Changes in Storage and the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types in Northeastern China
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Area
2.2. Soil Sampling and Analysis
2.3. Calculations and Statistical Analysis
3. Results
3.1. Changes in Soil Bulk Density under Different Vegetation Types
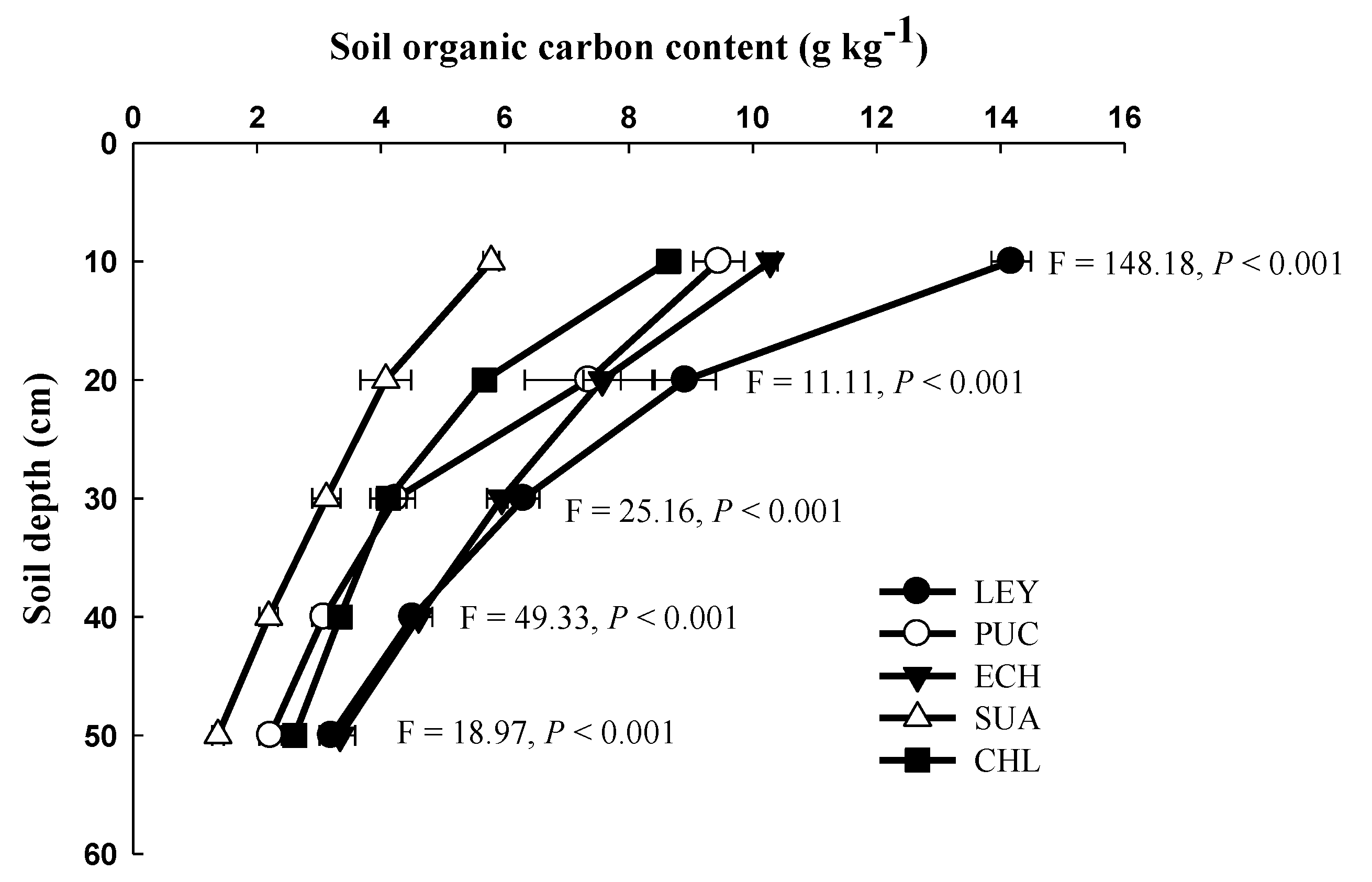
3.2. Changes in Concentrations and Storage of Soil Organic Carbon under Different Vegetation Types
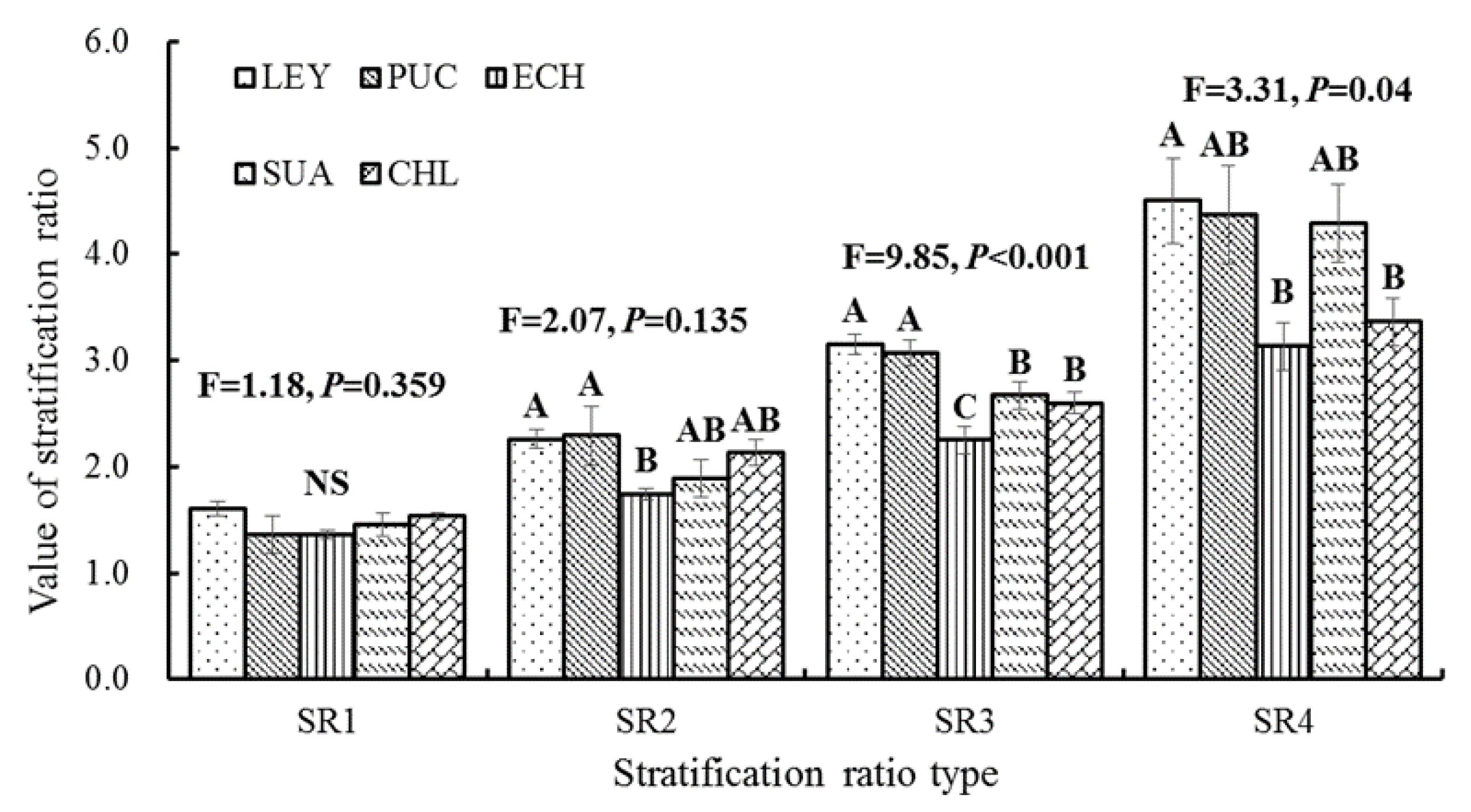
3.3. Changes in the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types
4. Discussion
4.1. Effect of Vegetation Type on SOC Concentration and Storage
4.2. Effect of Vegetation Type on Stratification Ratios of SOC Concentration
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Paz-Ferreiro, J.; Medina-Roldan, E.; Ostle, N.J.; Mcnamara, N.P.; Bardgett, R.D. Grazing increases the temperature sensitivity of soil organic matter decomposition in a temperate grassland. Environ. Res. Lett. 2012, 7, 014027. [Google Scholar] [CrossRef]
- Conant, R.T.; Cerri, C.E.P.; Osborne, B.B.; Paustian, K. Grassland management impacts on soil organic carbon stocks: A new synthesis. Ecol. Appl. 2017, 27, 662–668. [Google Scholar] [CrossRef]
- Jobbacy, E.G.; Jackson, R.B. The vertical distribution of soil organic carbon and its relation to climate and vegetation. Ecol. Appl. 2000, 10, 423–436. [Google Scholar] [CrossRef]
- Novara, A.; Minacapilli, M.; Santoro, A.; Rodrigo-Comino, J.; Carrubba, A.; Sarno, M.; Venezia, G.; Gristina, L. Real cover crops contribution to soil orgnaic carbon sequestration in sloping vineyard. Sci. Total Environ. 2019, 652, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Munoz-Rojas, M.; Rosa, D.D.; Zavala, L.M.; Jordan, A.; Anaya-Romero, M. Changes in land cover and vegetation carbon stocks in Andalusia, Southern Spain (1956–2007). Sci. Total Environ. 2011, 409, 2796–2806. [Google Scholar] [CrossRef] [PubMed]
- Fang, J.Y.; Yang, Y.H.; Ma, W.H.; Mohammat, A.; Shen, H. Ecosystem carbon stocks and their changes in China’s grassland. Sci. China Life Sci. 2010, 53, 757–765. [Google Scholar] [CrossRef]
- Santos, C.A.; Rezende, C.P.; Machado, P.E.F.; Pereira, J.M.; Alves, B.J.R.; Urquoaga, S.; Boddey, R.M. Changes in soil carbon stocks after land-use change form native vegetation to pastures in the Atlantic forest region of Brazil. Geoderma 2019, 337, 394–401. [Google Scholar] [CrossRef]
- Deng, L.; Wang, K.B.; Zhu, G.Y.; Liu, Y.L.; Chen, L.; Shangguan, Z.P. Changes of soil carbon in five land use stages following 10 years of vegetation succession on the Loess Plateau, China. Catena 2018, 171, 185–192. [Google Scholar] [CrossRef]
- Yu, P.J.; Li, Q.; Jia, H.T.; Zheng, W.; Wang, M.L.; Zhou, D.W. Carbon stocks and storage potential as affected by vegetation in the Songnen grassland of northeast China. Quat. Int. 2013, 306, 114–120. [Google Scholar] [CrossRef]
- Shrestha, B.M.; Singh, B.R. Soil and vegetation carbon pools in a mountainous watershed of Nepal. Nutr. Cycl. Agroecosyst. 2008, 81, 179–191. [Google Scholar] [CrossRef]
- Li, N.; Shao, T.Y.; Zhu, T.S.; Long, X.H.; Gao, X.M.; Liu, Z.P.; Shao, H.B.; Rengel, Z. Vegetation succession influences soil carbon sequestration in coastal alkali-saline soils in southeast China. Sci. Rep. 2018, 8, 9728. [Google Scholar] [CrossRef] [PubMed]
- Lemenih, M.; Itanna, F. Soil carbon stocks and turnovers in various types and arable lands along an gradient in southern Ethiopia. Geoderma 2014, 123, 177–188. [Google Scholar] [CrossRef]
- Hu, P.L.; Liu, S.J.; Ye, Y.Y.; Zhang, W.; Wang, K.L.; Su, Y.R. Effects of environmental factors on soil organic carbon under natural or managed vegetation restoration. Land Degrad. Dev. 2018, 29, 387–397. [Google Scholar] [CrossRef]
- Parker, T.C.; Subke, J.A.; Wookey, P.A. Rapid carbon turnover beneath shrub and tree vegetation is associated with low soil carbon stocks at a subarctic treeline. Glob. Chang. Biol. 2015, 21, 2070–2081. [Google Scholar] [CrossRef] [PubMed]
- Loranty, M.M.; Lieberman-Cribbin, W.; Berner, L.T.; Natali, S.M.; Goetz, S.J.; Alexander, H.D.; Kholodov, A.L. Spatial variation in vegetation productivity trends, fire disturbance, and soil carbon across arctic-boreal permafrost ecosystems. Environ. Res. Lett. 2016, 11, 095008. [Google Scholar] [CrossRef]
- Yu, P.J.; Tang, X.G.; Liu, S.W.; Liu, W.X.; Zhang, A.C. Short term effects of revegetation on labile carbon and available nutrients of sodic soils in northeast China. Land 2020, 9, 10. [Google Scholar] [CrossRef]
- Lal, R. Soil carbon sequestration to mitigate climate change. Geoderma 2004, 123, 1–22. [Google Scholar] [CrossRef]
- Franzluebbers, A.J. Soil organic matter stratification ratio as an indicator of soil quality. Soil Tillage Res. 2002, 66, 95–106. [Google Scholar] [CrossRef]
- Melero, S.; Lopez-Garrido, R.; Murillo, J.M.; Moreno, F. Conservation tillage, short-and long-term effects on soil carbon fractions and enzymatic activities under Mediterranean conditions. Soil Tillage Res. 2009, 104, 292–298. [Google Scholar] [CrossRef]
- Melero, S.; Lopez-Bellido, R.J.; Luis-Bellido, L.; Munoz-Romero, V.; Moren, F.; Murillo, J.M.; Franzluebbers, A.J. Stratification ratios in a rainfed Mediterranean Vertisol in wheat under differnet tillage, rotation and N fertilization rates. Soil Tillage Res. 2012, 119, 7–12. [Google Scholar] [CrossRef]
- Lopez-Fando, C.; Pardo, M.T. Soil carbon storage and stratification under different tillage systems in a semiarid region. Soil Tillage Res. 2011, 111, 224–230. [Google Scholar] [CrossRef]
- Xu, M.X.; Wang, Z.; Zhao, Y.G. Stratification ratio of soil organic carbon as an indicator of carbon sequestration and soil quality in ecological restoration. Restor. Ecol. 2018, 26, 555–562. [Google Scholar] [CrossRef]
- Zhao, X.; Xue, J.F.; Zhang, X.Q.; Kong, F.L.; Chen, F.; Lal, R.; Zhang, H.L. Stratification and storage of soil organic carbon and nitrogen as affected by tillage practices in the North China Plain. PLoS ONE 2015, 10, e01288873. [Google Scholar] [CrossRef] [PubMed]
- Moraes Sa, J.C.; Lal, R. Stratification ratio of soil organic matter pools as an indicator of carbon sequestration in a tillage chronosequence on a Brazilian Oxisol. Soil Tillage Res. 2009, 103, 46–56. [Google Scholar]
- Marinho, M.A.; Pereira, M.W.M.; Vazquez, E.V.; Lado, M.; Gonzalez, A.P. Depth distribution of soil organic carbon in an Oxisol under different land uses, Stratification indices and multifractal analysis. Geoderma 2017, 287, 126–134. [Google Scholar] [CrossRef]
- Patra, S.; Julich, S.; Feger, K.H.; Jat, M.L.; Sharma, P.C.; Schwarzel, K. Effects of converison agriculture on stratification of osil organic matter udner cereal-based cropping systems. Arch. Agron. Soil Sci. 2019, 65, 2013–2028. [Google Scholar] [CrossRef]
- Li, Q.; Song, Y.T.; Zhou, D.W.; Wang, M.L.; Chen, X.Y. Effects of fencing and grazing on soil carbon, nitrogen, phosphorus storage in degraded alkali-saline grassland. Pratacultural Sci. 2014, 31, 1811–1819. [Google Scholar]
- Blake, G.R.; Hartage, K.H. Bulk density. In Methods of Soil Analysis. Part 1—Physical Mineralogical Methods; Klute, A., Ed.; ASA and SSSA: Madison, WI, USA, 1986; pp. 363–375. [Google Scholar]
- Ellert, B.H.; Bettany, J.R. Calculation of organic matter and nutrients stored in soils under contrastin management regimes. Can. J. Soil Sci. 1995, 75, 529–538. [Google Scholar] [CrossRef]
- Gmach, M.R.; Dias, B.O.; Silva, C.A.; Nobrega, J.C.A.; Lustosa-Filho, J.; Siqueira-Neto, M. Soil organic matter dynamics and land use change on Oxisols in the Cerrado, Brazil. Geoderma Reg. 2018, 14, e00178. [Google Scholar] [CrossRef]
- Wang, M.; Liu, X.T.; Zhang, J.T.; Li, X.J.; Wang, G.D.; Lu, X.R.; Li, X.Y. Spatio-temporal variations of soil respiration in five typical plant communities in the meadow steppe of the western Songnen Plain, China. Chin. J. Plant Ecol. 2014, 38, 396–404. [Google Scholar]
- Poeplau, C.; Don, A.; Vesterdal, L.; Leifeld, J.; Van Wesemael, B.; Schumacher, J.; Gensior, A. Temporal dynamics of soil organic carbon after land-use change in the temperate zone-carbon response functions as a model approach. Glob. Chang. Biol. 2011, 17, 2415–2427. [Google Scholar] [CrossRef]
- Ding, F.; Hu, Y.L.; Li, L.J.; Li, A.; Shi, S.W.; Lian, P.Y.; Zeng, D.H. Changes in soil organic carbon and total nitrogen stocks after conversion of meadow to cropland in Northeast China. Plant Soil 2013, 373, 659–672. [Google Scholar] [CrossRef]
- Lal, R. Digging deeper, a holistic perspective of factors affecting soil organic carbon sequestration in agroecosystems. Glob. Chang. Biol. 2018, 24, 3285–3301. [Google Scholar] [CrossRef] [PubMed]
- He, N.P.; Yu, Q.; Wu, L.; Wang, Y.S.; Han, X.G. Carbon and nitrogen store and storage potential as affected by land-use in a Leymus chinensis grassland of northern China. Soil Biol. Biochem. 2008, 40, 2952–2959. [Google Scholar] [CrossRef]
- Lozano-Garcia, B.; Parras-Alcantara, L.; Cantudo-Perez, M. Land use change effects on stratification and storage of soil carbon and nitrogen, Application to a Mediterranean nature reserve. Agric. Ecosyst. Environ. 2016, 231, 105–113. [Google Scholar] [CrossRef]
- Zhang, Z.Q.; Qiang, H.J.; McHugh, A.D.; He, J.; Li, H.W.; Wang, Q.J.; Lu, Z.Y. Effect of conservation farming practices on soil organic matter and stratification in a mono-cropping system of Northern China. Soil Tillage Res. 2016, 156, 173–181. [Google Scholar] [CrossRef]
- Deng, J.; Sun, P.S.; Zhao, F.Z.; Han, X.H.; Yang, G.H.; Feng, Y.Z.; Ren, G.X. Soil C, N, P and its stratification ratio affected by artificial vegetation in subsoil, Loess Plateau China. PLoS ONE 2016, 11, e0151446. [Google Scholar] [CrossRef]
- Fan, H.; Zhao, W.W.; Daryanto, S.; Fu, B.J.; Wang, S.; Wang, Y.P. Vertical distributions of soil organic carbon and its influencing factors under different land use types in the desert riparian zone of downstream Heihe River basin, China. J. Geophys. Res. Atmos. 2018, 123, 7741–7753. [Google Scholar] [CrossRef]

| Vegetation Type | Companion Species | Soil Salinity and Sodicity (0–20 cm) | Biomass (g m−2) | ||
|---|---|---|---|---|---|
| EC (μm cm−1) | pH | Aboveground | Belowground (0–50 cm) | ||
| LEY | Chloris virgata | 438 | 9.5 | 411 | 518 |
| PUC | Chloris virgata, Polygonum aviculare | 515 | 9.8 | 264 | 352 |
| ECH | Puccinellia tenuiflora, Scirpus triqueter | 281 | 9.4 | 287 | 405 |
| SUA | 1496 | 10.2 | 273 | 86 | |
| CHL | Puccinellia tenuiflora, Polygonum aviculare | 827 | 10.1 | 316 | 126 |
| Soil Depth (cm) | Bulk Density (g cm−3) | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| LEY | PUC | ECH | SUA | CHL | F | P | |
| 0–10 | 1.456 (±0.017) Bc | 1.530 (±0.014) Ac | 1.486 (±0.019) Bc | 1.564 (±0.009) Ac | 1.531 (±0.011) ABc | 8.805 | 0.001 |
| 10–20 | 1.530 (±0.010) Bb | 1.560 (±0.007) Ac | 1.529 (±0.009) Bc | 1.590 (±0.009) Ac | 1.555 (±0.015) ABbc | 5.658 | 0.006 |
| 20–30 | 1.561 (±0.028) Bb | 1.601 (±0.012) ABb | 1.578 (±0.017) ABb | 1.621 (±0.007) Ab | 1.586 (±0.008) ABb | 2.121 | 0.129 |
| 30–40 | 1.599 (±0.003) Bab | 1.613 (±0.010) ABb | 1.615 (±0.009) ABab | 1.635 (±0.009) Aab | 1.636 (±0.014) Aa | 1.817 | 0.178 |
| 40–50 | 1.630 (±0.011) Aa | 1.661 (±0.013) Aa | 1.639 (±0.014) Aa | 1.666 (±0.016) Aa | 1.658 (±0.011) Aa | 1.326 | 0.305 |
| Soil Depth (cm) | Storage of Soil Organic Carbon (Mg Ha−1) | ANOVA | |||||
|---|---|---|---|---|---|---|---|
| LEY | PUC | ECH | SUA | CHL | F | P | |
| 0–10 | 20.625 (±0.465) Aa | 14.453 (±0.632) Ba | 15.275 (±0.181) Ba | 9.035 (±0.209) Da | 13.225 (±0.109) Ca | 123.578 | <0.001 |
| 10–20 | 13.555 (±0.739) Ab | 11.470 (±1.599) Ab | 11.575 (±0.464) Ab | 6.458 (±0.653) Bb | 8.815 (±0.240) Bb | 10.042 | <0.001 |
| 20–30 | 9.838 (±0.400) Ac | 6.785 (±0.495) Bc | 9.390 (±0.380) Ac | 5.058 (±0.380) Cc | 6.525 (±0.455) Bc | 22.849 | <0.001 |
| 30–40 | 7.208 (±0.197) Ad | 4.973 (±0.099) Bcd | 7.453 (±0.361) Ad | 3.578 (±0.241) Cd | 5.460 (±0.202) Bd | 46.870 | <0.001 |
| 40–50 | 5.210 (±0.304) ABe | 3.680 (±0.298) Bd | 5.470 (±0.399) Ae | 2.283 (±0.151) Ce | 4.333 (±0.299) Be | 18.228 | <0.001 |
| 0–50 | 56.436 (±1.038) A | 41.361 (±1.928) C | 49.163 (±0.804) B | 26.412 (±0.858) D | 38.358 (±0.738) C | 95.897 | <0.001 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yu, P.; Liu, S.; Ding, Z.; Zhang, A.; Tang, X. Changes in Storage and the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types in Northeastern China. Agronomy 2020, 10, 290. https://doi.org/10.3390/agronomy10020290
Yu P, Liu S, Ding Z, Zhang A, Tang X. Changes in Storage and the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types in Northeastern China. Agronomy. 2020; 10(2):290. https://doi.org/10.3390/agronomy10020290
Chicago/Turabian StyleYu, Pujia, Shiwei Liu, Zhi Ding, Aichun Zhang, and Xuguang Tang. 2020. "Changes in Storage and the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types in Northeastern China" Agronomy 10, no. 2: 290. https://doi.org/10.3390/agronomy10020290
APA StyleYu, P., Liu, S., Ding, Z., Zhang, A., & Tang, X. (2020). Changes in Storage and the Stratification Ratio of Soil Organic Carbon under Different Vegetation Types in Northeastern China. Agronomy, 10(2), 290. https://doi.org/10.3390/agronomy10020290







