Response of Common Bean Cultivars and Lines to Aluminum Toxicity
Abstract
1. Introduction
2. Material and Methods
2.1. Stage 1: Determination of the Optimized Al Concentration
2.2. Stage 2: Screening of Mesoamerican and Andean Genotypes
3. Results
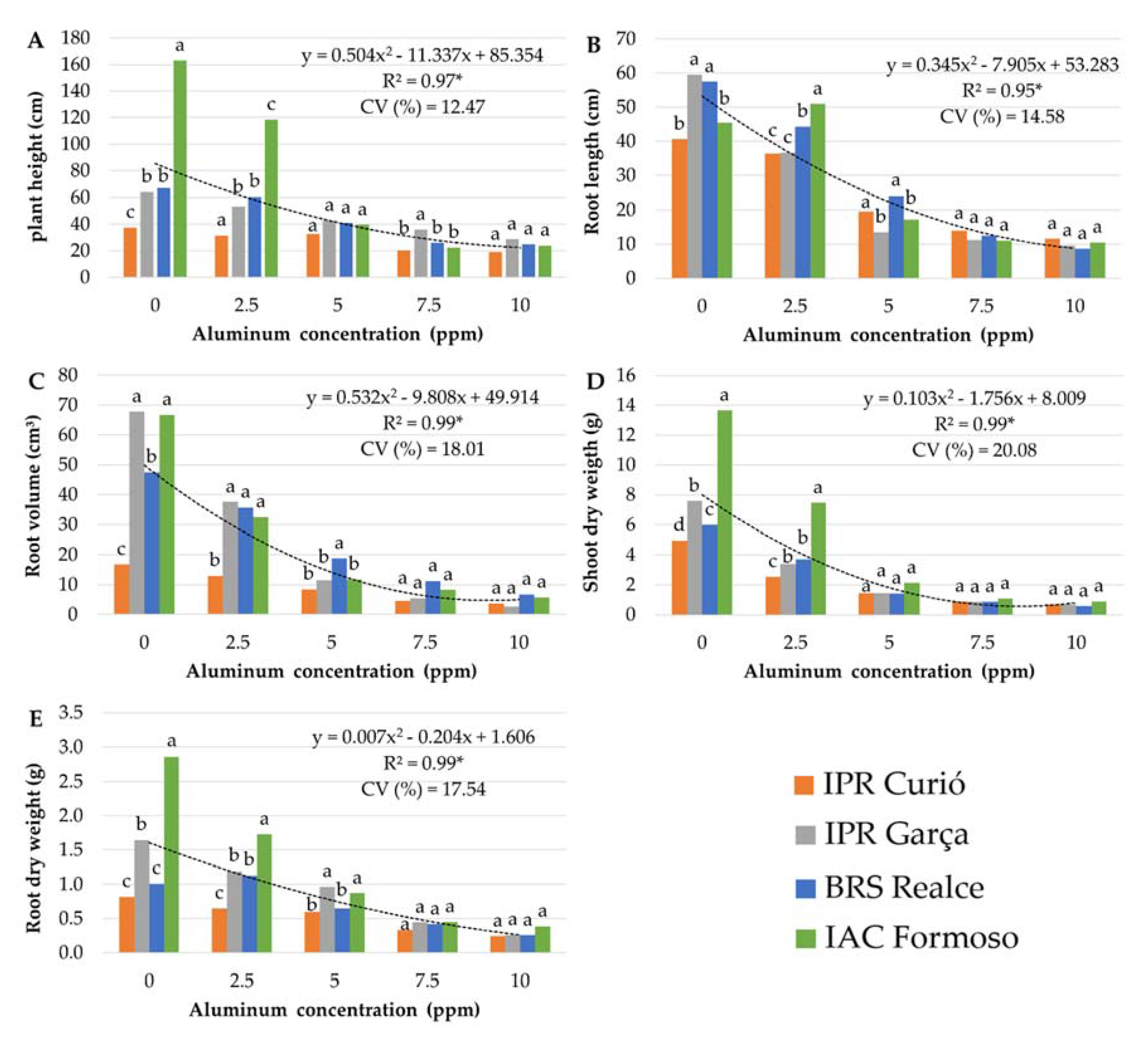
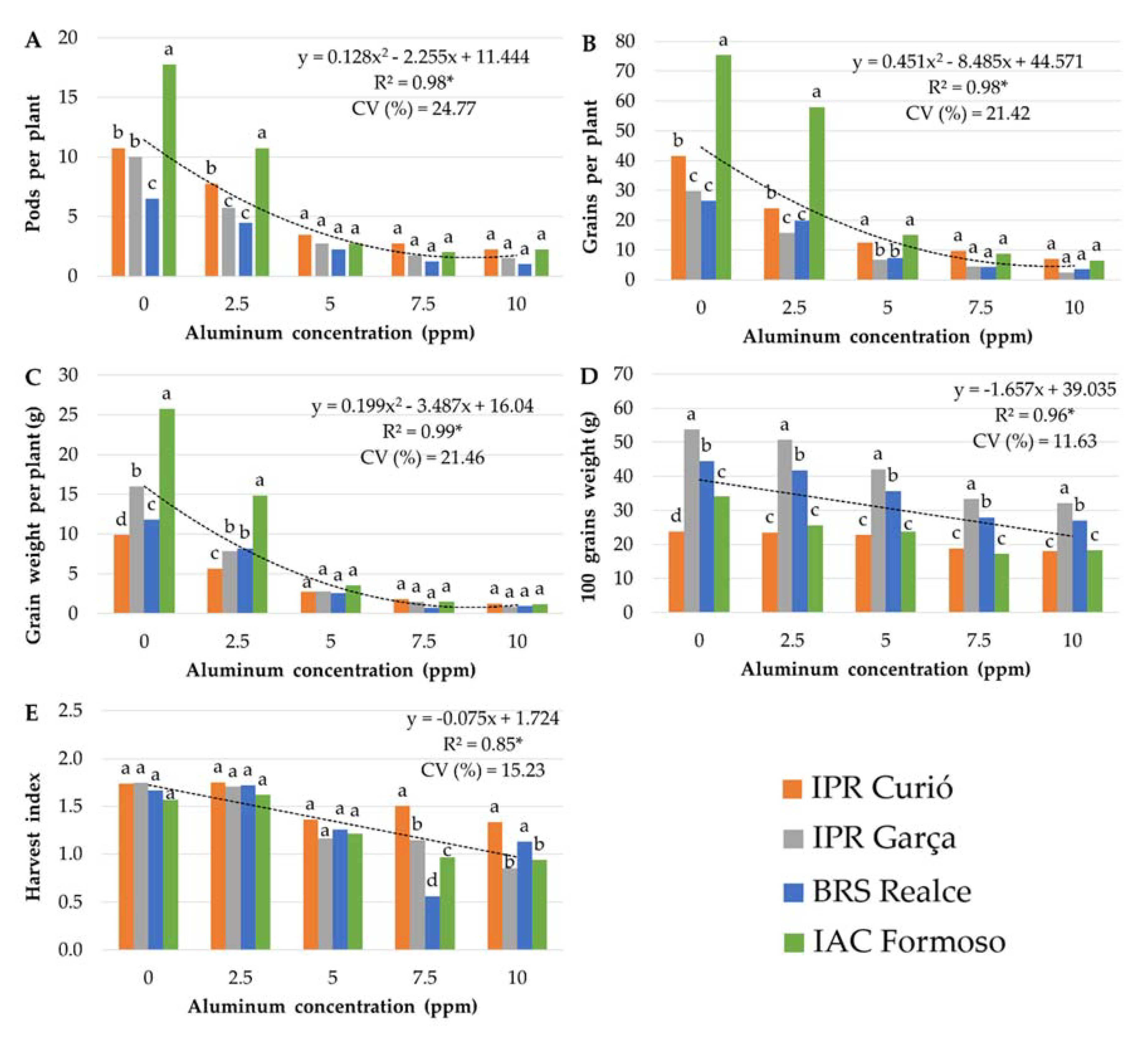
3.1. Stage 1: Determination of the Optimized Al Concentration
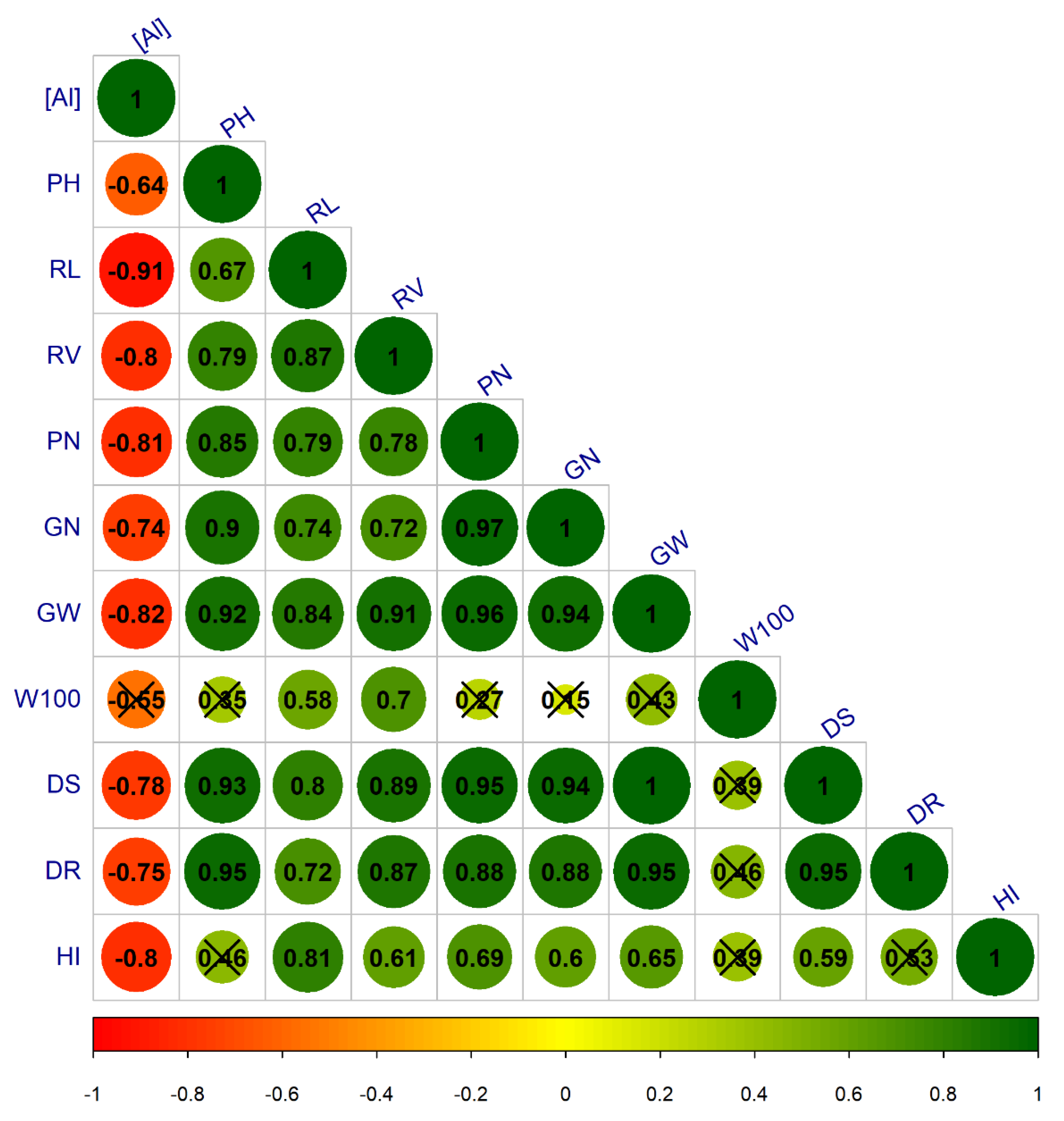
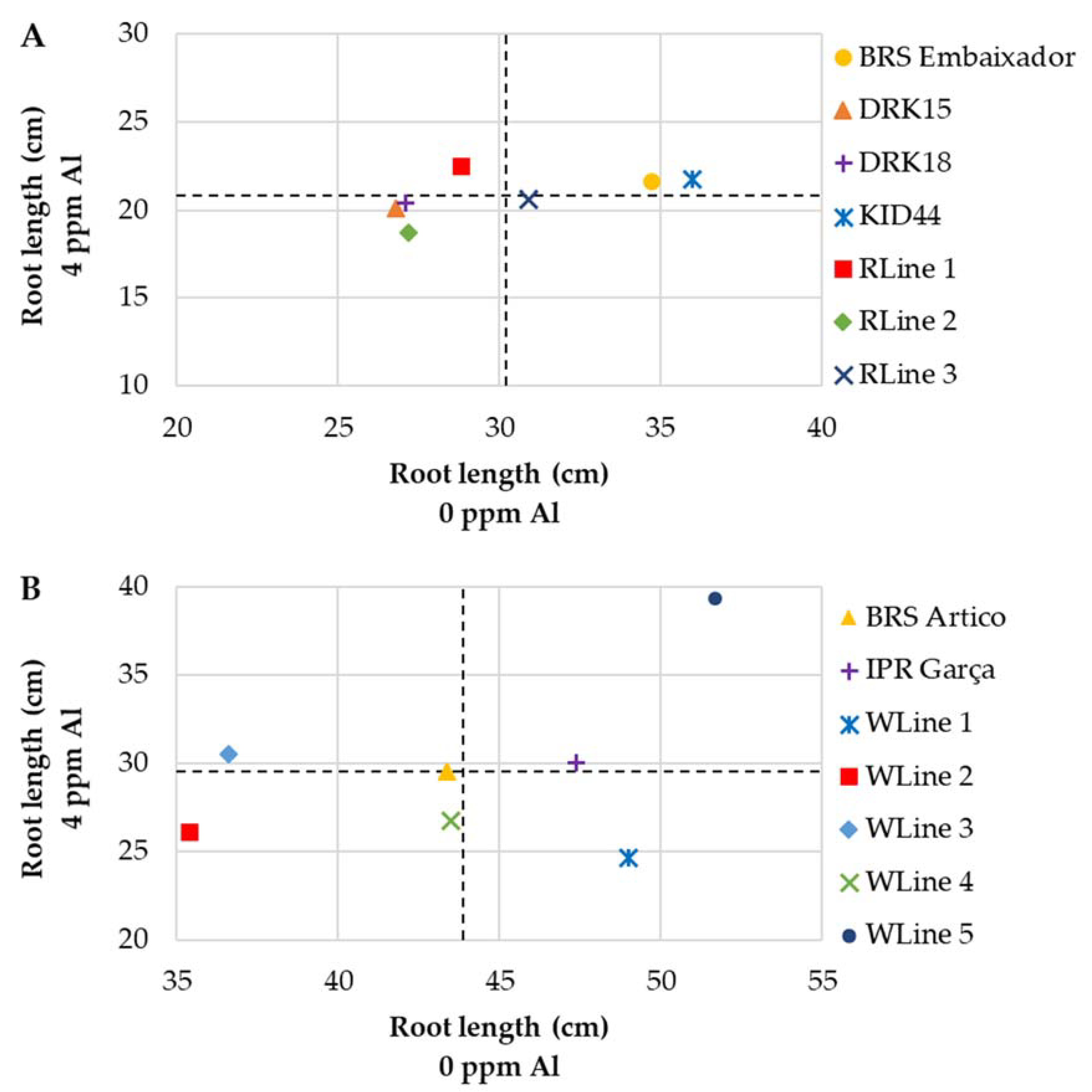
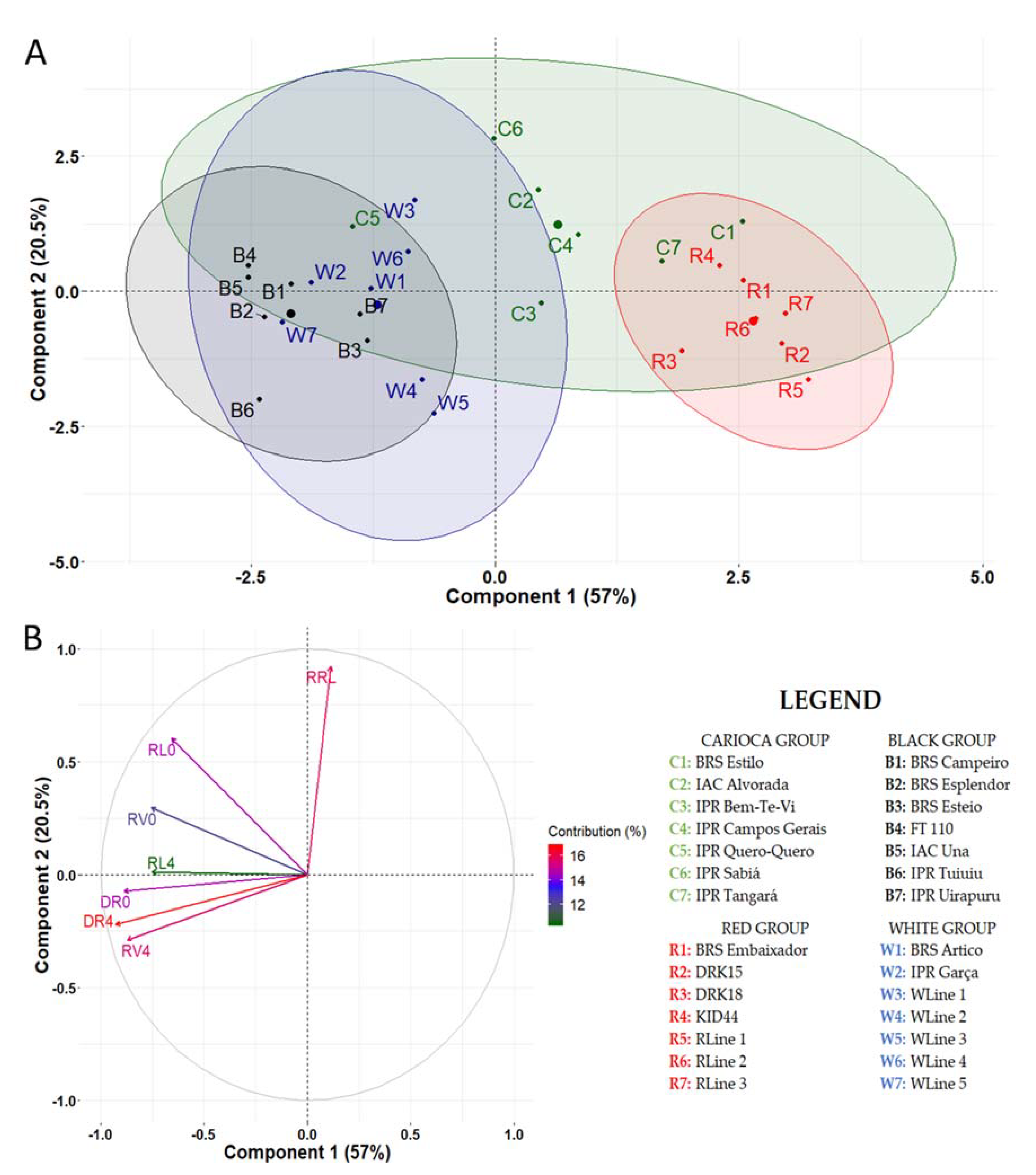
3.2. Stage 2: Genotype Screening
3.2.1. Mesoamerican Genotypes
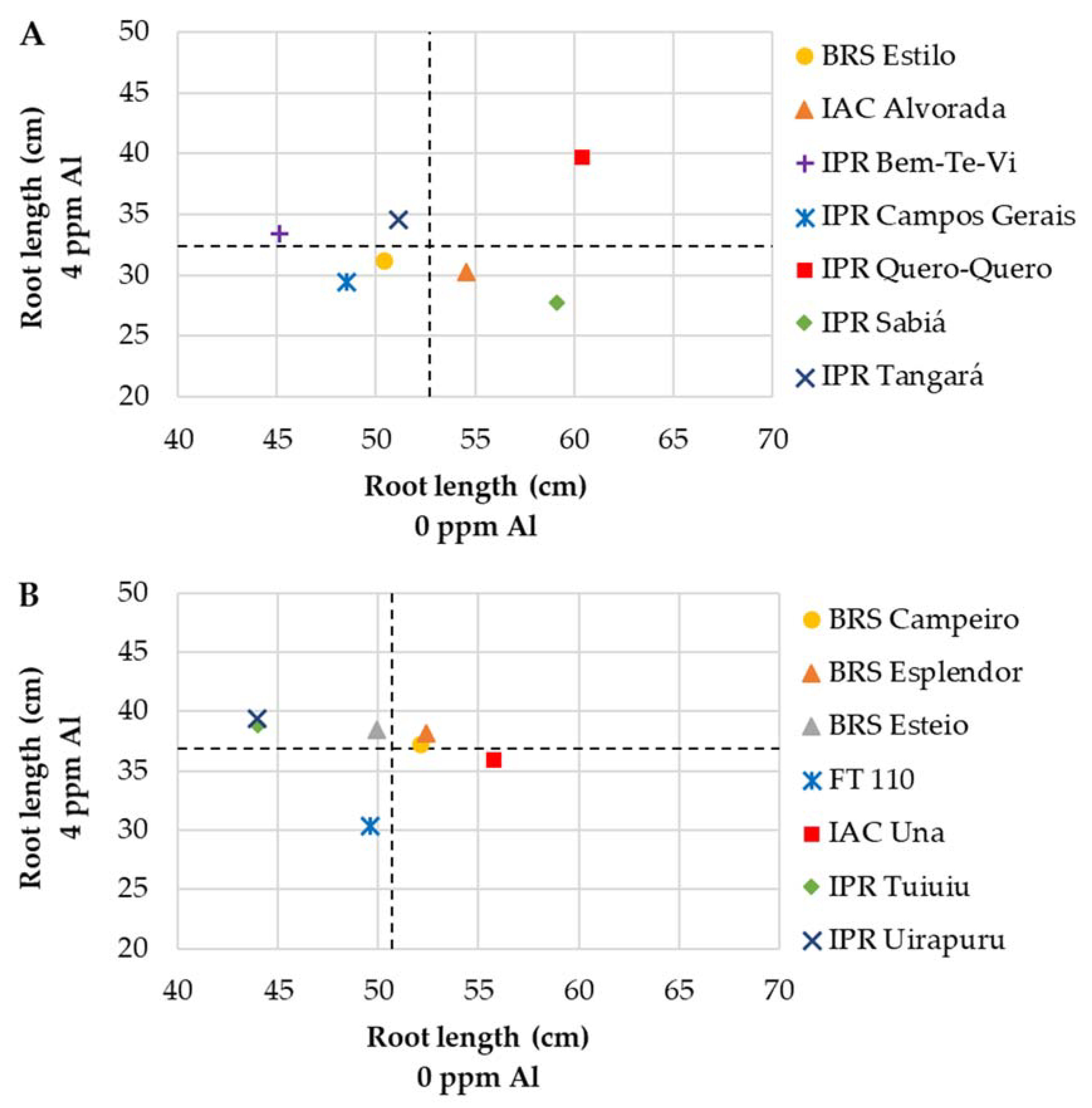
3.2.2. Andean Genotypes
3.2.3. Mesoamerican × Andean Genotypes
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Rezende, A.A.; Teresa, M.; Pacheco, B.; Sônia, V. Nutritional and protein quality of dry Brazilian beans (Phaseolus vulgaris L.). Food Sci. Technol. 2017, 2061, 1–7. [Google Scholar] [CrossRef]
- Campos-Vega, R.; Loarca-Piña, G.; Oomah, B.D. Minor components of pulses and their potential impact on human health. Food Res. Int. 2010, 43, 461–482. [Google Scholar] [CrossRef]
- CONAB, Companhia Nacional de Abastecimento. Acompanhamento da Safra Brasileira de Grãos. Available online: https://www.conab.gov.br/info-agro/safras (accessed on 16 June 2019).
- Souza, A.M.; Pereira, R.A.; Yokoo, E.M.; Levy, R.B.; Sichieri, R. Most consumed foods in Brazil: National dietary survey 2008–2009. Rev. Saude Publica 2013, 47, 190–199. [Google Scholar] [CrossRef] [PubMed]
- Tóth, B.; Grusak, M.A. Aluminum-toxicity responses in Phaseolus vulgaris L. genotypes. Columella 2017, 4, 95–100. [Google Scholar] [CrossRef]
- Broughton, W.J.; Hernández, G.; Blair, M.; Beebe, S.; Gepts, P.; Vanderleyden, J. Beans (Phaseolus spp.)—Model food legumes. Plant Soil 2003, 252, 55–128. [Google Scholar] [CrossRef]
- Giannakoula, A.; Moustakas, M.; Mylona, P.; Papadakis, I.; Yupsanis, T. Aluminum tolerance in maize is correlated with increased levels of mineral nutrients, carbohydrates and proline, and decreased levels of lipid peroxidation and Al accumulation. J. Plant Physiol. 2008, 165, 385–396. [Google Scholar] [CrossRef]
- Carvalho, M.D.C.S.; Nascente, A.S. Application of lime, phosphogypsum and fertilization rates affect soil fertility and common bean development in no-tillage system in a Cerrado Oxisol. Acta Sci. Agron. 2018, 40, 39322. [Google Scholar] [CrossRef]
- Freddi, O.S.; Tavanti, R.F.R.; Carvalho, M.P.; Montanari, R.; Andreotti, M. Restrictions of the Common Bean Productivity in Direct Seeding System in The Brazilian Cerrado. Nativa 2017, 5, 237–243. [Google Scholar] [CrossRef][Green Version]
- Hartwig, I.; Bertan, I.; Maia, L.C.; Fonseca, D.A.R. Associated mechanisms of aluminum tolerance in plants. Semin. Ciências Agrárias 2007, 28, 219–228. [Google Scholar] [CrossRef]
- Yang, Z.B.; Rao, I.M.; Horst, W.J. Interaction of aluminium and drought stress on root growth and crop yield on acid soils. Plant Soil 2013, 372, 3–25. [Google Scholar] [CrossRef]
- Blair, M.W.; López-Marín, H.D.; Rao, I.M. Identification of aluminum resistant Andean common bean (Phaseolus vulgaris L.) genotypes. Braz. J. Plant Physiol. 2009, 21, 291–300. [Google Scholar] [CrossRef]
- Domingues, A.M.; Silva, E.; Freitas, G.; Ganança, J.F.; Slaski, J.J.; Ângelo, M.; Carvalho, P. Aluminium tolerance in bean traditional cultivars from Madeira. Rev. Ciências Agrárias 2013, 36, 148–156. [Google Scholar]
- Akhter, A.; Wagatsuma, T.; Khan, M.S.H.; Tawaraya, K. Comparative Studies on Aluminum Tolerance Screening Techniques for Sorghum, Soybean and Maize in Simple Solution Culture. Am. J. Plant Physiol. 2009, 4, 1–8. [Google Scholar] [CrossRef]
- Butare, L.; Rao, I.; Lepoivre, P.; Polania, J.; Cajiao, C.; Cuasquer, J.; Beebe, S. New genetic sources of resistance in the genus Phaseolus to individual and combined aluminium toxicity and progressive soil drying stresses. Euphytica 2011, 181, 385–404. [Google Scholar] [CrossRef]
- Hoagland, D.R.; Arnon, D.I. The water-culture method for growing plants without soil. Circ. Calif. Agric. Exp. Stn. 1950, 347, 1–32. [Google Scholar]
- Pavan, M.A.; Bingham, F.T. Toxidez de alumínio em cafeeiros cultivados em solução nutritiva. Pesqui. Agropecu. Bras. 1982, 17, 1293–1302. [Google Scholar]
- Ferreira, D.F. Sisvar: A computer statistical analysis system. Ciência e Agrotecnologia 2011, 35, 1039–1042. [Google Scholar] [CrossRef]
- R Core Team. R: A Language and Environment for Statistical Computing; R Core Team: Vienna, Austria, 2017. [Google Scholar]
- Wei, T.; Simko, V. R Package “Corrplot”: Visualization of a Correlation Matrix (Version 0.84). 2017. Available online: https://github.com/taiyun/corrplot (accessed on 19 September 2019).
- Costa França, M.G.; Pham Thi, A.T.; Pimentel, C.; Pereyra, R.O.R.; Zuily-Fodil, Y.; Laffray, D. Differences in growth and water relations among Phaseolus vulgaris cultivars in response to induced drought stress. Environ. Exp. Bot. 2000, 43, 227–237. [Google Scholar] [CrossRef]
- Molina, J.C.; Moda-Cirino, V.; Júnior, N.D.S.F.; de Faria, R.T.; Destro, D.; Da, N.; Fonseca Júnior, S.; Teixeira, R.F.; Destro, D. Response of common bean cultivars and lines to water stress. Crop Breending Applies Biotechnol. 2001, 1, 363–372. [Google Scholar] [CrossRef]
- Kassambara, A.; Mundt, F. Factoextra: Extract and Visualize the Results of Multivariate Data Analyses (R Package Version 1.0.5). 2017. Available online: https://CRAN.R-project.org/package=factoextra (accessed on 19 September 2019).
- Sade, H.; Meriga, B.; Surapu, V.; Gadi, J.; Sunita, M.S.L.; Suravajhala, P.; Kavi Kishor, P.B. Toxicity and tolerance of aluminum in plants: Tailoring plants to suit to acid soils. BioMetals 2016, 29, 187–210. [Google Scholar] [CrossRef]
- Buol, S.W. Soils and agriculture in Central-West and North Brazil. Sci. Agric. 2009, 66, 697–707. [Google Scholar] [CrossRef]
- Hungria, M.; Vargas, M.A.T. Environmental factors affecting N2 fixation in grain legumes in the tropics, with an emphasis on Brazil. F. Crop. Res. 2000, 65, 151–164. [Google Scholar] [CrossRef]
- Mensack, M.M.; Fitzgerald, V.K.; Ryan, E.P.; Lewis, M.R.; Thompson, H.J.; Brick, M.A. Evaluation of diversity among common beans (Phaseolus vulgaris L.) from two centers of domestication using ‘omics’ technologies. BMC Genom. 2010, 11, 686. [Google Scholar] [CrossRef] [PubMed]
- Zeffa, D.M.; Filho, R.S.; Moda-Cirino, V.; Pavan, M.A. Genetic Variability for Tolerance to Aluminum Toxicity in Cultivars and Promising Lines of Beans. UNOPAR Científica Ciências Exatas e Tecnológicas 2011, 10, 21–28. [Google Scholar]
- Yang, Z.; Eticha, D.; Albacete, A.; Rao, I.M.; Roitsch, T.; Horst, W.J. Physiological and molecular analysis of the interaction between aluminium toxicity and drought stress in common bean (Phaseolus vulgaris). J. Exp. Bot. 2012, 63, 3109–3125. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Braun, H.P.; Horst, W.J. Aluminum resistance in common bean (Phaseolus vulgaris) involves induction and maintenance of citrate exudation from root apices. Physiol. Plant. 2010, 138, 176–190. [Google Scholar] [CrossRef]
- Rangel, A.F.; Rao, I.M.; Horst, W.J. Spatial aluminium sensitivity of root apices of two common bean (Phaseolus vulgaris L.) genotypes with contrasting aluminium resistance. J. Exp. Bot. 2007, 58, 3895–3904. [Google Scholar] [CrossRef]
- Gupta, N.; Gaurav, S.S.; Kumar, A. Molecular Basis of Aluminium Toxicity in Plants: A Review. Am. J. Plant Sci. 2013, 2013, 21–37. [Google Scholar] [CrossRef]
- Yang, Z.; Eticha, D.; Rao, I.M.; Horst, W.J. Alteration of cell-wall porosity is involved in osmotic stress-induced enhancement of aluminium resistance in common bean (Phaseolus vulgaris L.). J. Exp. Bot. 2010, 61, 3245–3258. [Google Scholar] [CrossRef]
- López-Marín, H.D.; Rao, I.M.; Blair, M.W. Quantitative trait loci for root morphology traits under aluminum stress in common bean (Phaseolus vulgaris L.). Theor. Appl. Genet. 2009, 119, 449–458. [Google Scholar] [CrossRef]
- Bojórquez-Quintal, E.; Escalante-Magaña, C.; Echevarría-Machado, I.; Martínez-Estévez, M. Aluminum, a Friend or Foe of Higher Plants in Acid Soils. Front. Plant Sci. 2017, 8, 1–18. [Google Scholar] [CrossRef] [PubMed]
- Bian, M.; Zhou, M.; Sun, D.; Li, C. Molecular approaches unravel the mechanism of acid soil tolerance in plants. Crop J. 2013, 1, 91–104. [Google Scholar] [CrossRef]
- Wang, H.; Huang, J.; Liang, W.; Liang, X.; Bi, Y. Involvement of putrescine and nitric oxide in aluminum tolerance by modulating citrate secretion from roots of red kidney bean. Plant Soil 2013, 366, 479–490. [Google Scholar] [CrossRef]
- Zhang, M.; Ma, Y.; Horst, W.J.; Yang, Z.B. Spatial-temporal analysis of polyethylene glycol-reduced aluminium accumulation and xyloglucan endotransglucosylase action in root tips of common bean (Phaseolus vulgaris). Ann. Bot. 2016, 118, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Eticha, D.; Zahn, M.; Bremer, M.; Yang, Z.; Rangel, A.F.; Rao, I.M.; Horst, W.J. Transcriptomic analysis reveals differential gene expression in response to aluminium in common bean (Phaseolus vulgaris) genotypes. Ann. Bot. 2010, 105, 1119–1128. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, D.P.; Vieira, N.M.B.; Souza, H.C.; De Morais, A.R.; Pereira, J.; Andrade, M.J.B. Qualidade tecnológica de grãos de cultivares de feijão-comum na safra das águas. Semin. Agrar. 2012, 33, 1831–1838. [Google Scholar] [CrossRef]
- Butare, L.; Rao, I.; Lepoivre, P.; Cajiao, C.; Polania, J.; Cuasquer, J.; Beebe, S. Phenotypic evaluation of interspecific recombinant inbred lines (RILs) of Phaseolus species for aluminium resistance and shoot and root growth response to aluminium-toxic acid soil. Euphytica 2012, 186, 715–730. [Google Scholar] [CrossRef]
| Cultivar | Institution 1 | Origin | Group | Growth Habit | Cycle |
|---|---|---|---|---|---|
| BRS Realce | Embrapa | Andean | Red | Determinate | Early |
| IAC Formoso | IAC | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Curió | IAPAR | Mesoamerican | Carioca | Determinate | Early |
| IPR Garça | IAPAR | Andean | White | Determinate | Early |
| Cultivars | Institution 1 | Origin | Group | Growth Habit | Cycle |
|---|---|---|---|---|---|
| BRS Estilo | Embrapa | Mesoamerican | Carioca | Indeterminate | Medium |
| IAC Alvorada | IAC | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Bem-Te-Vi | IAPAR | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Campos Gerais | IAPAR | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Quero-Quero | IAPAR | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Sabiá | IAPAR | Mesoamerican | Carioca | Indeterminate | Medium |
| IPR Tangará | IAPAR | Mesoamerican | Carioca | Indeterminate | Medium |
| BRS Campeiro | Embrapa | Mesoamerican | Black | Indeterminate | Medium |
| BRS Esplendor | Embrapa | Mesoamerican | Black | Indeterminate | Medium |
| BRS Esteio | Embrapa | Mesoamerican | Black | Indeterminate | Medium |
| FT 110 | FT Sementes | Mesoamerican | Black | Indeterminate | Medium |
| IAC Una | IAC | Mesoamerican | Black | Indeterminate | Medium |
| IPR Tuiuiú | IAPAR | Mesoamerican | Black | Indeterminate | Medium |
| IPR Uirapuru | IAPAR | Mesoamerican | Black | Indeterminate | Medium |
| BRS Embaixador | Embrapa | Andean | Red | Determinate | Early |
| DRK15 | CIAT | Andean | Red | Determinate | Early |
| DRK18 | CIAT | Andean | Red | Determinate | Early |
| KID44 | CIAT | Andean | Red | Determinate | Early |
| RLine 1 | IAPAR | Andean | Red | Determinate | Early |
| RLine 2 | IAPAR | Andean | Red | Determinate | Early |
| RLine 3 | IAPAR | Andean | Red | Determinate | Early |
| BRS Ártico | Embrapa | Andean | White | Determinate | Early |
| IPR Garça | IAPAR | Andean | White | Determinate | Early |
| WLine 1 | IAPAR | Andean | White | Determinate | Early |
| WLine 2 | IAPAR | Andean | White | Determinate | Early |
| WLine 3 | IAPAR | Andean | White | Determinate | Early |
| WLine 4 | IAPAR | Andean | White | Determinate | Early |
| WLine 5 | IAPAR | Andean | White | Determinate | Early |
| Genotypes | Root Length (cm) | Root Volume (cm3) | Root Dry Weight (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 4 ppm | RR(%) | 0 ppm | 4 ppm | RR(%) | 0 ppm | 4 ppm | RR(%) | |
| Carioca Group | |||||||||
| BRS Estilo | 50.4 Ab | 31.2 Bb | 38 | 4.5 Ab | 3.4 Bd | 26 | 0.22 Ac | 0.18 Ac | 16 |
| IAC Alvorada | 54.6 Aa | 30.3 Bb | 44 | 5.8 Aa | 4.9 Bb | 15 | 0.28 Ab | 0.26 Aa | 5 |
| IPR Bem-Te-Vi | 45.1 Ab | 33.4 Ba | 26 | 5.4 Aa | 4.7 Ab | 13 | 0.32 Aa | 0.27 Aa | 15 |
| IPR Campos Gerais | 48.5 Ab | 29.4 Bb | 39 | 5.4 Aa | 4.9 Ab | 10 | 0.27 Ab | 0.27 Aa | 0 |
| IPR Quero-Quero | 60.4 Aa | 39.8 Ba | 34 | 6.4 Aa | 6.0 Aa | 6 | 0.30 Aa | 0.31 Aa | 0 |
| IPR Sabiá | 59.1 Aa | 27.8 Bb | 53 | 5.9 Aa | 4.4 Bc | 26 | 0.36 Aa | 0.31 Aa | 14 |
| IPR Tangará | 51.1 Ab | 34.6 Ba | 32 | 4.4 Ab | 4.1 Ac | 7 | 0.22 Ac | 0.24 Ab | 0 |
| CV(%) | 16.49 | 14.14 | 18.56 | ||||||
| Black Group | |||||||||
| BRS Campeiro | 52.1 Aa | 37.3 Ba | 29 | 6.4 Aa | 5.7 Ab | 11 | 0.42 Aa | 0.41 Ab | 3 |
| BRS Esplendor | 52.4 Aa | 38.1 Ba | 27 | 5.6 Ab | 6.2 Aa | 0 | 0.44 Aa | 0.46 Aa | 0 |
| BRS Esteio | 49.9 Aa | 38.5 Ba | 23 | 5.2 Ab | 5.9 Ab | 0 | 0.34 Ab | 0.44 Ba | 0 |
| FT 110 | 49.6 Aa | 30.4 Bb | 39 | 6.4 Aa | 6.2 Aa | 3 | 0.46 Aa | 0.52 Ba | 0 |
| IAC Una | 55.7 Aa | 36.0 Ba | 35 | 5.7 Ab | 6.7 Ba | 0 | 0.43 Aa | 0.46 Aa | 0 |
| IPR Tuiuiu | 44.0 Ab | 38.9 Aa | 12 | 6.0 Aa | 6.2 Aa | 0 | 0.45 Aa | 0.45 Aa | 0 |
| IPR Uirapuru | 51.1 Aa | 39.4 Ba | 23 | 5.7 Ab | 5.2 Ab | 9 | 0.40 Ab | 0.38 Ab | 4 |
| CV(%) | 13.81 | 12.52 | 13.30 | ||||||
| Genotypes | Root Length (cm) | Root Volume (cm3) | Root Dry Weigth (g) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 0 ppm | 4 ppm | RR(%) | 0 ppm | 4 ppm | RR(%) | 0 ppm | 4 ppm | RR(%) | |
| Red group | |||||||||
| BRS Embaixador | 34.7 Aa | 21.6 Ba | 38 | 4.7 Ab | 4.0 Ab | 15 | 0.28 Aa | 0.25 Aa | 11 |
| DRK15 | 26.8 Ab | 20.1 Ba | 25 | 5.1 Aa | 3.8 Bb | 26 | 0.25 Aa | 0.23 Aa | 9 |
| DRK18 | 27.1 Ab | 20.4 Ba | 25 | 5.7 Aa | 5.0 Aa | 12 | 0.26 Aa | 0.25 Aa | 4 |
| KID44 | 36.0 Aa | 21.7 Ba | 40 | 5.1 Aa | 4.7 Aa | 8 | 0.24 Aa | 0.23 Aa | 2 |
| RLine 1 | 28.8 Ab | 22.5 Ba | 22 | 4.0 Ab | 4.6 Aa | 0 | 0.23 Aa | 0.20 Aa | 13 |
| RLine 2 | 27.2 Ab | 18.7 Ba | 31 | 5.2 Aa | 4.2 Bb | 20 | 0.29 Aa | 0.21 Ba | 26 |
| RLine 3 | 30.9 Ab | 20.6 Ba | 33 | 4.5 Ab | 4.3 Ab | 6 | 0.25 Aa | 0.22 Aa | 13 |
| CV(%) | 14.58 | 16.24 | 17.64 | ||||||
| White group | |||||||||
| BRS Artico | 43.4 Ab | 29.5 Bb | 32 | 6.6 Aa | 6.0 Ab | 9 | 0.38 Aa | 0.38 Aa | 1 |
| IPR Garça | 47.4 Aa | 30.0 Bb | 37 | 6.1 Aa | 6.6 Aa | 0 | 0.41 Aa | 0.45 Aa | 0 |
| WLine 1 | 49.0 Aa | 24.6 Bb | 50 | 6.1 Aa | 5.6 Ab | 8 | 0.43 Aa | 0.36 Aa | 16 |
| WLine 2 | 35.4 Ac | 26.1 Bb | 26 | 5.1 Ab | 6.6 Ba | 0 | 0.40 Aa | 0.45 Aa | 0 |
| WLine 3 | 36.6 Ac | 30.5 Bb | 17 | 5.0 Ab | 6.5 Ba | 0 | 0.36 Aa | 0.42 Aa | 0 |
| WLine 4 | 43.5 Ab | 26.7 Bb | 39 | 6.6 Aa | 5.5 Bb | 17 | 0.41 Aa | 0.35 Aa | 15 |
| WLine 5 | 51.7 Aa | 39.3 Ba | 24 | 6.1 Aa | 6.8 Ba | 0 | 0.35 Aa | 0.41 Aa | 0 |
| CV(%) | 14.96 | 15.93 | 21.83 | ||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
dos Santos Neto, J.; Delfini, J.; Willian Silva, T.; Akihide Hirose, A.; Marcos Novais, J.; Simões Azeredo Gonçalves, L.; Moda-Cirino, V. Response of Common Bean Cultivars and Lines to Aluminum Toxicity. Agronomy 2020, 10, 296. https://doi.org/10.3390/agronomy10020296
dos Santos Neto J, Delfini J, Willian Silva T, Akihide Hirose A, Marcos Novais J, Simões Azeredo Gonçalves L, Moda-Cirino V. Response of Common Bean Cultivars and Lines to Aluminum Toxicity. Agronomy. 2020; 10(2):296. https://doi.org/10.3390/agronomy10020296
Chicago/Turabian Styledos Santos Neto, José, Jessica Delfini, Tiago Willian Silva, Anderson Akihide Hirose, João Marcos Novais, Leandro Simões Azeredo Gonçalves, and Vânia Moda-Cirino. 2020. "Response of Common Bean Cultivars and Lines to Aluminum Toxicity" Agronomy 10, no. 2: 296. https://doi.org/10.3390/agronomy10020296
APA Styledos Santos Neto, J., Delfini, J., Willian Silva, T., Akihide Hirose, A., Marcos Novais, J., Simões Azeredo Gonçalves, L., & Moda-Cirino, V. (2020). Response of Common Bean Cultivars and Lines to Aluminum Toxicity. Agronomy, 10(2), 296. https://doi.org/10.3390/agronomy10020296





