Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production
Abstract
1. Introduction
2. Materials and Methods
2.1. Characteristics of the Materials
2.2. Physical and Chemical Characterization of the Growth Media
2.3. Phytotoxicity of the Growth Media
2.4. Stability to Microbial Degradation of the Growth Media
2.5. Experimental Design, Plant Growing Conditions, and Plant Analysis
2.6. Data Analyses
3. Results
3.1. Physical Properties of the Growth Media
3.2. Chemical Characteristics and Germination Index of the Growth Media
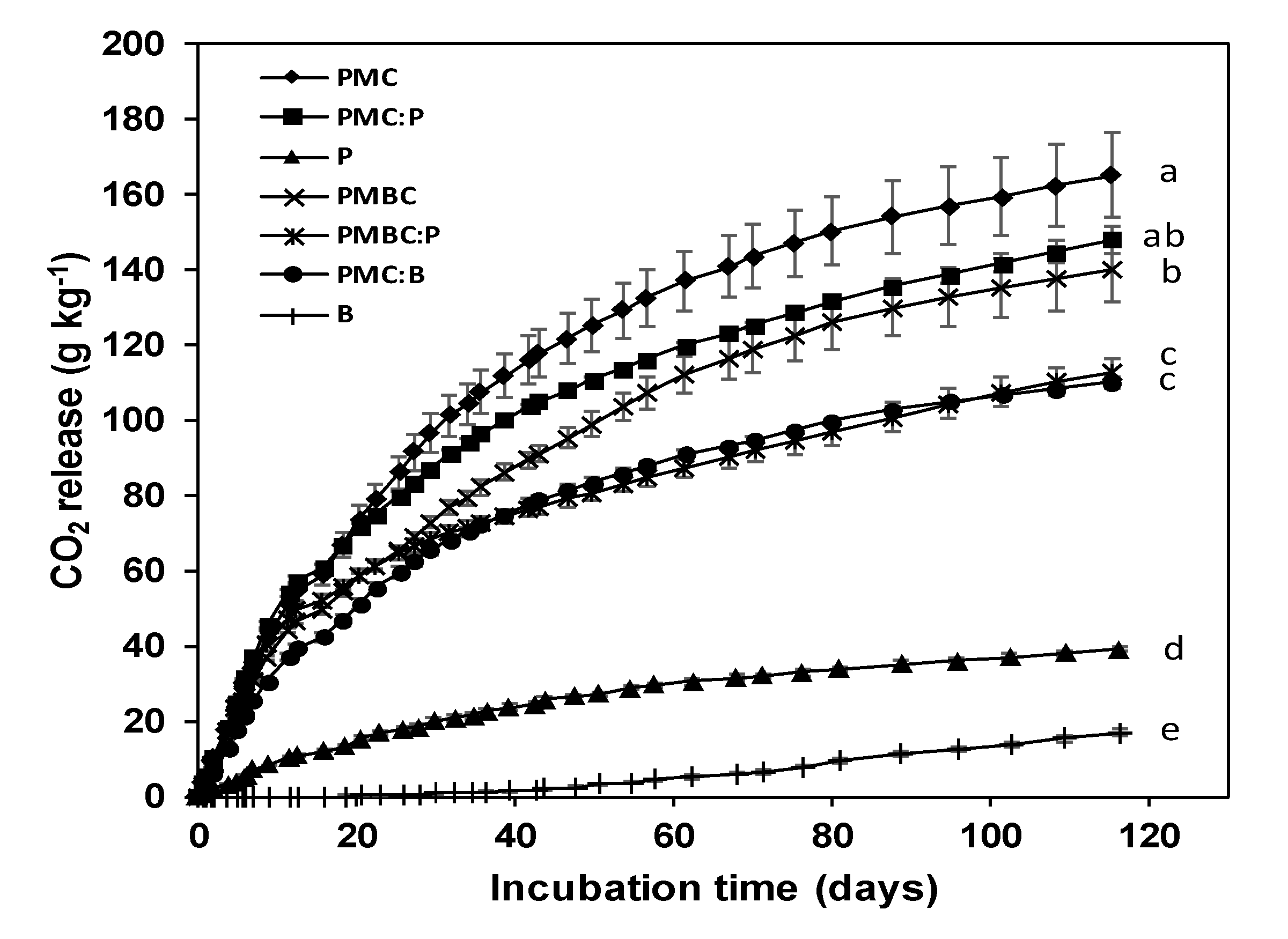
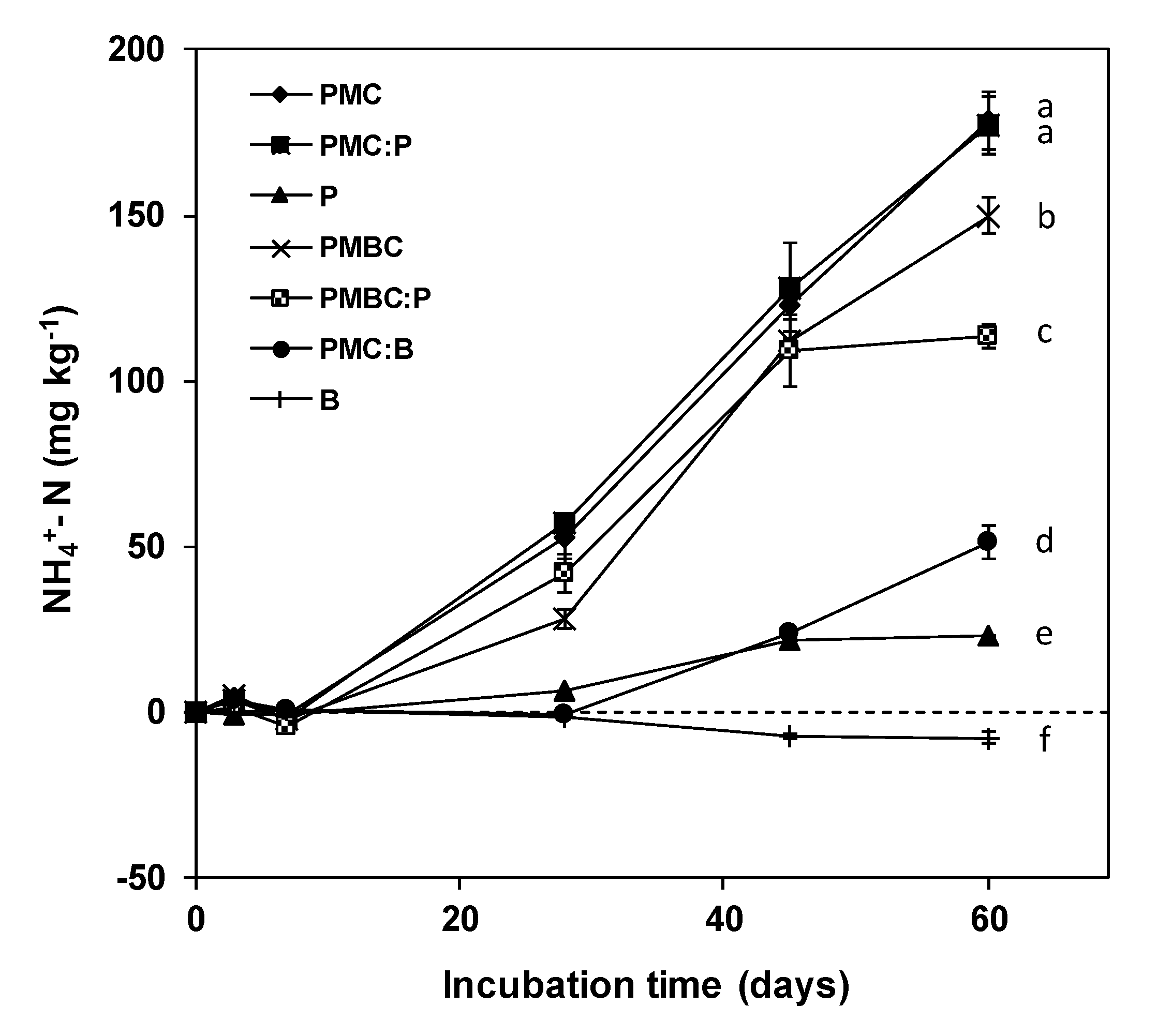
3.3. Stability to Microbial Degradation of the Growth Media
3.4. Experiment I: Comparison of Poultry Compost (PMC) Versus Poultry Manure Compost Co-Composted with Biochar (PMBC)
3.5. Experiment II. Comparison of Biochar (B) versus Peat (P) in Mixes with Poultry Manure Compost (PMC)
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kern, J.; Tammeorg, P.; Shanskiy, M.; Sakrabani, R.; Knicker, H.; Kammann, C.; Tuhkanen, E.M.; Smidt, G.; Prasad, M.; Tiilikkala, K.; et al. Synergistic use of peat and charred material in growing media—an option to reduce the pressure on peatlands? J. Environ. Eng. Landsc. Manag. 2017, 25, 160–174. [Google Scholar] [CrossRef]
- Tiemeyer, B.; Albiac Borraz, E.; Augustin, J.; Bechtold, M.; Beetz, S.; Beyer, C.; Drösler, M.; Ebli, M.; Eickenscheidt, T.; Fiedler, S.; et al. High emissions of greenhouse gases from grasslands on peat and other organic soils. Glob. Chang. Biol. 2016, 22, 4134–4149. [Google Scholar] [CrossRef]
- Raviv, M. Production of high-quality composts for horticultural purposes: A mini-review. Horttechnology 2005, 15, 52–57. [Google Scholar] [CrossRef]
- Carmona, E.; Abad, M. Aplicación Del Compost en Viveros y Semilleros. In Compostaje; Moreno, J., Moral, R., Eds.; Ediciones Mundi-Prensa: Madrid, Spain, 2008. [Google Scholar]
- Wu, T.Y.; Lim, S.L.; Lim, P.N.; Shak, K.P.Y. Biotransformation of biodegradable solid wastes into organic fertilizers using composting or/and vermicomposting. Chem. Eng. Trans. 2014, 39, 1579–1584. [Google Scholar]
- García de la Fuente, R.; Carrión, C.; Botella, S.; Fornes, F.; Noguera, V.; Abad, M. Biological oxidation of elemental sulphur added to three composts from different feedstocks to reduce their pH for horticultural purposes. Bioresour. Technol. 2007, 98, 3561–3569. [Google Scholar] [CrossRef]
- Alburquerque, J.A.; González, J.; García, D.; Cegarra, J. Measuring detoxification and maturity in compost made from ‘alperujo’, the solid by-product of extracting olive oil by the two-phase centrifugation system. Chemosphere 2006, 64, 470–477. [Google Scholar] [CrossRef]
- Wang, P.; Changa, C.M.; Watson, M.E.; Dick, W.A.; Chen, Y.; Hoitihk, H.A.J. Maturity indices for composted dairy and pig manures. Soil Biol. Biochem. 2004, 36, 767–776. [Google Scholar] [CrossRef]
- Sáez, J.A.; Belda, R.M.; Bernal, M.P.; Fornes, F. Biochar improves agro-environmental aspects of pig slurry compost as a substrate for crops with energy and remediation uses. Ind. Crops Prod. 2016, 94, 97–106. [Google Scholar] [CrossRef]
- Fitzpatrick, G.E. Composts Utilization in Ornamental and Nursery Crop Production Systems. In Compost Utilization in Horticultural Cropping Systems; Stoffella, P.J., Kahn, B.A., Eds.; CRC Press: Boca Raton, FL, USA, 2001; pp. 135–150. [Google Scholar]
- Kelleher, B.P.; Leahy, J.J.; Henihan, A.M.; O’Dwyer, T.F.; Sutton, D.; Leahy, M.J. Advances in poultry litter disposal technology—A review. Bioresour. Technol. 2002, 83, 27–36. [Google Scholar] [CrossRef]
- Dede, O.H.; Dede, G.; Ozdemir, S. Agricultural and municipal wastes as container media component for ornamental nurseries. Int. J. Environ. Res. 2010, 4, 193–200. [Google Scholar]
- Atiyeh, R.M.; Subler, S.; Edwards, C.A.; Bachman, G.; Metzger, J.D.; Shuster, W. Effects of vermicomposts and composts on plant growth in horticultural container media and soil. Pedobiologia 2000, 44, 579–590. [Google Scholar] [CrossRef]
- Steiner, C.; Harttung, T. Biochar as a growing media additive and peat substitute. Solid Earth 2014, 5, 995–999. [Google Scholar] [CrossRef]
- Lehmann, J.; Joseph, S. Biochar for environmental management: An Introduction. In Biochar for Environmental Management. Science and Technology; Lehmann, J., Joseph, S., Eds.; Earthscan: London, UK, 2009; pp. 1–12. [Google Scholar]
- Woolf, D.; Amonette, J.; Street-Perrott, F.; Lehmann, J.; Joseph, S. Sustainable biochar to mitigate global climate change. Nat. Commun. 2010, 1, 56. [Google Scholar] [CrossRef] [PubMed]
- Fornes, F.; Belda, R.M. Biochar versus hydrochar as growth media constituents for ornamental plant cultivation. Sci. Agric. 2018, 75, 304–312. [Google Scholar] [CrossRef]
- Tian, Y.; Sun, X.; Li, S.; Wang, H.; Wang, L.; Cao, J.; Zhang, L. Biochar made from green waste as peat substitute in growth media for Calathea rotundifola cv. Fasciata. Sci. Hortic. 2012, 143, 15–18. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Fernández de Córdova, P.; Cebolla-Cornejo, J. Assesment of biochar and hydrochar as minor to major constituents of growing media for containerized tomato production. J. Sci. Food Agric. 2017, 97, 3675–3684. [Google Scholar] [CrossRef]
- Petruccelli, R.; Bonetti, A.; Traversi, M.L.; Faraloni, C.; Valagussa, M.; Pozzi, A. Influence of biochar application on nutritional quality of tomato (Lycopersicon sculentum). Crop Past. Sci. 2015, 66, 747–755. [Google Scholar] [CrossRef]
- Belda, R.M.; Lidón, A.; Fornes, F. Biochars and hydrochars as substrate constituents for soilless growth of myrtle and mastic. Ind. Crops Prod. 2016, 94, 132–142. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M. Use of raw and acidified biochars as constituents of growth media for forest seedling production. New For. 2019, 50, 1063–1086. [Google Scholar] [CrossRef]
- Huang, L.; Niu, G.; Feagley, S.E.; Gu, M. Evaluation of a hardwood biochar and two composts mixes as replacements for a peat-based commercial substrate. Ind. Crops Prod. 2019, 129, 549–560. [Google Scholar] [CrossRef]
- Alvarez, J.M.; Pasian, C.; Lal, R.; López, R.; Fernández, M. Vermicompost and biochar as substitutes of growing media in ornamental-plant production. J. Appl. Hortic. 2017, 19, 205–214. [Google Scholar]
- Steiner, C.; Das, K.C.; Melear, N.; Lakly, D. Reducing nitrogen loss during poultry litter composting using biochar. J. Environ. Qual. 2010, 39, 1236–1242. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Lu, H.; Dong, D.; Deng, H.; Strong, P.J.; Wang, H.; Wu, W. Insight into the effects of biochar on manure composting: Evidence supporting the relationship between N2O emission and denitrifying community. Environ. Sci. Technol. 2013, 47, 7341–7349. [Google Scholar] [CrossRef]
- Wang, Y.; Villamil, M.B.; Davidson, P.C.; Akdeniz, N. A quantitative understanding of the role of co-composted biochar in plant growth using meta-analysis. Sci. Total Environ. 2019, 685, 741–752. [Google Scholar] [CrossRef]
- Sánchez-García, M.; Alburquerque, J.A.; Sánchez-Monedero, M.A.; Roig, A.; Cayuela, M.L. Biochar accelerates organic matter degradation and enhances N mineralization during composting of poultry manure without a relevant impact on gas emissions. Bioresour. Technol. 2015, 192, 272–279. [Google Scholar] [CrossRef]
- Maroušek, J.; Hašková, S.; Zeman, R.; Žák, J.; Vaníčková, R.; Maroušková, A.; Váchal, J.; Myšková, K. Polemics on ethical aspects in the compost business. Sci. Eng. Ethics 2016, 22, 581–590. [Google Scholar] [CrossRef]
- Abad, M.; Fornes, F.; Carrión, C.; Noguera, V.; Noguera, P.; Maquieira, A.; Puchades, R. Physical properties of various coconut coir dusts compared to peat. Hortscience 2005, 40, 2138–2144. [Google Scholar] [CrossRef]
- Laird, D.; Fleming, P.; Wang, B.; Horton, R.; Karlen, D. Biochar impact on nutrient leaching from a Midwestern agricultural soil. Geoderma 2010, 158, 436–442. [Google Scholar] [CrossRef]
- Jaiswal, A.K.; Elad, Y.; Paudel, I.; Graber, E.R.; Cytryn, E.; Frenkel, O. Linking the belowground microbial composition, diversity and activity to soilborne disease suppression and growth promotion of tomato amended with biochar. Sci. Rep. 2018, 7, 44382. [Google Scholar] [CrossRef]
- Elad, Y.; David, D.R.; Harel, Y.M.; Borenshtein, M.; Ben Kalifa, H.; Silber, A.; Graber, E.R. Induction of systemic resistance in plants by Biochar, a soil-applied carbon sequestering agent. Phytopathology 2010, 100, 913–921. [Google Scholar] [CrossRef]
- Graber, E.R.; Harel, Y.M.; Kolton, M.; Cytryn, E.; Silber, A.; David, D.R.; Tsechansky, M.B.; Elad, Y. Biochar impact on development and productivity of pepper and tomato grown in fertigated soilless media. Plant Soil 2010, 337, 481–496. [Google Scholar] [CrossRef]
- Peng, D.; Gu, M.; Zhao, Y.; Yu, F.; Choi, H. Effects of biochar mixes with peat-moss based substrates on growth and development of horticultural crops. Hort. Sci. Technol. 2018, 36, 501–512. [Google Scholar]
- Handreck, K.; Black, N. Growing Media for Ornamental Plants and Turf, 3rd Edition Reprinted with Revisions; New South Wales University Press: Randwick, NSW, Australia, 2005. [Google Scholar]
- Bunt, A.C. Media and Mixes for Container-Grown Plants: A Manual on the Preparation and Use of Growing Media for Pot Plants, 2nd ed.; Unwin Hyman: London, UK, 1988. [Google Scholar]
- Maronek, D.M.; Studebaker, D.; Oberly, B. Improving media aeration in liner and container production. Comb. Proc. Int. Plant Prop. Soc. 1985, 35, 591–597. [Google Scholar]
- EN-European Standards. [EN 13041, 1999. Determination of physical properties. Dry Bulk Density, Air Volume, Water Volume, Srinkage Value and Total Pore Space]. [EN 13037, 1999. Determination of pH, 11]. [EN 13038, 1999. Determination of Electrical Conductivity]. [EN 13652, 2001. Extraction of Water Soluble Nutrients and Elements]. In Soil Improvers and Growing Media; European Committee for Standardization (CEN): Brussels, Belgium, 1999. [Google Scholar]
- Zucconi, F.; Forte, M.; Monaco, A.; De Bertoldi, M. Biological evaluation of compost maturity. Biocycle 1981, 22, 27–29. [Google Scholar]
- Choi, S.R.; Lee, C.H.; Lee, H.S.; Shin, W.K.; Lee, Y.S. The effects of electrical conductivity of soil solution on the germination and regrowth of leaves and roots of several vegetable crops. Res. Rep. Rural Develop. Adm. Soils Fertil. 1989, 31, 56–82. [Google Scholar]
- Fornes, F.; Belda, R.M.; Lidón, A. Analysis of two biochars and one hydrochar from different feedstock: Focus set on environmental, nutritional and horticultural considerations. J. Clean Prod. 2015, 86, 40–48. [Google Scholar] [CrossRef]
- Fornes, F.; Belda, R.M.; Carrión, C.; Noguera, V.; García-Agustín, P.; Abad, M. Pre-conditioning ornamental plants to drought by means of saline water irrigation as related to salinity tolerance. Sci. Hortic. 2007, 113, 52–59. [Google Scholar] [CrossRef]
- Moran, R. Formulae for determination of chlorophyllous pigments extracted with N, N-dimethylformamide. Plant Physiol. 1982, 69, 1376–1381. [Google Scholar] [CrossRef]
- Mendoza-Hernández, D.; Fornes, F.; Belda, R.M. Compost and vermicompost of horticultural waste as substrates for cutting rooting and growth of rosemary. Sci. Hortic. 2014, 178, 192–202. [Google Scholar] [CrossRef]
- Fornes, F.; Mendoza-Hernández, D.; Belda, R.M. Compost versus vermicompost as substrate constituents for rooting shrub cuttings. Span. J. Agric. Res. 2013, 11, 518–528. [Google Scholar] [CrossRef]
- Esteban, R.; Ariz, I.; Cruz, C.; Moran, J.F. Mechanisms of ammonium toxicity and the quest for tolerance. Plant Sci. 2016, 248, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Domínguez-Valdivia, M.D.; Aparicio-Tejo, P.M.; Lamsfus, C.; Cruz, C.; Marins-Louçao, M.A.; Moran, J.F. Nitrogen nutrition and antioxidant metabolism in ammonium-tolerant and -sensitive plants. Physiol. Plant. 2008, 132, 359–369. [Google Scholar] [CrossRef] [PubMed]
- Britto, D.T.; Kronzucker, H.J. NH4+ toxicity in higher plants: A critical review. J. Plant Physiol. 2002, 159, 567–584. [Google Scholar] [CrossRef]
- Working Document. Biological Treatment of Biowaste (2nd Draft); European Commission: Brussels, Belgium, 2001; Annex III; p. 18. [Google Scholar]
- Hartmann, H.T.; Kester, D.E.; Davies, F.T., Jr.; Geneve, R.L. Techniques of Propagation by Cuttings. In Hartmann and Kester’s Plant Propagation: Principles and Practices, 8th ed.; Hartmann, H.T., Kester, D.E., Eds.; Pearsons Prentice Hall: Essex, UK, 2014; pp. 295–360. [Google Scholar]
- Fornes, F.; Carrión, C.; García de la Fuente, R.; Puchades, R.; Abad, M. Leaching composted lignocellulosic wastes to prepare container media: Feasibility and environmental concerns. J. Environ. Manag. 2010, 91, 1747–1755. [Google Scholar] [CrossRef] [PubMed]
- Peterson, J.C. Modify your pH perspective. Flor. Rev. 1981, 169, 34–35, 92, 94. [Google Scholar]
- Gastón, A.; Soriano, C.; Gómez-Miguel, V. Lithologic data improve plant species distribution models based on coarse-grained occurrence data. For. Syst. 2009, 18, 42–49. [Google Scholar]
- Mills, H.A.; Jones, J.B., Jr. Plant Analysis Handbook II. A Practical Sampling, Preparation, Analysis, and Interpretation Guide; Micromacro Publishing: Athens, Greece, 1996. [Google Scholar]
| Experiment I | ||||||||
| Cutting rooting (CR) assay | Plant growth (PG) assay | |||||||
| Ratio (%v:v) | Ratio (%v:v) | |||||||
| Substrate | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | 50:50 | 25:75 | 0:100 |
| PMC:P | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 |
| PMBC:P | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 |
| Experiment II | ||||||||
| Cutting rooting (CR) assay | Plant growth (PG) assay | |||||||
| Ratio (%v:v) | Ratio (%v:v) | |||||||
| Substrate | 100:0 | 75:25 | 50:50 | 25:75 | 0:100 | 50:50 | 25:75 | 0:100 |
| PMC:P | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 |
| PMC:B | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 | R1 R2 R3 |
| Substrate | Ratios (% v:v) | DB (kg m−3) | WHC (%) | PT (%) | Vair (%) | Vwater (%) | Shrinkage (%) |
|---|---|---|---|---|---|---|---|
| PMC:B | 100:0 | 396b | 169e | 80ef | 14fg | 65cd | 18bcd |
| 75:25 | 380bc | 159e | 80ef | 20de | 60de | 10de | |
| 50:50 | 370c | 155e | 79ef | 21cd | 58e | 5ef | |
| 25:75 | 377c | 153e | 78ef | 20cde | 58e | 4ef | |
| 0:100 | 323e | 147e | 81de | 33a | 47f | 1f | |
| PMC:P | 100:0 | 393b | 167e | 79de | 14fg | 65cd | 18bcd |
| 75:25 | 318e | 233d | 84d | 11g | 73a | 28a | |
| 50:50 | 290f | 282c | 84d | 14fg | 70abc | 27a | |
| 25:75 | 184h | 368b | 89b | 17ef | 72ab | 24ab | |
| 0:100 | 115i | 585a | 93a | 26b | 66bcd | 19bc | |
| PMBC:P | 100:0 | 440a | 163e | 77f | 6h | 71abc | 17bcd |
| 75:25 | 350d | 211d | 81de | 7h | 74a | 16bcd | |
| 50:50 | 267g | 275c | 85cd | 13fg | 72abc | 15cd | |
| 25:75 | 200h | 363b | 88bc | 16ef | 72ab | 17bcd | |
| 0:100 | 111i | 581a | 91ab | 24bc | 68abc | 19bc | |
| Main effects | |||||||
| Mix | PMC:B | 369A | 156C | 80C | 22A | 58B | 8C |
| PMC:P | 260C | 327A | 86A | 17B | 69A | 23A | |
| PMBC:P | 273B | 319B | 84B | 13C | 71A | 17B | |
| Ratio | 100:0 | 409A | 166E | 79D | 11D | 67A | 18A |
| 75:25 | 349B | 201D | 82C | 13CD | 69A | 18A | |
| 50:50 | 309C | 237C | 83C | 16BC | 66A | 16AB | |
| 25:75 | 253D | 295B | 85B | 18B | 67A | 15AB | |
| 0:100 | 183E | 438A | 88A | 28A | 60B | 13B | |
| Significance | |||||||
| Mix | *** | *** | *** | *** | *** | *** | |
| Ratio | *** | *** | *** | *** | ** | *** | |
| M × R | *** | *** | *** | * | * | *** |
| Substrate | Ratios (% v:v) | pH | EC (dS m−1) | OM (%) | NO3—N (mg L−1) | NH4+-N (mg L−1) | P (mg L−1) | K (g L−1) | Ca (mg L−1) | Mg (mg L−1) | Cress GI (%) | Lettuce GI (%) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| PMC:B | 100:0 | 9.2ef | 10.6ab | 45h | 198b | 233a | 978b | 17.7b | 277b | 156b | 21d | 25d |
| 75:25 | 9.5cd | 8.6c | 54g | 171cd | 175b | 704d | 13.1d | 212c | 120c | 40cd | 40cd | |
| 50:50 | 9.7bc | 7.0e | 63e | 144e | 117d | 528f | 9.3f | 159d | 86d | 60bc | 55bc | |
| 25:75 | 9.8ab | 3.3gh | 71d | 116f | 58e | 266g | 4.6h | 86e | 52e | 100a | 110a | |
| 0:100 | 10.0a | 0.8i | 79c | 89g | 1g | 20h | 0.3i | 32f | 3f | 110a | 120a | |
| PMC:P | 100:0 | 9.2def | 10.8a | 44h | 195b | 230a | 1000b | 17.8b | 280b | 160ab | 21d | 25d |
| 75:25 | 8.6g | 8.7c | 58f | 152de | 173b | 705d | 13.1d | 212c | 120c | 45bcd | 43cd | |
| 50:50 | 7.0j | 6.9e | 72d | 110f | 115d | 530f | 9.3f | 164d | 85d | 65b | 60bc | |
| 25:75 | 6.8j | 3.6g | 85b | 67h | 58e | 260g | 4.6h | 90e | 51e | 120a | 110a | |
| 0:100 | 4.2k | 0.1j | 98a | 24i | 1g | 1i | 0.01i | 2g | 5f | 125a | 125a | |
| PMBC:P | 100:0 | 9.5cde | 10.3b | 45h | 316a | 150c | 1100a | 19.1a | 308a | 176a | 21d | 55bc |
| 75:25 | 9.1f | 7.7d | 57fg | 185bc | 110d | 770c | 14.3c | 227c | 130c | 50bc | 65bc | |
| 50:50 | 8.2h | 5.7f | 73d | 153de | 70e | 575e | 10.1e | 172d | 89d | 70b | 80b | |
| 25:75 | 7.5i | 3.2h | 86b | 82gh | 32f | 283g | 5.0g | 98e | 64e | 120a | 110a | |
| 0:100 | 4.2k | 0.1j | 97a | 25i | 1g | 1i | 0.01i | 3g | 4f | 125a | 125a | |
| Main effects | ||||||||||||
| Mix | PMC:B | 9.7A | 6.1A | 62B | 144B | 117A | 499B | 9.0B | 154B | 83B | 75A | 70B |
| PMC:P | 7.2C | 6.1A | 71A | 111C | 115A | 499B | 9.0B | 150B | 84B | 66A | 73B | |
| PMBC:P | 7.7B | 5.4B | 71A | 152A | 73B | 546A | 9.7A | 162A | 93A | 77A | 87A | |
| Ratio | 100:0 | 9.3A | 10.6A | 44E | 237A | 204A | 1026A | 18.2A | 288A | 164A | 21D | 35C |
| 75:25 | 9.1B | 8.4B | 56D | 171B | 153B | 726B | 13.5B | 218B | 123B | 45C | 49BC | |
| 50:50 | 8.3C | 6.5C | 69C | 136C | 101C | 544C | 9.6C | 165C | 87C | 65B | 65B | |
| 25:75 | 8.1D | 3.4D | 80B | 88D | 50D | 270D | 4.7D | 91D | 56D | 113A | 110A | |
| 0:100 | 6.1E | 0.3E | 91A | 46E | 1E | 7E | 0.1E | 12E | 4E | 120A | 123A | |
| Significance | ||||||||||||
| Mix | *** | *** | *** | *** | *** | *** | *** | *** | *** | Ns | ** | |
| Ratio | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | *** | |
| M × R | *** | *** | *** | *** | *** | *** | *** | *** | Ns | Ns | Ns |
| Substrate | Ratios (% v:v) | Experiment I.CR Cutting Rooting | Experiment I.PG Plant Growth | ||||
|---|---|---|---|---|---|---|---|
| Rooted Cuttings (%) | Shoot Dry Weight (mg) | Root Dry Weight (mg) | Shoot Length (cm) | Shoot Dry Weight (mg) | Root Size (Visual Rating Score; 1–4) | ||
| PMC:P | 100:0 | 9cd | 70fg | 3cd | |||
| 75:25 | 10cd | 85de | 1d | ||||
| 50:50 | 53b | 112bc | 20b | 29a | 2000a | 2.1b | |
| 25:75 | 89a | 110c | 35a | 29a | 2180a | 2.7ab | |
| 0:100 | 67ab | 98cd | 20b | 21bc | 540b | 1.2c | |
| PMBC:P | 100:0 | 4d | 60g | 1d | |||
| 75:25 | 22c | 79ef | 7c | ||||
| 50:50 | 78ab | 125ab | 36a | 26ab | 1870a | 2.2b | |
| 25:75 | 100a | 128a | 35a | 24abc | 1890a | 2.9a | |
| 0:100 | 69ab | 100c | 22b | 19c | 500b | 1.3c | |
| Main effects | |||||||
| Material | PMC | 46B | 95A | 16B | 26A | 1573A | 2.0A |
| PMBC | 55A | 98A | 20A | 23A | 1420A | 2.1A | |
| Ratio | 100:0 | 7C | 65D | 2C | |||
| 75:25 | 16C | 82C | 4C | ||||
| 50:50 | 66B | 119A | 28AB | 28A | 1935A | 2.1B | |
| 25:75 | 95A | 119A | 35A | 27A | 2035A | 2.8A | |
| 0:100 | 68B | 99B | 21B | 20B | 520B | 1.3C | |
| Significance | |||||||
| Material | ** | Ns | * | Ns | Ns | Ns | |
| Ratio | *** | *** | *** | ** | *** | *** | |
| M × R | * | * | * | Ns | Ns | Ns | |
| Substrate | Ratios (% v:v) | Experiment II.CR Cutting Rooting | Experiment II.PG Plant growth | ||||
|---|---|---|---|---|---|---|---|
| Rooted Cuttings (%) | Shoot Dry Weight (mg) | Root Dry Weight (mg) | Shoot Length (cm) | Shoot Dry Weight (mg) | Root Size (visual rating Score; 1–4) | ||
| PMC:P | 100:0 | 9e | 72d | 4d | |||
| 75:25 | 11e | 87cd | 1d | ||||
| 50:50 | 55d | 115ab | 22bc | 30a | 2020a | 2.0ab | |
| 25:75 | 90a | 111abc | 33ab | 28a | 2100a | 2.6a | |
| 0:100 | 68bc | 99bc | 22bc | 20bc | 520b | 1.3b | |
| PMC:B | 100:0 | 10e | 71d | 4d | |||
| 75:25 | 51cd | 96bcd | 19c | ||||
| 50:50 | 50d | 106abc | 28abc | 26ab | 1580a | 2.0ab | |
| 25:75 | 85ab | 130a | 36a | 26ab | 1530a | 2.3a | |
| 0:100 | 88a | 118ab | 37a | 14c | 320b | 2.0ab | |
| Main effects | |||||||
| Material | P | 47B | 97B | 16B | 26A | 1547A | 2.0A |
| B | 57A | 104A | 25A | 22B | 1143B | 2.1A | |
| Ratio | 100:0 | 10D | 72C | 4C | |||
| 75:25 | 31C | 91B | 10C | ||||
| 50:50 | 53B | 111A | 25B | 28A | 1800A | 2.0AB | |
| 25:75 | 87A | 121A | 35A | 27A | 1815A | 2.5A | |
| 0:100 | 78A | 109AB | 29AB | 17B | 420B | 1.7B | |
| Significance | |||||||
| Material | * | * | *** | ** | * | Ns | |
| Ratio | *** | *** | *** | *** | *** | ** | |
| M × R | ** | Ns | ** | Ns | Ns | Ns | |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fornes, F.; Liu-Xu, L.; Lidón, A.; Sánchez-García, M.; Cayuela, M.L.; Sánchez-Monedero, M.A.; Belda, R.M. Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production. Agronomy 2020, 10, 261. https://doi.org/10.3390/agronomy10020261
Fornes F, Liu-Xu L, Lidón A, Sánchez-García M, Cayuela ML, Sánchez-Monedero MA, Belda RM. Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production. Agronomy. 2020; 10(2):261. https://doi.org/10.3390/agronomy10020261
Chicago/Turabian StyleFornes, Fernando, Luisa Liu-Xu, Antonio Lidón, María Sánchez-García, María Luz Cayuela, Miguel A. Sánchez-Monedero, and Rosa María Belda. 2020. "Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production" Agronomy 10, no. 2: 261. https://doi.org/10.3390/agronomy10020261
APA StyleFornes, F., Liu-Xu, L., Lidón, A., Sánchez-García, M., Cayuela, M. L., Sánchez-Monedero, M. A., & Belda, R. M. (2020). Biochar Improves the Properties of Poultry Manure Compost as Growing Media for Rosemary Production. Agronomy, 10(2), 261. https://doi.org/10.3390/agronomy10020261






