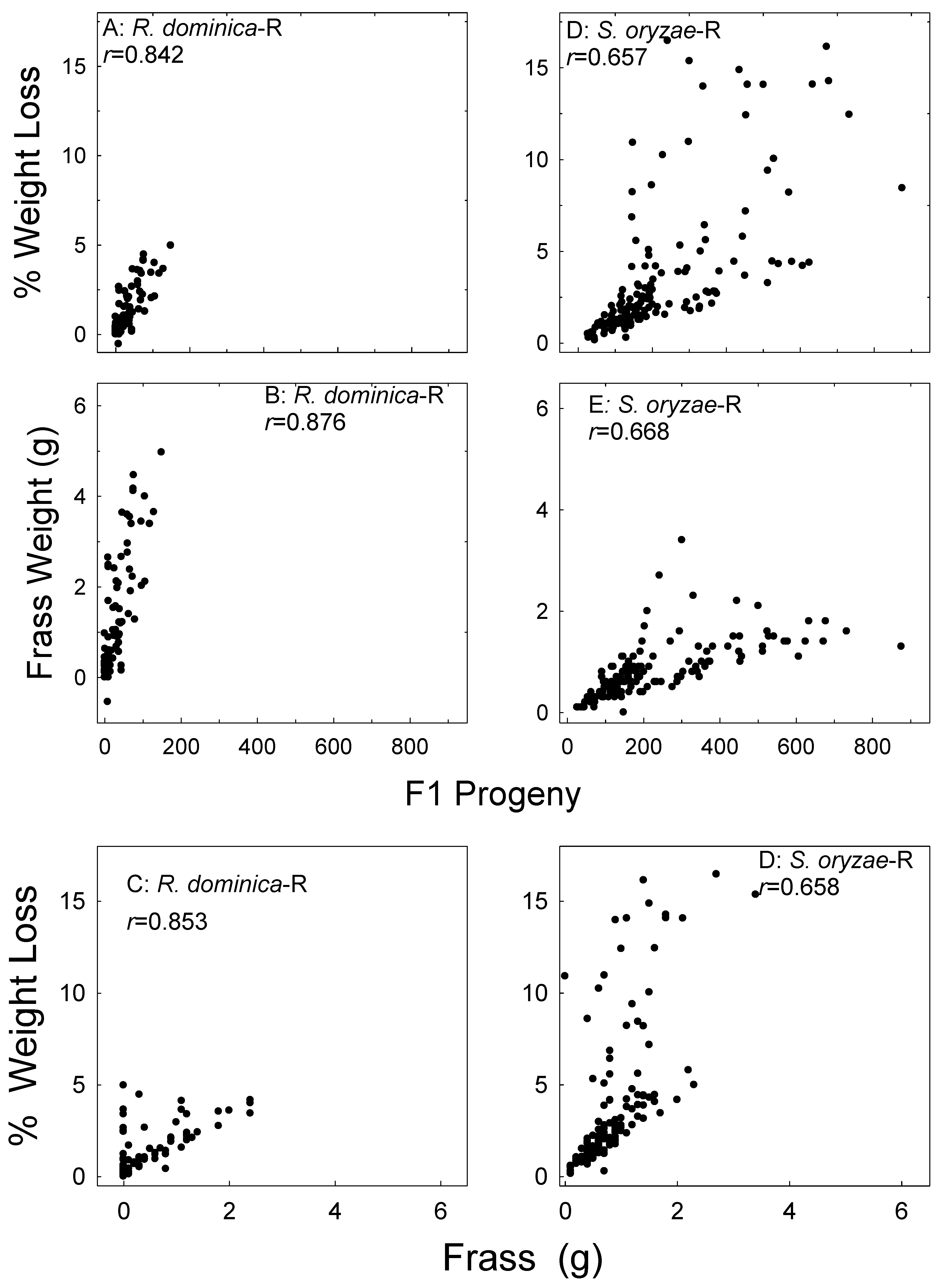
Figure 3.
Correlation of wheat (W) sample weight loss and frass weight with F1 progeny of R. dominica (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. oryzae (D,E), and sample weight loss and frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
Figure 3.
Correlation of wheat (W) sample weight loss and frass weight with F1 progeny of R. dominica (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. oryzae (D,E), and sample weight loss and frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
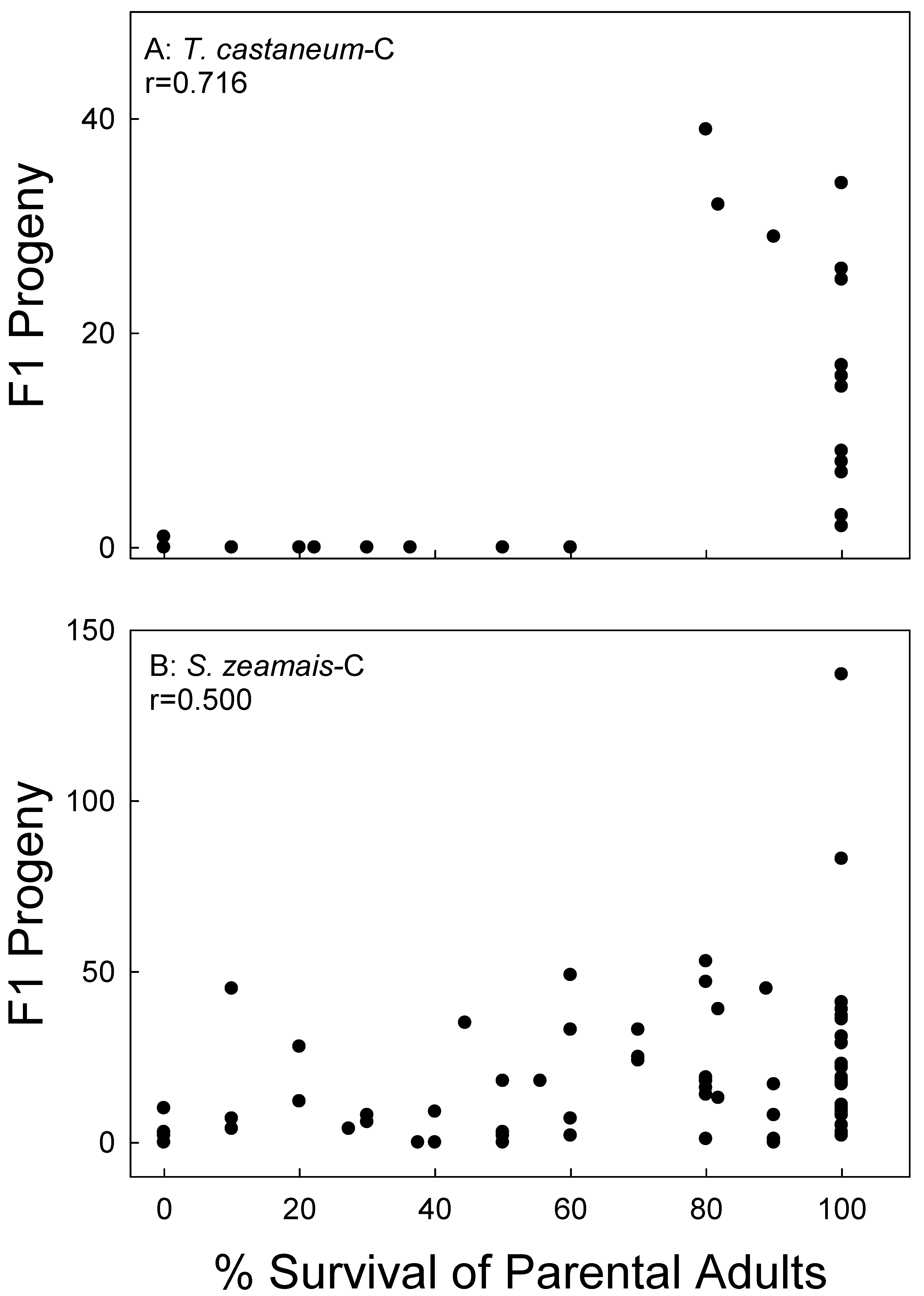
Figure 4.
Correlation of parental survival with F1 progeny production of T. castaneum (A) and S. zeamais (B) on corn (C). Data combined for treatments and all bioassay months.
Figure 4.
Correlation of parental survival with F1 progeny production of T. castaneum (A) and S. zeamais (B) on corn (C). Data combined for treatments and all bioassay months.
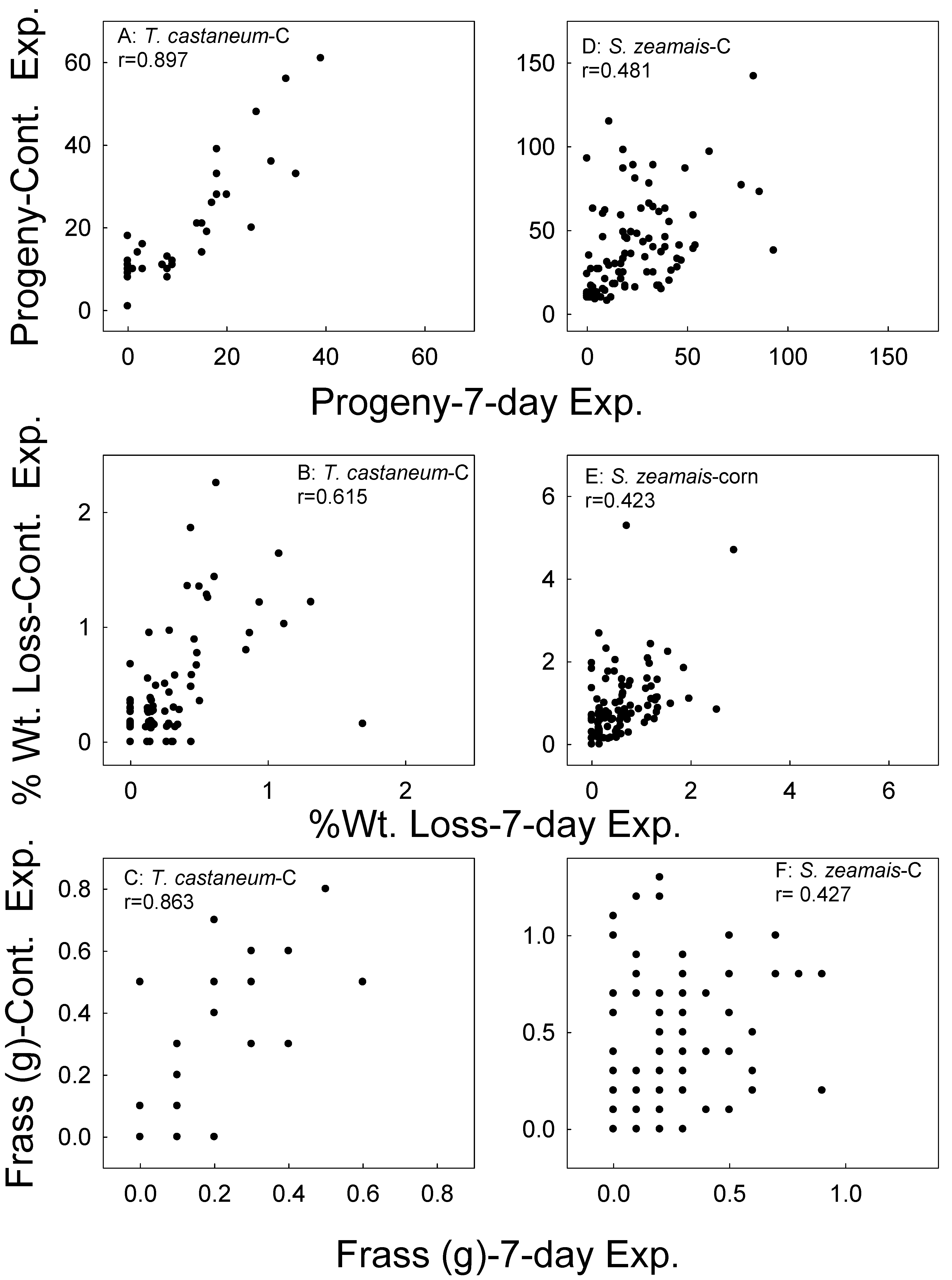
Figure 5.
Correlation of F1 progeny, sample weight loss, and frass weight in the corn (C) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for T. castaneum (A–C) and S. zeamais (D–F). Data combined for all treatments and all bioassay months.
Figure 5.
Correlation of F1 progeny, sample weight loss, and frass weight in the corn (C) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for T. castaneum (A–C) and S. zeamais (D–F). Data combined for all treatments and all bioassay months.
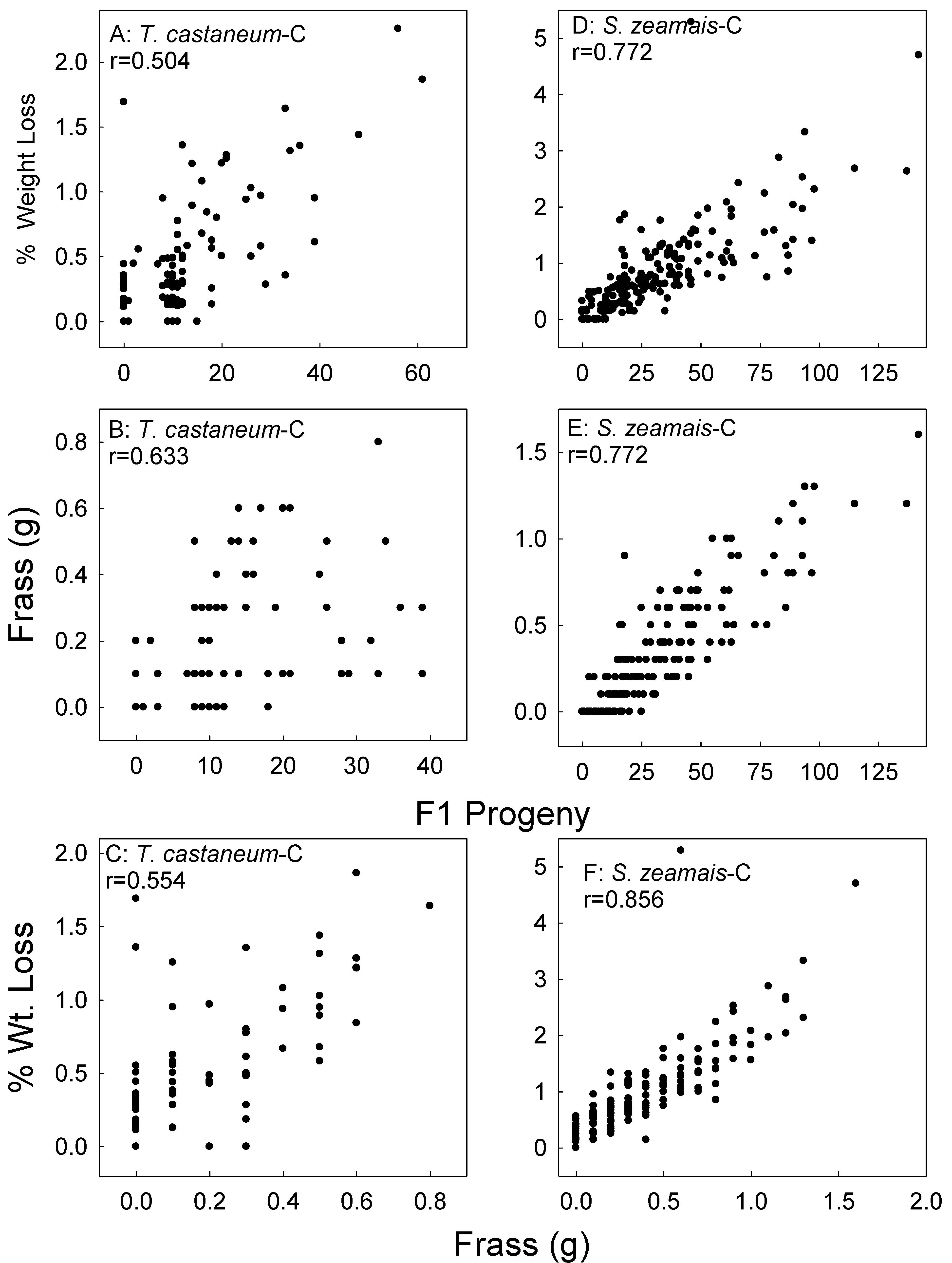
Figure 6.
Correlation of corn (C) sample weight loss and frass weight with F1 progeny of T. castaneum (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. zeamais (D,E), and sample weight loss with frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
Figure 6.
Correlation of corn (C) sample weight loss and frass weight with F1 progeny of T. castaneum (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. zeamais (D,E), and sample weight loss with frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
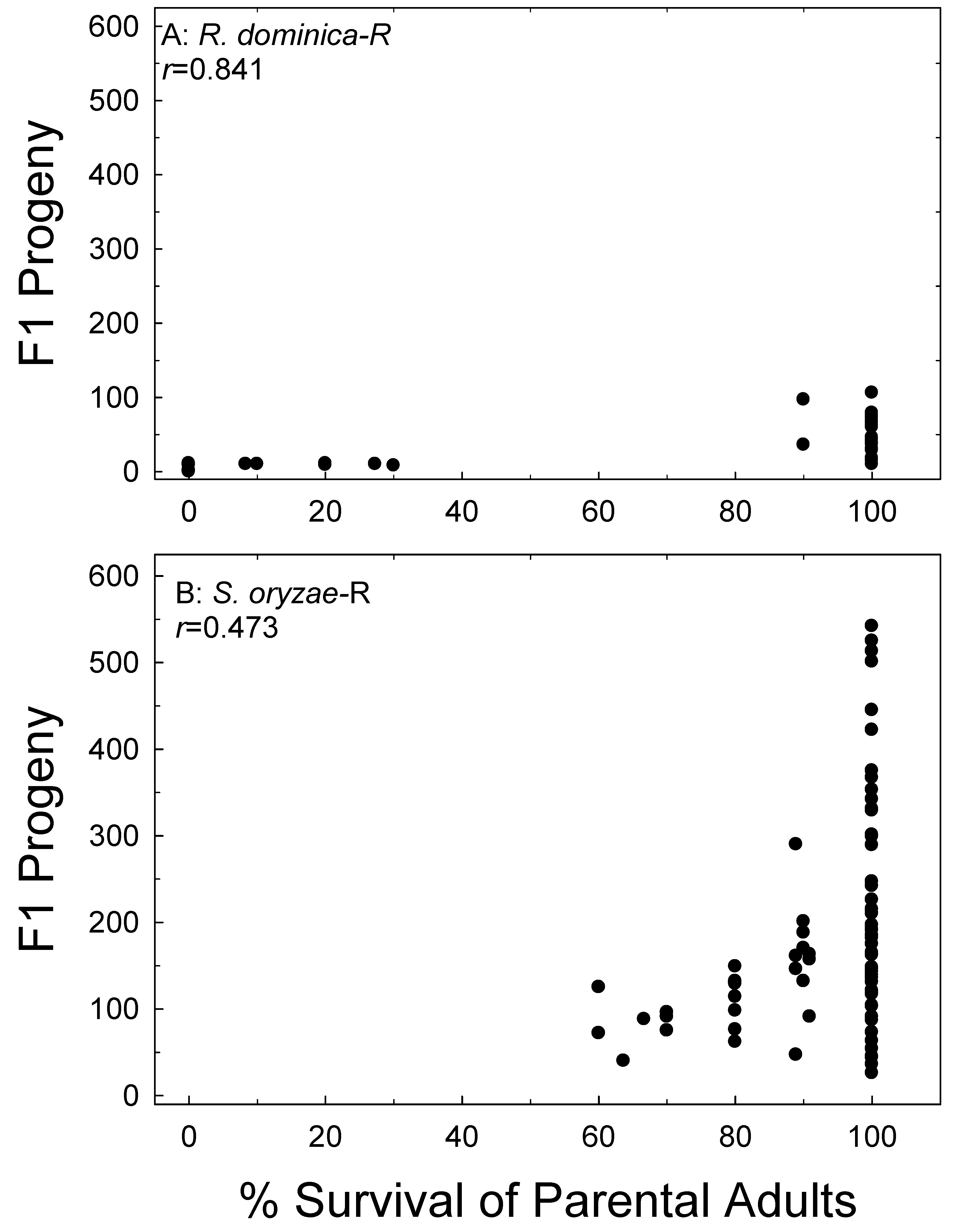
Figure 7.
Correlation of parental survival with F1 progeny production of R. dominica (A) and S. oryzae (B) on brown rice (R). Data combined for all treatments and all bioassay months.
Figure 7.
Correlation of parental survival with F1 progeny production of R. dominica (A) and S. oryzae (B) on brown rice (R). Data combined for all treatments and all bioassay months.
Figure 8.
Correlation of F1 progeny, sample weight loss, and feeding damage (frass weight) in the brown rice (R) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for R. dominica (A–C) and S. oryzae (D–F). Data combined for all treatments and all bioassay months.
Figure 8.
Correlation of F1 progeny, sample weight loss, and feeding damage (frass weight) in the brown rice (R) samples whereby parental adults were exposed for 7 days versus the continual exposures of parental adults for R. dominica (A–C) and S. oryzae (D–F). Data combined for all treatments and all bioassay months.
Figure 9.
Correlation of brown rice (R) sample weight loss and frass weight with F1 progeny of R. dominica (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. oryzae (D,E), and sample weight loss with frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
Figure 9.
Correlation of brown rice (R) sample weight loss and frass weight with F1 progeny of R. dominica (A,B), sample weight loss with frass weight (C), sample weight loss and frass weight with F1 progeny of S. oryzae (D,E), and sample weight loss with frass weight (F). Data for the two parental exposure regimes combined, data for all treatments and all bioassay months combined.
Table 1.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, the low and high rates of Diacon IGR+, and the Gravista formulation) for the percentage (means ± SE) of adult R. dominica and S. oryae that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on treated wheat.
Table 1.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, the low and high rates of Diacon IGR+, and the Gravista formulation) for the percentage (means ± SE) of adult R. dominica and S. oryae that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on treated wheat.
| Species | Response | Variable | F | df | p |
|---|
| R. dominica | Live | MO | 1.9 | 4, 79 | 0.114 |
| | | TRT | 3160.3 | 3, 79 | <0.001 |
| | | MO*TRT | 1.1 | 12, 79 | 0.392 |
| | KD | MO | 11.6 | 4, 79 | <0.001 |
| | | TRT | 2.5 | 3, 79 | 0.064 |
| | | MO*TRT | 2.3 | 12, 79 | 0.014 |
| | Dead | MO | 12.1 | 4,79 | <0.001 |
| | | TRT | 605.9 | 3, 79 | <0.001 |
| | | MO*TRT | 1.9 | 12, 79 | 0.046 |
| S. oryzae | Live | MO | 20.7 | 4,79 | <0.001 |
| | | TRT | 58.5 | 3, 79 | <0.001 |
| | | MO*TRT | 17.0 | 12, 79 | <0.001 |
| | KD | MO | 57.3 | 4, 159 | <0.001 |
| | | TRT | 15.3 | 3, 159 | <0.001 |
| | | MO*TRT | 14.3 | 12, 159 | <0.001 |
| | Dead | MO | 32.1 | 4, 159 | <0.001 |
| | | TRT | 42.2 | 3, 159 | <0.001 |
| | | MO*TRT | 17.8 | 12, 159 | <0.001 |
Table 2.
Percentage (means ± SE) of R. dominica and S. oryzae alive (Live), knocked down (KD), or dead (means ± SE) seven days after exposure on untreated wheat (UTC) or wheat treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm + 2.5 ppm methoprene), or the new Gravista formulation (methoprene + deltamethrin + piperonyl butoxide synergist). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, 9, and 12 months after treatment 1.
Table 2.
Percentage (means ± SE) of R. dominica and S. oryzae alive (Live), knocked down (KD), or dead (means ± SE) seven days after exposure on untreated wheat (UTC) or wheat treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm + 2.5 ppm methoprene), or the new Gravista formulation (methoprene + deltamethrin + piperonyl butoxide synergist). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, 9, and 12 months after treatment 1.
| Month | Treatment | Species | % Live | % KD | % Dead |
|---|
| 0 | UTC | R. dominica | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | Gravista | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 95.0 ± 5.0 a | 0.0 ± 0.0 a | 5.0 ± 5.0 b |
| | HDiacon IGR+ | | 100.0 ± 5.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b |
| | Gravista | | 4.0 ± 4.0 b | 5.0 ± 5.0 a | 91.0 ± 5.7 a |
| 3 | UTC | R. dominica | 96.2 ± 2.3 a | 0.0 ± 0.0 a | 3.8 ± 2.3 b |
| | LDiacon IGR+ | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | Gravista | | 0.0 ± 0.0 b | 3.8 ± 2.3 a | 96.2 ± 2.3 a |
| | UTC | S. oryzae | 100.0 ± 0.0a | 0.0 ± 0.0 a | 0.0 ± 0.0 c |
| | LDiacon IGR+ | | 64.6 ± 8.1 b | 1.8 ± 1.8 a | 33.6 ± 6.5 b |
| | HDiacon IGR+ | | 42.0 ± 9.1 c | 2.0 ± 2.0 a | 56.0 ± 10.3 a |
| | Gravista | | 48.0 ± 13.6 bc | 4.0 ± 4.0 a | 48.0 ± 12.8 ab |
| 6 | UTC | R. dominica | 95.8 ± 2.6 a | 0.0 ± 0.0 a | 4.2 ± 2.6 b |
| | LDiacon IGR+ | | 2.0 ± 2.0 b | 0.0 ± 0.0 a | 98.0 ± 2.0 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 0.0 ± 0.0 a | 100.0 ± 0.0 a |
| | Gravista | | 0.0 ± 0.0 b | 3.8 ± 2.3 a | 96.2 ± 2.3 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 98.2 ± 1.8 a | 0.0 ± 0.0 a | 1.8 ± 1.8 b |
| | HDiacon IGR+ | | 37.0 ± 4.3 b | 0.0 ± 0.0 a | 68.0 ± 8.6 a |
| | Gravista | | 51.0 ± 16.1 b | 0.0 ± 0.0 a | 48.9 ± 16.6 a |
| 9 | UTC | R. dominica | 92.0 ± 5.8 a | 8.0 ± 5.8 a | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 1.8 ± 1.8 b | 0.0 ± 0.0 a | 98.2 ± 2.3 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 3.9 ± 2.4 a | 96.2 ± 2.3 a |
| | Gravista | | 0.0 ± 0.0 b | 5.8 ± 3.9 a | 94.2 ± 3.9 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 c | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 79.6 ± 4.8 b | 18.6 ± 3.4 b | 1.8 ± 1.8 a |
| | HDiacon IGR+ | | 40.7 ± 6.3 c | 59.3 ± 6.4 a | 0.0 ± 0.0 |
| | Gravista | | 75.7 ± 8.6 b | 24.2 ± 8.6 b | 0.0 ± 0.0 a |
| 12 | UTC | LGB | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 2.0 ± 2.0 b | 14.2 ± 3.9 b | 83.8 ± 3.9 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 16.0 ± 5.1 a | 84.0 ± 5.1 a |
| | Gravista | | 5.7 ± 3.9 b | 29.3 ± 12.8 a | 65.0 ± 16.3 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | HDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | Gravista | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
Table 3.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult R. dominica exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated wheat (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month. No progeny development on wheat treated with low and high rates of Diacon IGR+ or the Gravista formulation; no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
Table 3.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult R. dominica exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated wheat (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month. No progeny development on wheat treated with low and high rates of Diacon IGR+ or the Gravista formulation; no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
| Month | Treatment | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | UTC | Cont. | 47.6 ± 8.8 a | 2.51 ± 0.41 | 1.16 ± 0.32 a |
| | | 7 day | 68.2 ± 21.0 a | 1.65 ± 0.36 | 0.80 ± 0.21 a |
| 3 | UTC | Cont. | 235.4 ± 33.0 a | 6.27 ± 0.58 a | 2.96 ± 0.40 a |
| | | 7 day | 165.2 ± 29.9 b | 3.83 ± 0.63 b | 1.76 ± 0.35 b |
| 6 | UTC | Cont. | 333.8 ± 48.1 a | 5.74 ± 0.58 a | 3.42 ± 0.21 a |
| | | 7 day | 173.2 ± 42.5 b | 4.60 ± 0.99 a | 1.78 ± 0.35 b |
| 9 | UTC | Cont. | 108.4 ± 9.5 a | 4.61 ± 0.22 a | 2.78 ± 0.18 a |
| | | 7 day | 89.4 ± 13.0 a | 2.46 ± 0.47 b | 1.42 ± 0.26 b |
| 12 | UTC | Cont. | 134.0 ± 5.7 a | 6.39 ± 0.39 a | 4.51 ± 0.39 a |
| | | 7 day | 158.8 ± 9.4 a | 3.54 ± 0.21 b | 2.56 ± 0.13 b |
Table 4.
ANOVA Table for main effects month (MO), Treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation product), and count (CT, parental insects removed after 7 days or left continuously on the wheat for 8 weeks), and all associated interactions, for S. oryzae F1 progeny, % of sample weight loss, and weight in grams of feeding damage.
Table 4.
ANOVA Table for main effects month (MO), Treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation product), and count (CT, parental insects removed after 7 days or left continuously on the wheat for 8 weeks), and all associated interactions, for S. oryzae F1 progeny, % of sample weight loss, and weight in grams of feeding damage.
| Response | Variable | F | df | p |
|---|
| F1 Progeny | MO | 8.4 | 4, 159 | <0.001 |
| | TRT | 30.1 | 3, 159 | <0.001 |
| | CT | 56.2 | 1, 159 | <0.001 |
| | MO*TRT | 4.1 | 12, 159 | <0.001 |
| | MO*CT | 5.7 | 4, 159 | <0.001 |
| | TRT*CT | 2.2 | 3, 159 | 0.092 |
| | MO*TRT*CT | 2.4 | 12, 159 | 0.009 |
| % Weight Loss | MO | 7.2 | 4, 159 | <0.001 |
| | TRT | 30.4 | 3, 159 | <0.001 |
| | CT | 17.7 | 1, 159 | <0.001 |
| | MO*TRT | 1.8 | 12, 159 | 0.060 |
| | MO*CT | 0.7 | 4, 159 | 0.616 |
| | TRT*CT | 0.9 | 3, 159 | 0.454 |
| | MO*TRT*CT | 0.8 | 12, 159 | 0.629 |
| Frass Weight (g) | MO | 11.4 | 4, 159 | <0.001 |
| | TRT | 32.4 | 3, 159 | <0.001 |
| | CT | 7.6 | 1, 159 | 0.007 |
| | MO*TRT | 2.0 | 12, 159 | 0.032 |
| | MO*CT | 1.4 | 4, 159 | 0.221 |
| | TRT*CT | 0.3 | 3, 159 | 0.796 |
| | MO*TRT*CT | 0.8 | 12, 159 | 0.666 |
Table 5.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. oryzae exposed on ~80 g of untreated wheat (UTC) wheat or wheat treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm+2.5 ppm methoprene), or the Gravista. Parental adults were either continuously exposed for about 8 weeks (Cont.) or were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1,2.
Table 5.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. oryzae exposed on ~80 g of untreated wheat (UTC) wheat or wheat treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm+2.5 ppm methoprene), or the Gravista. Parental adults were either continuously exposed for about 8 weeks (Cont.) or were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1,2.
| Month | Treatment | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | UTC | Cont. | 191.0 ± 16.2 a | 2.28 ± 0.43 a | 0.38 ± 0.09 a |
| | LDiacon IGR+ | | 138.0 ± 39.7 a | 1.66 ± 0.48 ab | 0.16 ± 0.07 b |
| | HDiacon IGR+ | | 61.4 ± 36.9 b | 0.74 ± 0.37 b | 0.08 ± 0.06 b |
| | Gravista | | 43.0 ± 17.1 b | 0.37 ± 0.18 b | 0.04 ± 0.02 b |
| | UTC | 7 day | 114.8 ± 15.5 a | 1.52 ± 0.22 a | 0.29 ± 0.13 a |
| | LDiacon IGR+ | | 160.8 ± 34.3 a | 1.43 ± 0.34 a | 0.36 ± 0.11 a |
| | HDiacon IGR+ | | 53.8 ± 19.1 b | 0.43 ± 0.14 b | 0.06 ± 0.04 b |
| | Gravista | | 12.6 ± 7.7 bc | 0.17 ± 0.18 b | 0.00 ± 0.00 b |
| 3 | UTC | Cont. | 296.4 ± 56.8 a | 3.08 ± 0.55 a | 0.60 ± 0.15 a |
| | LDiacon IGR+ | | 270.8 ± 39.9 a | 2.60 ± 0.43 ab | 0.56 ± 0.07 a |
| | HDiacon IGR+ | | 81.6 ± 24.8 b | 1.32 ± 0.36 b | 0.18 ± 0.09 b |
| | Gravista | | 97.2 ± 59.8 b | 1.30 ± 0.52 b | 0.18 ± 0.16 b |
| | UTC | 7 day | 175.8 ± 35.1 a | 3.00 ± 0.68 ab | 0.56 ± 0.13 a |
| | LDiacon IGR+ | | 107.6 ± 21.1 a | 1.80 ± 0.39 b | 0.44 ± 0.13 a |
| | HDiacon IGR+ | | 42.0 ± 20.3 b | 0.92 ± 0.27 b | 0.16 ± 0.09 b |
| | Gravista | | 51.4 ± 28.3 b | 1.05 ± 0.30 ab | 0.14 ± 0.12 b |
| 6 | UTC | Cont. | 128.8 ± 41.7 b | 1.65 ± 0.45 b | 0.54 ± 0.20 a |
| | LDiacon IGR+ | | 401.7 ± 90.7 a | 3.11 ± 0.58 a | 0.90 ± 0.15 a |
| | HDiacon IGR+ | | 124.0 ± 28.7 b | 1.13 ± 0.26 b | 0.38 ± 0.10 a |
| | Gravista | | 262.8 ± 75.9 ab | 1.77 ± 0.59 b | 0.48 ± 0.14 a |
| | UTC | 7 day | 125.4 ± 14.1 a | 1.74 ± 0.28 a | 0.56 ± 0.07 a |
| | LDiacon IGR+ | | 131.2 ± 12.5 a | 1.61 ± 0.29 a | 0.72 ± 0.10 a |
| | HDiacon IGR+ | | 22.8 ± 6.3 b | 0.38 ± 0.18 b | 0.10 ± 0.04 b |
| | Gravista | | 61.0 ± 15.3 ab | 0.56 ± 0.16 b | 0.14 ± 0.05 b |
| 9 | UTC | Cont. | 146.6 ± 23.5 a | 1.94 ± 0.36 a | 0.60 ± 0.13 a |
| | LDiacon IGR+ | | 145.8 ± 26.1 a | 2.04 ± 0.49 a | 0.46 ± 0.10 a |
| | HDiacon IGR+ | | 55.8 ± 14.1 b | 0.60 ± 0.10 b | 0.16 ± 0.04 b |
| | Gravista | | 49.6 ± 13.5 b | 0.76 ± 0.14 b | 0.24 ± 0.08 b |
| | UTC | 7 day | 97.0 ± 21.1 a | 1.52 ± 0.58 a | 0.46 ± 0.05 a |
| | LDiacon IGR+ | | 79.6 ± 9.7 ab | 0.87 ± 0.07 a | 0.28 ± 0.06 ab |
| | HDiacon IGR+ | | 32.8 ± 7.6 b | 0.59 ± 0.16 a | 0.06 ± 0.02 b |
| | Gravista | | 58.5 ± 13.4 ab | 0.67 ± 0.16 a | 0.15 ± 0.06 b |
| 12 | UTC2 | Cont. | . | . | . |
| | LDiacon IGR+ | | 129.8 ± 6.5 a | 1.39 ± 0.16 a | 0.40 ± 0.03 a |
| | HDiacon IGR+ | | 88.6 ± 17.3 a | 1.32 ± 0.39 a | 0.24 ± 0.06 b |
| | Gravista | | 103.8 ± 17.4 a | 1.12 ± 0.21 a | 0.38 ± 0.05 ab |
| | UTC2 | 7 day | . | . | . |
| | LDiacon IGR+ | | 88.8 ± 18.1 a | 1.17 ± 0.16 a | 0.34 ± 0.02 a |
| | HDiacon IGR+ | | 61.6 ± 9.7 a | 0.75 ± 0.09 ab | 0.30 ± 0.03 ab |
| | Gravista | | 61.1 ± 8.1 a | 0.65 ± 0.13 b | 0.22 ± 0.04 b |
Table 6.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, two rates of Diacon IGR+, and the Gravista formulation for the percentage of adult T. castaneum and S. zeamais that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on corn.
Table 6.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, two rates of Diacon IGR+, and the Gravista formulation for the percentage of adult T. castaneum and S. zeamais that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on corn.
| Species | Response | Variable | F | df | p |
|---|
| T. castaneum | Live | MO | 2.6 | 3, 61 | 0.059 |
| | | TRT | 438.2 | 3, 61 | <0.001 |
| | | MO*TRT | 3.5 | 12, 61 | 0.002 |
| | KD | MO | 60.6 | 3, 61 | <0.001 |
| | | TRT | 77.5 | 3, 61 | <0.001 |
| | | MO*TRT | 13.7 | 9, 61 | <0.001 |
| | Dead | MO | 53.8 | 3, 61 | <0.001 |
| | | TRT | 42.7 | 3, 61 | <0.001 |
| | | MO*TRT | 11.3 | 9, 61 | <0.001 |
| S. zeamais | Live | MO | 23.2 | 3, 60 | <0.001 |
| | | TRT | 47.9 | 3, 60 | <0.001 |
| | | MO*TRT | 7.0 | 9, 60 | <0.001 |
| | KD | MO | 10.6 | 3, 60 | <0.001 |
| | | TRT | 7.8 | 3, 60 | <0.001 |
| | | MO*TRT | 6.0 | 9, 60 | <0.001 |
| | Dead | MO | 30.1 | 3, 60 | <0.001 |
| | | TRT | 32.2 | 3, 60 | <0.001 |
| | | MO*TRT | 9.6 | 9, 60 | <0.001 |
Table 7.
Percentage (means ± SE) of T. castaneum and S. zeamais alive (Live), knocked down (KD), or dead (means ± SE) seven days after exposure on untreated corn (UTC) or on corn treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm + 2.5 ppm), or the Gravista (methoprene + deltamethrin + piperonyl butoxide synergist). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, 9, and 12 months after treatment 1,2.
Table 7.
Percentage (means ± SE) of T. castaneum and S. zeamais alive (Live), knocked down (KD), or dead (means ± SE) seven days after exposure on untreated corn (UTC) or on corn treated with the low rate of Diacon IGR+ (LDiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (HDiacon IGR+, 1.0 ppm + 2.5 ppm), or the Gravista (methoprene + deltamethrin + piperonyl butoxide synergist). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, 9, and 12 months after treatment 1,2.
| Month | Treatment | Species | % Live | % KD | % Dead |
|---|
| 0 | UTC | T. castaneum | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 17.7 ± 6.1 b | 68.1 ± 6.4 a | 14.2 ± 9.2 ab |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 74.9 ± 5.9 a | 25.1 ± 5.9 a |
| | Gravista | | 0.0 ± 0.0 b | 76.0 ± 6.0 a | 24.0 ± 6.0 a |
| | UTC | S. zeamais | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 c |
| | LDiacon IGR+ | | 53.5 ± 7.7 b | 29.0 ± 7.0 a | 17.5 ± 7.7 bc |
| | HDiacon IGR+ | | 29.4 ± 7.1 b | 29.6 ± 9.6 a | 40.9 ± 14.3 b |
| | Gravista | | 0.0 ± 0.0 c | 0.0 ± 0.0 b | 100.0 ± 0.0 a |
| 3 | UTC | T. castaneum | 100.0 ± 0.0 a | 0.0 ± 0.0 c | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 4.4 ± 4.4 b | 83.5 ± 9.3 a | 12.0 ± 9.7 b |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 22.9 ± 5.7 bc | 77.1 ± 5.7 a |
| | Gravista | | 0.0 ± 0.0 b | 14.2 ± 11.6 bc | 85.8 ± 11.6 a |
| | UTC | S. zeamais | 100.0 ± 0.0 a | 0.0 ± 0.0 c | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 60.4 ± 6.8 b | 39.6 ± 6.8 b | 0.0 ± 0.0 b |
| | HDiacon IGR+ | | 77.8 ± 12.3 b | 20.0 ± 13.0 b | 2.2 ± 2.2 b |
| | Gravista | | 2.0 ± 2.0 c | 69.1 ± 9.2 a | 28.0 ± 8.9 a |
| 6 | UTC | T. castaneum | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 8.0 ± 3.7 b | 0.0 ± 0.0 b | 92.0 ± 3.7 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 c | 98.0 ± 2.0 a | 2.0 ± 2.0 b |
| | Gravista | | 0.0 ± 0.0 c | 98.0 ± 2.0 a | 2.0 ± 2.0 b |
| | UTC | S. zeamais | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | HDiacon IGR+ | | 70.0 ± 13.0 a | 10.0 ± 7.7 a | 2.0 ± 2.0 b |
| | Gravista | | 88.0 ± 7.3 a | 0.0 ± 0.0 b | 48.9 ± 16.6 a |
| 12 | UTC | T. castaneum | 87.9 ± 4.6 a | 2.3 ± 2.3 a | 9.8 ± 4.1 c |
| | LDiacon IGR+ | | 23.2 ± 11.4 b | 19.9 ± 10.5 a | 56.9 ± 12.0 b |
| | HDiacon IGR+ | | 25.0 ± 10.4 b | 25.0 ± 13.2 a | 50.1 ± 4.1 b |
| | Gravista | | 0.0 ± 0.0 b | 10.0 ± 5.4 a | 90.0 ± 5.4 a |
| | UTC | S. zeamais | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 82.4 ± 4.9 a | 0.0 ± 0.0 a | 17.6 ± 4.9 b |
| | HDiacon IGR+ | | 62.5 ± 15.5 a | 0.0 ± 0.0 a | 37.5 ± 15.5 a |
| | Gravista | | 48.0 ± 13.2 b | 18.2 ± 12.0 a | 34.0 ± 7.0 a |
Table 8.
ANOVA Table for main effects month (MO), treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation), and count (CT, parental insects removed after 7 days or left continuously on the corn for 8 weeks), and all associated interactions, for T. castaneum and S. zeamais F1 progeny, % of sample weight loss, and weight in grams of feeding damage.
Table 8.
ANOVA Table for main effects month (MO), treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation), and count (CT, parental insects removed after 7 days or left continuously on the corn for 8 weeks), and all associated interactions, for T. castaneum and S. zeamais F1 progeny, % of sample weight loss, and weight in grams of feeding damage.
| Species | Response | Variable | F | df | p |
|---|
| T. castaneum | F1 Progeny | MO | 23.9 | 4, 159 | <0.001 |
| | | TRT | 192.3 | 3, 159 | <0.001 |
| | | CT | 323.2 | 1, 159 | <0.001 |
| | | MO*TRT | 21.4 | 12, 159 | <0.001 |
| | | MO*CT | 3.2 | 4, 159 | 0.013 |
| | | TRT*CT | 0.6 | 3, 159 | 0.652 |
| | | MO*TRT*CT | 1.9 | 12, 159 | 0.043 |
| | % Weight Loss | MO | 1.6 | 4, 159 | 0.162 |
| | | TRT | 23.8 | 3, 159 | <0.001 |
| | | CT | 8.3 | 1, 159 | 0.005 |
| | | MO*TRT | 2.1 | 12, 159 | 0.020 |
| | | MO*CT | 0.6 | 4, 159 | 0.654 |
| | | TRT*CT | 2.5 | 3, 159 | 0.059 |
| | | MO*TRT*CT | 1.9 | 12, 159 | 0.038 |
| | Frass Weight | MO | 15.3 | 4, 159 | <0.001 |
| | | TRT | 270.8 | 3, 159 | <0.001 |
| | | CT | 20.5 | 1, 159 | <0.001 |
| | | MO*TRT | 15.4 | 12, 159 | <0.001 |
| | | MO*CT | 2.9 | 4, 159 | 0.023 |
| | | TRT*CT | 20.8 | 3, 159 | <0.001 |
| | | MO*TRT*CT | 1.9 | 12, 159 | 0.033 |
| S. zeamais | F1 Progeny | MO | 37.1 | 4, 155 | <0.001 |
| | | TRT | 26.3 | 3, 155 | <0.001 |
| | | CT | 37.7 | 1, 155 | <0.001 |
| | | MO*TRT | 2.6 | 12, 155 | 0.004 |
| | | MO*CT | 4.4 | 4, 155 | 0.002 |
| | | TRT*CT | 0.4 | 3, 155 | 0.724 |
| | | MO*TRT*CT | 1.8 | 12,155 | 0.053 |
| | % Weight Loss | MO | 23.6 | 4, 155 | <0.001 |
| | | TRT | 30.7 | 3, 155 | <0.001 |
| | | CT | 25.2 | 1, 155 | <0.001 |
| | | MO*TRT | 2.1 | 12, 155 | 0.024 |
| | | MO*CT | 2.0 | 4, 155 | 0.102 |
| | | TRT*CT | 1.9 | 3, 155 | 0.132 |
| | | MO*TRT*CT | 2.7 | 12, 155 | 0.002 |
| | Frass Weight | MO | 36.9 | 4, 159 | <0.001 |
| | | TRT | 33.0 | 3, 159 | <0.001 |
| | | CT | 41.0 | 1, 159 | <0.001 |
| | | MO*TRT | 1.7 | 12, 159 | 0.075 |
| | | MO*CT | 6.3 | 4, 159 | <0.001 |
| | | TRT*CT | 1.8 | 3, 159 | 0.158 |
| | | MO*TRT*CT | 2.1 | 12, 159 | 0.022 |
Table 9.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult T. castaneum exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated corn (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). No progeny development on corn treated with either rate of Diacon IGR+ or the Gravista formulation, no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
Table 9.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult T. castaneum exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated corn (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). No progeny development on corn treated with either rate of Diacon IGR+ or the Gravista formulation, no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
| Month | Treatment | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | UTC | Cont. | 11.4 ± 3.3 a | 1.18 ± 0.14 a | 0.56 ± 0.08 a |
| | | 7 day | 21.4 ± 3.6 a | 1.06 ± 0.08 a | 0.46 ± 0.04 b |
| 3 | UTC | Cont. | 0.4 ± 0.7 b | 0.66 ± 0.10 a | 0.34 ± 0.05 a |
| | | 7 day | 8.2 ± 0.4 a | 0.51 ± 0.10 a | 0.20 ± 0.04 b |
| 6 | UTC | Cont. | 4.8 ± 1.8 a | 0.78 ± 0.14 a | 0.48 ± 0.05 a |
| | | 7 day | 6.2 ± 2.4 a | 0.33 ± 0.10 b | 0.18 ± 0.06 b |
| 9 | UTC | Cont. | 18.8 ± 3.8 a | 0.68 ± 0.18 a | 0.08 ± 0.02 a |
| | | 7 day | 14.0 ± 3.6 a | 0.33 ± 0.09 b | 0.06 ± 0.06 a |
| 12 | UTC | Cont. | 35.8 ± 6.1 a | 1.58 ± 0.22 a | 0.46 ± 0.09 a |
| | | 7 day | 28.8 ± 3.4 a | 0.49 ± 0.06 b | 0.20 ± 0.04 b |
Table 10.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. zeamais exposed on ~80 g of untreated corn (UTC) wheat or corn treated with the low rate of Diacon IGR+ (Ldiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (Hdiacon IGR+, 1.0 ppm+2.5 ppm methoprene), or the Gravista formulation (methoprene + deltamethrin + piperonyl butoxide synergist). Parental adults were either continuously exposed for about 8 weeks (Cont.) or were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1,2.
Table 10.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. zeamais exposed on ~80 g of untreated corn (UTC) wheat or corn treated with the low rate of Diacon IGR+ (Ldiacon IGR+, 0.5 ppm deltamethrin + 1.25 ppm methoprene), the high rate of Diacon IGR+ (Hdiacon IGR+, 1.0 ppm+2.5 ppm methoprene), or the Gravista formulation (methoprene + deltamethrin + piperonyl butoxide synergist). Parental adults were either continuously exposed for about 8 weeks (Cont.) or were removed after 1 week and samples were held for an additional 8 weeks (7 day). Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1,2.
| Month | Treatment | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | UTC | Cont. | 23.2 ± 6.3 a | 1.86 ± 0.86 a | 0.46 ± 0.08 a |
| | Ldiacon IGR+ | | 4.0 ± 1.6 b | 0.44 ± 0.10 ab | 0.05 ± 0.03 b |
| | Hdiacon IGR+ | | 2.0 ± 1.8 b | 0.25 ± 0.06 b | 0.02 ± 0.02 b |
| | Gravista | | 0.4 ± 0.2 b | 0.29 ± 0.07 b | 0.00 ± 0.00 b |
| | UTC | 7 day | 14.6 ± 5.8 a | 0.54 ± 0.13 a | 0.20 ± 0.07 a |
| | Ldiacon IGR+ | | 7.6 ± 3.2 b | 0.39 ± 0.07 ab | 0.02 ± 0.02 b |
| | Hdiacon IGR+ | | 2.0 ± 1.1 b | 0.22 ± 0.04 b | 0.00 ± 0.00 b |
| | Gravista | | 0.0 ± 0.0 b | 0.09 ± 0.03 b | 0.00 ± 0.00 b |
| 3 | UTC | Cont. | 14.6 ± 4.3 a | 0.87 ± 0.16 ab | 0.32 ± 0.09 b |
| | Ldiacon IGR+ | | 11.8 ± 3.2 a | 1.42 ± 0.19 a | 0.52 ± 0.07 a |
| | Hdiacon IGR+ | | 13.2 ± 4.0 a | 0.52 ± 0.11 b | 0.10 ± 0.05 c |
| | Gravista | | 6.0 ± 3.9 a | 0.41 ± 0.52 b | 0.12 ± 0.04 c |
| | UTC | 7 day | 27.6 ± 14.2 a | 0.97 ± 0.12 a | 0.42 ± 0.07 a |
| | Ldiacon IGR+ | | 21.6 ± 3.8 ab | 0.56 ± 0.15 ab | 0.22 ± 0.04 b |
| | Hdiacon IGR+ | | 18.2 ± 7.1 ab | 0.58 ± 0.20 ab | 0.02 ± 0.02 c |
| | Gravista | | 4.8 ± 1.6 b | 0.23 ± 0.0.8 b | 0.08 ± 0.05 c |
| 6 | UTC | Cont. | 64.6 ± 18.6 a | 1.80 ± 0.43 a | 0.94 ± 0.24 a |
| | Ldiacon IGR+ | | 36.6 ± 3.4 ab | 0.90 ± 0.18 b | 0.54 ± 0.09 ab |
| | Hdiacon IGR+ | | 14.0 ± 6.7 b | 0.37 ± 0.16 b | 0.24 ± 0.10 b |
| | Gravista | | 15.8 ± 3.4 b | 0.52 ± 0.14 b | 0.34 ± 0.04 b |
| | UTC | 7 day | 13.0 ± 3.4 a | 0.26 ± 0.08 ab | 0.14 ± 0.04 a |
| | Ldiacon IGR+ | | 14.4 ± 5.3 a | 0.33 ± 0.13 a | 0.12 ± 0.06 ab |
| | Hdiacon IGR+ | | 4.4 ± 1.2 a | 0.06 ± 0.04 b | 0.00 ± 0.00 b |
| | Gravista | | 4.6 ± 2.2 a | 0.08 ± 0.06 ab | 0.04 ± 0.04 ab |
| 9 | UTC | Cont. | 43.0 ± 11.5 ab | 0.85 ± 0.10 ab | 0.40 ± 0.12 ab |
| | Ldiacon IGR+ | | 67.2 ± 7.3 a | 1.08 ± 0.14 a | 0.58 ± 0.09 a |
| | Hdiacon IGR+ | | 14.0 ± 6.6 b | 0.94 ± 0.21 ab | 0.44 ± 0.16 ab |
| | Gravista | | 15.8 ± 3.5 b | 0.65 ± 0.07 b | 0.24 ± 0.02 b |
| | UTC | 7 day | 58.6 ± 13.9 a | 1.40 ± 0.29 a | 0.48 ± 0.13 a |
| | Ldiacon IGR+ | | 41.0 ± 6.7 ab | 0.90 ± 0.13 ab | 0.34 ± 0.04 ab |
| | Hdiacon IGR+ | | 29.0 ± 4.1 b | 0.40 ± 0.09 b | 0.16 ± 0.06 b |
| | Gravista | | 28.2 ± 6.1 b | 0.35 ± 0.11 b | 0.12 ± 0.06 b |
| 12 | UTC | Cont. | 94.7 ± 16.8 a | 3.18 ± 0.56 a | 1.15 ± 0.18 a |
| | Ldiacon IGR+ | | 47.8 ± 5.4 b | 1.48 ± 0.20 b | 0.80 ± 0.07 a |
| | Hdiacon IGR+ | | 53.8 ± 17.1 b | 1.18 ± 0.37 b | 0.75 ± 0.22 ab |
| | Gravista | | 36.6 ± 4.4 b | 1.19 ± 0.13 b | 0.40 ± 0.10 b |
| | UTC | 7 day | 82.0 ± 21.7 a | 2.06 ± 0.41 a | 0.85 ± 0.20 a |
| | Ldiacon IGR+ | | 35.6 ± 4.9 b | 1.45 ± 0.12 b | 0.56 ± 0.11 ab |
| | Hdiacon IGR+ | | 22.2 ± 8.9 b | 0.76 ± 0.23 c | 0.28 ± 0.13 b |
| | Gravista | | 36.8 ± 5.3 b | 1.19 ± 0.24 bc | 0.38 ± 0.09 b |
Table 11.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, two rates of Diacon IGR+, and the Gravista product) for the percentage of adult R. dominica and S. oryzae that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on brown rice.
Table 11.
ANOVA Table for main effects month (MO), treatment (TR, untreated controls, two rates of Diacon IGR+, and the Gravista product) for the percentage of adult R. dominica and S. oryzae that were alive (Live), knocked down (KD), or dead (D) after 7 days of exposure on brown rice.
| Species | Response | Variable | F | df | p |
|---|
| R. dominica | Live | MO | 4.4 | 4, 79 | 0.003 |
| | | TRT | 1396.3 | 3, 79 | <0.001 |
| | | MO*TRT | 2.6 | 12, 79 | 0.006 |
| | KD | MO | 1.9 | 4, 79 | 0.115 |
| | | TRT | 21.9 | 3, 79 | <0.001 |
| | | MO*TRT | 2.4 | 12, 79 | 0.011 |
| | Dead | MO | 2.6 | 4, 79 | 0.048 |
| | | TRT | 297.0 | 3, 79 | <0.001 |
| | | MO*TRT | 1.3 | 12, 79 | 0.210 |
| S. oryzae | Live | MO | 16.7 | 4, 78 | <0.001 |
| | | TRT | 3.5 | 3, 78 | 0.012 |
| | | MO*TRT | 2.4 | 12, 78 | 0.012 |
| | KD | MO | 14.3 | 4, 78 | <0.001 |
| | | TRT | 2.4 | 3, 78 | 0.076 |
| | | MO*TRT | 2.4 | 12, 78 | 0.010 |
| | Dead | MO | 6.0 | 4, 78 | <0.001 |
| | | TRT | 3.6 | 3, 78 | 0.016 |
| | | MO*TRT | 1.2 | 12, 78 | 0.287 |
Table 12.
Percentage (means ± SE) of R. dominica and S. oryzae live, knocked down (KD), or dead (means ± SE) seven days after exposure on untreated brown rice (UTC) or brown rice treated with 0.5 ppm deltamethrin + 1.25 ppm methoprene (0.5 ppm D + 1.25 ppm M), 1.0 ppm + 2.5 ppm methoprene (1.5 ppm D + 2.5 ppm M), or the Gravista (methoprene (M) + deltamethrin (D) + piperonyl butoxide synergist (PBO). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, and 12 months after treatment 1.
Table 12.
Percentage (means ± SE) of R. dominica and S. oryzae live, knocked down (KD), or dead (means ± SE) seven days after exposure on untreated brown rice (UTC) or brown rice treated with 0.5 ppm deltamethrin + 1.25 ppm methoprene (0.5 ppm D + 1.25 ppm M), 1.0 ppm + 2.5 ppm methoprene (1.5 ppm D + 2.5 ppm M), or the Gravista (methoprene (M) + deltamethrin (D) + piperonyl butoxide synergist (PBO). Data are separated by month, bioassays were done at 0 months (1 day), and 3, 6, and 12 months after treatment 1.
| Month | Treatment | Species | % Live | % KD | % Dead |
|---|
| 0 | UTC | R. dominica | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 0.0 ± 0.0 b | 29.7 ± 3.4 a | 70.3 ± 3.4 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 18.0 ± 6.6 a | 82.0 ± 6.6 a |
| | Gravista | | 0.0 ± 0.0 b | 29.3 ± 5.1 a | 70.7 ± 5.1 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 94.2 ± 3.9 a | 0.0 ± 0.0 a | 5.8 ± 3.9 a |
| | HDiacon IGR+ | | 92.0 ± 5.8 a | 0.0 ± 0.0 a | 8.0 ± 1.4 a |
| | Gravista | | 93.7 ± 2.6 a | 0.0 ± 0.0 a | 6.3 ± 2.6 a |
| 3 | UTC | R. dominica | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 0.0 ± 0.0 b | 30.0 ± 8.4 a | 70.0 ± 8.4 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 16.0 ± 5.1 a | 84.0 ± 5.1 a |
| | Gravista | | 0.0 ± 0.0 b | 18.0 ± 3.7 a | 82.0 ± 3.7 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | LDiacon IGR+ | | 78.0 ± 2.0 b | 8.0 ± 3.7 a | 14.0 ± 2.4 a |
| | HDiacon IGR+ | | 74.1 ± 7.3 b | 18.1 ± 6.0 a | 7.8 ± 5.8 ab |
| | Gravista | | 75.8 ± 4.9 b | 10.0 ± 6.3 a | 12.2 ± 2.4 a |
| | UTC | | | | |
| 6 | LDiacon IGR+ | R. dominica | 100.0 ± 0.0 a | 0.0 ± 0.0 b | 0.0 ± 0.0 b |
| | HDiacon IGR+ | | 12.0 ± 12.0 b | 36.9 ± 11.5 a | 51.1 ± 7.5 a |
| | Gravista | | 0.0 ± 0.0 b | 23.5 ± 5.7 a | 76.5 ± 5.7 a |
| | | | 0.0 ± 0.0 b | 25.5 ± 5.7 a | 74.5 ± 5.7 a |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | HDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | Gravista | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| 9 | UTC | R. dominica | 87.9 ± 4.6 a | 2.3 ± 2.3 b | 9.8 ± 4.1 b |
| | LDiacon IGR+ | | 21.4 ± 3.4 b | 2.0 ± 2.0 b | 76.5 ± 3.8 a |
| | HDiacon IGR+ | | 0.0 ± 0.0 c | 6.0 ± 4.0 b | 94.0 ± 4.0 a |
| | Gravista | | 3.7 ± 2.3 c | 37.0 ± 10.4 a | 71.3 ± 8.1 a |
| | UTC | S. oryzae | 97.8 ± 2.2 a | 0.0 ± 0.0 a | 2.2 ± 2.2 a |
| | LDiacon IGR+ | | 98.0 ± 2.0 a | 0.0 ± 0.0 a | 2.0 ± 2.0 a |
| | HDiacon IGR+ | | 96.0 ± 2.4 a | 0.0 ± 0.0 a | 4.0 ± 2.4 a |
| | Gravista | | 96.0 ± 4.0 a | 0.0 ± 0.0 a | 4.0 ± 4.0 a |
| 12 | UTC | R. dominica | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 0.0 ± 0.0 b | 26.0 ± 4.0 b | 74.0 ± 0.0 b |
| | HDiacon IGR+ | | 0.0 ± 0.0 b | 16.0 ± 5.1 b | 84.0 ± 5.1 b |
| | Gravista | | 0.0 ± 0.0 b | 20.0 ± 7.1 b | 80.0 ± 7.1 b |
| | UTC | S. oryzae | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | LDiacon IGR+ | | 98.0 ± 2.0 a | 0.0 ± 0.0 a | 2.0 ± 2.0 a |
| | HDiacon IGR+ | | 100.0 ± 0.0 a | 0.0 ± 0.0 a | 0.0 ± 0.0 a |
| | Gravista | | 96.0 ± 4.0a | 0.0 ± 0.0a | 4.0 ± 4.0a |
Table 13.
ANOVA Table for main effects month (MO), treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation), and count (CT, parental insects removed after 7 days or left continuously on the corn for 8 weeks), and all associated interactions, for R. dominica and S. oryzae F1 progeny, % of sample weight loss, and weight in grams of feeding damage 1.
Table 13.
ANOVA Table for main effects month (MO), treatment (untreated controls, two rates of Diacon IGR+, and the Gravista formulation), and count (CT, parental insects removed after 7 days or left continuously on the corn for 8 weeks), and all associated interactions, for R. dominica and S. oryzae F1 progeny, % of sample weight loss, and weight in grams of feeding damage 1.
| Species | Response | Variable | F | df | p |
|---|
| R. dominica | F1 Progeny | MO | 2.2 | 4, 149 | 0.067 |
| | | TRT | 128.5 | 3, 149 | <0.001 |
| | | CT | 9.0 | 1, 149 | 0.003 |
| | | MO*TRT | 5.6 | 12, 149 | <0.001 |
| | | MO*CT | 2.7 | 4, 149 | 0.035 |
| | | TRT*CT | 0.2 | 3, 149 | 0.997 |
| | | MO*TRT*CT | 2.9 | 12, 149 | 0.001 |
| | % Weight Loss | MO | 9.3 | 4, 148 | <0.001 |
| | | TRT | 109.5 | 3, 148 | <0.001 |
| | | CT | 12.6 | 1, 148 | <0.001 |
| | | MO*TRT | 2.9 | 12, 148 | 0.001 |
| | | MO*CT | 2.7 | 4, 148 | 0.059 |
| | | TRT*CT | 2.5 | 3, 148 | 0.063 |
| | | MO*TRT*CT | 4.4 | 12, 148 | <0.001 |
| | Frass Weight | MO | 3.4 | 4, 149 | 0.013 |
| | | TRT | 115.2 | 3, 149 | <0.001 |
| | | CT | 16.2 | 1, 149 | <0.001 |
| | | MO*TRT | 3.0 | 12, 149 | <0.001 |
| | | MO*CT | 2.3 | 4, 149 | 0.001 |
| | | TRT*CT | 15.1 | 3, 149 | <0.001 |
| | | MO*TRT*CT | 2.2 | 12, 149 | 0.013 |
| S. oryzae | F1 Progeny | MO | 37.5 | 3, 119 | <0.001 |
| | | TRT | 0.3 | 3, 119 | 0.856 |
| | | CT | 14.1 | 1, 155 | <0.001 |
| | | MO*TRT | 5.5 | 9, 119 | 0.004 |
| | | MO*CT | 12.4 | 3, 119 | 0.002 |
| | | TRT*CT | 0.2 | 3, 119 | 0.885 |
| | | MO*TRT*CT | 1.9 | 9, 119 | 0.056 |
| | % Weight Loss | MO | 78.3 | 3, 115 | <0.001 |
| | | TRT | 2.0 | 3, 115 | 0.118 |
| | | CT | 7.4 | 1, 115 | 0.007 |
| | | MO*TRT | 3.9 | 9, 115 | 0.001 |
| | | MO*CT | 12.6 | 3, 115 | <0.001 |
| | | TRT*CT | 2.9 | 3, 115 | 0.037 |
| | | MO*TRT*CT | 4.8 | 9, 115 | <0.001 |
| | Frass Weight | MO | 22.7 | 3, 118 | <0.001 |
| | | TRT | 1.6 | 3, 118 | 0.186 |
| | | CT | 0.0 | 1, 118 | 0.973 |
| | | MO*TRT | 9.0 | 9, 118 | <0.001 |
| | | MO*CT | 2.0 | 3, 118 | 0.121 |
| | | TRT*CT | 3.4 | 3, 118 | 0.032 |
| | | MO*TRT*CT | 5.6 | 9, 118 | <0.001 |
Table 14.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult R. dominica exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated brown rice (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). No progeny development on brown rice treated with low and high rates of Diacon IGR+ or the Gravista formulation, no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
Table 14.
Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult R. dominica exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated brown rice (UTC), or parental adults were removed after 1 week and samples were held for an additional 8 weeks (7 day). No progeny development on brown rice treated with low and high rates of Diacon IGR+ or the Gravista formulation, no overall ANOVA done, only comparison between the two parental exposure regimes. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9, and 12 months after treatment 1.
| Month | Treatment | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | UTC | Cont. | 53.0 ± 7.7 a | 2.74 ± 0.53 a | 1.64 ± 0.29 a |
| | | 7 day | 74.8 ± 9.8 a | 3.11 ± 0.49 a | 0.86 ± 0.21 a |
| 3 | UTC | Cont. | 28.4 ± 2.5 a | 1.26 ± 0.27 a | 0.44 ± 0.12 a |
| | | 7 day | 38.8 ± 8.9 a | 1.26 ± 0.33 a | 0.52 ± 0.19 a |
| 6 | UTC | Cont. | 73.6 ± 12.0 a | 3.29 ± 0.31 a | 1.74 ± 0.30 a |
| | | 7 day | 43.8 ± 13.2 a | 0.83 ± 0.25 a | 0.46 ± 0.14 b |
| 9 | UTC | Cont. | 25.4 ± 1.4 a | 1.38 ± 0.33 a | 0.86 ± 0.18 a |
| | | 7 day | 37.4 ± 7.6 a | 1.73 ± 0.66 a | 0.58 ± 0.19 a |
| 12 | UTC | Cont. | 94.0 ± 23.4 a | 2.82 ± 0.77 a | 1.84 ± 0.53 a |
| | | 7 day | 50.0 ± 14.3 a | 1.22 ± 0.30 b | 0.60 ± 0.19 b |
Table 15.
Data for S. oryzae combined for treatments since overall ANOVA showed no treatment effect. Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. oryzae exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated brown rice, or adults removed after 7 days of exposure (7 day). Only comparison between the two parental exposure regimes were done. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9 months after treatment 1.
Table 15.
Data for S. oryzae combined for treatments since overall ANOVA showed no treatment effect. Number of F1 adult progeny, % of sample weight loss due to insect feeding, and feeding damage (frass) (mean ± SE for all) from exposure of ten mixed-sex parental adult S. oryzae exposed continuously for about 8 weeks (Cont.) on ~80 g of untreated brown rice, or adults removed after 7 days of exposure (7 day). Only comparison between the two parental exposure regimes were done. Data are separated by month, bioassays were done at 1 day (month 0), and 3, 6, 9 months after treatment 1.
| Month | Exposure | Progeny | %Wt. Loss | Frass (g) |
|---|
| 0 | Cont. | 462.0 ± 46.9 a | 10.44 ± 0.90 a | 1.18 ± 0.10 a |
| | 7 day | 208.2 ± 27.0 b | 5.85 ± 1.10 b | 1.17 ± 0.19 a |
| 3 | Cont. | 141.3 ± 14.2 a | 1.62 ± 0.24 a | 0.66 ± 0.15 a |
| | 7 day | 94.4 ± 6.5 b | 1.28 ± 0.12 a | 0.42 ± 0.04 a |
| 6 | Cont. | 346.6 ± 45.6 a | 2.88 ± 0.52 a | 0.88 ± 0.10 a |
| | 7 day | 304.0 ± 37.2 a | 3.01 ± 0.55 a | 0.79 ± 0.12 a |
| 9 | Cont. | 148.2 ± 8.7 a | 1.76 ± 0.17 a | 0.65 ± 0.07 a |
| | 7 day | 158.9 ± 11.2 a | 2.27 ± 0.23 b | 0.76 ± 0.09 a |