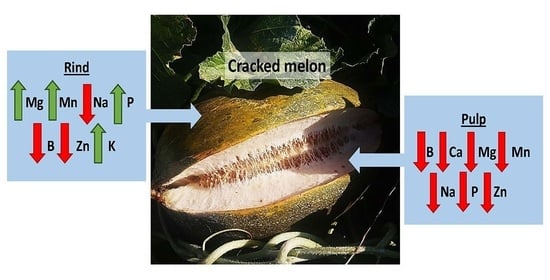
Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking
Abstract
1. Introduction
2. Material and Methods
2.1. Experimental Conditions and Foliar Treatments
2.2. Fresh Weight and Incidence of Cracking
2.3. Physiological Determinations
2.4. Analysis of Mineral Elements
2.5. Data Analysis
3. Results
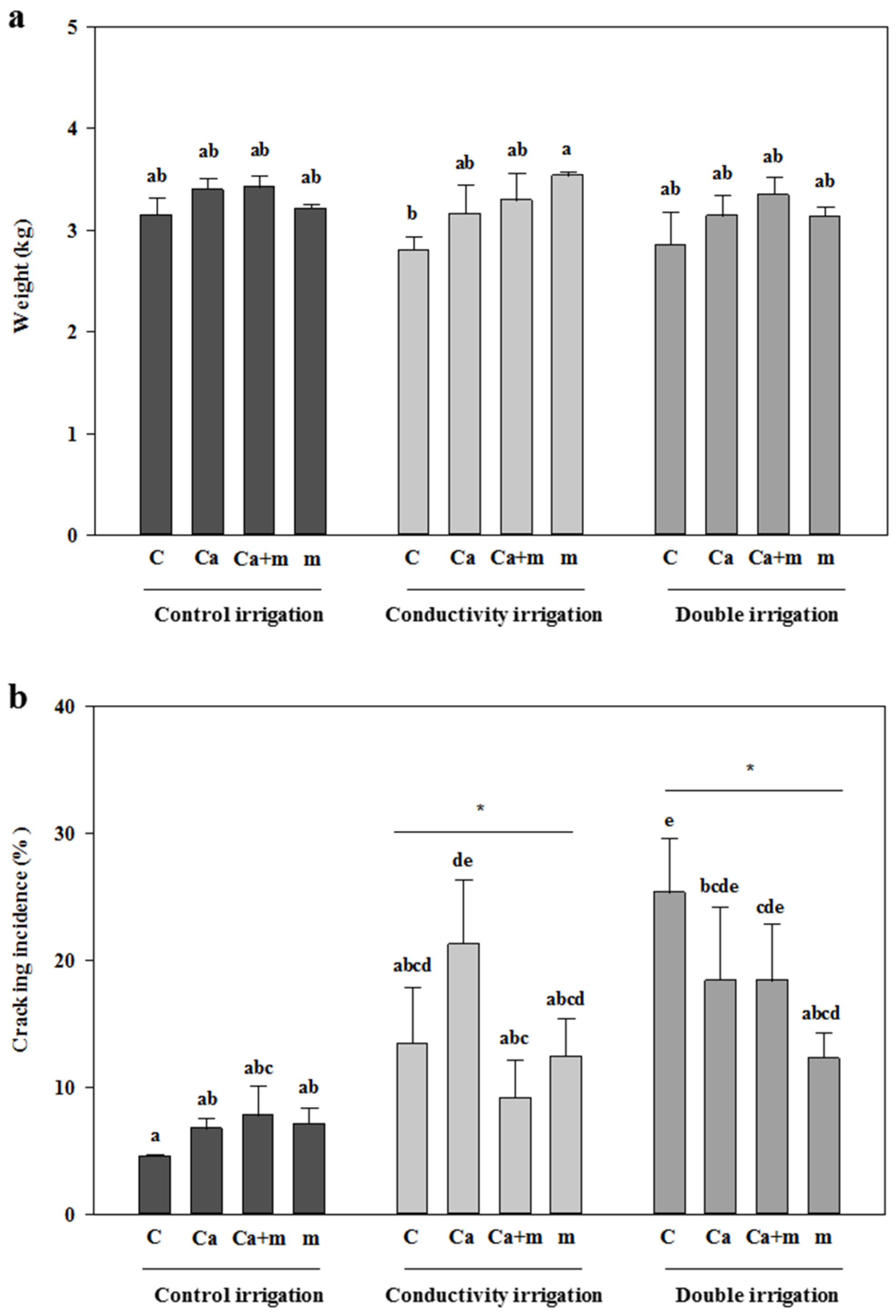
3.1. Fresh Weight and Incidence of Cracking
3.2. Physiological Determinations
3.3. Analysis of Mineral Elements in Leaves
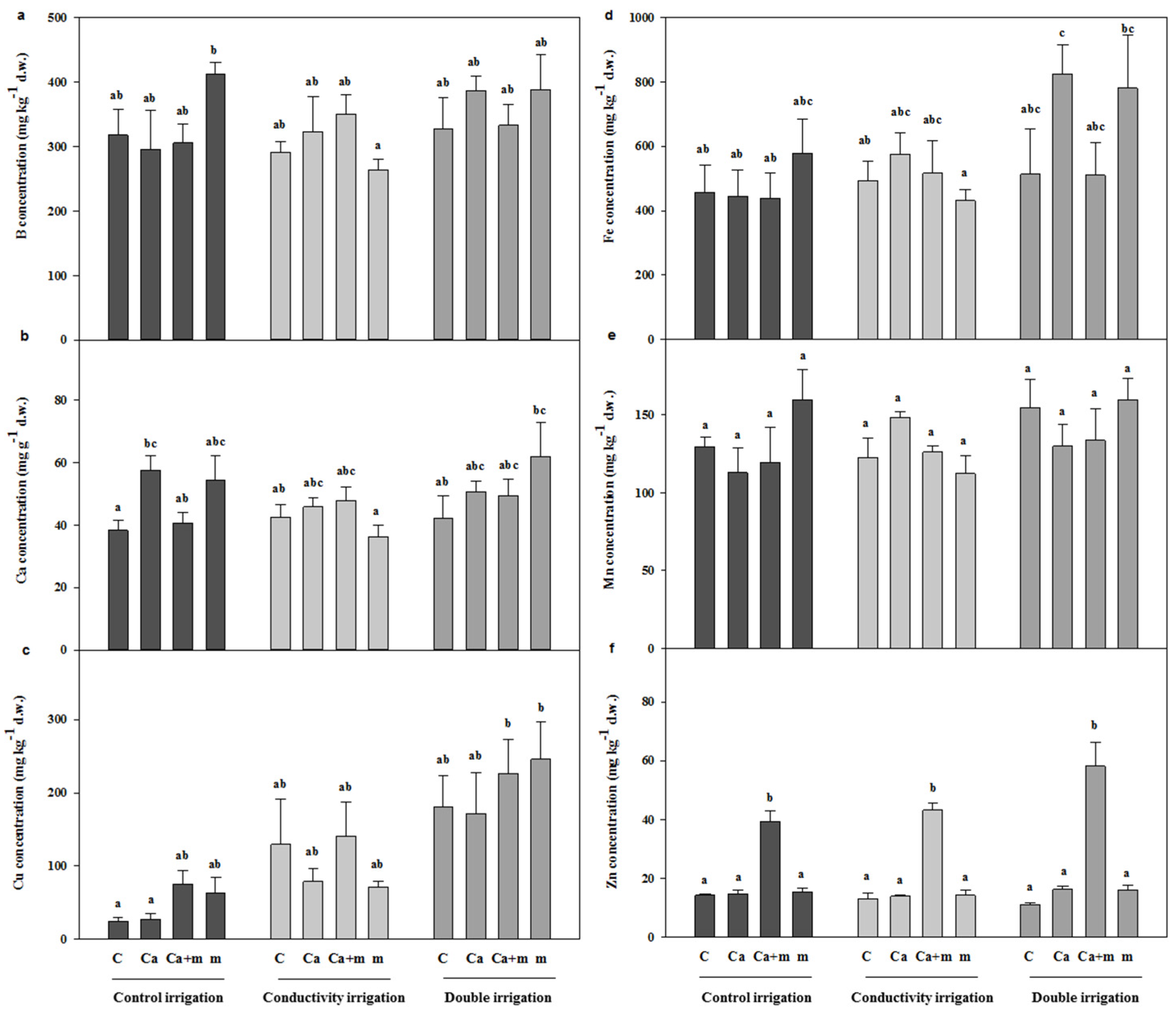
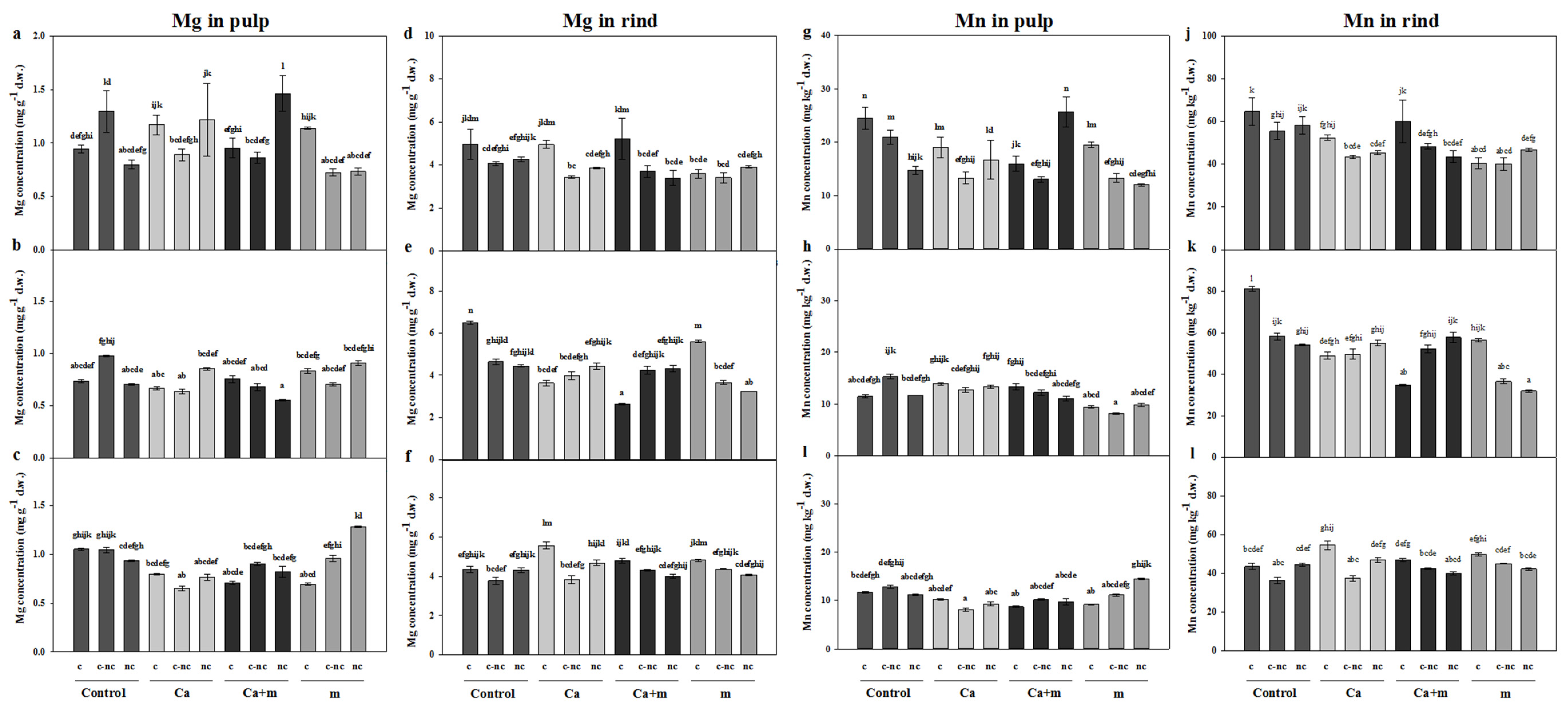
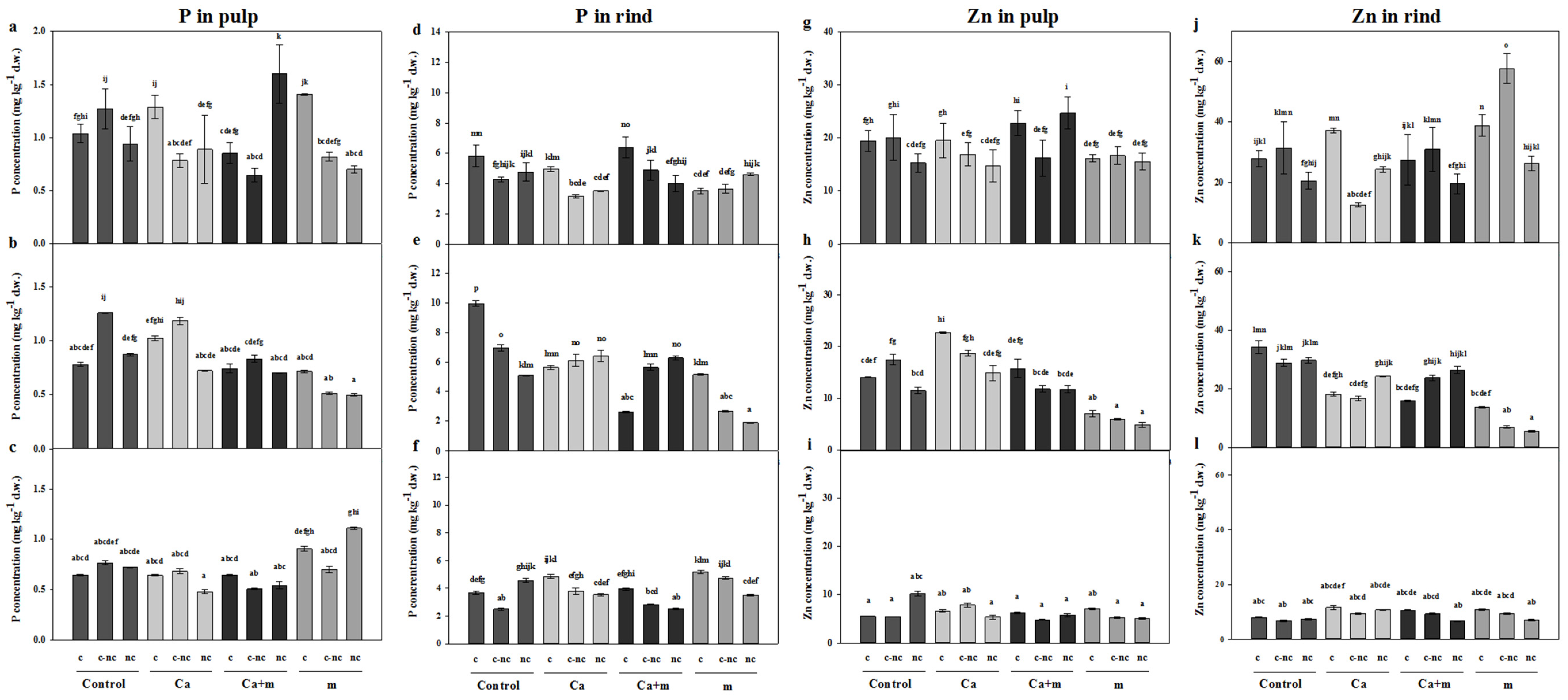
3.4. Analysis of Mineral Elements in Fruit
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Matas, A.J.; Cobb, E.D.; Paolillo, D.J.; Niklas, K.J. Crack resistance in cherry tomato fruit correlates with cuticular membrane thickness. HortScience 2004, 39, 1354–1358. [Google Scholar] [CrossRef]
- Peet, M.M. Fruit cracking in tomato. Horttechnology 1992, 2, 216–223. [Google Scholar] [CrossRef]
- Khadivi-Khub, A. Physiological and genetic factors influencing fruit cracking. Acta Physiologiae Plantarum 2015, 37, 1718. [Google Scholar] [CrossRef]
- Winkler, A.; Peschel, S.; Kohrs, K.; Knoche, M. Rain cracking in sweet cherries is not due to excess water uptake but to localized skin phenomena. J. Am. Soc. Hortic. Sci. 2016, 141, 653–660. [Google Scholar] [CrossRef]
- Li, J.; Chen, J. Citrus fruit-cracking: Causes and occurrence. Hortic. Plant J. 2017, 3, 255–260. [Google Scholar] [CrossRef]
- Winkler, A.; Knoche, M. Calcium and the physiology of sweet cherries: A review. Sci. Hortic. 2019, 245, 107–115. [Google Scholar] [CrossRef]
- Schumann, C.; Jürgen Schlege, H.; Grimm, E.; Knoche, M.; Lang, A. Water potential and its components in developing sweet cherry. J. Am. Soc. Hortic. Sci. 2014, 139, 349–355. [Google Scholar] [CrossRef]
- Cline, J.A.; Trought, M. Effect of gibberellic acid on fruit cracking and quality of bing and sam sweet cherries. Can. J. Plant Sci. 2007, 87, 545–550. [Google Scholar] [CrossRef]
- Joshi, M.; Baghel, R.S.; Fogelman, E.; Stern, R.A.; Ginzberg, I. Identification of candidate genes mediating apple fruit-cracking resistance following the application of gibberellic acids 4 + 7 and the cytokinin 6-benzyladenine. Plant Physiol. Biochem. 2018, 127, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Trujillo, J.P.; Lester, G.E.; Dos-Santos, N.; Juan, A.M.; Esteva, J.; Jifon, J.L.; Varó, P. Pre-and postharvest muskmelon fruit cracking: Causes and potential remedies. HortTechnology 2013, 23, 266–275. [Google Scholar] [CrossRef]
- Qi, Z.; Li, J.; Raza, M.A.; Zou, X.; Cao, L.; Rao, L.; Chen, L. Inheritance of fruit cracking resistance of melon (Cucumis Melo L.) fitting E-0 genetic model using major gene plus polygene inheritance analysis. Sci. Hortic. 2015, 189, 168–174. [Google Scholar] [CrossRef]
- Capel, C.; Yuste-Lisbona, F.J.; López-Casado, G.; Angosto, T.; Cuartero, J.; Lozano, R.; Capel, J. Multi-environment QTL mapping reveals genetic architecture of fruit cracking in a tomato RIL Solanum Lycopersicum × S. Pimpinellifolium population. Theor. Appl. Genet. 2017, 130, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Ren, Y.; Shen, L.-; Wang, X.; Yan, C.; Mao, L.; Mao, Y. Study on the related cracking-resistant genes in Chinese Jujube. Sci. Pap. Ser. B Hortic. 2017, 61, 155–164. [Google Scholar]
- Sharma, R.R.; Datta, S.C.; Varghese, E. Effect of Surround WP®, a Kaolin-based particle film on sunburn, fruit cracking and postharvest quality of ‘Kandhari’ pomegranates. Crop Prot. 2018, 114, 18–22. [Google Scholar] [CrossRef]
- Correia, S.; Santos, M.; Glińska, S.; Gapińska, M.; Matos, M.; Carnide, V.; Schouten, R.; Silva, A.P.; Gonçalves, B. Effects of exogenous compound sprays on cherry cracking: Skin properties and gene expression. J. Sci. Food Agric. 2020, 100, 2911–2921. [Google Scholar] [CrossRef] [PubMed]
- Lester, G.E.; Jifon, J.L.; Makus, D.J. Impact of potassium nutrition on postharvest fruit quality: Melon (Cucumis Melo L) case study. Plant Soil 2010, 335, 117–131. [Google Scholar] [CrossRef]
- Dinesh, K.; Rajesh, K.; Subhash, C.; Heerendra, S. Effect of foliar application of nutrients on fruit firmness, cracking and shelf life in litchi (Litchi Chinensis Sonn.) cultivar early large red. Environ. Ecol. 2017, 35, 2418–2422. [Google Scholar]
- Davarpanah, S.; Tehranifar, A.; Abadía, J.; Val, J.; Davarynejad, G.; Aran, M.; Khorassani, R. Foliar calcium fertilization reduces fruit cracking in pomegranate (Punica granatum cv. Ardestani). Sci. Hortic. 2018, 230, 86–91. [Google Scholar] [CrossRef]
- Bakshi, P. Effect of foliar nutrition and growth regulators on nutrient status and fruit quality of Eureka Lemon (Citrus Limon). Indian J. Agric. Sci. 2018, 88, 704–708. [Google Scholar]
- Marshall, D.A.; Spiers, J.M.; Curry, K.J. (373) use of calcium foliar feed fertilization to reduce rain-related splitting in rabbiteye and southern highbush blueberry. HortScience 2005, 40, 1059A-1059. [Google Scholar] [CrossRef]
- Breia, R.; Mósca, A.F.; Conde, A.; Correia, S.; Conde, C.; Noronha, H.; Soveral, G.; Gonçalves, B.; Gerós, H. Sweet cherry (Prunus Avium L.) PAPIP1; 4 is a functional aquaporin upregulated by pre-harvest calcium treatments that prevent cracking. Int. J. Mol. Sci. 2020, 21, 3017. [Google Scholar] [CrossRef] [PubMed]
- Vangdal, E.; Hovland, K.L.; Børve, J.; Sekse, L.; Slimestad, R. Foliar application of calcium reduces postharvest decay in sweet cherry fruit by various mechanisms. Acta Hortic. 2008, 768, 143–148. [Google Scholar] [CrossRef]
- Koutinas, N.; Sotiropoulos, T.; Petridis, A.; Almaliotis, D.; Deligeorgis, E.; Therios, I.; Voulgarakis, N. Effects of preharvest calcium foliar sprays on several fruit quality attributes and nutritional status of the kiwifruit cultivar Tsechelidis. HortScience 2010, 45, 984–987. [Google Scholar] [CrossRef]
- Khoravi Mashizi, M.; Sarcheshmehpour, M. Effect of foliar application of calcium and potassium on growth, fruit yield and some properties of two muskmelon cultivars (Cucumis Melo L.). J. Crop Prod. Process. 2015, 5, 295–310. [Google Scholar] [CrossRef]
- Chater, J.M.; Garner, L.C. Foliar nutrient applications to ‘wonderful’ pomegranate (Punica Granatum L.). II. Effects on leaf nutrient status and fruit split, yield and size. Sci. Hortic. 2018, 242, 207–213. [Google Scholar] [CrossRef]
- Hardiyanto, H.; Friyanti, D.N. Application of K, Ca, and Mg on peel thickness and fruit cracking incidence of citrus. Russ. J. Agric. Socio-Econ. Sci. 2019, 87, 45–56. [Google Scholar] [CrossRef]
- Schober, P.; Boer, C.; Schwarte, L.A. Correlation Coefficients: Appropriate Use and Interpretation. Anesth. Analg. 2018, 126, 1763–1768. [Google Scholar] [CrossRef]
- Cline, J.A.; Sekse, L.; Meland, M.; Webster, A.D. Rain-induced fruit cracking of sweet cherries: I. Influence of cultivar and rootstock on fruit water absorption, cracking and quality. Acta Agric. Scand. Sect. B Soil Plant Sci. 1995, 45, 213–223. [Google Scholar] [CrossRef]
- Miró, J.J.; Estrela, M.J.; Caselles, V.; Gómez, I. Spatial and temporal rainfall changes in the Júcar and Segura Basins (1955–2016): Fine-scale trends. Int. J. Climatol. 2018, 38, 4699–4722. [Google Scholar] [CrossRef]
- Maestre-Valero, J.F.; Gonzalez-Ortega, M.J.; Martinez-Alvarez, V.; Gallego-Elvira, B.; Conesa-Jodar, F.J.; Martin-Gorriz, B. Revaluing the nutrition potential of reclaimed water for irrigation in southeastern Spain. Agric. Water Manag. 2019, 218, 174–181. [Google Scholar] [CrossRef]
- Martínez-Ballesta, M.C.; Diaz, R.; Martínez, V.; Carvajal, M. Different blocking effects of HgCl2 and NaCl on aquaporins of pepper plants. J. Plant Physiol. 2003, 160, 1487–1492. [Google Scholar] [CrossRef]
- Sharma, P.K.; Hall, D.O. Interaction of salt stress and photoinhibition on photosynthesis in barley and sorghum. J. Plant Physiol. 1991, 138, 614–619. [Google Scholar] [CrossRef]
- Carvajal, M.; Del Amor, F.M.; Fernandez-Ballester, G.; Martínez, V.; Cerdá, A. Time course of solute accumulation and water relations in muskmelon plants exposed to salt during different growth stages. Plant Sci. 1998, 138, 103–112. [Google Scholar] [CrossRef]
- Nguyen, L.T.T.; Osanai, Y.; Anderson, I.C.; Bange, M.P.; Tissue, D.T.; Singh, B.K. Flooding and prolonged drought have differential legacy impacts on soil nitrogen cycling, microbial communities and plant productivity. Plant Soil 2018, 431, 371–387. [Google Scholar] [CrossRef]
- Bhusal, N.; Kim, H.S.; Han, S.G.; Yoon, T.M. Photosynthetic traits and plant–water relations of two apple cultivars grown as bi-leader trees under long-term waterlogging conditions. Environ. Exp. Bot. 2020, 176, 104111. [Google Scholar] [CrossRef]
- Lewis, D.H. Boron: The essential element for vascular plants that never was. N. Phytol. 2019, 221, 1685–1690. [Google Scholar] [CrossRef]
- Ishii, T.; Matsunaga, T. Isolation and characterization of a boron-rhamnogalacturonan-II complex from cell walls of sugar beet pulp. Carbohydr. Res. 1996, 284, 1–9. [Google Scholar] [CrossRef]
- Kobayashi, M.; Matoh, T.; Azuma, J.I. Two chains of rhamnogalacturonan II are cross-linked by borate-diol ester bonds in higher plant cell walls. Plant Physiol. 1996, 110, 1017–1020. [Google Scholar] [CrossRef]
- Goldbach, H.E.; Wimmer, M.A. Boron in plants and animals: Is there a role beyond cell-wall structure? J. Plant Nutr. Soil Sci. 2007, 39–48. [Google Scholar] [CrossRef]
- Saure, M.C. Calcium translocation to fleshy fruit: Its mechanism and endogenous control. Sci. Hortic. 2005, 105, 65–89. [Google Scholar] [CrossRef]
- Ortiz, A.; Graell, J.; Lara, I. Preharvest calcium applications inhibit some cell wall-modifying enzyme activities and delay cell wall disassembly at commercial harvest of “Fuji Kiku-8” apples. Postharvest Biol. Technol. 2011, 62, 161–167. [Google Scholar] [CrossRef]
- Cybulska, J.; Zdunek, A.; Konstankiewicz, K. Calcium effect on mechanical properties of model cell walls and apple tissue. J. Food Eng. 2011, 102, 217–223. [Google Scholar] [CrossRef]
- Guo, W.; Nazim, H.; Liang, Z.; Yang, D. Magnesium deficiency in plants: An urgent problem. Crop J. 2016, 4, 83–91. [Google Scholar] [CrossRef]
- Sperrazza, J.M.; Spremulli, L.L. Quantitation of cation binding to wheat germ ribosomes: Influences on submit association equilibria and ribosome activity. Nucl. Acids Res. 1983, 11, 2665–2679. [Google Scholar] [CrossRef]
- Heenan, D.P.; Campbell, L.C. Influence of potassium and manganese on growth and uptake of magnesium by soybeans (Glycine Max (L.) Merr. Cv. Bragg). Plant Soil 1981, 61, 447–456. [Google Scholar] [CrossRef]
- Cowan, J.A. Structural and catalytic chemistry of magnesium-dependent enzymes. BioMetals 2002, 15, 225–235. [Google Scholar] [CrossRef]
- Chen, Z.; Yan, W.; Sun, L.; Tian, J.; Liao, H. Proteomic analysis reveals growth inhibition of soybean roots by manganese toxicity is associated with alteration of cell wall structure and lignification. J. Proteom. 2016, 143, 151–160. [Google Scholar] [CrossRef]
- Lott, J.N.A.; Bojarski, M.; Kolasa, J.; Batten, G.D.; Campbell, L.C. A review of the phosphorus content of dry cereal and legume crops of the world. Int. J. Agric. Res. Gov. Ecol. 2009, 8, 351–370. [Google Scholar] [CrossRef]
- Ockenden, I.; Dorsch, J.A.; Reid, M.M.; Lin, L.; Grant, L.K.; Raboy, V.; Lott, J.N.A. Characterization of the storage of phosphorus, inositol phosphate and cations in grain tissues of four barley (Hordeum Vulgare L.) low phytic acid genotypes. Plant Sci. 2004, 167, 1131–1142. [Google Scholar] [CrossRef]
- Kasim, W.A. Physiological consequences of structural and ultra-structural changes induced by Zn stress in phaseolus vulgaris. I. Growth and photosynthetic apparatus. Int. J. Bot. 2007, 3, 15–22. [Google Scholar] [CrossRef]
- Dang, H.K.; Li, R.Q.; Sun, Y.H.; Zhang, X.W.; Li, Y.M. Absorption, accumulation and distribution of Zinc in highly-yielding winter wheat. Agric. Sci. China 2010, 9, 965–973. [Google Scholar] [CrossRef]
- Balbontín, C.; Ayala, H.; Rubilar, J.; Cote, J.; Figueroa, C.R. Transcriptional analysis of cell wall and cuticle related genes during fruit development of two sweet cherry cultivars with contrasting levels of cracking tolerance. Chil. J. Agric. Res. 2014, 74, 162–169. [Google Scholar] [CrossRef]
- Wang, J.; Gao, X.; Ma, Z.; Chen, J.; Liu, Y. Analysis of the molecular basis of fruit cracking susceptibility in litchi chinensis cv. baitangying by transcriptome and quantitative proteome profiling. J. Plant Physiol. 2019, 234, 106–116. [Google Scholar] [CrossRef] [PubMed]




| B | Ca | Cu | Fe | K | Mg | Mn | Mo | Na | P | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1.000 | 0.749 * | 0.676 * | 0.175 | 0.474 * | 0.556 * | 0.849 ** | 0.198 | 0.799 * | 0.735 * | 0.775 * |
| Ca | 0.749 * | 1.000 | 0.299 | 0.059 | 0.472 * | 0.832 ** | 0.843 ** | 0.140 | 0.477 * | 0.752 * | 0.495 * |
| Cu | 0.676 * | 0.299 | 1.000 | 0.246 | 0.376 | 0.162 | 0.603 * | 0.074 | 0.541 * | 0.508 * | 0.756 * |
| Fe | 0.175 | 0.059 | 0.246 | 1.000 | 0.074 | 0.036 | 0.179 | −0.008 | 0.196 | 0.087 | 0.138 |
| K | 0.474 * | 0.472 * | 0.376 | 0.074 | 1.000 | 0.673 * | 0.474 * | 0.069 | 0.512 * | 0.352 | 0.145 |
| Mg | 0.556 * | 0.832 ** | 0.162 | 0.036 | 0.673 * | 1.000 | 0.710 * | 0.148 | 0.431 * | 0.619 * | 0.198 |
| Mn | 0.849 ** | 0.843 ** | 0.603 * | 0.179 | 0.474 * | 0.710 * | 1.000 | 0.182 | 0.565 * | 0.803 ** | 0.682 * |
| Mo | 0.198 | 0.140 | 0.074 | −0.008 | 0.069 | 0.148 | 0.182 | 1.000 | 0.138 | 0.170 | 0.145 |
| Na | 0.799 * | 0.477 * | 0.541 * | 0.196 | 0.512 * | 0.431 * | 0.565 * | 0.138 | 1.000 | 0.442 * | 0.554 * |
| P | 0.735 * | 0.752 * | 0.508 * | 0.087 | 0.352 | 0.619 * | 0.803 ** | 0.170 | 0.442 * | 1.000 | 0.581 * |
| Zn | 0.775 * | 0.495 * | 0.756 * | 0.138 | 0.145 | 0.198 | 0.682 * | 0.145 | 0.554 * | 0.581 * | 1.000 |
| B | Ca | Cu | Fe | K | Mg | Mn | Mo | Na | P | Zn | |
|---|---|---|---|---|---|---|---|---|---|---|---|
| B | 1.000 | 0.510 * | 0.600 * | 0.423 * | −0.023 | 0.305 | 0.618 * | 0.706 ** | 0.590 * | 0.673 * | 0.615 * |
| Ca | 0.510 * | 1.000 | 0.435 * | 0.261 | 0.191 | 0.568 * | 0.659 * | 0.334 | 0.324 | 0.558 * | 0.172 |
| Cu | 0.600 * | 0.435 * | 1.000 | 0.318 | −0.037 | 0.500 * | 0.643 * | 0.362 | 0.226 | 0.602 * | 0.332 |
| Fe | 0.423 * | 0.261 | 0.318 | 1.000 | −0.172 | 0.180 | 0.414 | 0.444 * | 0.176 | 0.367 | 0.330 |
| K | −0.023 | 0.191 | −0.037 | −.172 | 1.000 | 0.131 | −0.096 | −0.173 | 0.408 * | −0.234 | −0.293 |
| Mg | 0.305 | 0.568 * | 0.500 * | 0.180 | 0.131 | 1.000 | 0.809 ** | 0.176 | −0.072 | 0.686 * | 0.131 |
| Mn | 0.618 * | 0.659 * | 0.643 * | 0.414 * | −0.096 | 0.809 ** | 1.000 | 0.524 * | 0.139 | 0.881 ** | 0.450 * |
| Mo | 0.706 * | 0.334 | 0.362 | 0.444 * | −0.173 | 0.176 | 0.524 * | 1.000 | 0.415 * | 0.541 * | 0.540 * |
| Na | 0.590 * | 0.324 | 0.226 | 0.176 | 0.408 * | −0.072 | 0.139 | 0.415 * | 1.000 | 0.146 | 0.161 |
| P | 0.673 * | 0.558 * | 0.602 * | 0.367 | −0.234 | 0.686 * | 0.881 ** | 0.541 * | 0.146 | 1.000 | 0.422 * |
| Zn | 0.615 * | 0.172 | 0.332 | 0.330 | −0.293 | 0.131 | 0.450 * | 0.540 * | 0.161 | 0.422 * | 1.000 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lopez-Zaplana, A.; Bárzana, G.; Agudelo, A.; Carvajal, M. Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking. Agronomy 2020, 10, 1815. https://doi.org/10.3390/agronomy10111815
Lopez-Zaplana A, Bárzana G, Agudelo A, Carvajal M. Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking. Agronomy. 2020; 10(11):1815. https://doi.org/10.3390/agronomy10111815
Chicago/Turabian StyleLopez-Zaplana, Alvaro, Gloria Bárzana, Agatha Agudelo, and Micaela Carvajal. 2020. "Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking" Agronomy 10, no. 11: 1815. https://doi.org/10.3390/agronomy10111815
APA StyleLopez-Zaplana, A., Bárzana, G., Agudelo, A., & Carvajal, M. (2020). Foliar Mineral Treatments for The Reduction of Melon (Cucumis melo L.) Fruit Cracking. Agronomy, 10(11), 1815. https://doi.org/10.3390/agronomy10111815