Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Growth Conditions
2.2. Treatment Applications
2.2.1. Experiment 1—Accumulation of Glycinebetaine (GB) under Various Abiotic Stresses
2.2.2. Experiment 2—Accumulation of GB under Different Temperatures
2.2.3. Experiment 3—The Effect of Exogenous GB Application on GB Accumulation in Ryegrass
2.2.4. Experiments 4 and 5—The Effect of Exogenous GB Application on Growth under Cold Temperatures
2.3. Quantification of Glycinebetaine
2.4. Quantification of Growth
2.5. Water Relations Measurements
2.6. Statistical Analyses
3. Results
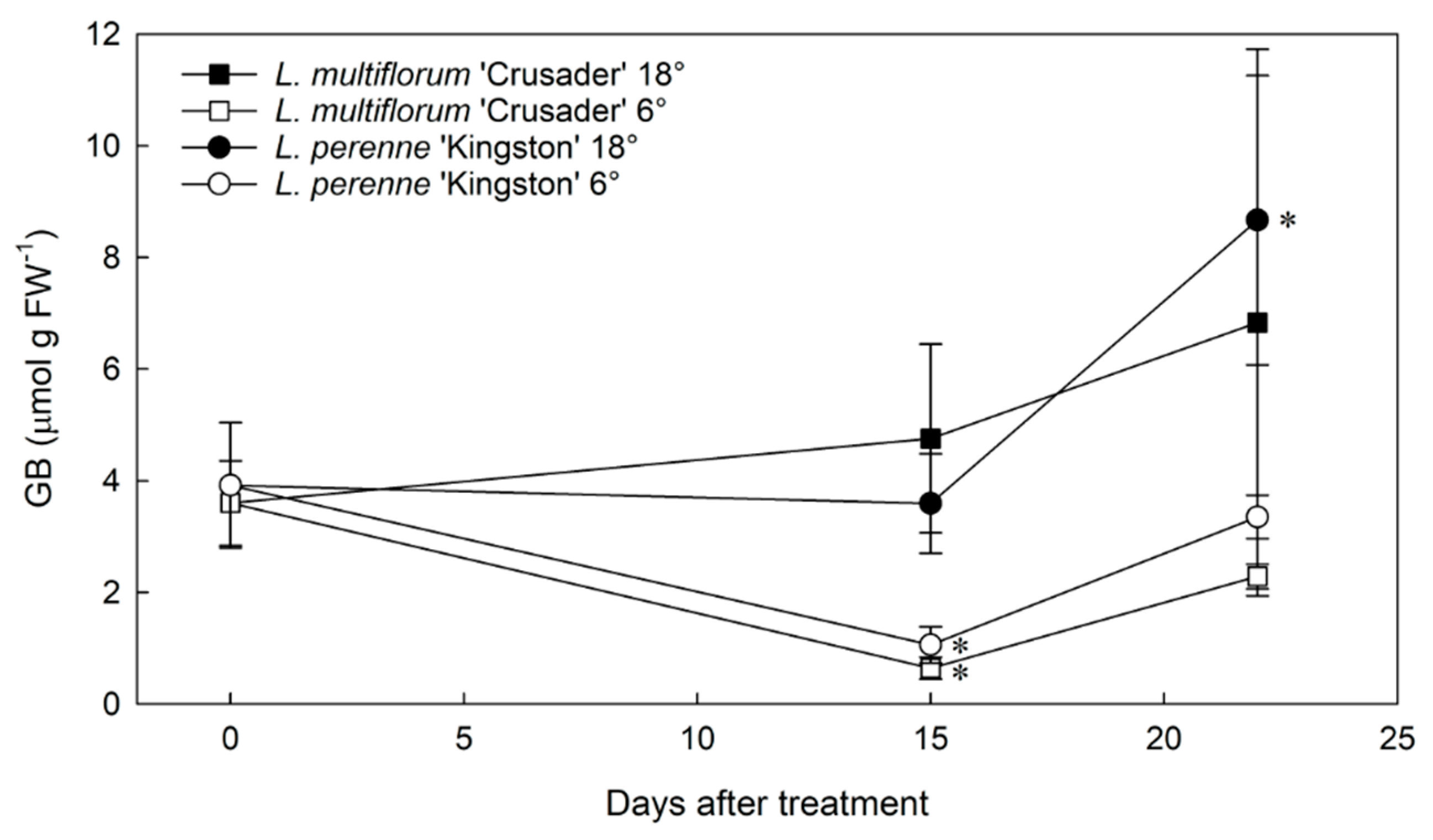
3.1. Glycinebetaine Concentrations in Two Lolium Species in Response to Various Stresses
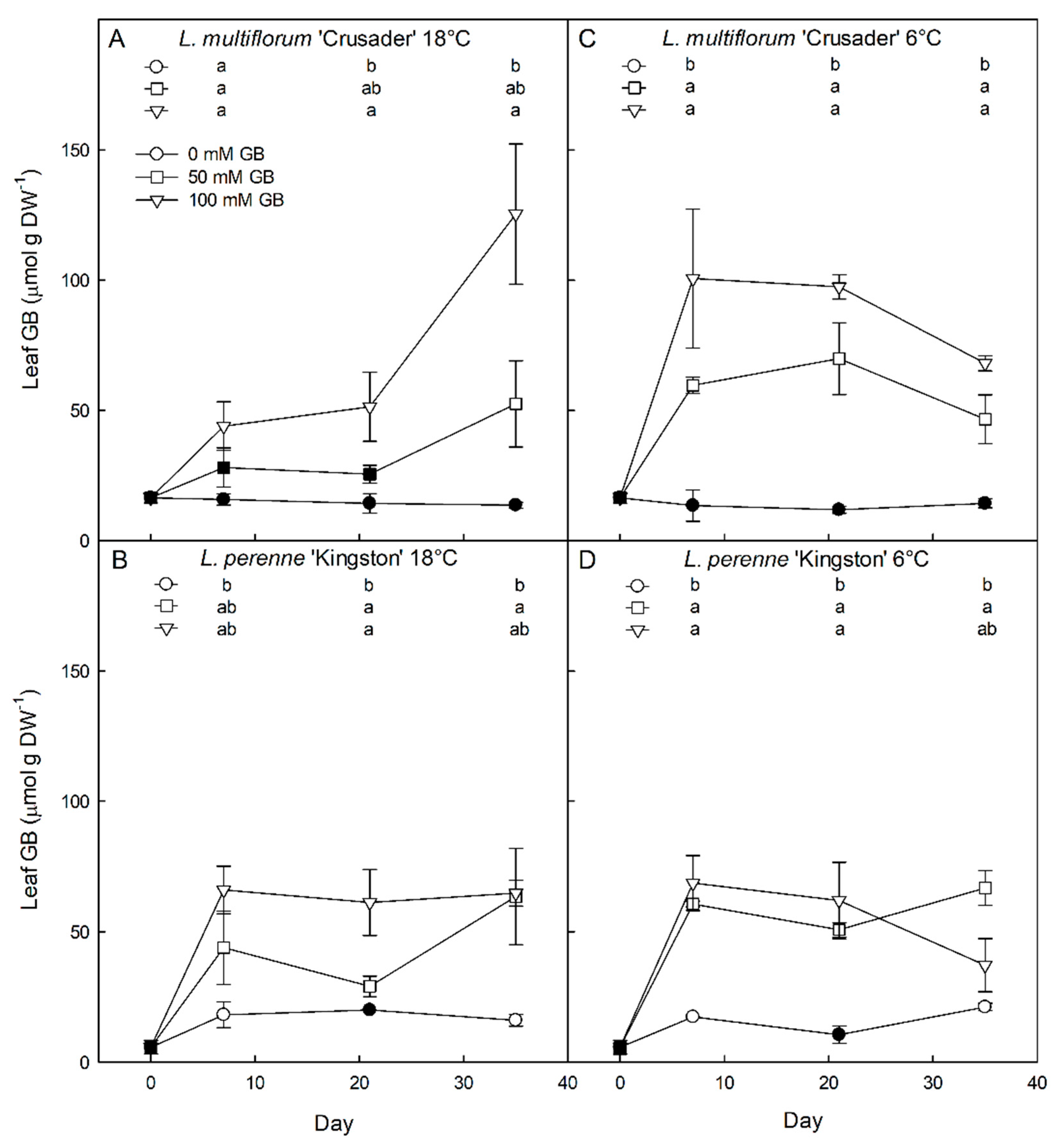
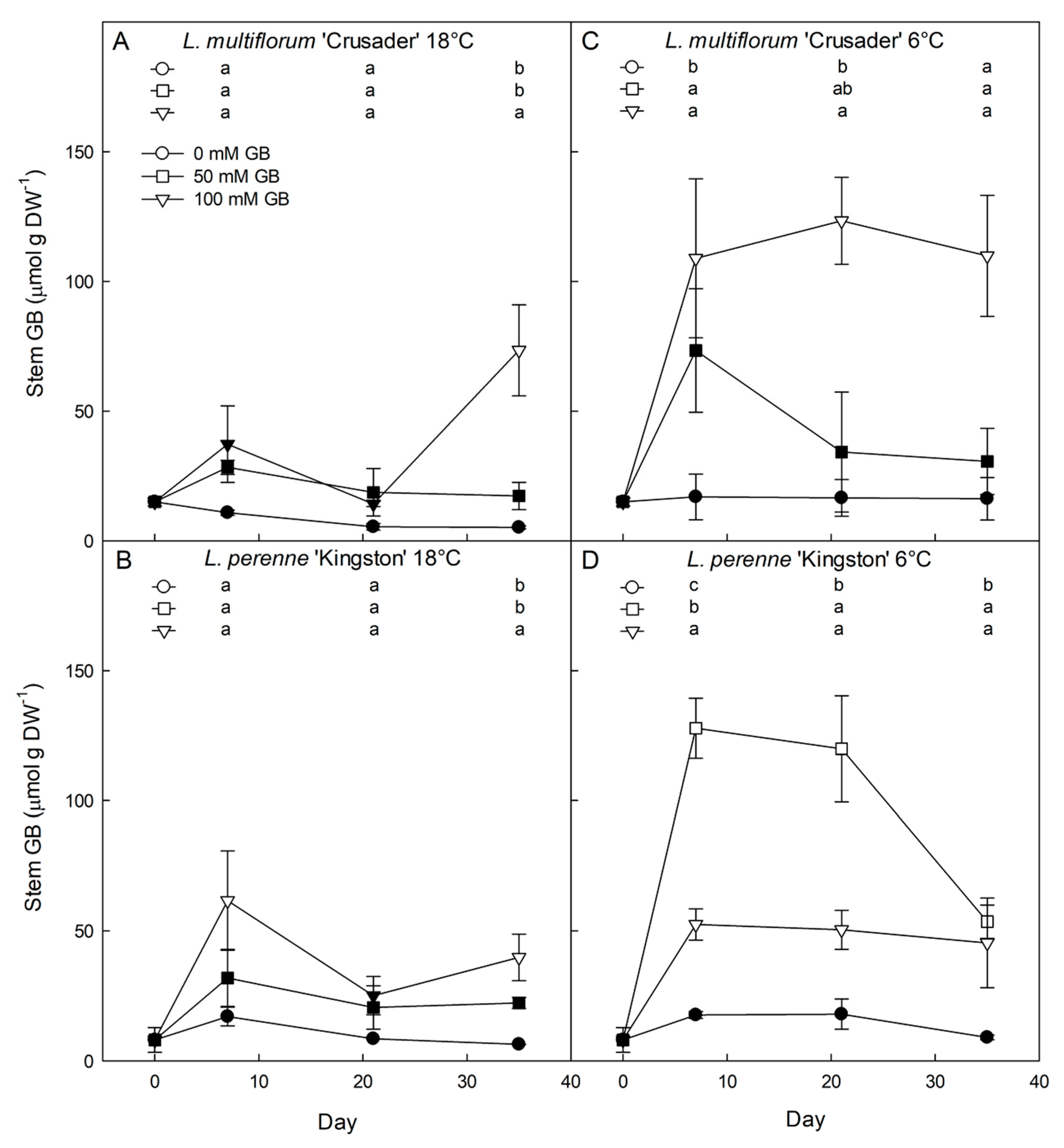
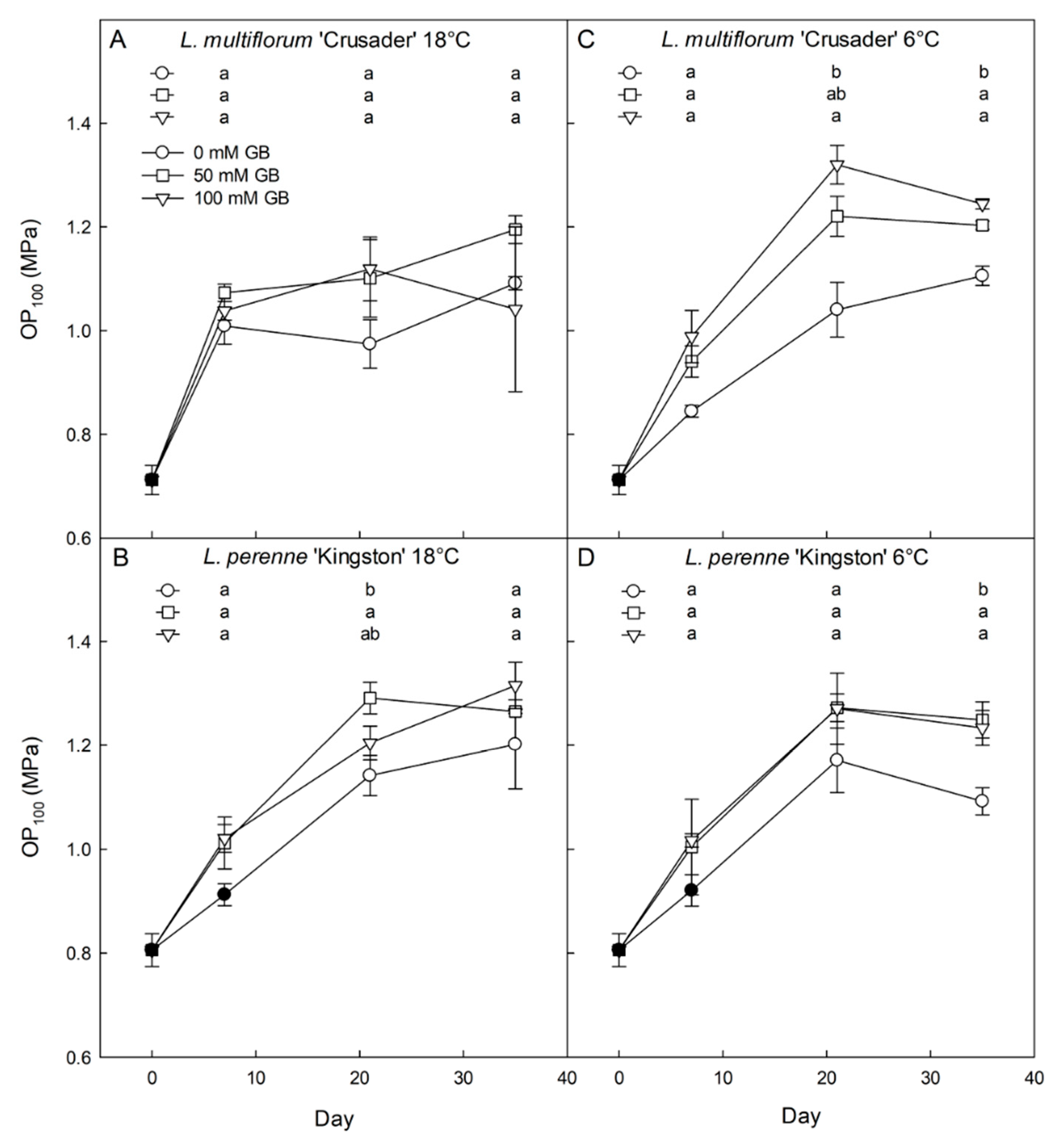
3.2. Effects of Exogenous Glycinebetaine Application on Glycinebetaine Accumulation, Growth, and Water Relations
4. Discussion
4.1. Glycinebetaine Concentrations Do Not Increase In Response to Cold Temperatures
4.2. Both L. perenne and L. multiflorum Have the Ability to Accumulate Exogenously Applied Glycinebetaine That Contributes to Reduced Osmotic Potential
4.3. Exogenous Application of Glycinebetaine Results in Lowered Solute Potential, But Inconsistently Affects Growth
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Humphreys, M.; Feuerstein, U.; Vandewalle, M.; Baert, J. Ryegrasses. In Fodder Crops and Amenity Grasses. Handbook of Plant Breeding; Boller, B., Posselt, U., Veronesi, F., Eds.; Springer: New York, NY, USA, 2010; Volume 5, pp. 211–260. [Google Scholar]
- John, R.; Anjum, N.A.; Sopory, S.K.; Akram, N.A.; Ashraf, M. Some key physiological and molecular processes of cold acclimation. Biol. Plantarum. 2016, 60, 603–618. [Google Scholar] [CrossRef]
- Thomas, H. Osmotic adjustment in Lolium perenne; Its heritability and the nature of solute accumulation. Ann. Bot. 1990, 66, 521–530. [Google Scholar] [CrossRef]
- Thomas, H. Accumulation and consumption of solutes in swards of Lolium perenne during drought and after rewatering. New Phytol. 1991, 118, 35–48. [Google Scholar] [CrossRef]
- Lou, Y.; Yang, Y.; Hu, L.; Liu, H.; Xu, Q. Exogenous glycinebetaine alleviates the detrimental effect of Cd stress on perennial ryegrass. Ecotoxicology 2015, 24, 1330–1340. [Google Scholar] [CrossRef] [PubMed]
- Pan, L.; Meng, C.; Wang, J.; Ma, X.; Fan, X.; Yang, Z.; Zhou, M.; Zhang, X. Integrated omics data of two annual ryegrass (Lolium multiflorum L.) genotypes reveals core metabolic processes under drought stress. BMC Plant Biol. 2018, 18, 26–55. [Google Scholar] [CrossRef] [PubMed]
- Annunziata, M.G.; Ciarmiello, L.F.; Woodrow, P.; Dell’Aversana, E.; Carillo, P. Spatial and temporal profile of glycine betaine accumulation in plants under abiotic stresses. Front. Plant Sci. 2019, 10, 230–261. [Google Scholar] [CrossRef]
- Takahashi, W.; Oishi, H.; Ebina, M.; Komatsu, T.; Takamizo, T. Production of Italian ryegrass expressing the betaine aldehyde dehydrogenase gene of zoyziagrass. Breed. Sci. 2010, 60, 279–285. [Google Scholar] [CrossRef]
- Bao, Y.; Zhao, R.; Li, F.; Tang, W.; Han, L. Simultaneous expression of Spinacia oleracea chloroplast choline monooxygenase (CMO) and betaine aldehyde dehydrogenase (BADH) genes contribute to dwarfism in transgenic Lolium perenne. Plant Mol. Biol. Rep. 2011, 29, 379–388. [Google Scholar] [CrossRef]
- Ashraf, M.; Foolad, M.R. Roles of glycine betaine and proline in improving plant abiotic stress resistance. Environ. Exp. Bot. 2007, 59, 206–216. [Google Scholar] [CrossRef]
- Hu, L.; Hu, T.; Zhang, T.; Pang, X.H.; Fu, J. Exogenous glycine betaine ameliorates the adverse effect of salt stress on perennial ryegrass. J. Amer. Soc. Hort. Sci. 2012, 137, 38–46. [Google Scholar] [CrossRef]
- Bergmann, H.; Eckert, H. Effect of glycinebetaine on water use efficiency of wheat (Triticum aestivum L.). Biol. Plant 1984, 26, 384–387. [Google Scholar] [CrossRef]
- Xing, W.; Rajashekar, C.B. Glycine betaine involvement in freezing tolerance and water stress in Arabidopsis thaliana. Environ. Exp. Bot. 2001, 46, 21–28. [Google Scholar] [CrossRef]
- Park, E.-J.; Jeknić, Z.; Chen, T.H.H. Exogenous application of glycinebetaine increases chilling tolerance in tomato plants. Plant Cell Physiol. 2006, 47, 706–714. [Google Scholar] [CrossRef] [PubMed]
- Somersalo, S.; Kyei-Boahen, S.; Pehu, E. Exogenous glycine betaine application as a possibility to increase low temperature tolerance of crop plants. Nord. Jordbr. 1996, 78, 102–114. [Google Scholar]
- Naidu, B.P.; Stevenson, G.; Page, R.; Munford, S. Glycinebetaine foliar application increases pasture winter growth and milk yield. In Proceedings of the 11th Australian Agronomy Conference, Geelong, Australia, 2–6 February 2003. [Google Scholar]
- Lee, J.M.; Elborough, K.; Catto, W.D.; Donaghy, D.J.; Roche, J.R. Effect of surface applied glycine betaine on herbage production and quality of perennial ryegrass-white clover pastures. Aust. J. Exp. Agric. 2008, 48, 687–694. [Google Scholar] [CrossRef][Green Version]
- Rhodes, D.; Rich, P.J.; Myers, A.C.; Reuter, C.R.; Jamieson, G.C. Determination of betaines by fast atom bombardment mass spectrometry: Identification of glycine betaine deficient genotypes of Zea mays. Plant Physiol. 1987, 84, 781–788. [Google Scholar] [CrossRef]
- Rhodes, D.; Rich, P.J. Preliminary genetic studies of the phenotype of betaine deficiency in Zea mays L. Plant Physiol. 1988, 88, 102–108. [Google Scholar] [CrossRef]
- Brunk, D.G.; Rich, P.J.; Rhodes, D. Genotypic variation for glycinebetaine among public inbred lines of maize. Plant Physiol. 1989, 91, 1122–1125. [Google Scholar] [CrossRef]
- Rhodes, D.; Rich, P.J.; Brunk, D.G.; Ju, G.C.; Rhodes, J.C.; Pauly, M.H.; Hanson, L.A. Development of two isogenic sweet corn hybrids differing for glycinebetaine content. Plant Physiol. 1989, 91, 1112–1121. [Google Scholar] [CrossRef]
- Ladyman, J.A.R.; Ditz, K.M.; Grumet, R.; Hanson, A.D. Genotypic variation for glycinebetaine accumulation by cultivated and wild barley in relation to water stress. Crop Sci. 1983, 23, 465–468. [Google Scholar] [CrossRef]
- Lerma, C.; Rich, P.J.; Ju, G.C.; Yang, W.-J.; Hanson, A.D.; Rhodes, D. Betaine deficiency in maize: Complementation tests and metabolic basis. Plant Physiol. 1991, 95, 1113–1119. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sima, N.A.K.K.; Askari, H.; Mirzaei, H.H.; Pessarakli, M. Genotype-dependent differential responses of three forage species to calcium supplement in saline conditions. J. Plant Nutr. 2009, 32, 579–597. [Google Scholar] [CrossRef]
- Raza, S.H.; Athar, H.R.; Ashraf, M.; Hameed, A. Glycinebetaine-induced modulation of antioxidant enzymes activities and ion accumulation in two wheat cultivars differing in salt tolerance. Environ. Exp. Bot. 2007, 60, 368–376. [Google Scholar] [CrossRef]
- Yang, W.-J.; Rich, P.J.; Axtell, J.D.; Wood, K.V.; Bonham, C.C.; Ejeta, G.; Mickelbart, M.V.; Rhodes, D. Genotypic variation for glycinebetaine in sorghum. Crop Sci. 2003, 43, 162–169. [Google Scholar] [CrossRef]
- Abernathy, G.A.; McManus, M.T. Biochemical responses to an imposed water deficit in mature leaf tissue of Festuca arundinaceae. Environ. Exp. Bot. 1998, 40, 17–28. [Google Scholar] [CrossRef]
- Naidu, B.P.; Paleg, L.G.; Aspinall, D.; Jennings, A.C.; Jones, G.P. Rate of imposition of water stress alters accumulation of nitrogen-containing solutes by wheat seedlings. Aust. J. Plant Physiol. 1990, 17, 653–664. [Google Scholar] [CrossRef]
- Wang, G.P.; Li, F.; Zhang, J.; Zhao, M.R.; Hui, Z.; Wang, W. Overaccumulation of glycine betaine enhances tolerance of the photosynthetic apparatus to drought and heat stress in wheat. Photosynthetica 2010, 48, 30–41. [Google Scholar] [CrossRef]
- Rivero, R.M.; Mestre, T.C.; Mittler, R.; Rubio, F.; Garcia-Sanchez, F.; Martinez, V. The combined effect of salinity and heat reveals a specific physiological, biochemical and molecular response in tomato plants. Plant Cell Environ. 2014, 37, 1059–1073. [Google Scholar] [CrossRef]
- Ochiai, K.; Matoh, T. Mechanism of salt tolerance in the grass species, Anneurolepidium chinense. 1. Growth response to salinity and osmotic adjustment. Soil Sci. Plant Nutr. 2001, 47, 579–585. [Google Scholar] [CrossRef]
- Naidu, B.P.; Paleg, L.G.; Aspinall, D.; Jennings, A.C.; Jones, G.P. Amino acid and glycine betaine accumulation in cold-stressed wheat seedlings. Phytochemistry 1991, 30, 407–409. [Google Scholar] [CrossRef]
- Mäkelä, P.; Mantila, J.; Hinkkanen, R.; Pehu, E.; Peltonen-Sainio, P. Effect of foliar applications of glycinebetaine on stress tolerance, growth, and yield of spring cereals and summer turnip rape in Finland. J. Agron. Crop Sci. 1996, 176, 223–234. [Google Scholar] [CrossRef]
- Mäkelä, P.; Peltonen-Sainio, P.; Jokinen, K.; Setälä, H.; Hinkkanen, R.; Somersalo, S. Uptake and translocation of foliar-applied glycinebetaine in crop plants. Plant Sci. 1996, 121, 221–230. [Google Scholar] [CrossRef]
- Mickelbart, M.V.; Chapman, P.; Collier-Christian, L. Endogenous levels and exogenous application of glycinebetaine to grapevines. Sci. Hort. 2006, 111, 7–16. [Google Scholar] [CrossRef]
- Lorenzetti, F.; Tyler, B.F.; Cooper, J.P.; Breese, E.L. Cold tolerance and winter hardiness in Lolium perenne. 1. Development of screening techniques for cold tolerance and survey of geographical location. J. Agric. Sci. Camb. 1971, 76, 199–209. [Google Scholar] [CrossRef]
- Cyriac, D.; Hofmann, R.W.; Stewart, A.; Sathish, P.; Winefield, C.S.; Moot, D.J. Intraspecific differences in long-term drought tolerance in perennial ryegrass. PLoS ONE 2018, 13, e0194977. [Google Scholar] [CrossRef] [PubMed]
- Nio, S.A.; Cawthray, G.R.; Wade, L.J.; Colmer, T.D. Pattern of solutes accumulated during leaf osmotic adjustment as related to duration of water deficit for wheat at the reproductive stage. Plant Physiol. Biochem. 2011, 49, 1126–1137. [Google Scholar] [CrossRef]
- Ollerenshaw, J.H.; Baker, R.H. Low temperature growth in a controlled environment of Lolium perenne L. ecotypes from northern latitudes. J. Agric. Sci. 1982, 99, 85–90. [Google Scholar] [CrossRef]
- Mäkelä, P.; Kleemoia, J.; Jokinen, K.; Pehu, E.; Peltonen-Sainio, P. Growth response of pea and summer turnip rape to foliar application of glycinebetaine. Acta Agric. Scand. 1997, 47, 168–175. [Google Scholar] [CrossRef]
- Mahdavi, S.; Kafi, M.; Fallahi, E.; Shokrpour, M.; Tabrizi, L. Drought and biostimulant impacts on mineral nutrients, ambient and reflected light-based chlorophyll index, and performance of perennial ryegrass. J. Plant Nutr. 2017, 40, 2248–2258. [Google Scholar] [CrossRef]
- Campbell, J.A.; Naidu, B.P.; Wilson, J.R. The effect of glycinebetaine application on germination and early growth of sugarcane. Seed Sci. Technol. 1999, 27, 747–752. [Google Scholar]
- Demiral, T.; Türkan, I. Exogenous glycinebetaine affects growth and proline accumulation and retards senescence in two rice cultivars under NaCl stress. Environ. Exp. Bot. 2006, 56, 72–79. [Google Scholar] [CrossRef]

| Species | Applied GB | DW | RWC | Ψπ | Ψπ100 | Suc | GB | GBC |
|---|---|---|---|---|---|---|---|---|
| mM | g | % | MPa | MPa | nmol | % | ||
| L. m. | 0 | 1.64 | 91.8 | −1.27 | −1.15 | 6.50 | 1.23 | 2.74 |
| 100 | 2.38 | 91.6 | −1.40 | −1.27 * | 6.88 | 5.93 * | 11.98 * | |
| L. p. | 0 | 1.53 | 93.5 | −1.37 | −1.28 | 6.12 | 1.42 | 3.34 |
| 100 | 1.10 | 93.5 | −1.50 * | −1.38 * | 5.86 | 5.50 * | 10.57 * | |
| Sig z | GB | n.s. | n.s. | ** | ** | n.s. | *** | *** |
| Species | * | ** | ** | ** | *** | n.s. | n.s. | |
| GB × Species | * | n.s. | n.s. | n.s. | ** | n.s. | * |
| Temperature | Species | Applied GB | Growth Rate | Growth Rate |
|---|---|---|---|---|
| mM | mg FW day−1 | mg DW day−1 | ||
| 18 °C | L. m. | 0 | 19.15 | 6.23 |
| 50 | 13.76 | 4.41 | ||
| 100 | 32.91 *** | 7.04 ** | ||
| L. p. | 0 | 14.19 | 4.89 | |
| 50 | 12.24 | 4.15 | ||
| 100 | 22.13 | 5.94 | ||
| 6 °C | L. m. | 0 | 6.32 | 2.61 |
| 50 | 5.76 | 2.53 | ||
| 100 | 10.33 | 2.10 | ||
| L. p. | 0 | 5.84 | 1.22 | |
| 50 | 14.16 * | 4.17 *** | ||
| 100 | 18.75 *** | 4.30 *** |
| Temperature | Species | Applied GB | RWC | Suc | Ψπ | Ψπ100 | GBC |
|---|---|---|---|---|---|---|---|
| °C | mM | % | MPa | MPa | % | ||
| 18 | L. m. | 0 | 92.8 | 3.98b | −1.12b | −1.03 | 10.0b |
| 50 | 92.6 | 4.19b | −1.22ab | −1.15 | 23.2a | ||
| 100 | 88.9 | 4.93a | −1.29a | −1.14 | 33.4a | ||
| L. p. | 0 | 92.3 | 3.91b | −1.28b | −1.17 | 11.4b | |
| 50 | 89.0 | 4.17ab | −1.46a | −1.28 | 25.1ab | ||
| 100 | 86.7 | 4.84a | −1.48a | −1.26 | 31.0a | ||
| Sig z | GB | ** | * | *** | * | *** | |
| Species | n.s. | n.s. | *** | *** | n.s. | ||
| GB × Species | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| 6 | L. m. | 0 | 85.5 | 4.07ab | −1.28b | −1.07b | 7.8b |
| 50 | 84.4 | 3.95b | −1.47a | −1.21a | 31.5a | ||
| 100 | 85.8 | 4.36a | −1.52a | −1.28a | 38.0a | ||
| L. p. | 0 | 85.3 | 4.36 | −1.35b | −1.13b | 8.4b | |
| 50 | 85.2 | 4.19 | −1.51a | −1.26a | 29.4a | ||
| 100 | 84.6 | 4.22 | −1.51a | −1.25a | 24.9a | ||
| Sig z | GB | n.s. | n.s. | *** | *** | *** | |
| Species | n.s. | n.s. | n.s. | n.s. | n.s. | ||
| GB × Species | n.s. | n.s. | n.s. | n.s. | n.s. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mickelbart, M.V.; Boine, B. Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures. Agronomy 2020, 10, 210. https://doi.org/10.3390/agronomy10020210
Mickelbart MV, Boine B. Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures. Agronomy. 2020; 10(2):210. https://doi.org/10.3390/agronomy10020210
Chicago/Turabian StyleMickelbart, Michael V., and Barbara Boine. 2020. "Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures" Agronomy 10, no. 2: 210. https://doi.org/10.3390/agronomy10020210
APA StyleMickelbart, M. V., & Boine, B. (2020). Glycinebetaine Enhances Osmotic Adjustment of Ryegrass under Cold Temperatures. Agronomy, 10(2), 210. https://doi.org/10.3390/agronomy10020210





