Bees Occurring in Corn Production Fields Treated with Atoxigenic Aspergillus flavus (Texas, USA)
Abstract
1. Introduction
2. Materials and Methods
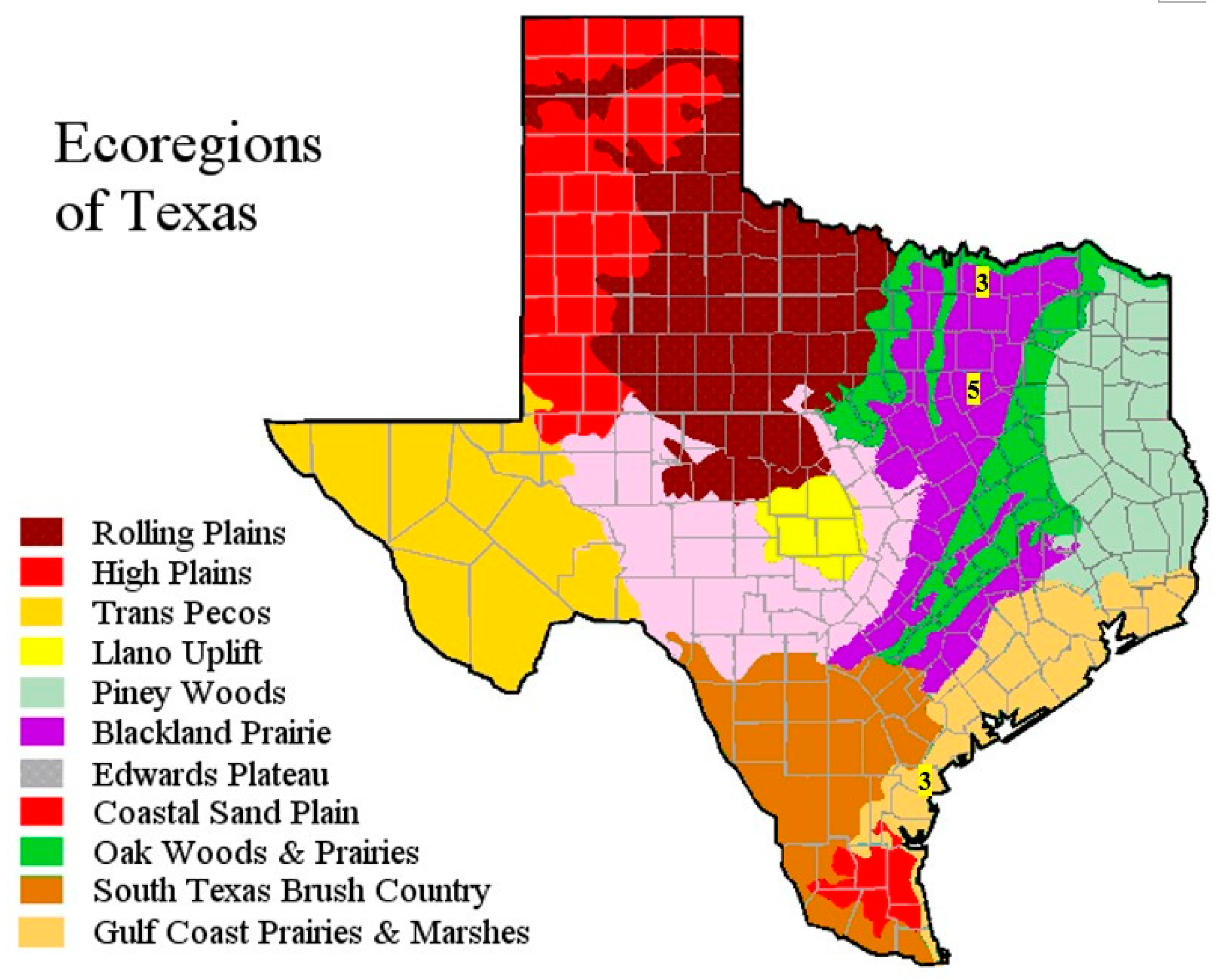
2.1. Description of Field Sites
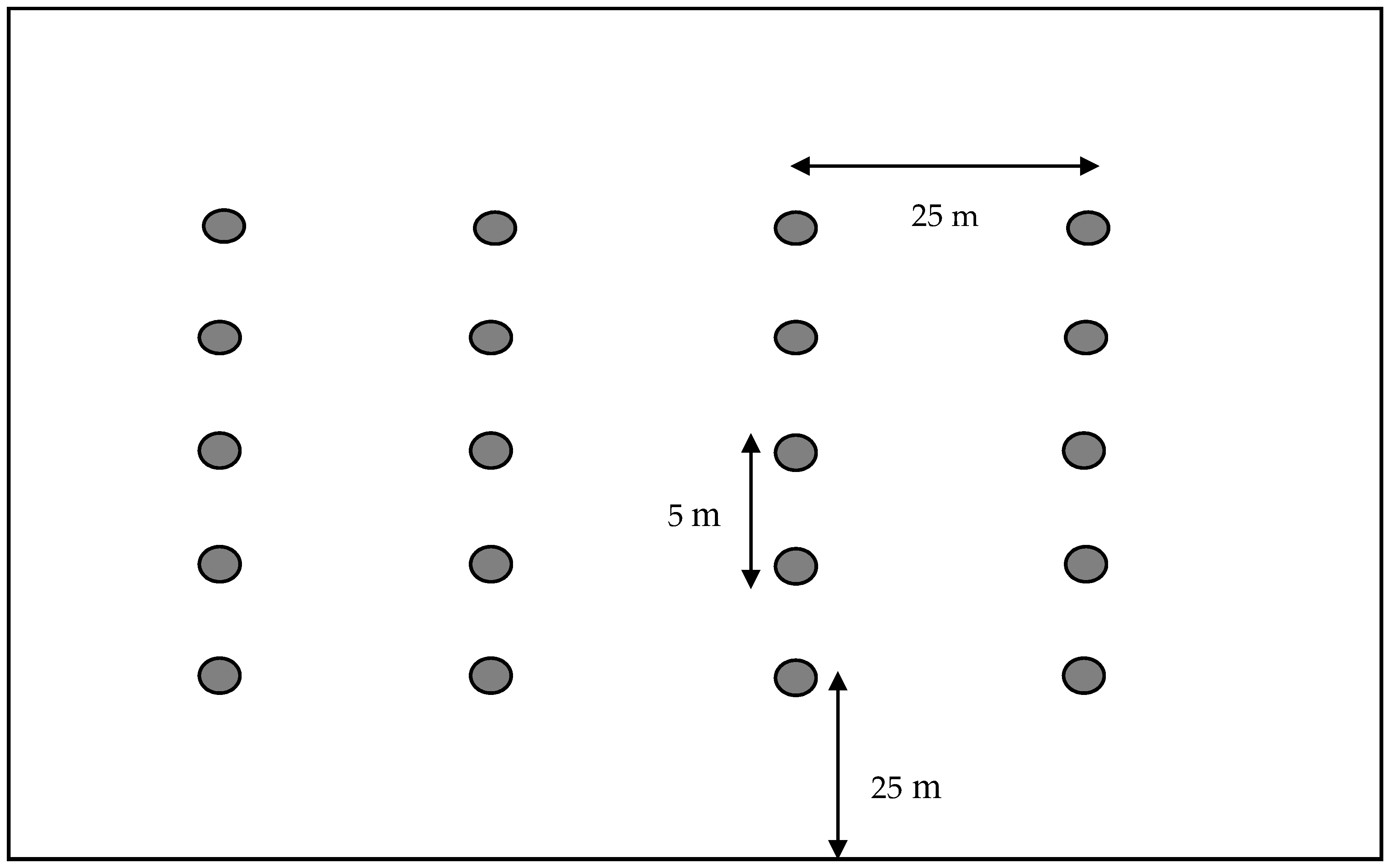
2.2. Bee Bowl Procedure
2.3. Statistical Analyses
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Mehl, H.L.; Cotty, P.J. Variation in competitive ability among isolates of Aspergillus flavus from different vegetative compatibility groups during maize infection. Phytopathology 2010, 100, 150–159. [Google Scholar] [CrossRef] [PubMed]
- Cotty, P.J. Influence of field application of an atoxigenic strain of Aspergillus flavus on the populations of A. flavus infecting cotton bolls and on the aflatoxin content of cottonseed. Phytopathology 1994, 84, 1270–1277. [Google Scholar] [CrossRef]
- Klich, M.A. Aspergillus flavus: The major producer of aflatoxin. Mol Plant Pathol. 2007, 8, 713–722. [Google Scholar] [CrossRef] [PubMed]
- Bacon, C.W.; Nelson, P.E. Fumonisin production in corn by toxigenic strains of Fusarium moniliforme and Fusarium proliferatum. J. Food Prot. 1994, 57, 514–521. [Google Scholar] [CrossRef] [PubMed]
- Payne, G.A. Aflatoxin in maize. Crit. Rev. Plant Sci. 1992, 10, 423–440. [Google Scholar] [CrossRef]
- Nishie, K.; Cole, R.J.; Domer, J.W. Toxicity and neurophamacology of cyclopiazonic acid. Food Chem. Toxicol. 1985, 9, 831–839. [Google Scholar] [CrossRef]
- US EPA. Ecological Risk Assessment for Experimental Testing of the Microbial Pesticide End-Use Product, FourSure. M (A.I.: Aspergillus Flavus Strains TCI 6F, TC35C, TC38B and) to Control Aflatoxin-Producing Toxigenic Aspergillus Flavus; EPA File Symbol 91I63-EUP-R, Tolerance Petition #5E8397; Decision No. 508471; Submission No. 972674; DP Barcode No: 4 I 9535; E-Sub #: 8226; US EPA: Washington, DC, USA, 2016.
- Jensen, A.B.; Aronstein, K.; Flores, J.M.; Vojvodic, S.; Palacio, M.A.; Spivak, M. Standard methods for fungal brood disease research. J. Apic. Res. 2013, 52, 1–39. [Google Scholar] [CrossRef] [PubMed]
- US EPA. Environmental Hazard Assessment for the Microbial Pesticide, Aspergillus Flavus AF36 for Conditional Registration in Arizona and EUP Extension in Texas; EPA Reg. No. 071693-R.; Barcode Nos. D288777; D288782; D286708; US EPA: Washington, DC, USA, 2003.
- Bayman, P.; Cotty, P.J. Vegetative compatibility and genetic variation in the Aspergillus flavus population of a single field. Can. J. Bot. 1991, 69, 1707–1711. [Google Scholar] [CrossRef]
- Ehrlich, K.C. Non-aflatoxigenic Aspergillus flavus to prevent aflatoxin contamination in crops: Advantages and limitations. Front. Microbiol. 2014, 5, 50. [Google Scholar] [CrossRef] [PubMed]
- Bhandari, K.B.; West, C.P.; Acosta-Martinez, V.; Cotton, J.; Cano, A. Soil health indicators as affected by diverse forage species and mixtures in semi-arid pastures. Appl. Soil Ecol. 2018, 132, 179–186. [Google Scholar] [CrossRef]
- Wheelock, M.J.; O’Neal, M.E. Insect pollinators in Iowa cornfields: Community identification and trapping method analysis. PLoS ONE 2016, 11, e0143479. [Google Scholar] [CrossRef] [PubMed]
- Droege, S.; Engler, J.D.; Sellers, E.; O’Brien, L.E. National Protocol Framework for the Inventory and Monitoring of Bees. U.S. Fish and Wildlife Service, U.S. Department of the Interior, 2016. Available online: https://ecos.fws.gov/ServCat/DownloadFile/151922 (accessed on 4 March 2020).
- Littell, R.C.; Milliken, G.A.; Stroup, W.W.; Wilfinger, R.D.; Schabenberger, O. SAS for Mixed Models, 2nd ed.; SAS Institute: Cary, NC, USA, 2006. [Google Scholar]
- Krupke, C.H.; Hunt, G.J.; Eitzer, B.D.; Andino, G.; Given, K. Multiple routes of pesticide exposure for honey bees living near agricultural fields. PLoS ONE 2012, 7, e29268. [Google Scholar] [CrossRef] [PubMed]
- Keller, R.; Fluri, P.; Imdorf, A. Pollen nutrition and colony development in honey bees: Part: 1. Bee World 2005, 86, 3–10. [Google Scholar] [CrossRef]
- Tuell, J.K.; Isaacs, R. Elevated pan traps to monitor bees in flowering crop canopies. Entomol. Exp. Appl. 2009, 131, 93–98. [Google Scholar] [CrossRef]
- Bhandari, K.B.; West, C.P.; Longing, S.D.; Brown, C.P.; Green, P.E.; Barkowsky, E. Pollinator abundance in semi-arid pastures as affected by forage species. Crops Sci. 2018, 58, 2665–2671. [Google Scholar] [CrossRef]
- Warriner, M.; Hutchins, B. Management Recommendations for Native Insect Pollinators in Texas. Texas Parks and Wildlife Department, 2016. Available online: https://tpwd.texas.gov/publications/pwdpubs/media/pwd_bk_w7000_1813.pdf (accessed on 4 March 2020).
- Evans, E.; Smart, M.; Cariveau, D.; Spivak, M. Wild, native bees and managed honey bees benefit from similar agricultural land uses. Agric. Ecosyst. Environ. 2018, 268, 162–170. [Google Scholar] [CrossRef]
| Variable | Treatment | County | ||
|---|---|---|---|---|
| San Patricio | Ellis | Grayson | ||
| Dates of corn planting | FourSure | 14 and 21 February | 8 March and 1 April | 22 March |
| Control | 14 and 21 February | 8 March and 1 April | 22 March | |
| Dates of FourSure application | FourSure | 15 and 22 April | 19 May | 6 June |
| Control | - | - | - | |
| Dates of bee bowl | FourSure | 21 May | 11 June | 20 June |
| Control | 21 May | 11 June | 20 June | |
| Variable | County | |||
|---|---|---|---|---|
| San Patricio | Ellis | Grayson | ||
| Air temperature (°C) † | ||||
| Previous week of sampling | Maximum | 31.3 | 29.8 | 30.9 |
| Minimum | 23.8 | 20.5 | 20.7 | |
| Average | 27.6 | 25.2 | 25.8 | |
| Sampling date | Maximum | 34.4 | 26.1 | 31.4 |
| Minimum | 26.7 | 16.1 | 21.1 | |
| Average | 30.6 | 21.1 | 26.3 | |
| Rainfall (mm) † | Total | - | 34 | 35 |
| Family | Genus | Common Name | Abundance Count |
|---|---|---|---|
| Apidae | Melissodes | long-horned bee | 59 |
| Ceratina | small carpenter bee | 3 | |
| Diadasia | sunflower/chimney bee | 3 | |
| Apis | honey bee | 2 | |
| Svastra | long-horned bee | 1 | |
| Colletidae | Hylaeus | masked bee | 1 |
| Halictidae | Agapostemon | Texas striped sweat bee | 110 |
| Lasioglossum | metallic sweat bee | 57 | |
| Halictus | sweat bee | 9 | |
| Augochlorella | green sweat bee | 2 | |
| Megachilidae | Megachile | leafcutter bee | 1 |
| Bees | Treatment | County | Mean | ||
|---|---|---|---|---|---|
| San Patricio | Ellis | Grayson | |||
| (Number Per Field) | |||||
| Total | FourSure | 29.3 | 2.4 | 1.6 | 11.1 |
| Control | 36.7 | 4.8 | 3.0 | 14.8 | |
| Treatment effect | p = 0.30 | p = 0.09 | p = 0.27 | p = 0.07 † | |
| n | 3 | 5 | 3 | ||
| Treatment x County | p = 0.53 † | ||||
| Treatment | Agapostemon texanus | Melissodes spp. | Lasioglossum spp. |
|---|---|---|---|
| (Number Per Field) | |||
| FourSure | 16.0 | 7.3 | 4.7 |
| Control | 16.7 | 8.0 | 9.0 |
| Treatment effect | p = 0.80 | p = 0.63 | p = 0.30 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bhandari, K.B.; Longing, S.D.; West, C.P. Bees Occurring in Corn Production Fields Treated with Atoxigenic Aspergillus flavus (Texas, USA). Agronomy 2020, 10, 571. https://doi.org/10.3390/agronomy10040571
Bhandari KB, Longing SD, West CP. Bees Occurring in Corn Production Fields Treated with Atoxigenic Aspergillus flavus (Texas, USA). Agronomy. 2020; 10(4):571. https://doi.org/10.3390/agronomy10040571
Chicago/Turabian StyleBhandari, Krishna B., Scott D. Longing, and Charles P. West. 2020. "Bees Occurring in Corn Production Fields Treated with Atoxigenic Aspergillus flavus (Texas, USA)" Agronomy 10, no. 4: 571. https://doi.org/10.3390/agronomy10040571
APA StyleBhandari, K. B., Longing, S. D., & West, C. P. (2020). Bees Occurring in Corn Production Fields Treated with Atoxigenic Aspergillus flavus (Texas, USA). Agronomy, 10(4), 571. https://doi.org/10.3390/agronomy10040571





