Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation
Abstract
1. Introduction
2. Materials and Methods
2.1. Plasmid Construction for Plant Transformation
2.2. Generation of Transgenic Barley Plants
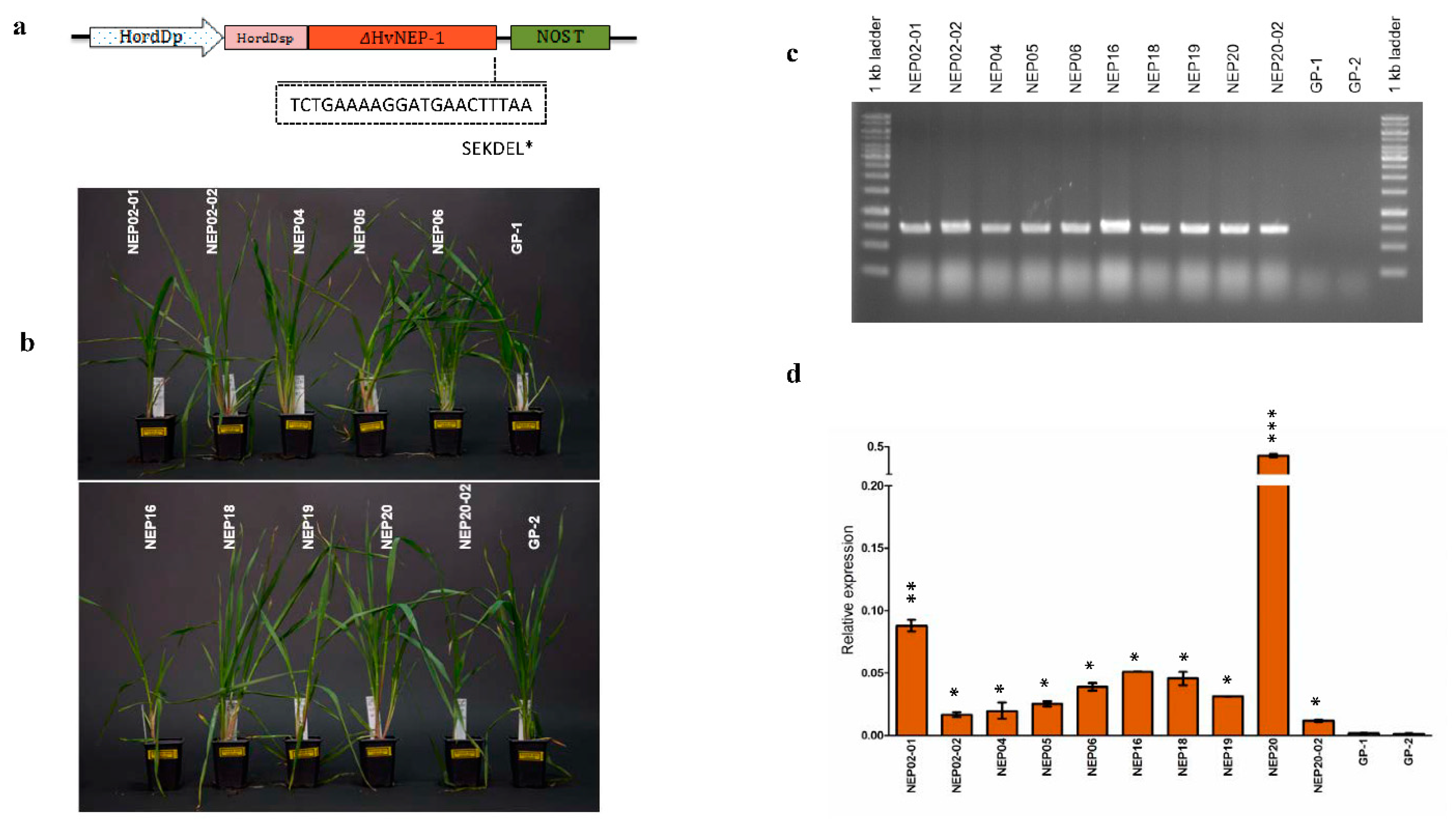
2.3. PCR Analysis of Transformed Barley Plants
2.4. Inoculation of Transgenic Barley Lines with Fusarium
2.5. Disease Scoring and Mycotoxin Analysis
2.6. HvNEP-1 Expression in the Transgenic Barley Lines
2.7. Field Soil Plot Analysis of Transgenic Barley Lines for Fusarium Resistance
3. Results
3.1. HvNEP-1-Overexpressing Barley Lines and Assessment of FHB Resistance
3.2. Mycotoxin Analysis of the HvNEP-1-Overexpressing Lines
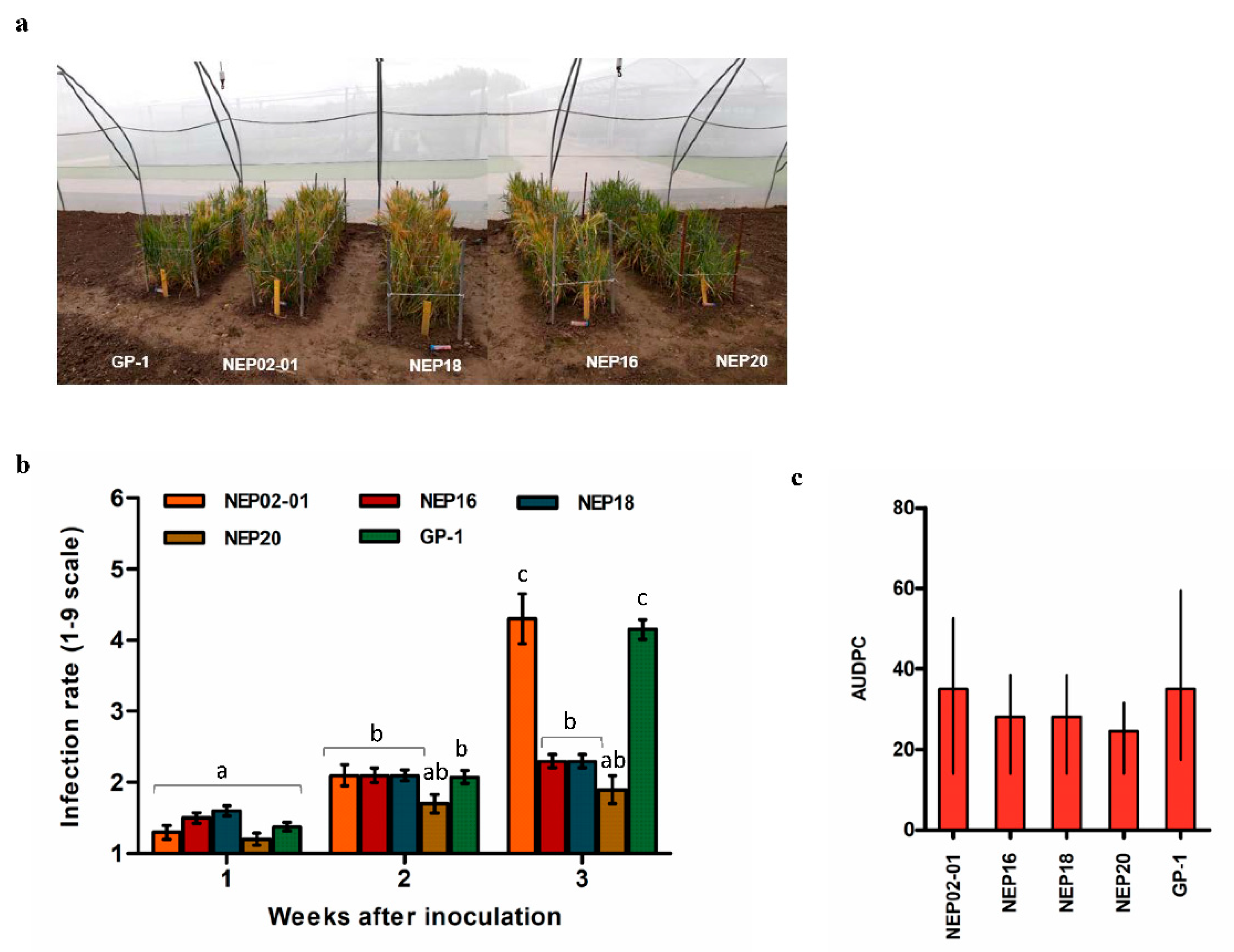
3.3. Semi-Field Analysis of the HvNEP-1 Transgenic Lines
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Parry, D.W.; Jenkinson, P.; McLeod, L. Fusarium ear blight (scab) in small-grain cereals—A review. Plant Pathol. 1995, 44, 207–238. [Google Scholar] [CrossRef]
- Stenglein, S.A. Fusarium Poae: A pathogen that needs more attention. J. Plant Pathol. 2009, 91, 25–36. [Google Scholar]
- Toth, B.; Mesterhazy, A.; Horvath, Z.; Bartok, T.; Varga, M.; Varga, J. Genetic variability of central European isolates of the Fusarium graminearum species complex. Eur. J. Plant Pathol. 2005, 113, 35–45. [Google Scholar] [CrossRef]
- Nganje, W.E.; Bangsund, D.A.; Leistritz, F.L.; Wilson, W.W.; Tiapo, N.M. Regional economic impacts of Fusarium Head Blight in wheat and barley. Rev. Agric. Econ. 2004, 26, 332–347. [Google Scholar] [CrossRef]
- Roth, G. Mid-Atlantic Soft Winter Wheat Region; Wheat and Barley Scab Initiative: Fusarium Focus; Academic: Milwaukee, WI, USA, 2010; Volume 10, pp. 1–8. [Google Scholar]
- Hollandbeck, G.F.; Dewolf, E.; Todd, T. Preliminary 2019 Kansas Wheat Disease Loss Estimates; Kansas cooperative plant disease survey report; Kansas Department of Agriculture: Manhattan, KS, USA, 2019.
- McMullen, M.; Bergstrom, G.; De Wolf, E.; Dill-Macky, R.; Hershman, D.; Shaner, G.; Van Sanford, D. A Unified Effort to Fight an Enemy of Wheat and Barley: Fusarium Head Blight. Plant Dis. 2012, 96, 1712–1728. [Google Scholar] [CrossRef]
- Desjardins, A.E. Fusarium Mycotoxins: Chemistry, Genetics and Biology. Plant Pathol. 2007, 56, 337. [Google Scholar]
- Harris, L.J.; Desjardins, A.E.; Plattner, R.D.; Nicholson, P.; Butler, G.; Young, J.C.; Weston, G.; Proctor, R.H.; Hohn, T.M. Possible role of trichothecene mycotoxins in virulence of Fusarium graminearum on maize. Plant Dis. 1999, 83, 954–960. [Google Scholar] [CrossRef]
- Proctor, R.H.; Hohn, T.M.; McCormick, S.P. Reduced virulence of Gibberella-zeae caused by disruption of a trichothecene toxin biosynthetic gene. Mol. Plant-Microbe Interact. 1995, 8, 593–601. [Google Scholar] [CrossRef]
- Placinta, C.M.; D’Mello, J.P.F.; Macdonald, A.M.C. A review of worldwide contamination of cereal grains and animal feed with Fusarium mycotoxins. Anim. Feed Sci. Technol. 1999, 78, 21–37. [Google Scholar] [CrossRef]
- Bai, G.H.; Shaner, G. Management and resistance in wheat and barley to Fusarium head blight. Annu. Rev. Phytopathol. 2004, 42, 135–161. [Google Scholar] [CrossRef] [PubMed]
- Buerstmayr, H.; Ban, T.; Anderson, J.A. QTL mapping and marker-assisted selection for Fusarium head blight resistance in wheat: A review. Plant Breed. 2009, 128, 1–26. [Google Scholar] [CrossRef]
- Jin, F.; Zhang, D.D.; Bockus, W.; Baenziger, P.S.; Carver, B.; Bai, G.H. Fusarium Head Blight Resistance in US Winter Wheat Cultivars and Elite Breeding Lines. Crop Sci. 2013, 53, 2006–2013. [Google Scholar] [CrossRef]
- Li, G.; Zhou, J.; Jia, H.; Gao, Z.; Fan, M.; Luo, Y.; Zhao, P.; Xue, S.; Li, N.; Yuan, Y.; et al. Mutation of a histidine-rich calcium-binding-protein gene in wheat confers resistance to Fusarium head blight. Nat. Genet. 2019, 51, 1106–1112. [Google Scholar] [CrossRef] [PubMed]
- Su, Z.; Bernardo, A.; Tian, B.; Chen, H.; Wang, S.; Ma, H.; Cai, S.; Liu, D.; Zhang, D.; Li, T.; et al. A deletion mutation in TaHRC confers Fhb1 resistance to Fusarium head blight in wheat. Nat. Genet. 2019, 51, 1099–1105. [Google Scholar] [CrossRef]
- Rawat, N.; Pumphrey, M.O.; Liu, S.; Zhang, X.; Tiwari, V.K.; Ando, K.; Trick, H.N.; Bockus, W.W.; Akhunov, E.; Anderson, J.A.; et al. Wheat Fhb1 encodes a chimeric lectin with agglutinin domains and a pore-forming toxin-like domain conferring resistance to Fusarium head blight. Nat. Genet. 2016, 48, 1576–1580. [Google Scholar] [CrossRef]
- Gadaleta, A.; Colasuonno, P.; Giove, S.L.; Blanco, A.; Giancaspro, A. Map-based cloning of QFhb.mgb-2A identifies a WAK2 gene responsible for Fusarium Head Blight resistance in wheat. Sci. Rep. 2019, 9, 6929. [Google Scholar] [CrossRef]
- Liu, S.Y.; Hall, M.D.; Griffey, C.A.; McKendry, A.L. Meta-Analysis of QTL Associated with Fusarium Head Blight Resistance in Wheat. Crop Sci. 2009, 49, 1955–1968. [Google Scholar] [CrossRef]
- Loffler, M.; Schon, C.C.; Miedaner, T. Revealing the genetic architecture of FHB resistance in hexaploid wheat (Triticum aestivum L.) by QTL meta-analysis. Mol. Breed. 2009, 23, 473–488. [Google Scholar] [CrossRef]
- Simoes, I.; Faro, C. Structure and function of plant aspartic proteinases. Eur. J. Biochem. 2004, 271, 2067–2075. [Google Scholar] [CrossRef]
- Simoes, I.; Faro, R.; Bur, D.; Faro, C. Characterization of recombinant CDR1, an Arabidopsis aspartic proteinase involved in disease resistance. J. Biol. Chem. 2007, 282, 31358–31365. [Google Scholar] [CrossRef]
- Rawlings, N.D.; Alan, J.; Thomas, P.D.; Huang, X.D.; Bateman, A.; Finn, R.D. The MEROPS database of proteolytic enzymes, their substrates and inhibitors in 2017 and a comparison with peptidases in the PANTHER database. Nucleic Acids Res. 2018, 46, D624–D632. [Google Scholar] [CrossRef] [PubMed]
- Vines, S.H. The Proteolytic Enzyme of Nepenthes. Ann. Bot. 1897, 11, 563–584. [Google Scholar] [CrossRef]
- Vines, S.H. The Proteolytic Enzyme of Nepenthes. Ann. Bot. 1901, 15, 563–573. [Google Scholar] [CrossRef][Green Version]
- Owen, T.P.; Lennon, K.A. Structure and development of the pitchers from the carnivorous plant Nepenthes alata (Nepenthaceae). Am. J. Bot. 1999, 86, 1382–1390. [Google Scholar] [CrossRef]
- Amagase, S.; Nakayama, S.; Tsugita, A. Acid protease in nepenthes 2. Study on specificity of nepenthesin. J. Biochem. 1969, 66, 431–439. [Google Scholar] [CrossRef]
- Amagase, S. Digestive enzymes in insectivorous plants part 3 Acid proteases in the genus Nepenthes and Drosera-peltata. J. Biochem. 1972, 72, 73–81. [Google Scholar] [CrossRef]
- Nakayama, S.; Amagase, S. Acid protease in nepenthes - partial purification and properties of enzyme. Proc. Jpn. Acad. 1968, 44, 358. [Google Scholar] [CrossRef][Green Version]
- An, C.I.; Fukusaki, E.; Kobayashi, A. Aspartic proteinases are expressed in pitchers of the carnivorous plant Nepenthes alata Blanco. Planta 2002, 214, 661–667. [Google Scholar] [CrossRef]
- Athauda, S.B.P.; Matsumoto, K.; Rajapakshe, S.; Kuribayashi, M.; Kojima, M.; Kubomura-Yoshida, N.; Iwamatsu, A.; Shibata, C.; Inoue, H.; Takahashi, K. Enzymic and structural characterization of nepenthesin, a unique member of a novel subfamily of aspartic proteinases. Biochem. J. 2004, 381, 295–306. [Google Scholar] [CrossRef]
- Kadek, A.; Mrazek, H.; Halada, P.; Rey, M.; Schriemer, D.C.; Man, P. Aspartic Protease Nepenthesin-1 as a Tool for Digestion in Hydrogen/Deuterium Exchange Mass Spectrometry. Anal. Chem. 2014, 86, 4287–4294. [Google Scholar] [CrossRef] [PubMed]
- Lee, L.; Zhang, Y.; Ozar, B.; Sensen, C.W.; Schriemer, D.C. Carnivorous Nutrition in Pitcher Plants ( Nepenthes spp.) via an Unusual Complement of Endogenous Enzymes. J. Proteome Res. 2016, 15, 3108–3117. [Google Scholar] [CrossRef] [PubMed]
- Buch, F.; Kaman, W.E.; Bikker, F.J.; Yilamujiang, A.; Mithöfer, A.; Mithoefer, A. Nepenthesin Protease Activity Indicates Digestive Fluid Dynamics in Carnivorous Nepenthes Plants. PLoS ONE 2015, 10, 3. [Google Scholar] [CrossRef] [PubMed]
- Bekalu, Z.E.E.; Madsen, C.K.K.; Dionisio, G.; Brinch-Pedersen, H. Aspergillus ficuum phytase activity is inhibited by cereal grain components. PLoS ONE 2017, 12, 13. [Google Scholar] [CrossRef] [PubMed]
- Bekalu, Z.E.E.; Dionisio, G.; Krogh Madsen, C.; Etzerodt, T.; Brinch-Pedersen, H. A novel barley nepenthesin-like aspartic protease HvNEP-1 inhibits fungal phytases and suppress fungal growth and toxin production. Status (Unpublished, under preparation).
- Bekalu, Z.E.; Dionisio, G.; Madsen, C.K.; Holme, I.B.; Etzerodt, T.P.; Fomsgaard, I.; Jørgensen, L.N.; Brinch-Pedersen, H. Nepenthesin-1 Derived Resistance to Fungal Pathogens in Major Crop Plants; WO 2019/057845 A1, filed 20 September 2018, and issued 28 March 2019; World Intellectual Property Organization: Geneva, Switzerland, 2019. [Google Scholar]
- Zhu, B.; Cai, G.; Hall, E.O.; Freeman, G.J. In-fusion assembly: Seamless engineering of multidomain fusion proteins, modular vectors, and mutations. Biotechniques 2007, 43, 354–359. [Google Scholar] [CrossRef] [PubMed]
- An, G.; Ebert, P.R.; Mitra, A.; Ha, S.B. Binary vectors. In Plant Molecular Biology Manual; Springer: Dordrecht, The Netherlands, 2014; pp. 45–63. [Google Scholar]
- Jones, H.D.; Doherty, A.; Wu, H. Review of methodologies and a protocol for the Agrobacterium-mediated transformation of wheat. Plant Methods. 2005, 1, 5. [Google Scholar] [CrossRef]
- Holme, I.B.; Dionisio, G.; Brinch-Pedersen, H.; Wendt, T.; Madsen, C.K.; Vincze, E.; Holm, P.B. Cisgenic barley with improved phytase activity. Plant Biotechnol. J. 2012, 10, 237–247. [Google Scholar] [CrossRef]
- Bartlett, J.G.; Alves, S.C.; Smedley, M.; Snape, J.W.; Harwood, W.A. High-throughput Agrobacterium-mediated barley transformation. Plant Methods 2008, 4, 22. [Google Scholar] [CrossRef]
- Doyle, J.J.; Doyle, J.L. Isolation of plant DNA from fresh tissue. Focus (Madison) 1990, 12, 13–15. [Google Scholar]
- Etzerodt, T.; Maeda, K.; Nakajima, Y.; Laursen, B.; Fomsgaard, I.S.; Kimura, M. 2,4-Dihydroxy-7-methoxy-2H-1,4-benzoxazin-3(4H)-one (DIMBOA) inhibits trichothecene production by Fusarium graminearum through suppression of Tri6 expression. Int. J. Food Microbiol. 2015, 214, 123–128. [Google Scholar] [CrossRef]
- Zadoks, J.C.; Changi, T.T.; Konzak, C.F. A decimal code for the growth stages of cereals. Weed Res. 1974, 14, 415–421. [Google Scholar] [CrossRef]
- Etzerodt, T.; Gislum, R.; Laursen, B.B.; Heinrichson, K.; Gregersen, P.L.; Jorgensen, L.N.; Fomsgaard, I.S. Correlation of Deoxynivalenol Accumulation in Fusarium-Infected Winter and Spring Wheat Cultivars with Secondary Metabolites at Different Growth Stages. J. Agric. Food Chem. 2016, 64, 4545–4555. [Google Scholar] [CrossRef] [PubMed]
- Chomczynski, P.; Sacchi, N. The single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction: Twenty-something years on. Nat. Protoc. 2006, 1, 581–585. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, H.W.; Christensen, J.J. Factors affecting resistance of wheat to scab caused by Gibberella zeae. Phytopathology 1963, 53, 831. [Google Scholar]
- Miller, J.D.; Young, J.C.; Sampson, D.R. Deoxynivalenol and fusarium head blight resistance in spring cereals. Phytopathol. Zeitschrift J. Phytopathol. 1985, 113, 359–367. [Google Scholar] [CrossRef]
- Antonissen, G.; Martel, A.; Pasmans, F.; Ducatelle, R.; Verbrugghe, E.; Vandenbroucke, V.; Li, S.; Haesebrouck, F.; Van Immerseel, F.; Croubels, S. The impact of Fusarium Mycotoxins on human and animal host susceptibility to infectious diseases. Toxins (Basel) 2014, 6, 430–452. [Google Scholar] [CrossRef]
- Cui, N.; He, Y.; Yao, S.; Zhang, H.; Ren, J.; Fang, H.; Yu, Y. Tebuconazole induces triazole-resistance in Aspergillus fumigatus in liquid medium and soil. Sci. Total Environ. 2019, 648, 1237–1243. [Google Scholar] [CrossRef]
- Mortensen, K.L.; Mellado, E.; Lass-Flörl, C.; Rodriguez-Tudela, J.L.; Johansen, H.K.; Arendrup, M.C. Environmental study of azole-resistant Aspergillus fumigatus and other aspergilli in Austria, Denmark, and Spain. Antimicrob. Agents Chemother. 2010, 54, 4545–4549. [Google Scholar] [CrossRef]

| HvNEP-1 transgenic lines and GP controls | ||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| NEP02-01 | NEP02-02 | NEP04 | NEP05 | NEP06 | NEP16 | NEP18 | NEP19 | NEP20 | NEP20-02 | GP-1 | GP-2 | |||||
| Samples used for analysis | FC | FHB+ | 15938 | 794 | 1627 | 1231 | 9198 | 925 | 1257 | <50 | 25043 | 462 | 7264 | 77 | NIV | Mycotoxins (µg/kg DW) |
| <50 | <50 | <50 | <50 | 52 | <50 | <50 | <50 | 152 | <50 | 37091 | 2324 | DON | ||||
| <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 186 | 55 | ZEA | ||||
| FHB– | 77 | <50 | <50 | 55 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | NIV | |||
| <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 142 | 855 | DON | ||||
| <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 15 | ZEA | ||||
| FG | FHB+ | 35510 | 6243 | 1543 | 16377 | 29727 | 597 | 97 | <50 | 53 | 2399 | 13050 | <50 | NIV | ||
| 5295 | <50 | 56 | <50 | <50 | <50 | <50 | 8170 | 40073 | <50 | 6022 | 1664 | DON | ||||
| <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | 256 | <5 | <5 | 38 | ZEA | ||||
| FHB– | 124 | <50 | <50 | <50 | 171 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | NIV | |||
| <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | 569 | 240 | DON | ||||
| <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | ZEA | ||||
| MQ | <50 | 52 | <50 | <50 | <50 | 163 | <50 | <50 | <50 | <50 | <50 | <50 | NIV | |||
| <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | <50 | DON | ||||
| <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | <5 | ZEA | ||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bekalu, Z.E.; Krogh Madsen, C.; Dionisio, G.; Bæksted Holme, I.; Jørgensen, L.N.; S. Fomsgaard, I.; Brinch-Pedersen, H. Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation. Agronomy 2020, 10, 203. https://doi.org/10.3390/agronomy10020203
Bekalu ZE, Krogh Madsen C, Dionisio G, Bæksted Holme I, Jørgensen LN, S. Fomsgaard I, Brinch-Pedersen H. Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation. Agronomy. 2020; 10(2):203. https://doi.org/10.3390/agronomy10020203
Chicago/Turabian StyleBekalu, Zelalem Eshetu, Claus Krogh Madsen, Giuseppe Dionisio, Inger Bæksted Holme, Lise Nistrup Jørgensen, Inge S. Fomsgaard, and Henrik Brinch-Pedersen. 2020. "Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation" Agronomy 10, no. 2: 203. https://doi.org/10.3390/agronomy10020203
APA StyleBekalu, Z. E., Krogh Madsen, C., Dionisio, G., Bæksted Holme, I., Jørgensen, L. N., S. Fomsgaard, I., & Brinch-Pedersen, H. (2020). Overexpression of Nepenthesin HvNEP-1 in Barley Endosperm Reduces Fusarium Head Blight and Mycotoxin Accumulation. Agronomy, 10(2), 203. https://doi.org/10.3390/agronomy10020203






