Effect of Manure and Urea Fertilization on Yield, Carbon Speciation and Greenhouse Gas Emissions from Vegetable Production Systems of Nigeria and Republic of Benin: A Phytotron Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Sites
2.2. Measurement of GHG Emissions
2.3. FTIR Spectroscopy
2.4. Statistical Analysis
3. Results
3.1. Soil pH and Vegetable Yield
3.2. Soil N, P and organic C content
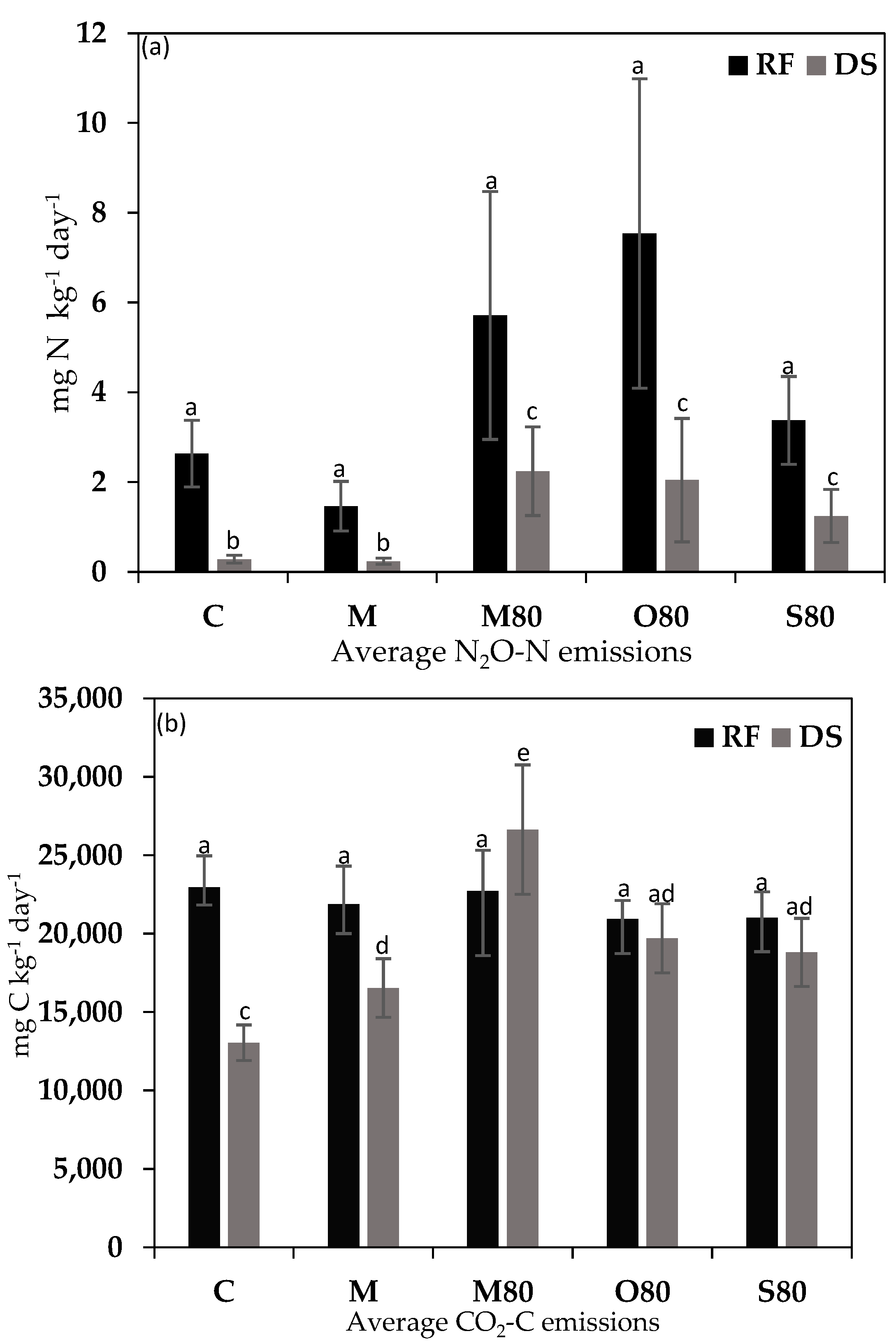
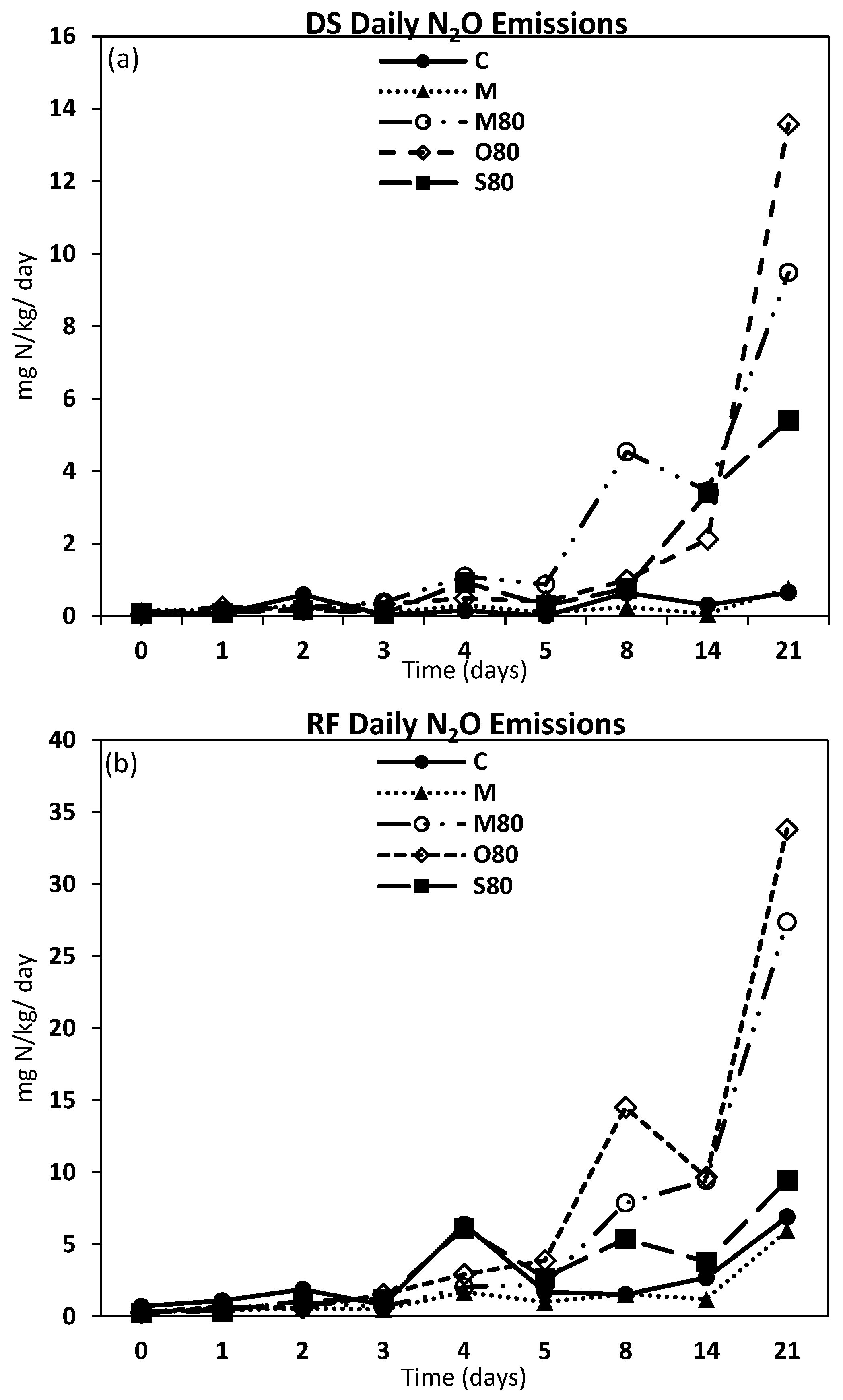
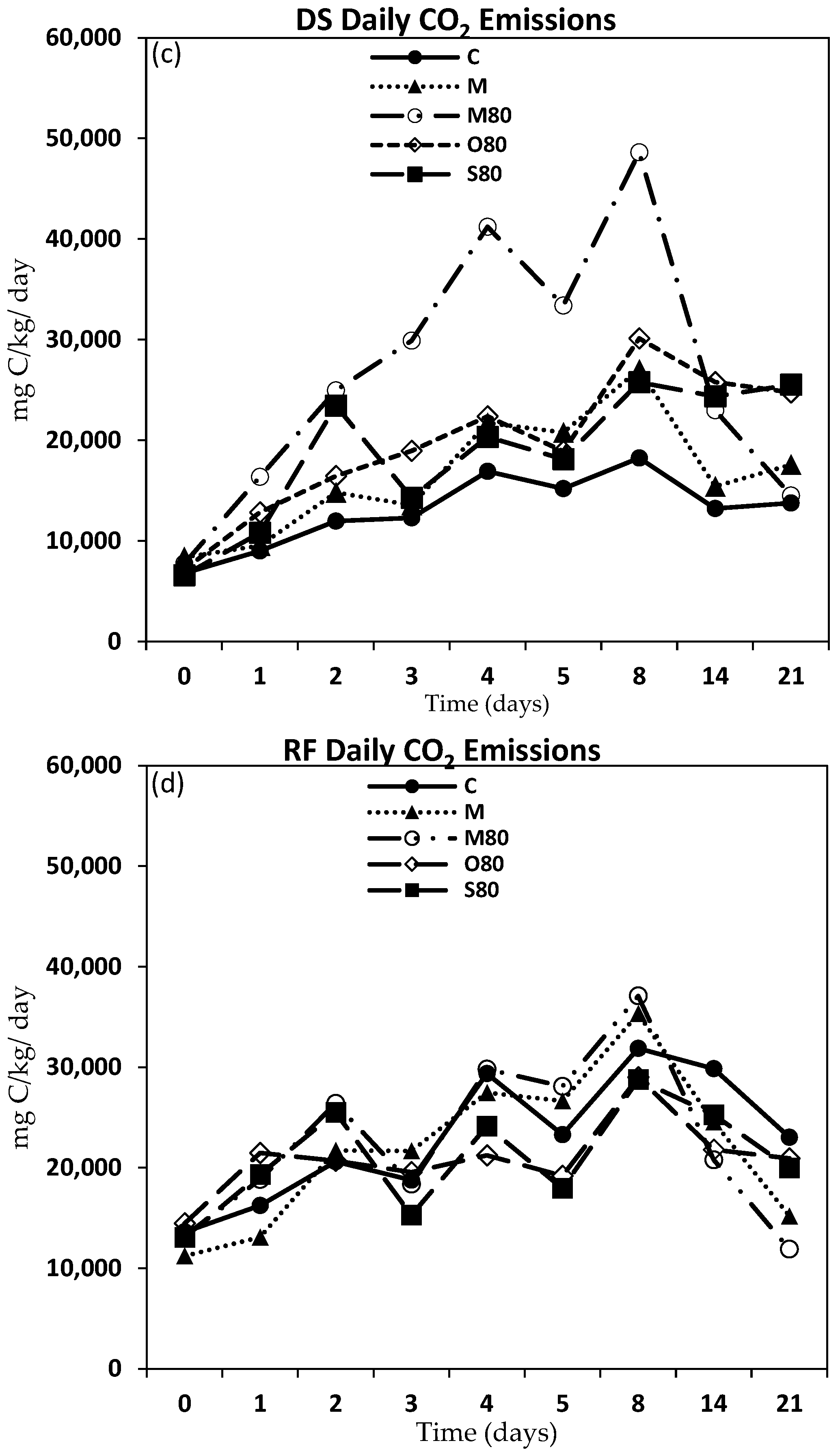
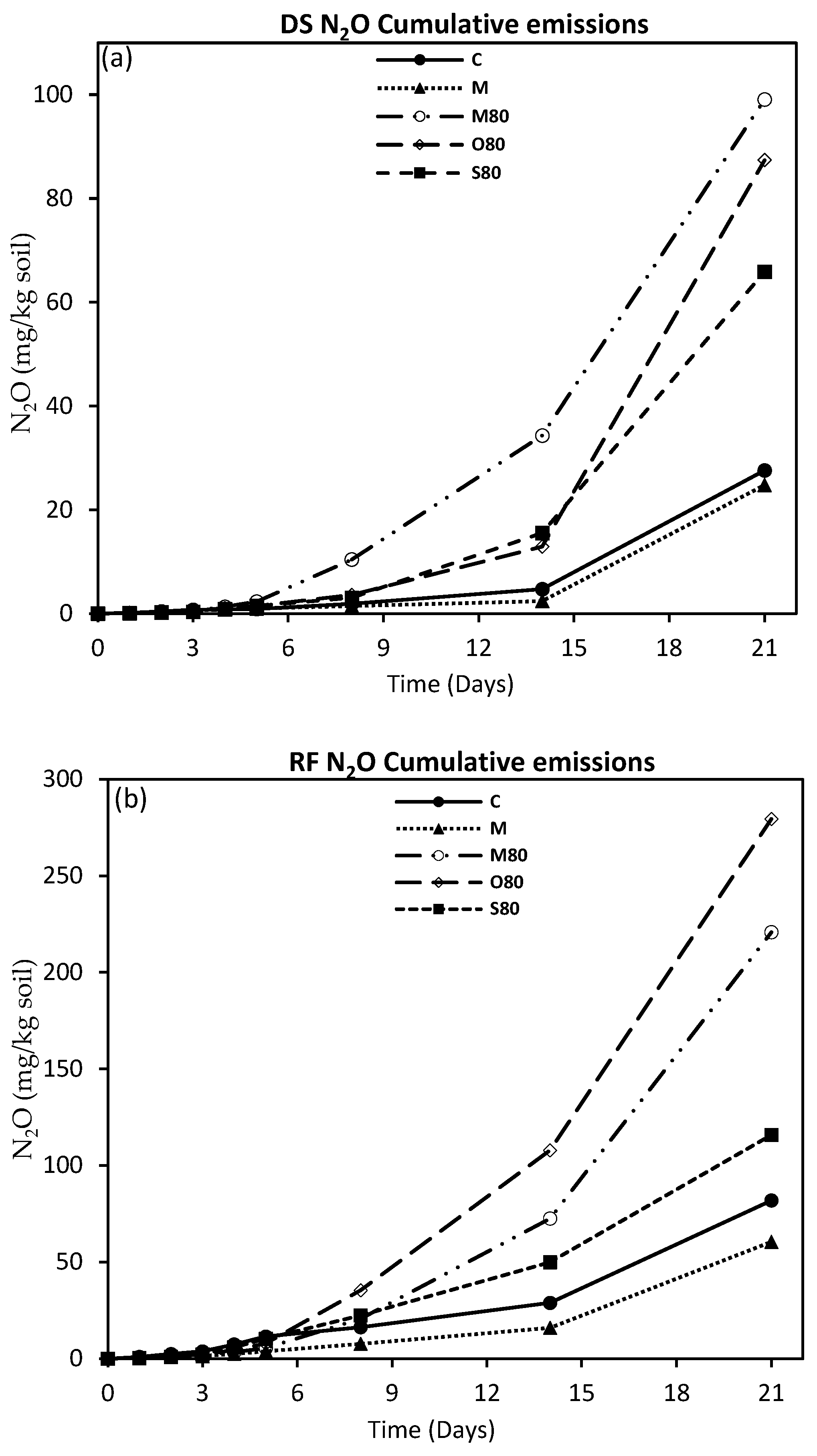
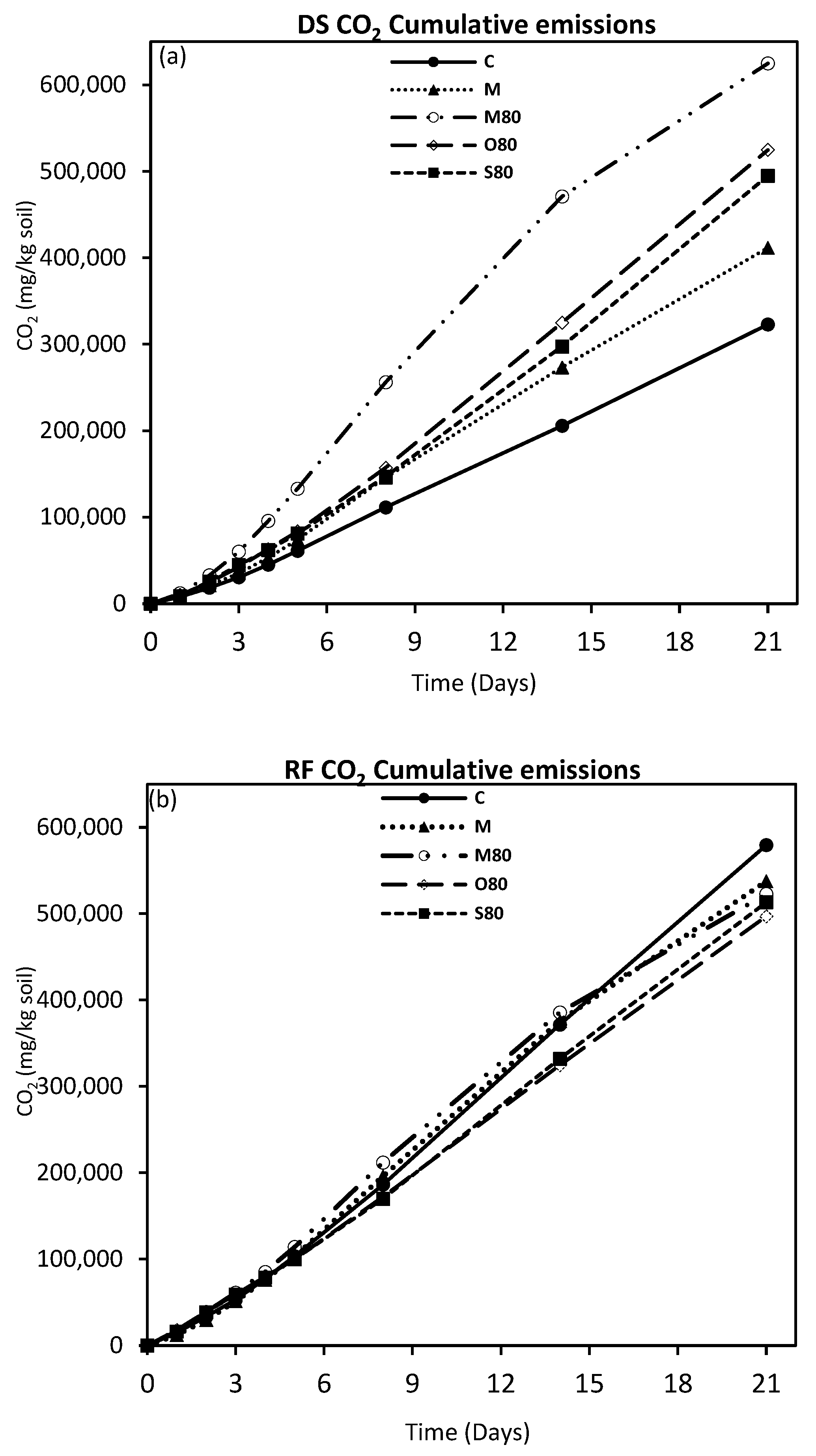
3.3. GHG Emissions
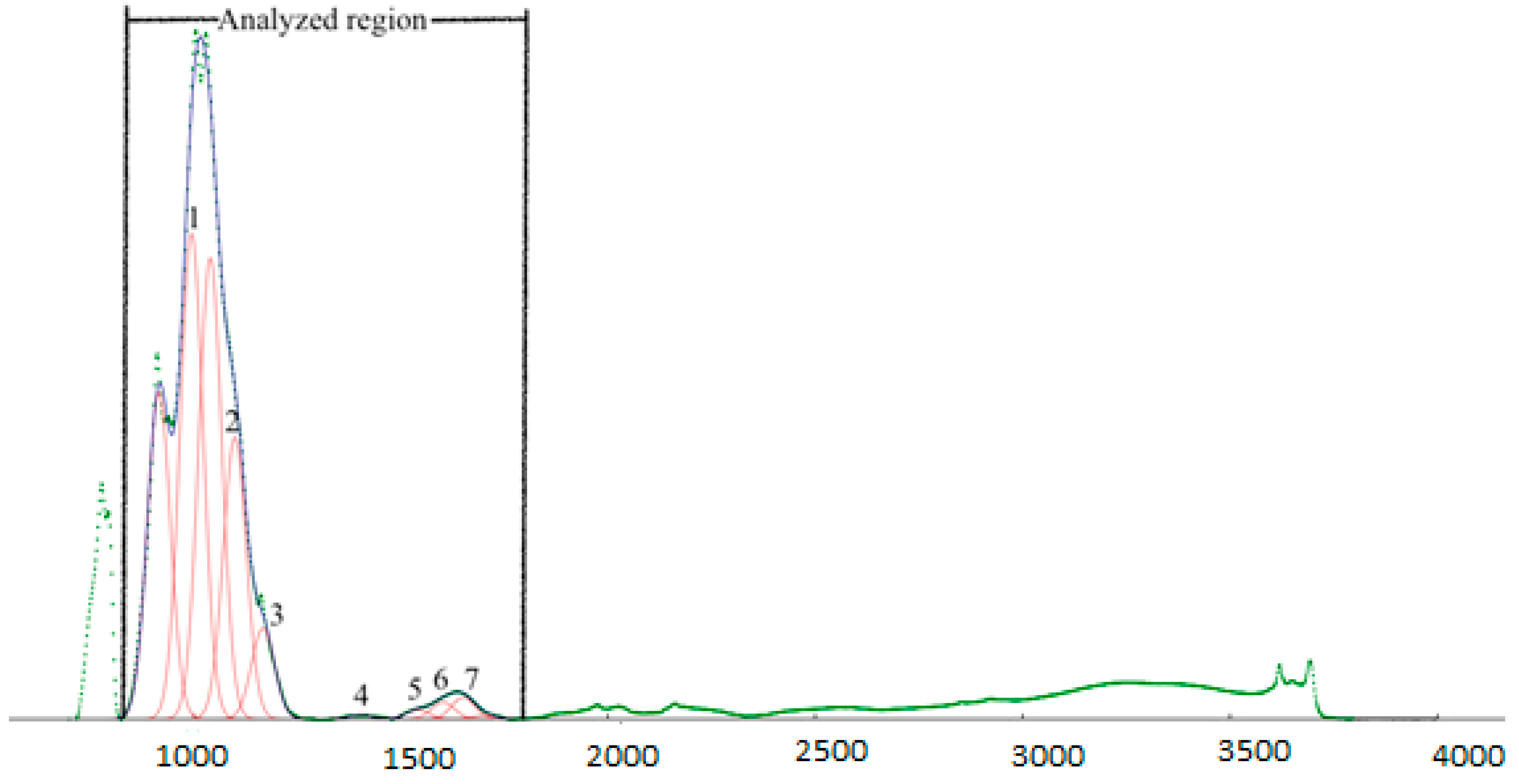
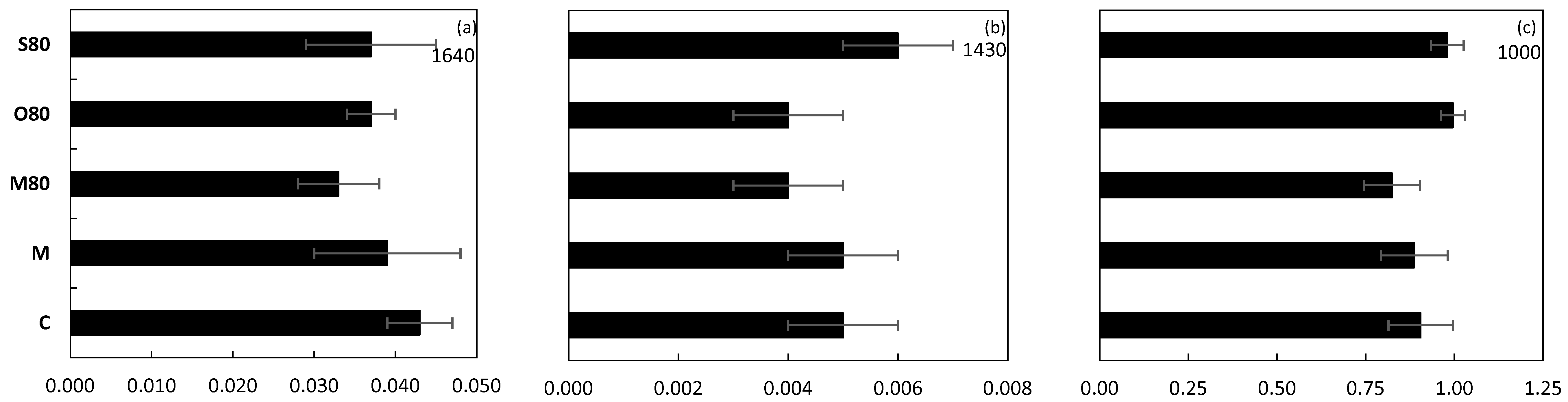
3.4. ATR-FTIR Spectroscopy
4. Discussion
4.1. Soil pH and Vegetable Yield
4.2. GHG Emissions
4.3. Treatment Effect on SOC Composition
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Appendix A
| Band (cm−1) | 1000 | 1100 | 1160 | 1430 | 1540 | 1600 | 1640 |
|---|---|---|---|---|---|---|---|
| Dry Savanna | |||||||
| C | |||||||
| M | 1.023 | 0.811 | 0.249 | 0.007 | 0.020 | 0.036 | 0.034 |
| M80 | 0.803 | 1.312 | 0.463 | 0.007 | 0.012 | 0.036 | 0.030 |
| O80 | 0.889 | 0.535 | 0.118 | 0.008 | 0.020 | 0.032 | 0.043 |
| S80 | 0.877 | 0.578 | 0.157 | 0.008 | 0.021 | 0.041 | 0.055 |
| Rainforest | |||||||
| C | 0.708 | 0.737 | 0.266 | 0.005 | 0.007 | 0.020 | 0.021 |
| M | 0.689 | 0.719 | 0.245 | 0.003 | 0.006 | 0.017 | 0.017 |
| M80 | 0.784 | 0.961 | 0.366 | 0.002 | 0.009 | 0.024 | 0.020 |
| O80 | 1.156 | 0.558 | 0.202 | 0.001 | 0.004 | 0.008 | 0.012 |
| S80 | 0.000 | 1.406 | 0.475 | 0.004 | 0.009 | 0.030 | 0.025 |
| Band (cm−1) | 1000 | 1100 | 1160 | 1430 | 1540 | 1600 | 1640 |
|---|---|---|---|---|---|---|---|
| Dry Savanna | |||||||
| C | 0.602 | 0.716 | 0.265 | 0.002 | 0.006 | 0.019 | 0.014 |
| M | 0.582 | 0.667 | 0.278 | 0.006 | 0.013 | 0.033 | 0.028 |
| M80 | 0.629 | 0.738 | 0.288 | 0.012 | 0.017 | 0.046 | 0.038 |
| O80 | 0.697 | 1.058 | 0.375 | 0.005 | 0.011 | 0.031 | 0.026 |
| S80 | 0.623 | 1.016 | 0.369 | 0.004 | 0.008 | 0.022 | 0.022 |
| Rainforest | |||||||
| C | 0.905 | 1.069 | 0.364 | 0.005 | 0.020 | 0.039 | 0.043 |
| M | 0.887 | 0.837 | 0.233 | 0.005 | 0.016 | 0.029 | 0.039 |
| M80 | 0.824 | 0.772 | 0.252 | 0.004 | 0.014 | 0.030 | 0.033 |
| O80 | 0.996 | 0.692 | 0.231 | 0.004 | 0.017 | 0.031 | 0.037 |
| S80 | 0.980 | 0.633 | 0.194 | 0.006 | 0.018 | 0.029 | 0.037 |
References
- Smith, P.; Martino, Z.; Cai, D. Agriculture. In Climate Change 2007: Mitigation; Metz, B., Davidson, O.R., Bosch, P.R., Dave, R., Meyer, L.A., Eds.; Contribution of Working Group III to the Fourth Assessment Report of the Intergovernmental Panel on Climate Change, Cambridge University Press: Cambridge, UK; New York, NY, USA, 2007. [Google Scholar]
- Snyder, C.S.; Bruulsema, T.W.; Jensen, T.L.; Fixen, P.E. Review of greenhouse gas emissions from crop production systems and fertilizer management effects. Agric. Ecosyst. Environ. 2009, 133, 247–266. [Google Scholar] [CrossRef]
- Blandford, D.; Hassapoyannes, K. The role of agriculture in global GHG mitigation. In OECD Food, Agriculture and Fisheries Papers; No. 112; OECD Publishing: Paris, France, 2018. [Google Scholar] [CrossRef]
- Vitousek, P.M.; Naylor, R.; Crews, T.; David, M.B.; Drinkwater, L.E.; Holland, E.; Johnes, P.J.; Katzenberger, J.; Martinelli, L.A.; Matson, P.A.; et al. Nutrient imbalances in agricultural development. Science 2009, 324, 1519–1520. [Google Scholar] [CrossRef]
- Bouwman, A.F.; Boumans, L.J.M.; Batjes, N.H. Emissions of N2O and NO from fertilized fields: Summary of available measurement data. Glob. Biogeochem. Cycl. 2002, 16, 6:1–6:13. [Google Scholar] [CrossRef]
- Nziguheba, G.; Palm, C.A.; Berhe, T.; Denning, G.; Dicko, A.; Diouf, O.; Diru, W.; Flor, R.; Frimpong, F.; Harawa, R.; et al. The African green revolution: Results from the millennium villages project. In Advances in Agronomy; Academic Press: San Diego, CA, USA, 2010; Volume 109, pp. 75–115. [Google Scholar]
- Sanchez, P.A. Soil fertility and hunger in Africa. Science 2002, 295, 2019–2020. [Google Scholar] [CrossRef]
- Alliance for a Green Revolution in Africa (AGRA). Building on the New Momentum in African Agriculture: AGRA in 2008; Alliance for a Green Revolution in Africa: Nairobi, Kenya, 2009. [Google Scholar]
- Nyamadzawo, G.; Shi, Y.; Chirinda, N.; Olesen, J.E.; Mapanda, F.; Wuta, M.; Wu, W.; Meng, F.; Oelofse, M.; de Neergaard, A.; et al. Combining organic and inorganic nitrogen fertilisation reduces N2O emissions from cereal crops: A comparative analysis of China and Zimbabwe. Mitig. Adapt. Strateg. Glob. Chang. 2017, 22, 233–245. [Google Scholar] [CrossRef]
- Mosier, A.; Kroeze, C.; Nevison, C.; Oenema, O.; Seitzinger, S.; van Cleemput, O. Closing the global atmospheric N2O budget: Nitrous oxide emissions through the agricultural nitrogen cycle. (OECD/IPCC/IEA Phase II Development of IPCC Guidelines for National Greenhouse Gas Inventories). In Dissipation of N from the Human N-cycle, and Its Role in Present and Future N2O Emissions to the Atmosphere; Int. Workshop: Oslo, Norway, 1997; p. 4. [Google Scholar]
- Ruser, R.; Flessa, H.; Russow, R.; Schmidt, G.; Buegger, F.; Munch, J.C. Emission of N2O, N2 and CO2 from soil fertilized with nitrate: Effect of compaction, soil moisture and rewetting. Soil Biol. Biochem. 2006, 38, 263–274. [Google Scholar] [CrossRef]
- Chirinda, N.; Carter, M.S.; Albert, K.R.; Ambus, P.; Olesen, J.E.; Porter, J.R.; Petersen, S.O. Emissions of nitrous oxide from arable organic and conventional cropping systems on two soil types. Agric. Ecosyst. Environ. 2010, 136, 199–208. [Google Scholar] [CrossRef]
- Dick, J.; Kaya, B.; Soutoura, M.; Skiba, U.; Smith, R.; Niang, A.; Tabo, R. The contribution of agricultural practices to nitrous oxide emissions in semi-arid Mali. Soil Use Manag. 2008, 24, 292–301. [Google Scholar] [CrossRef]
- Efthimiadou, A.; Bilalis, D.; Karkanis, A.; Froud-Williams, B. Combined organic/inorganic fertilization enhance soil quality and increased yield, photosynthesis and sustainability of sweet maize crop. Australian J. Crop. Sci. 2010, 4, 722. [Google Scholar]
- Huang, Y.; Zou, J.; Zheng, X.; Wang, Y.; Xu, X. Nitrous oxide emissions as influenced by amendment of plant residues with different C:N ratios. Soil Biol. Biochem. 2004, 36, 973–981. [Google Scholar] [CrossRef]
- Johnson, J.M.F.; Franzluebbers, A.J.; Weyers, S.L.; Reicosky, D.C. Agricultural opportunities to mitigate greenhouse gas emissions. Environ. Pollut. 2007, 150, 107–124. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Silva, N.; Luna-Guido, M.; Fernández-Luqueño, F.; Marsch, R.; Dendooven, L. Emission of greenhouse gases from an agricultural soil amended with urea: A laboratory study. Appl. Soil Ecol. 2011, 47, 92–97. [Google Scholar] [CrossRef]
- Ramirez, K.S.; Craine, J.M.; Fierer, N. Consistent effects of nitrogen amendments on soil microbial communities and processes across biomes. Glob. Chang. Biol. 2012, 18, 1918–1927. [Google Scholar] [CrossRef]
- Tan, H.; Barret, M.; Mooij, M.J.; Rice, O.; Morrissey, J.P.; Dobson, A.; Griffiths, B.; O’Gara, F. Long-term phosphorus fertilisation increased the diversity of the total bacterial community and the phoD phosphorus mineraliser group in pasture soils. Biol. Fertil. Soil. 2013, 49, 661–672. [Google Scholar] [CrossRef]
- Fraser, T.; Lynch, D.H.; Entz, M.H.; Dunfield, K.E. Linking alkaline phosphatase activity with bacterial phoD gene abundance in soil from a long-term management trial. Geoderma 2015, 257, 115–122. [Google Scholar] [CrossRef]
- Singh, S.N.; Verma, A. The potential of nitrification inhibitors to manage the pollution effect of nitrogen fertilizers in agricultural and other soils: A review. Environ. Pract. 2007, 9, 266. [Google Scholar] [CrossRef]
- Artz, R.R.; Chapman, S.J.; Robertson, A.J.; Potts, J.M.; Laggoun-Défarge, F.; Gogo, S.; Comont, L.; Disnar, J.R.; Francez, A.J. FTIR spectroscopy can be used as a screening tool for organic matter quality in regenerating cutover peatlands. Soil Biol. Biochem. 2008, 40, 515–527. [Google Scholar] [CrossRef]
- Lehmann, J.; Solomon, D. Organic carbon chemistry in soils observed by synchrotron-based spectroscopy. In Developments in Soil Science; Elsevier: Amsterdam, The Netherlands, 2010; Volume 34, pp. 289–312. [Google Scholar]
- Dhillon, G.S.; Gillespie, A.; Peak, D.; Van Rees, K.C. Spectroscopic investigation of soil organic matter composition for shelterbelt agroforestry systems. Geoderma 2017, 298, 1–13. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Kinyangi, J.; Liang, B.; Schäfer, T. Carbon K-edge NEXAFS and FTIR-ATR spectroscopic investigation of organic carbon speciation in soils. Soil Sci. Soc. Am. J. 2005, 69, 107–119. [Google Scholar] [CrossRef]
- Solomon, D.; Lehmann, J.; Kinyangi, J.; Amelung, W.; Lobe, I.; Pell, A.; Riha, S.; Ngoze, S.; Verchot, L.; Mbugua, D. Long-term impacts of anthropogenic perturbations on dynamics and speciation of organic carbon in tropical forest and subtropical grassland ecosystems. Glob. Chang. Biol. 2007, 13, 511–530. [Google Scholar] [CrossRef]
- Jones, A.; Breuning-Madsen, H.; Brossard, M.; Dampha, A.; Deckers, J.; Dewitte, O.; Gallali, T.; Hallett, S.; Jones, R.; Kilasara, M.; et al. Zougmoré. In Soil Atlas of Africa; European Commission, Publications Office of the European Union: Luxembourg, France, 2013; p. 176. [Google Scholar]
- Carter, M.R.; Gregorich, E.G. Soil Sampling and Methods of Analysis, 2nd ed.; CRC Press, Taylor & Francis Group: Boca Raton, FL, USA, 2008. [Google Scholar]
- Birch, H.F. The effect of soil drying on humus decomposition and nitrogen availability. Plant Soil 1958, 10, 9–31. [Google Scholar] [CrossRef]
- Haberhauer, G.; Feigl, B.; Gerzabek, M.H.; Cerri, C. FT-IR spectroscopy of organic matter in tropical soils: Changes induced through deforestation. Appl. Spectrosc. 2000, 54, 221–224. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Gerke, H.H.; Bachmann, J.; Goebel, M.O. Composition of organic matter fractions for explaining wettability of three forest soils. Soil Sci. Soc. Am. J. 2005, 69, 57–66. [Google Scholar] [CrossRef]
- Wojdyr, M. Fityk: A general-purpose peak fitting program. J. Appl. Crystallogr. 2010, 43, 1126–1128. [Google Scholar] [CrossRef]
- Ellerbrock, R.H.; Höhn, A.; Gerke, H.H. Characterization of soil organic matter from a sandy soil in relation to management practice using FT-IR spectroscopy. Plant Soil 1999, 213, 55–61. [Google Scholar] [CrossRef]
- Calderón, F.; Haddix, M.; Conant, R.; Magrini-Bair, K.; Paul, E. Diffuse-reflectance Fourier-transform mid-infrared spectroscopy as a method of characterizing changes in soil organic matter. Soil Sci. Soc. Am. J. 2013, 77, 1591–1600. [Google Scholar]
- Parikh, S.J.; Goyne, K.W.; Margenot, A.J.; Mukome, F.N.; Calderón, F.J. Soil chemical insights provided through vibrational spectroscopy. In Advances in Agronomy; Academic Press: San Diego, CA, USA, 2014; Volume 126, pp. 1–148. [Google Scholar]
- Bornemann, L.; Welp, G.; Amelung, W. Particulate organic matter at the field scale: Rapid acquisition using mid-infrared spectroscopy. Soil Sci. Soc. Am. J. 2010, 74, 1147–1156. [Google Scholar] [CrossRef]
- Janik, L.J.; Merry, R.H.; Forrester, S.; Lanyon, D.; Rawson, A. Rapid prediction of soil water retention using mid infrared spectroscopy. Soil Sci. Soc. Am. J. 2007, 71, 507–514. [Google Scholar] [CrossRef]
- Janik, L.J.; Skjemstad, J.; Shepherd, K.; Spouncer, L. The prediction of soil carbon fractions using mid-infrared-partial least square analysis. Aust. J. Soil Res. 2007, 45, 73–81. [Google Scholar] [CrossRef]
- Olaleye, A.; Oyedele, D.; Akponikpe, P.; Kar, G.; Peak, D. Molecular Scale Studies of Phosphorus Speciation and Transformation in Manure Amended and Microdose Fertilized Indigenous Vegetable Production Systems of Nigeria and Republic of Benin. Soil Syst. 2020, 4, 5. [Google Scholar] [CrossRef]
- Mando, A.; Bonzi, M.; Wopereis, M.C.S.; Lompo, F.; Stroosnijder, L. Long-term effects of mineral and organic fertilization on soil organic matter fractions and sorghum yield under Sudano-Sahelian conditions. Soil Use Manag. 2005, 21, 396–401. [Google Scholar] [CrossRef]
- Tovihoudji, P.G.; Akponikpè, P.I.; Agbossou, E.K.; Bertin, P.; Bielders, C.L. Fertilizer microdosing enhances maize yields but may exacerbate nutrient mining in maize cropping systems in northern Benin. Field crop. Res. 2017, 213, 130–142. [Google Scholar] [CrossRef]
- Detchinli, K.S.; Sogbedji, J.M. Yield performance and economic return of maize as affected by nutrient management strategies on ferralsols in coastal western Africa. Eur. Sci. J. 2015, 11, 27. [Google Scholar]
- Potter, C.S.; Matson, P.A.; Vitousek, P.M.; Davidson, E.A. Process modeling of controls on nitrogen trace gas emissions from soils worldwide. J. Geophys. Res. Atmos. 1996, 101, 1361–1377. [Google Scholar] [CrossRef]
- Rees, R.M.; Wuta, M.; Furley, P.A.; Li, C. Nitrous oxide fluxes from savanna (miombo) woodlands in Zimbabwe. J. Biogeogr. 2006, 33, 424–437. [Google Scholar] [CrossRef]
- Malhi, S.S.; Lemke, R.; Wang, Z.H.; Chhabra, B.S. Tillage, nitrogen and crop residue effects on crop yield, nutrient uptake, soil quality, and greenhouse gas emissions. Soil Tillage Res. 2006, 90, 171–183. [Google Scholar] [CrossRef]
- Kachanoski, R.G.; O’Halloran, I.; Rochette, P. Site-Specific Application of Fertilizer N for Reducing Greenhouse Gas Emissions; Climate Change Funding Initiative in Agriculture; Canadian Agri-Food Research Council: Ottawa, ON, Canada, 2003. [Google Scholar]
- Vallejo, A.; García-Torres, L.; Díez, J.A.; Arce, A.; López-Fernández, S. Comparison of N losses (NO−3, N2O, NO) from surface applied, injected or amended (DCD) pig slurry of an irrigated soil in a Mediterranean climate. Plant Soil 2005, 272, 313–325. [Google Scholar] [CrossRef]
- Mapanda, F.; Wuta, M.; Nyamangara, J.; Rees, R.M. Effects of organic and mineral fertilizer nitrogen on greenhouse gas emissions and plant-captured carbon under maize cropping in Zimbabwe. Plant Soil 2011, 343, 67–81. [Google Scholar] [CrossRef]
- Chapuis-Lardy, L.Y.D.I.E.; Wrage, N.; Metay, A.; Chotte, J.L.; Bernoux, M. Soils, a sink for N2O? A review. Glob. Chang. Biol. 2007, 13, 1–17. [Google Scholar] [CrossRef]
- Peng, Q.; Qi, Y.; Dong, Y.; Xiao, S.; He, Y. Soil nitrous oxide emissions from a typical semiarid temperate steppe in inner Mongolia: Effects of mineral nitrogen fertilizer levels and forms. Plant Soil 2011, 342, 345–357. [Google Scholar] [CrossRef]


| Treatment | pH | Total P (%) | Organic C (%) | Total N (%) | ||||
|---|---|---|---|---|---|---|---|---|
| BP | AH | BP | AH | BP | AH | BP | AH | |
| Rainforest | ||||||||
| C | 4.7 (0.02) | 4.7 (0.02) | 0.08 (0.003) | 0.07 (0.001) | 1.75 (0.04) | 1.81 (0.05) | 0.20 (0.01) | 0.12 (0.02) |
| M | 5.2 (0.02) | 5.0 (0.02) | 0.07 (0.002) | 0.07 (0.002) | 1.70 (0.01) | 1.80 (0.08) | 0.21 (0.02) | 0.20 (0.03) |
| M80 | 4.9 (0.04) | 5.2 (0.04) | 0.07 (0.006) | 0.07 (0.002) | 1.66 (0.09) | 1.84 (0.05) | 0.18 (0.01) | 0.20 (0.05) |
| O80 | 5.9 (0.06) | 5.2 (0.05) | 0.07 (0.002) | 0.06 (0.002) | 1.87 (0.02) | 1.84 (0.02) | 0.22 (0.03) | 0.22 (0.02) |
| S80 | 4.8 (0.05) | 5.2 (0.03) | 0.06 (0.006) | 0.07 (0.002) | 1.81 (0.03) | 1.71 (0.11) | 0.13 (0.01) | 0.16 (0.03) |
| Dry Savanna | ||||||||
| C | 5.5 (0.06) | 5.2 (0.05) | 0.04 (0.001) | 0.04 (0.002) | 0.56 (0.01) | 0.58 (0.02) | 0.08 (0.01) | 0.08 (0.01) |
| M | 6.4 (0.04) | 5.8 (0.04) | 0.04 (0.001) | 0.04 (0.001) | 0.62 (0.01) | 0.63 (0.01) | 0.10 (0.01) | 0.08 (0.01) |
| M80 | 6.3 (0.007) | 5.8 (0.02) | 0.04 (0.001) | 0.04 (0.001) | 0.56 (0.01) | 0.56 (0.01) | 0.09 (0.01) | 0.10 (0.02) |
| O80 | 5.6 (0.06) | 5.3 (0.06) | 0.04 (0.001) | 0.04 (0.001) | 0.62 (0.02) | 0.60 (0.02) | 0.08 (0.01) | 0.10 (0.01) |
| S80 | 4.4 (0.06) | 4.7 (0.02) | 0.06 (0.006) | 0.04 (0.001) | 0.57 (0.01) | 0.55 (0.01) | 0.09 (0.01) | 0.07 (0.01) |
| pH | Total P | Org. C | Total N | Yield | |
|---|---|---|---|---|---|
| Rainforest | |||||
| Treatments | 0.002 | 0.208 | 0.432 | 0.105 | 0.018 |
| Time | 0.243 | 0.533 | 0.212 | 0.487 | |
| Treatments*Time | 0.012 | 0.098 | 0.327 | 0.299 | |
| Dry Savanna | |||||
| Treatments | 0.002 | 0.607 | 0.006 | 0.607 | 0.005 |
| Time | 0.006 | 0.816 | 0.996 | 0.816 | |
| Treatments*Time | 0.003 | 0.394 | 0.538 | 0.394 | |
| B1000 | B1100 | B1160 | B1430 | B1540 | B1600 | B1640 | |
|---|---|---|---|---|---|---|---|
| Dry Savanna | |||||||
| pH | −0.111 | −0.418 | −0.359 | 0.597 * | 0.644 ** | 0.613 * | 0.516 * |
| Total P | 0.003 | 0.035 | 0.011 | −0.044 | −0.038 | −0.074 | 0.016 |
| Org C | −0.558 * | 0.114 | 0.146 | −0.092 | 0.092 | 0.051 | 0.010 |
| Total N | 0.225 | −0.193 | −0.300 | 0.008 | −0.123 | −0.068 | −0.125 |
| Rainforest | |||||||
| pH | 0.067 | −0.407 | −0.419 | −0.125 | −0.267 | −0.543 * | −0.289 |
| Total P | −0.059 | 0.019 | −0.045 | 0.259 | −0.053 | 0.063 | 0.183 |
| Org C | −0.534 * | 0.340 | 0.389 | −0.541 * | −0.445 | −0.264 | −0.577 * |
| Total N | −0.227 | 0.051 | 0.062 | −0.528 * | −0.620 * | −0.625 * | −0.556 * |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olaleye, A.; Peak, D.; Shorunke, A.; Dhillon, G.; Oyedele, D.; Adebooye, O.; Akponikpe, P.B.I. Effect of Manure and Urea Fertilization on Yield, Carbon Speciation and Greenhouse Gas Emissions from Vegetable Production Systems of Nigeria and Republic of Benin: A Phytotron Study. Agronomy 2020, 10, 400. https://doi.org/10.3390/agronomy10030400
Olaleye A, Peak D, Shorunke A, Dhillon G, Oyedele D, Adebooye O, Akponikpe PBI. Effect of Manure and Urea Fertilization on Yield, Carbon Speciation and Greenhouse Gas Emissions from Vegetable Production Systems of Nigeria and Republic of Benin: A Phytotron Study. Agronomy. 2020; 10(3):400. https://doi.org/10.3390/agronomy10030400
Chicago/Turabian StyleOlaleye, Abimfoluwa, Derek Peak, Akeem Shorunke, Gurbir Dhillon, Durodoluwa Oyedele, Odunayo Adebooye, and P.B. Irenikatche Akponikpe. 2020. "Effect of Manure and Urea Fertilization on Yield, Carbon Speciation and Greenhouse Gas Emissions from Vegetable Production Systems of Nigeria and Republic of Benin: A Phytotron Study" Agronomy 10, no. 3: 400. https://doi.org/10.3390/agronomy10030400
APA StyleOlaleye, A., Peak, D., Shorunke, A., Dhillon, G., Oyedele, D., Adebooye, O., & Akponikpe, P. B. I. (2020). Effect of Manure and Urea Fertilization on Yield, Carbon Speciation and Greenhouse Gas Emissions from Vegetable Production Systems of Nigeria and Republic of Benin: A Phytotron Study. Agronomy, 10(3), 400. https://doi.org/10.3390/agronomy10030400






