The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant and Soil Analyses
2.2. Tomatoes Samples Preparation
2.3. Chemical Analysis
2.3.1. Extract Preparation
2.3.2. Reagents and Equipment
TAC Evaluation
TPC Determination
AsA Content
Determination of Lyc Content
2.4. Statistical Analysis
3. Results
3.1. Fruit Morphological Traits
3.2. Antioxidants and Nutraceutical Component Analysis
3.2.1. Assessment of TAC
3.2.2. Assessment of TPC, Lyc, and AsAContent
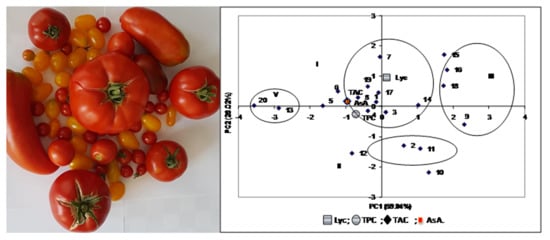
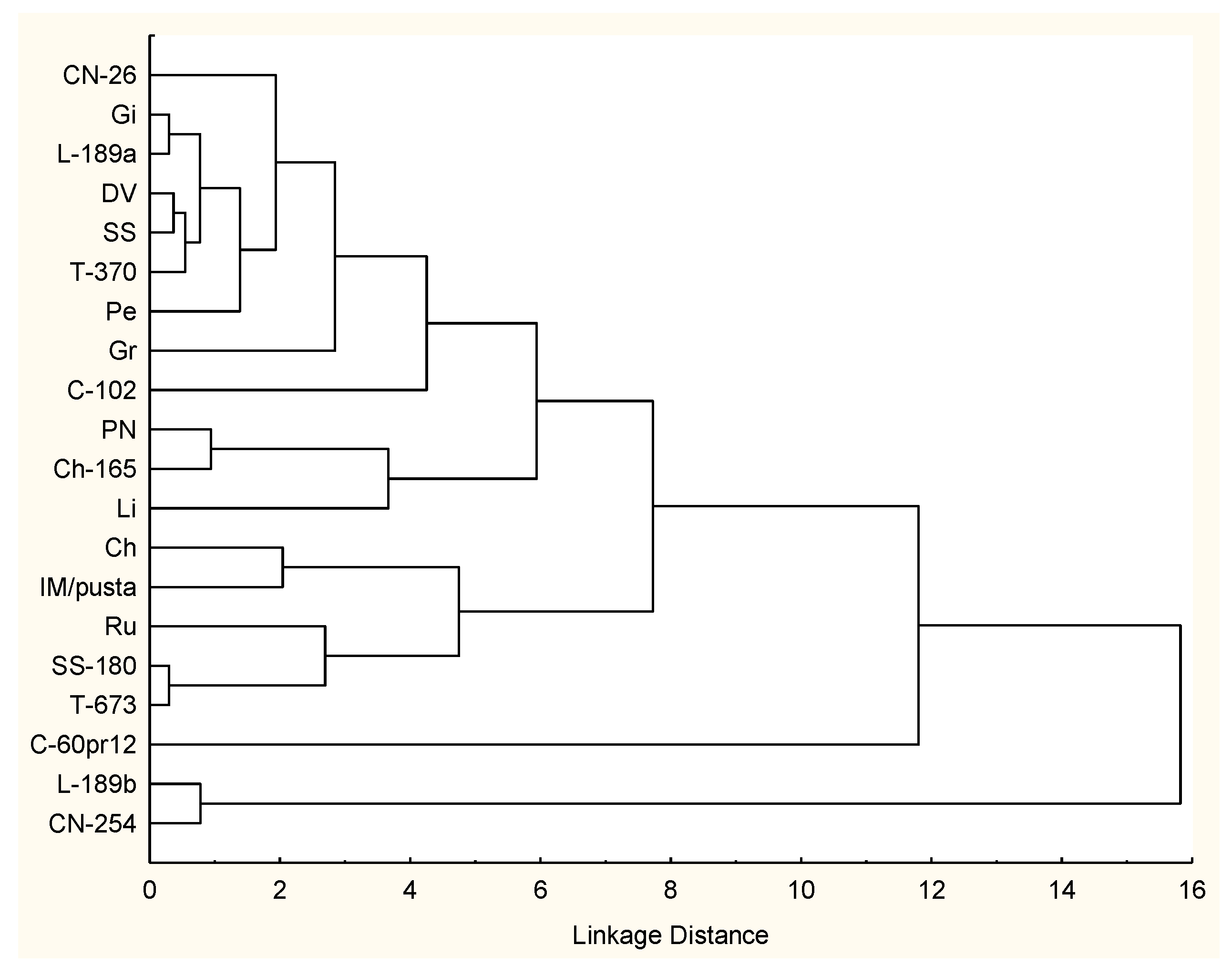
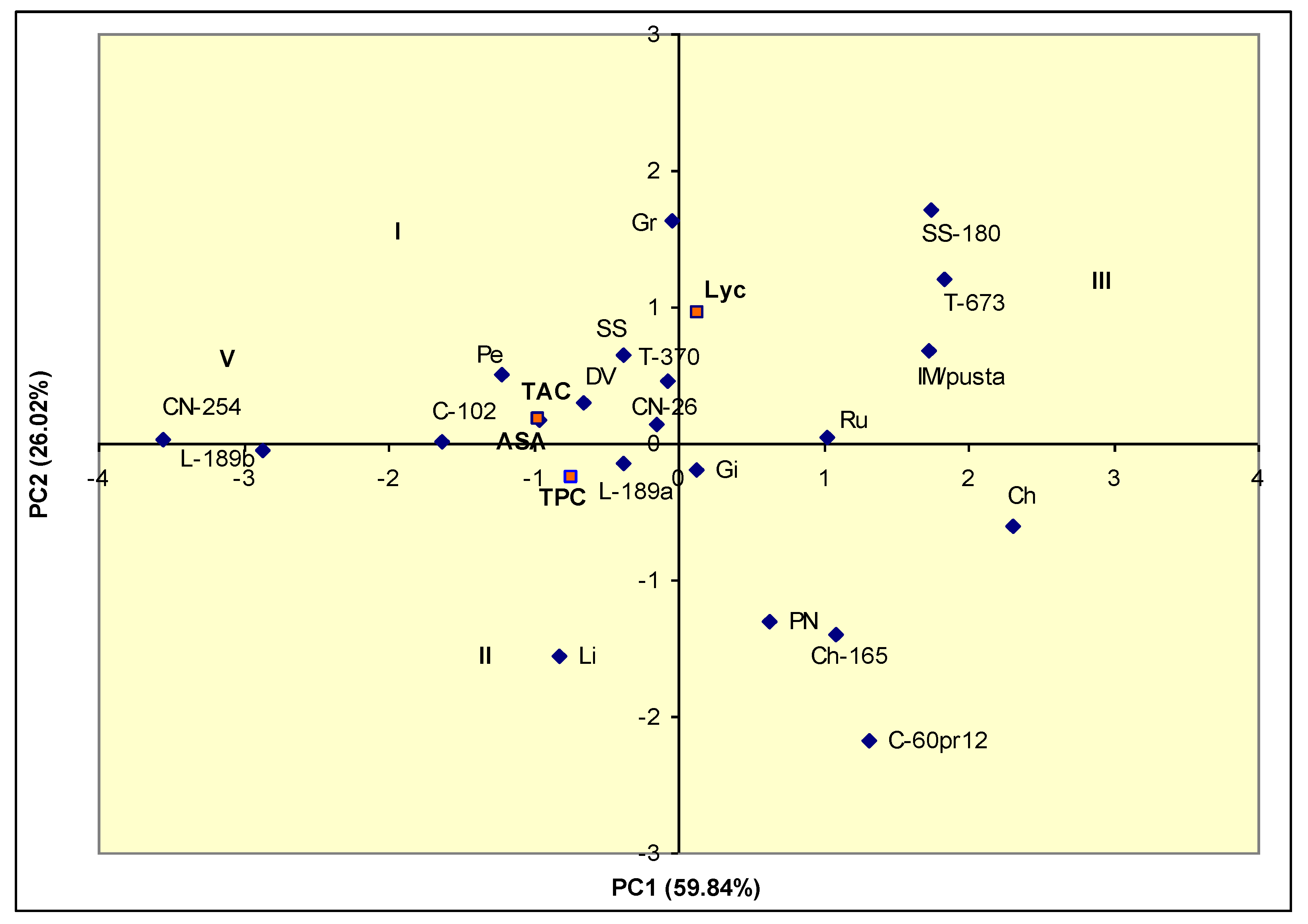
3.3. Comparison of Tomato Landraces for Nutraceutical Traits
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Díez, M.J.; Nuez, F. Vegetables II Fabaceae, Liliaceae, Solanaceae, and Umbelliferae; Prohens, J., Nuez, F., Eds.; Springer: New York, NY, USA, 2008; pp. 249–323. [Google Scholar] [CrossRef]
- Sardaro, M.L.S.; Marmiroli, M.; Maestri, E.; Marmiroli, N. Genetic characterization of Italian tomato varieties and their traceability in tomatofood products. Food Sci. Nutr. 2013, 1, 54–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shaye, N.A.; Migdadi, H.; Charbaji, A.; Alsayegh, S.; Daoud, S.; Al-Anazi, W.; Alghamdi, S. Genetic variation among Saudi tomato (Solanum lycopersicum L.) landraces studied using SDS-PAGE and SRAP markers. Saudi J. Biol. Sci. 2018, 25, 1007–1015. [Google Scholar] [CrossRef] [PubMed]
- Abete, I.; Perez-Cornago, A.; Navas-Carretero, S.; Bondia-Pons, I.; Zulet, M.A.; Martinez, J.A. A regular lycopene enriched tomato sauce consumption influences antioxidant status of healthy young-subjects: A crossover study. J. Funct. Foods 2013, 5, 28–35. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fita, A.; García-Martínez, M.D.; Casanova, C.; Borràs, D.; Plazas, M.; Andújar, I.; Soler, S. Characterization of composition traits related to organoleptic and functional quality for the differentiation, selection and enhancement of local varieties of tomato from different cultivar groups. Food Chem. 2015, 187, 517–524. [Google Scholar] [CrossRef] [Green Version]
- Scalzo, J.; Politi, A.; Pellegrini, N.; Mezzetti, B.; Battino, M. Plant genotype affects total antioxidant capacity and phenolic contents in fruit. Nutrition 2005, 21, 207–213. [Google Scholar] [CrossRef]
- Figàs, M.R.; Prohens, J.; Raigón, M.D.; Fernández-de-Córdova, P.; Fita, A.; Soler, S. Characterization of a collection of local varieties of tomato (Solanum lycopersicum L.) using conventional descriptors and the high-throughput phenomics tool Tomato Analyzer. Genet. Resour. Crop Evol. 2015, 62, 189–204. [Google Scholar] [CrossRef] [Green Version]
- Schreiner, M. Vegetable crop management strategies to increase the quantity of phytochemicals. Eur. J. Nutr. 2005, 44, 85–94. [Google Scholar] [CrossRef]
- Ceccarelli, S. Landraces: Importance and use in breeding and environmentally friendly agronomic systems. In Agrobiodiversity Conservation: Securing the Diversity of Crop Wild Relatives and Landraces; Maxted, N., EhsanDulloo, M., Ford-Lloyd, B.V., Frese, L., Iriondo, J.M., Pinheiro de Carvalho, M.A.A., Eds.; CAB International: Wallingford, UK, 2012; Chapter 15; pp. 103–117. [Google Scholar] [CrossRef]
- Zoran, I.S.; Nikolaos, K.; Ljubomir, Š. Tomato Fruit Quality from Organic and Conventional Production. In Organic Agriculture towards Sustainability; Pilipavicius, V., Ed.; In Tech: Dubrovnik, Croatia, 2014; Chapter 7; pp. 17–169. [Google Scholar] [CrossRef] [Green Version]
- Jacobo-Velázquez, D.A.; Cisneros-Zevallos, L. An alternative use of horticultural crops: Stressed plants as biofactories of bioactive phenolic compounds. Agriculture 2012, 2, 259–271. [Google Scholar] [CrossRef] [Green Version]
- Rozema, J.; Schat, H. Salt tolerance of halophytes, research questions reviewed in the perspective of saline agriculture. Environ. Exp. Bot. 2013, 92, 83–95. [Google Scholar] [CrossRef] [Green Version]
- Mazzucato, A.; Ficcadenti, N.; Caioni, M.; Mosconi, P.; Piccinini, E.; Rami, V.; Sanampudi, R.; Sestili, S.; Ferrari, V. Genetic diversity and distinctiveness in tomato (Solanum lycopersicum L.) landraces: The Italian case study of ‘A peraAbruzzese’. Sci. Hortic. 2010, 125, 55–62. [Google Scholar] [CrossRef]
- Terzopoulos, P.J.; Bebeli, P.J. Phenotypic diversity in Greek tomato (Solanum lycopersicum L.) landraces. Sci. Hortic. 2010, 126, 138–144. [Google Scholar] [CrossRef]
- Sacco, A.; Ruggieri, V.; Parisi, M.; Festa, G.; Rigano, M.M.; Picarella, M.E.; Mazzucato, A.; Barone, A. Exploring a Tomato Landraces Collection for Fruit-Related Traits by the Aid of a High-Throughput Genomic Platform. PLoS ONE 2015, 10, e0137139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beckles, D.M. Factors affecting the postharvest soluble solids and sugar content of tomato (Solanum lycopersicum L.) fruit. Postharvest Biol. Technol. 2012, 63, 129–140. [Google Scholar] [CrossRef]
- Oliveira, S.M.; Brandao, T.R.S.; Silva, C.L.M. Influence of drying processes and pretreatments on nutritional and bioactive characteristics of dried vegetables: A review. Food Eng. Rev. 2016, 8, 134–163. [Google Scholar] [CrossRef]
- Causse, M.; Friguet, C.; Coiret, C.; Lépicier, M.; Navez, B.; Lee, M.; Holthuysen, L.; Sinesio, F.; Moneta, E.; Grandillo, S. Consumer preferences for fresh tomato at the European scale: A common segmentation on taste and firmness. J. Food Sci. 2010, 75, S531–S541. [Google Scholar] [CrossRef]
- Siddiqui, M.W.; Ayala-Zavala, J.F.; Dhua, R.S. Genotypic variation intomatoes affecting processing and antioxidant properties. Crit. Rev. Food Sci.Nutr. 2015, 55, 1819–1835. [Google Scholar] [CrossRef]
- Garg, N.; Cheema, D.S. Assessment of fruit quality attributes of tomato hybrids involving ripening mutants under high temperature conditions. Sci. Hortic. 2011, 131, 29–38. [Google Scholar] [CrossRef]
- Fernandez-Ruiz, V.; Olives, A.I.; Camara, M.; Sanchez-Mata, M.C.; Torija, M.E. Mineral and trace elements content in 30 accessions of tomato fruits (Solanum lycopersicum L.) and wild relatives (Solanum pimpinellifolium L., Solanum cheesmaniae L. Riley, and Solanum habrochaites S. Knapp & D.M. Spooner). Biol. Trace Elem. Res. 2011, 141, 329–339. [Google Scholar] [CrossRef]
- Antunes, M.D.C.; Rodrigues, D.; Pantazis, V.; Cavaco, A.M.; Siomos, A.; Miguel, G. Nutritional quality changes of fresh-cut tomato during shelf life. Food Sci. Biotechnol. 2013, 22, 1–8. [Google Scholar] [CrossRef]
- Hala, E.M.A.; Ghada, S.M.I. Tomato fruit quality as influenced by salinity and nitric oxide. Turk. J. Bot. 2014, 38, 122–129. [Google Scholar] [CrossRef]
- Barros, L.; Duenas, M.; Pinela, J.; Carvalho, A.M.; Buelga, C.S.; Ferreira, I.C. Characterization and quantification of phenolic compounds in four tomato (Lycopersicon esculentum L.) farmers’ varieties in northeastern Portugal homegardens. Plant Foods Hum. Nutr. 2012, 67, 229–234. [Google Scholar] [CrossRef] [PubMed]
- Doncean, A.; Sumalan, R.M.; Beinsan, C.; Gergen, I.; Sumalan, R.L. Influence of different types and mixtures of composts on quality of tomatoes fruits (Solanum lycopersicum L.). J. Hyg. Eng. Des. 2015, 8, 40–47. [Google Scholar]
- Gitenay, D.; Lyan, B.; Rambeau, M.; Mazur, A.; Rock, E. Comparison of lycopene and tomato effects on biomarkers of oxidative stress in vitamin E deficient rats. Eur. J. Nutr. 2007, 46, 468–475. [Google Scholar] [CrossRef] [PubMed]
- Pinela, J.; Barros, L.; Carvalho, A.M.; Ferreira, I.C. Nutritional composition and antioxidant activity of four tomato (Lycopersicon esculentum L.) farmer’ varieties in Northeastern Portugal homegardens. Food Chem. Toxicol. 2012, 50, 829–834. [Google Scholar] [CrossRef]
- Khoo, H.E.; Prasad, K.N.; Kong, K.W.; Jiang, Y.; Ismail, A. Carotenoids and their isomers: Color pigments in fruits and vegetables. Molecules 2011, 16, 1710–1738. [Google Scholar] [CrossRef]
- Srivastava, S.; Srivastava, A.K. Lycopene; chemistry, biosynthesis, metabolism and degradation under various abiotic parameters. J. Food Sci. Technol. 2015, 52, 41–53. [Google Scholar] [CrossRef]
- Chiva-Blanch, G.; Visioli, F. Polyphenols and health: Moving beyond antioxidants. J. Berry Res. 2012, 2, 63–71. [Google Scholar] [CrossRef] [Green Version]
- Raiola, A.; Del Giudice, R.; Monti, D.M.; Tenore, G.C.; Barone, A.; Rigano, M.M. Bioactive compound content and cytotoxic effect on human cancer cells of fresh and processed yellow tomatoes. Molecules 2015, 21, 33–47. [Google Scholar] [CrossRef] [Green Version]
- Opara, U.L.; Al-Ani, M.R.; Al-Rahbi, M. Effect of fruit ripening stage on physico-chemical properties, nutritional composition and antioxidant components of tomato (Lycopersicum esculentum) cultivars. Food Bioprocess Technol. 2012, 5, 3236–3243. [Google Scholar] [CrossRef]
- Corwin, D.L.; Lesch, S.M. Application of soil electrical conductivity to precision agriculture: Theory principles and guideline. Agron. J. 2003, 95, 455–471. [Google Scholar] [CrossRef]
- Sumalan, R.L.; Popescu, I.; Schmidt, B.; Sumalan, R.M.; Popescu, C.; Gaspar, S. Salt tolerant tomatoes local landraces from Romania–Preserving the genetic resources for future sustainable agriculture. J. Biotehnol. 2015, 208, S18. [Google Scholar] [CrossRef]
- IUSS Working Group WRB. World Reference Base for Soil Resources 2014, update 2015. In International Soil Classification System for Naming Soils and Creating Legends for Soil Maps; World Soil Resources Reports No. 106; FAO: Rome, Italy, 2015. [Google Scholar]
- UPOV—International Union for the Protection of New Varieties of Plants–Geneva. TOMATO UPOV Code: SOLAN_LYC Solanum lycopersicum L. Guidelines for the Conduct of Tests for Distinctness, Uniformity and Stability. 2018. Available online: https://www.upov.int/edocs/tgdocs/en/tg044.pdf (accessed on 27 March 2020).
- Benzie, I.F.; Strain, J.J. Ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- AOAC. Vitamin C (ascorbic acid) in vitamin preparations and juices. In AOAC: Official Methods of Analysis, 15th ed.; Helrich, K., Ed.; Association of Official Analytical Chemists, Inc.: Arlington County, VA, USA, 1990; Volume 1, pp. 1058–1059. [Google Scholar]
- Sharma, S.K.; Le Maguer, M. Lycopene in tomatoes and tomato pulp fractions. Ital. J. Food Sci. 1996, 8, 107–113. [Google Scholar]
- Toor, R.K. Influence of different types of fertilizers on the major antioxidant components of tomatoes. J. Food Compos. Anal. 2006, 19, 20–27. [Google Scholar] [CrossRef]
- Ciulca, S. Metodologii de Experimentare în Agricultura şi Biologie; Editura Agroprint: Timisoara, Romania, 2006; pp. 33–53. [Google Scholar]
- Felsenstein, J. PHYLIP (Phylogeny Inference Package) Version 3.5c; Distributed by the author; Department of Genetics, University of Washington: Seattle, WA, USA, 1993. [Google Scholar]
- Singh, R.K.; Chaudhary, B.D. Biometrical Methods in Quantitative Genetic Analysis; Kalyani Publishers: Ludhiana, India, 1979; pp. 191–200. [Google Scholar]
- Yan, W.; Hunt, L.A.; Sheng, Q.; Szlavnics, Z. Cultivar evaluation and mega-environment investigation based on the GGE biplot. Crop Sci. 2000, 40, 597–605. [Google Scholar] [CrossRef]
- Yan, W.; Kang, M.S. GGE Biplot Analysis: A Graphical Tool for Breeders, Geneticists, and Agronomists, 1st ed.; CRC Press: Boca Raton, FL, USA, 2002; p. 144. [Google Scholar]
- Corrado, G.; Piffanelli, P.; Caramante, M.; Coppola, M.; Rao, R. SNP genotyping reveals genetic diversity between cultivated landraces and contemporary varieties of tomato. BMC Genom. 2013, 14, 835–848. [Google Scholar] [CrossRef] [Green Version]
- Cebola-Cornejo, J.; Rosello, S.; Nuez, F. Phenotypic and genetic diversity of Spanish tomato landraces. Sci. Hortic. 2013, 162, 150–164. [Google Scholar] [CrossRef] [Green Version]
- Knapp, S.; Peralta, I.E. The tomato (Solanum lycopersicum L. Solanaceae) and its botanical relatives. In The Tomato Genome (Compendium of Plant Genomes); Causse, M., Giovannoni, J., Bouzayen, M., Zouine, M., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 7–21. [Google Scholar] [CrossRef]
- Corrado, G.; Caramante, M.; Piffanelli, P.; Rao, R. Genetic diversity in Italian tomato landraces: Implications for the development of a core collection. Sci. Hortic. 2014, 168, 138–144. [Google Scholar] [CrossRef]
- Tomescu, D.; Sumalan, R.L.; Copolovici, L.; Copolovici, D. The influence of soil salinity on volatile organic compounds emission and photosynthetic parameters of Solanum lycopersicum L. varieties. Open Life Sci. 2017, 12, 135–142. [Google Scholar] [CrossRef]
- Salim, M.M.R.; Rashid, M.H.; Hossain, M.M.; Zakaria, M. Morphological characterization of tomato (Solanum lycopersicum L.) genotypes. J. Saudi Soc. Agric. Sci. 2018, in press. [Google Scholar] [CrossRef]
- Tanksley, S.D. The genetic, developmental, and molecular bases of fruit size and shape variation in tomato. Plant Cell 2004, 16, S181–S189. [Google Scholar] [CrossRef]
- Miller, G.; Suzuki, N.; Ciftci-Yilmaz, S.; Mittler, R. Reactive oxygen species homeostasis and signaling during drought and salinity stresses. Plant Cell Environ. 2010, 33, 453–467. [Google Scholar] [CrossRef]
- Passam, H.C.; Karapanos, I.C.; Bebeli, P.J.; Savvas, D. A review of recent research on tomato nutrition, breeding and post-harvest technology with reference to fruit quality. Eur. J. Plant Sci. Biotechnol. 2007, 1, 1–21. [Google Scholar]
- García-Valverde, V.; Navarro-González, I.; García-Alonso, J.; Periago, M.J. Antioxidant bioactive compounds in selected industrial processing and fresh consumption tomato cultivars. Food Bioprocess Technol. 2013, 6, 391–402. [Google Scholar] [CrossRef]
- Deng, G.-F.; Lin, X.; Xu, X.-R.; Gao, L.-L.; Xie, J.-F.; Li, H.-B. Antioxidant capacities and total phenolic contents of 56 vegetables. J. Funct. Foods 2013, 5, 260–266. [Google Scholar] [CrossRef]
- Ilahy, R.; Hdider, C.; Lenucci, M.S.; Tlili, I.; Dalessandro, G. Antioxidant activity and bioactive compound changes during fruit ripening of high-lycopene tomato cultivars. J. Food Compos. Anal. 2011, 24, 588–595. [Google Scholar] [CrossRef]
- Martinez-Valverde, I.; Periago, M.J.; Provan, G.; Chesson, A. Phenolic compounds, lycopene and antioxidant activity in commercial varieties of tomato (Lycopersicum esculentum). J. Sci. Food Agric. 2002, 82, 323–330. [Google Scholar] [CrossRef]
- Drakou, M.; Birmpa, A.; Koutelidakis, A.E.; Komaitis, M.; Panagou, E.Z.; Kapsokefalou, M. Total antioxidant capacity, total phenolic content and iron and zinc dialyzability in selected Greek varieties of table olives, tomatoes and legumes from conventional and organic farming. Int. J. Food Sci. Nutr. 2015, 66, 197–202. [Google Scholar] [CrossRef] [PubMed]
- Hernández, V.; Hellín, P.; Fenoll, J.; Flores, P.J. Increased temperature produces changes in the bioactive composition of tomato, depending on its developmental stage. J. Agric. Food Chem. 2015, 63, 2378–2382. [Google Scholar] [CrossRef]
- Lairon, D. Nutritional quality and safety of organic food. A review. Agron. Sustain. Dev. 2010, 30, 33–41. [Google Scholar] [CrossRef] [Green Version]
- Massaretto, I.L.; Albaladejo, I.; Purgatto, E.; Flores, F.B.; Plasencia, F.; Egea-Fernanadez, J.M.; Bolarin, M.C.; Egea, I. Recovering tomato landraces to simultaneously fruit yield and nutritional quality against salt stress. Front. Plant Sci. 2018, 9, 1–17. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alba, R.; Cordonnier-Pratt, M.M.; Pratt, L.H. Fruit-localized phytochromes regulate lycopene accumulation independently of ethylene production in tomato. Plant Physiol. 2000, 123, 363–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rosati, C.; Aquilani, R.; Dharmapuri, S.; Pallara, P.; Marusic, C.; Tavazza, R.; Bouvier, F.; Camara, B.; Giuliano, G. Metabolic engineering of beta-carotene and lycopene content in tomato fruit. Plant J. 2000, 24, 413–419. [Google Scholar] [CrossRef] [PubMed]
- Giovannetti, M.; Avio, L.; Barale, L.; Ceccarelli, N.; Cristofani, R.; Iezzi, A.; Mignolli, F.; Picciarelli, P.; Pinto, B.; Reali, D.; et al. Nutraceutical value and safety of tomato fruits produced by mycorrhizal plants. Br. J. Nutr. 2012, 107, 242–251. [Google Scholar] [CrossRef] [Green Version]
- Perveen, R.; Rasul-Suleria, H.A.; Anjum, F.M.; Butt, M.S.; Pasha, I.; Sarfraz, A. Tomato (Solanumlycopersicum) carotenoids and lycopenes chemistry; metabolism, absorption, nutrition, and allied health claims—A comprehensive review. Crit. Rev. Food Sci. Nutr. 2015, 55, 919–929. [Google Scholar] [CrossRef]
- Kavitha, P.; Shivashankara, K.S.; Rao, V.K.; Sadashiva, A.T.; Ravishankar, K.V.; Sathish, G.J. Genotypic variability for antioxidant and quality parameters among tomato cultivars, hybrids, cherry tomatoes and wild species. J. Sci. Food Agric. 2014, 94, 993–999. [Google Scholar] [CrossRef]
- Borghesi, E.; González-Miret, M.L.; Escudero-Gilete, M.L.; Malorgio, F.; Heredia, F.J.; Meléndez-Martínez, A.J. Effects of Salinity Stress on Carotenoids, Anthocyanins, and Color of Diverse Tomato Genotypes. J. Agric. Food Chem. 2011, 59, 11676–11682. [Google Scholar] [CrossRef]
- Giannakoula, A.E.; Ilias, A.F. The effect of water stress and salinity on growth and physiology of tomato (Lycopersicon Esculentum Mill.). Arch. Biol. Sci. 2013, 65, 611–620. [Google Scholar] [CrossRef]
- Ehret, D.L.; Usher, K.; Helmer, T.; Block, G.; Steinke, D.; Frey, B.; Kuang, T.; Diarra, M. Tomato Fruit Antioxidants in Relation to Salinity and Greenhouse Climate. J. Agric. Food Chem. 2013, 61, 1138–1145. [Google Scholar] [CrossRef]
- Van Meulebroek, L.; Hanssens, J.; Steppe, K.; Vanhaecke, L. Metabolic Fingerprinting to Assess the Impact of Salinity on Carotenoid Content in Developing Tomato Fruits. Int. J. Mol. Sci. 2016, 17, 821. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, S.; Das, M. Functional foods: An overview. Food Sci. Biotechnol. 2011, 20, 861–875. [Google Scholar] [CrossRef]
- Rao, A.V.; Agarwal, S. Role of antioxidant lycopene in cancer and heart disease. J. Am. Coll. Nutr. 2000, 19, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, B.; Asada, K.; Kramer, D.M.; Edwards, G. Characterization of photosynthetic electron transport in bundle sheath cells of maize. I. Ascorbate effectively stimulates cyclic electron flow around PSI. Planta 2005, 220, 572–581. [Google Scholar] [CrossRef]
- Shao, H.B.; Chu, L.Y.; Shao, M.A.; Jaleel, C.A.; Mi, H.M. Higher plant antioxidants and redox signaling under environmental stresses. Comptes Rendus Biol. 2008, 331, 433–441. [Google Scholar] [CrossRef]
- Bodnarescu, F.; Sumalan, R.M.; Ciulca, S.; Copolovici, L.; Sumalan, R.L. The influence of parental lines on lycopene and ß-carotene content in tomato F1 hybrids (Solanum lycopersicum L.). Res. J. Agric. Sci. 2018, 50, 90–97. [Google Scholar]
- Rossi, F.; Godani, F.; Bertuzzi, T.; Trevisan, M.; Ferrari, F.; Gatti, S. Health-promoting substances and heavy metal content in tomatoes grown with different farming techniques. Eur. J. Nutr. 2008, 47, 266–272. [Google Scholar] [CrossRef]
- Carnovale, E. La qualitanutrizionaledeiprodottidell’agricolturabiologica. Italus Hortus 1999, 6, 41–44. [Google Scholar]
- Lucarini, M.; Carbonaro, M.; Nicoli, S.; Aguzzi, A.; Cappelloni, M.; Ruggeri, S.; DiLullo, G.; Gambelli, L.; Carnovale, E. Endogenous markers for organic versus conventional plant products. In Agrifood Quality II: Quality Managements of Fruits and Vegetables, Session VI: Quality Assessment; Hagg, M., Ahvenainen, R., Evers, A.M., Tiilikkala, K., Eds.; Royal Society of Chemistry: Cambridge, UK, 1999; pp. 306–310. [Google Scholar]
- Asami, D.K.; Hong, Y.J.; Barrett, D.M.; Mitchell, A.E. Comparison of the total phenolic and ascorbic acid content of freeze-dried and air-dried marionberry, strawberry and corn grown using conventional, organic and sustainable agricultural practices. J. Agric. Food Chem. 2003, 51, 1237–1241. [Google Scholar] [CrossRef]
- Caris-Veyrat, C.; Amiot, M.J.; Tyssandier, V.; Grasselly, D.; Buret, M.; Mikolajczak, M.; Guilland, J.C.; Bouteloup-Demange, C.; Borel, P. Influence of organic versus conventional agricultural practice on the antioxidant microconstituent content of tomatoes and derived pureed; consequences on antioxidant plasma status in humans. J. Agric. Food Chem. 2004, 52, 6503–6509. [Google Scholar] [CrossRef]
- Auclair, L.; Zee, J.A.; Karam, A.; Rochat, E. Valeur nutritive qualitéorganoleptique et productivite des tomatoes de serre en function de leur mode de production: Biologique-conventionel-hydroponique. Sci. Aliment. 1995, 15, 511–528. [Google Scholar]
- Dumas, Y.; Dadomo, A.; Di Lucca, G.; Grolier, P. Effects of environmental factors and agricultural techniques on antioxidant content of tomatoes. J. Sci. Food Agric. 2003, 83, 369–382. [Google Scholar] [CrossRef]
- Dorais, M.; Ehret, D.L.; Papadopoulos, A.P. Tomato (Solanum lycopersicum) health components: From the seed to the consumer. Phytochem. Rev. 2008, 7, 231–250. [Google Scholar] [CrossRef]
- Fanasca, S.; Martino, A.; Heuvelink, E.; Stanghellini, C. Effect of electrical conductivity, fruit pruning, and truss position on quality in greenhouse tomato fruit. J. Hort. Sci. Biotechnol. 2007, 82, 488–494. [Google Scholar] [CrossRef]
- Helyes, L.; Pék, Z.; Lugasi, A. Function of the variety technological traits and growing conditions on fruit components of tomato (Lycopersicon Lycopersicum L Karsten). Acta Aliment. 2008, 37, 427–436. [Google Scholar] [CrossRef]
- Hallmann, E.; Lipowski, J.; Marszałek, K.; Rembiałkowska, E. The seasonal variation in bioactive compounds content in juice from organic and non-organic tomatoes. Plant Foods Hum. Nutr. 2013, 68, 171–176. [Google Scholar] [CrossRef] [Green Version]
| Genotype Code | Site | GPS Coordinates (lat/long) | Soil EC(dSm−1) | Fruit Shape 1 | Tomatoes Weight Average (g) 2 | Full Ripeness Color 3 |
|---|---|---|---|---|---|---|
| CN26 | Crai Nou | 45°29′17″N/21°0′1″E | 6.86 | flattened | 352.47 | light red |
| PN | Peciu Nou | 45°36′54″N/21°01′54″E | 5.63 | flattened | 184.66 | light red |
| Gi | Giera | 45°25′21″N/20°57′25″E | 5.25 | circular | 124.18 | red |
| L-189a | Lovrin | 45°57′03″N/20°46′32″E | 5.02 | obovate | 73.75 | light red |
| C-102 | Cruceni | 45°28′23″N/20°52′44″E | 7.04 | circular | 133.55 | light red |
| Pe | Periam | 46°01′41″N/20°53′35″E | 6.86 | flattened | 295.76 | red |
| Gr | Gradinari | 45°06′16″N/21°34′59″E | 4.38 | circular | 273.00 | light red |
| DV | Dudestii Vechi | 46°04′55″N20°26′55″E | 5.80 | obovate | 164.92 | red |
| Ch | Cheglevici | 46°6′40″N/20°26′56″E | 6.04 | flattened | 264.54 | red |
| C-60pr12 | Cherestur | 46°7′60″N/20°22′60″E | 5.65 | ovate | 150.64 | light red |
| Ch-165 | Cheglevici | 46°6′40″N/20°26′56″E | 6.29 | circular | 105.44 | light red |
| Li | Livezile | 45°23′09″N/21°02′43″E | 6.44 | cilindric | 133.62 | yellow |
| L-189b | Lovrin | 21°02′43″E/20°46′32″E | 6.58 | flattened | 136.11 | light red |
| Ru | Rudna | 45°29′54″N/21°0′31″E | 4.50 | obovate | 81.73 | red |
| SS180 | Sanmartinu Sarbesc | 45°36′23″N/20°57′38″E | 4.47 | cordate | 303.09 | red |
| T673 | Tarnova | 45°20′06″N/22°00′08″E | 4.11 | flattened | 309.57 | red |
| T370 | Tarnova | 45°20′06″N/22°00′08″E | 4.23 | flattened | 348.42 | red |
| IM/pusta | Iecea Mare/Pusta | 45°50′51″N/20°54′08″E | 4.18 | flattened | 392.60 | red |
| SS | Sanmartinu Sarbesc | 45°36′23″N/20°57′38″E | 4.30 | flattened | 185.59 | light red |
| CN-254 | Crai Nou | 45°29′17″N/21°0′1″E | 7.21 | obovate | 150.33 | red |
| Genotype Code | FRAP µM Fe2± 100 g−1 FW |
|---|---|
| CN-26 | 413.27 ± 3.63 d |
| PN | 305.44 ± 2.99 g |
| Gi | 349.03 ± 3.76 f |
| L-189a | 386.49 ± 3.91 e |
| C-102 | 420.97 ± 2.13 d |
| Pe | 451.76 ± 1.36 c |
| Gr | 384.03 ± 3.19 e |
| DV | 411.36 ± 2.02 |
| Ch | 274.00 ± 1.53 i |
| C-60pr12 | 240.75 ± 1.51 j |
| Ch-165 | 304.67 ± 1.65 g |
| Li | 415.14 ± 1.99 d |
| L-189b | 506.51 ± 2.65 b |
| Ru | 300.05 ± 2.42 gh |
| SS-180 | 289.21 ± 1.58 h |
| T-673 | 287.27 ± 1.60 hi |
| T-370 | 407.56 ± 2.31 d |
| IM/pusta | 307.69 ± 1.62 g |
| SS | 387.05 ± 1.92 e |
| CN-254 | 561.61 ± 4.37 a |
| Mean | 370.19 ± 10.48 |
| Cochran’s C Test Bartlett’s Test LSD5% | 0.145; p = 1.00 |
| 1.300; p = 0.974 | |
| 13.78 |
| Genotype Code | Lycopene mg 100 g−1 FW | Ascorbic Acid mg 100 g−1 FW | Total Phenols mg GAE 100 g−1 FW |
|---|---|---|---|
| CN26 | 10.72 ± 0.19 hi | 16.93 ± 0.20 de | 69.15 ± 1.28 f |
| PN | 7.93 ± 0.12 k | 15.21 ± 0.14 ij | 92.88 ± 1.83 c |
| Gi | 11.43 ± 0.17 g | 15.94 ± 0.17 h | 93.22 ± 1.71 c |
| L-189a | 11.20 ± 0.13 gh | 16.44 ± 0.19 fg | 93.68 ± 2.18 c |
| C-102 | 12.57 ± 0.12 f | 17.37 ± 0.16 bc | 123.32 ± 2.10 a |
| Pe | 12.51 ± 0.10 f | 17.70 ± 0.18 b | 91.76 ± 2.19 cd |
| Gr | 17.37 ± 0.14b | 16.56 ± 0.14 ef | 86.58 ± 1.67 cd |
| DV | 12.29 ± 0.11 f | 16.99 ± 0.16 cde | 91.37 ± 1.70 cd |
| Ch | 9.30 ± 0.08 j | 13.98 ± 0.16 l | 53.06 ± 0.73 g |
| C-60pr12 | 6.81 ± 0.06 l | 13.17 ± 0.12 m | 112.96 ± 1.74 b |
| Ch-165 | 6.79 ± 0.07 l | 15.07 ± 0.13 ij | 75.19 ± 1.53 ef |
| Li | 5.30 ± 0.07 m | 17.06 ± 0.11 cd | 88.26 ± 2.12 cd |
| L-189b | 10.73 ± 0.12 hi | 19.23 ± 0.16 a | 117.77 ± 2.53 ab |
| Ru | 12.87 ± 0.17 ef | 14.81 ± 0.13 jk | 89.29 ± 2.09 cd |
| SS-180 | 18.43 ± 0.15 a | 14.54 ± 0.11 kl | 73.19 ± 1.17 f |
| T-673 | 16.61 ± 0.13c | 14.31 ± 0.13 l | 71.79 ± 1.01 f |
| T-370 | 12.83 ± 0.11 ef | 16.10 ± 0.18 gh | 82.69 ± 1.83 de |
| IM/pusta | 13.23 ± 0.10 e | 15.30 ± 0.13 i | 51.49 ± 1.19 g |
| SS | 14.00 ± 0.13 d | 16.72 ± 0.15 def | 93.48 ± 1.46 c |
| CN-254 | 10.18 ± 0.08 i | 20.15 ± 0.17 a | 114.89 ± 2.16 ab |
| means | 11.65 ± 0.44 | 16.18 ± 0.22 | 88.30 ± 2.49 |
| Cochran’s C Test Bartlett’s Test LSD5% | 0.128; p = 1.00 | 0.086; p = 1.00 | 0.102; p = 1.00 |
| 1.191; p = 0.998 | 1.057; p = 1.00 | 1.162; p = 0.999 | |
| 0.65 | 1.56 | 9.49 |
| Traits | Clusters | Times Ranked First | Contribution to Divergence (%) | ||||
|---|---|---|---|---|---|---|---|
| I | II | III | IV | V | |||
| TAC | 401.28 | 341.75 | 291.77 | 240.75 | 534.06 | 35 | 17.5 |
| TPC | 91.79 | 85.44 | 67.76 | 112.96 | 116.33 | 90 | 45.0 |
| Lyc | 12.77 | 6.67 | 14.09 | 6.81 | 10.46 | 48 | 24.0 |
| AsA | 16.75 | 15.78 | 14.59 | 13.17 | 19.69 | 27 | 13.5 |
| Cluster | Landraces | I | II | III | IV | V |
|---|---|---|---|---|---|---|
| I | CN-26; Gi; L-189a; DV; SS; T-370; Pe; Gr; C102 | 2.30 | 5.94 | 7.42 | 13.22 * | 8.71 |
| II | PN; Ch-165; Li | 2.76 | 8.63 | 6.70 | 15.42 ** | |
| III | Ch; IM/pusta; Ru; SS180; T-673 | 3.62 | 12.31 * | 26.36 *** | ||
| IV | Ch-60pr12 | 0.00 | 28.31 *** | |||
| V | L-189b; CN254 | 0.78 |
| Traits | PC1 | PC2 | PC3 | PC4 |
|---|---|---|---|---|
| TAC | −0.956 | 0.171 | 0.225 | −0.081 |
| TPC | −0.737 | −0.253 | −0.627 | 0.000 |
| Lyc | 0.144 | 0.959 | −0.244 | 0.000 |
| AsA | −0.957 | 0.169 | 0.221 | 0.082 |
| Eigen value | 2.394 | 1.041 | 0.552 | 0.013 |
| Cumulative eigen value | 2.394 | 3.435 | 3.987 | 4.000 |
| Proportion variance | 59.84 | 26.02 | 13.80 | 0.33 |
| Cumulative variance | 59.84 | 85.86 | 99.67 | 100.00 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sumalan, R.M.; Ciulca, S.I.; Poiana, M.A.; Moigradean, D.; Radulov, I.; Negrea, M.; Crisan, M.E.; Copolovici, L.; Sumalan, R.L. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy 2020, 10, 500. https://doi.org/10.3390/agronomy10040500
Sumalan RM, Ciulca SI, Poiana MA, Moigradean D, Radulov I, Negrea M, Crisan ME, Copolovici L, Sumalan RL. The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy. 2020; 10(4):500. https://doi.org/10.3390/agronomy10040500
Chicago/Turabian StyleSumalan, Renata M., Sorin I. Ciulca, Mariana A. Poiana, Diana Moigradean, Isidora Radulov, Monica Negrea, Manuela E. Crisan, Lucian Copolovici, and Radu L. Sumalan. 2020. "The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value" Agronomy 10, no. 4: 500. https://doi.org/10.3390/agronomy10040500
APA StyleSumalan, R. M., Ciulca, S. I., Poiana, M. A., Moigradean, D., Radulov, I., Negrea, M., Crisan, M. E., Copolovici, L., & Sumalan, R. L. (2020). The Antioxidant Profile Evaluation of Some Tomato Landraces with Soil Salinity Tolerance Correlated with High Nutraceuticaland Functional Value. Agronomy, 10(4), 500. https://doi.org/10.3390/agronomy10040500