1. Introduction
Globally, an estimated 33% of soils are degraded [
1]. In response, a global soil partnership was formed to converge both policy and research to identify and integrate pillars of action and priorities. Cover crops were identified to have considerable potential to address such priorities by both enhancing and stabilising stores of soil organic matter and reducing the use of nitrogen (N) and phosphorous (P) [
1]. There is increasing evidence that cover crops can provide weed, pest and disease control [
2,
3,
4,
5,
6] and reduce nutrient loss [
7,
8,
9]. However, harnessing the benefits from cover crops, for both soil and commercial crops, is highly dependent on species choice and management [
10] and both quantity and quality of the biomass produced [
11]. Maximising the biomass of cover crops is desirable as it has been found that their benefits to soil biology are associated with the amount of biomass produced [
12,
13]. Cover crops also offer weed suppression through smothering [
14] and allelopathy [
15] which is key to sustainable weed management. Exploiting the natural evapotranspiration of cover crops has been highlighted as an effective strategy against rainfall variability when in excess and also when limiting to plant growth: through improved storage, field water capacity and plant available water [
16,
17]. Limited research has evaluated cover crops for their potential to use water through evapotranspiration and how this varies between different species. Cover crops are also an effective way to add organic matter to soil and can help slow down the decline of carbon (C) stocks, which occurs during tillage and intensive crop production [
6,
18]. Cover crop biomass also affects overall C and N return and cycling [
19]. Maximising this could offer many benefits. However, when the cover crops are destroyed the residue that is added to the soil can introduce additional variability in nutrient supply to the commercial crop, specifically N, with a possibility of immobilisation [
20]. Nutrient mineralisation rates of residue are affected by factors including soil type, temperature, residue quality, which is species dependent, and other factors [
3,
21]. N immobilisation increases when the quantity of biomass is increased [
22]. This means cover crops can negatively affect grain yields due to low N mineralisation from the residue [
23], although species selection can overcome this to enhance N supply to the commercial crop and increase yields [
24,
25]. A legume cover crop of field beans (
Vicia faba L.) in Switzerland managed to biologically fix an estimated maximum of 172 kg N/ha whereas chickpea (
Cicer arietinum L.) fixed2 kg N/ha [
26]. Therefore, the correct legume species is crucial to can enhance soil N. Cover crop species choice is influenced by site-specific soil and climatic conditions [
7], making identification of suitable families and species critical.
Spring barley in Northern Ireland (NI) accounts for over 40% of the total cereal area of 33,500 ha. This results in a large proportion of land devoted to cereals lying fallow over-winter for nearly half the year. Other spring-sown cereals and vegetable crops also add to this fallow area [
27]. This fallow land is at greater risk of degradation compared to land with a growing crop, with the risk predominantly caused by high rainfall, and lack of ground cover to protect the soil from erosion [
28,
29]. Additionally, the animal farming sector in NI) creates a vast quantity of organic manure estimated at 10 million tonnes annually [
30]. This is a cost-effective source of nutrients essential for farming and environmental sustainability, the recycling of nutrients required for crop growth and to decrease dependence on inorganic fertiliser requirements [
31]. The Department of Agriculture, Environment and Rural Affairs (DAERA) Nitrates Action Programme (NAP) restricts the spreading of manures over winter (“closed period” is 15th October–31st January) in NI [
32]. The high capital cost required for storage, estimated at around 70–80 €/m
3 [
33], consequently means that these manures are often applied as late as possible to the “closed period” to reduce storage cost. Nitrogen (N) uptake in the subsequent crop following autumn applications is around 5–35% depending on type and rate of manure, whereas spring applications have higher efficiencies of 50–60% [
34]. Autumn applications are applied to either stubble, preceding winter crops, or grassland where uptake and storage by the crop can be poor due to limited growth. The low efficiency is primarily due to leaching caused by excess rainfall, which washes nutrients below the rhizosphere [
35]. Whilst this is a regional issue highlighted, it is not a localised problem, as Cambardella, Moorman [
36] highlighted that over one billion tonnes of N is excreted from swine manure annually in the United States [
37] and similar problems of N leaching and P loss are experienced. Cover crops have been found to reduce leaching on average by 43% and, on a clay soil, a cover crop of perennial ryegrass (
Lolium perenne L.) reduced N leaching by 85 to 89% [
7]. N immobilisation in cover crop biomass can directly reduce soil mineral N (SMN) which minimises the downward leaching of N [
38]. Therefore, cover crops offer considerable potential to reduce N leaching in a high winter rainfall climate [
39]. Studies on integrating organic manures and cover crops have shown increased biomass growth of the cover crops [
40] and a subsequent increase in cover crop N uptake [
40,
41,
42] and reduction in SMN over-winter [
36,
43]. However, these studies have shown that the selection of species that are best suited to region/climate to be critical [
43].
The integration of cover crops and slurry requires research to find responsive species in terms of not only increased N uptake but also if there is a beneficial effect on commercial crop yield and N uptake/supply. Using a mesocosm trial to help understand the complex plant–soil–slurry interaction will help facilitate identifying species that provide benefits such weed suppression, increased N supply to the commercial crop to increase grain yield. This could lead to increased cover crop use by farmers, so indirectly implementing the many benefits they provide. Thus, an array of species from a range of families should be evaluated to find best-suited species to this practice along with the effects on N cycling and commercial crop yield. It is hypothesised that: (i) legumes will lead to increased spring barley yield over non-leguminous species due to greater N cycling (defined as total N offtake in the grain and straw), and (ii) slurry integrated with cover crops will be more beneficial than when applied to the control in terms of grain yield and overall N offtake.
2. Materials and Method
A pot experiment was conducted with 16 species of cover crop and a control (no cover crop) in response to an application of pig slurry. These species were evaluated for growth, effect on weeds, water usage, N and C accumulation as well as the effect on the grain and straw yield and N content of the subsequent crop of spring barley.
2.1. Experimental Design
The experimental design was a randomised, split plot, with four replicates of 17 treatments (16 species plus the control) with and without slurry. Species are listed in the
Appendix Table A1 along with their suggested sowing rates recommended from literature and guides for Ireland and the UK [
44]. Species were chosen from those that breeders and merchants in the UK are currently recommending. Of all species in this trial, a subset of the best performing species from different families was selected for further analysis of N determination of the spring barley grain and straw plus chaff (non-stem or grain proportions of the spring barley) fractions and also SMN. This subset was decided by selecting cover crops from a range of families that produced large/sufficient amounts of biomass and led to a high spring barley grain yield.
2.2. Mesocosm Set Up
Twenty two kilograms of soil (35.8% sand, 38.6% silt and 25.8% clay), which is relatively typical of NI arable soils) at 67.8% dry matter (DM), was added to large pots measuring 50 cm tall with a diameter of 22.5 cm, giving a soil volume of 17,137 cm
−3 and a bulk density of 0.87 kg/l DM or 1.28 kg/l fresh weight. Soil nutrient analysis was conducted in accordance with standard ADAS methods [
45] (
Table 1). The mesocosms were designed to allow ample depth for sufficient root growth and the large diameter provided ample area to sow the cover crops at representative sowing rates.
One application of 180 mL of pig slurry (
Table 2), representing a field application rate of 45.2 m
3/ha (50 m
3/ha NI legislative maximum individual application), was applied to half the pots, with the other half receiving 180 mL of tap water as a control. The top 5 cm of soil in all pots were then mixed using a trowel.
The species were sown according to their recommended seed rate (
Table A1) at advised depths (based on available recommendations). To maintain a water-holding capacity (WHC) of 70%, the pots were weighed with water applied using sprinkler nozzles to simulate rainfall to bring the pots back up to the original weight. Total water usage for each species was the total weight of water applied during the 100 days of cover crop growth to maintain 70% WHC.
2.3. Greenhouse Heat and Light
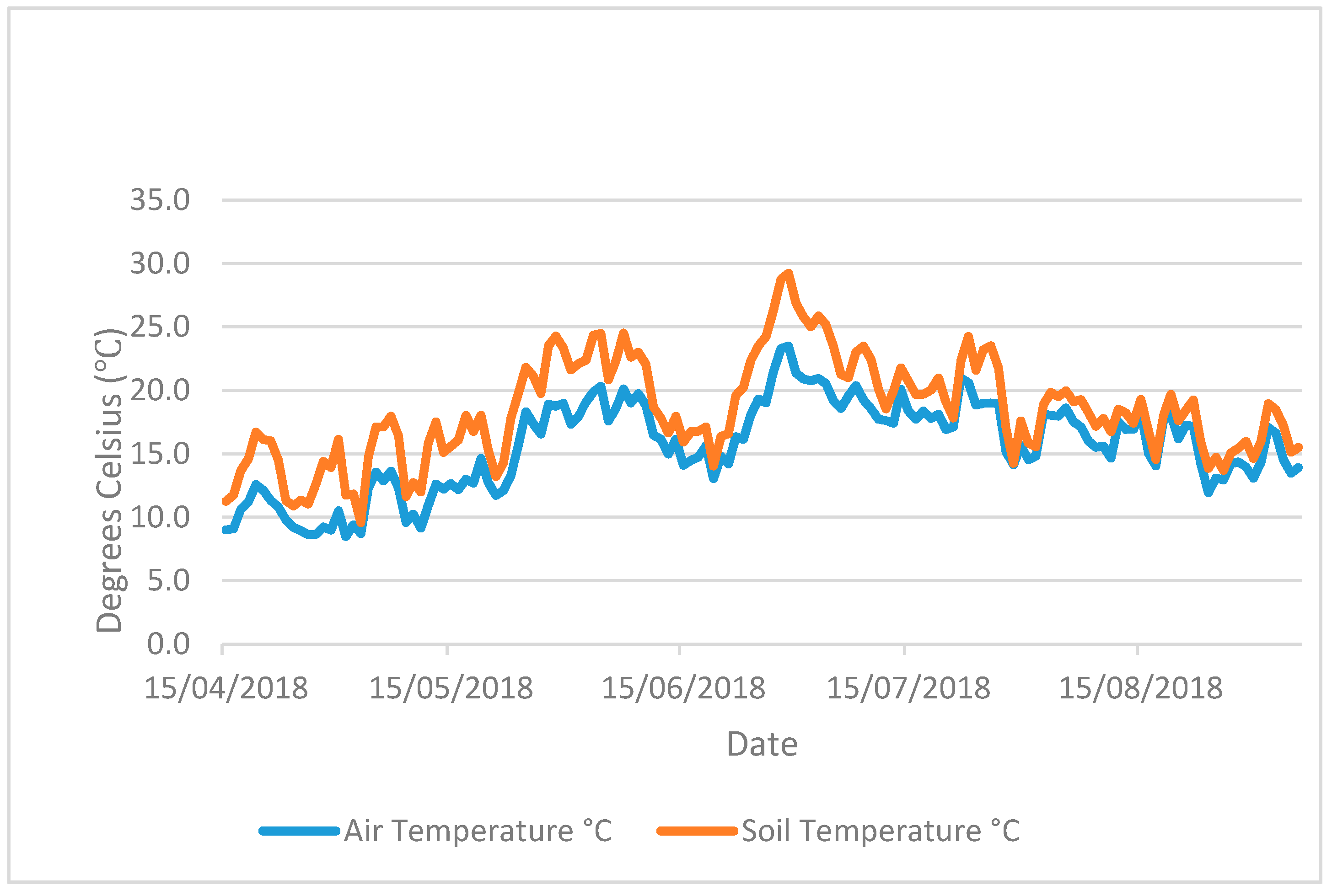
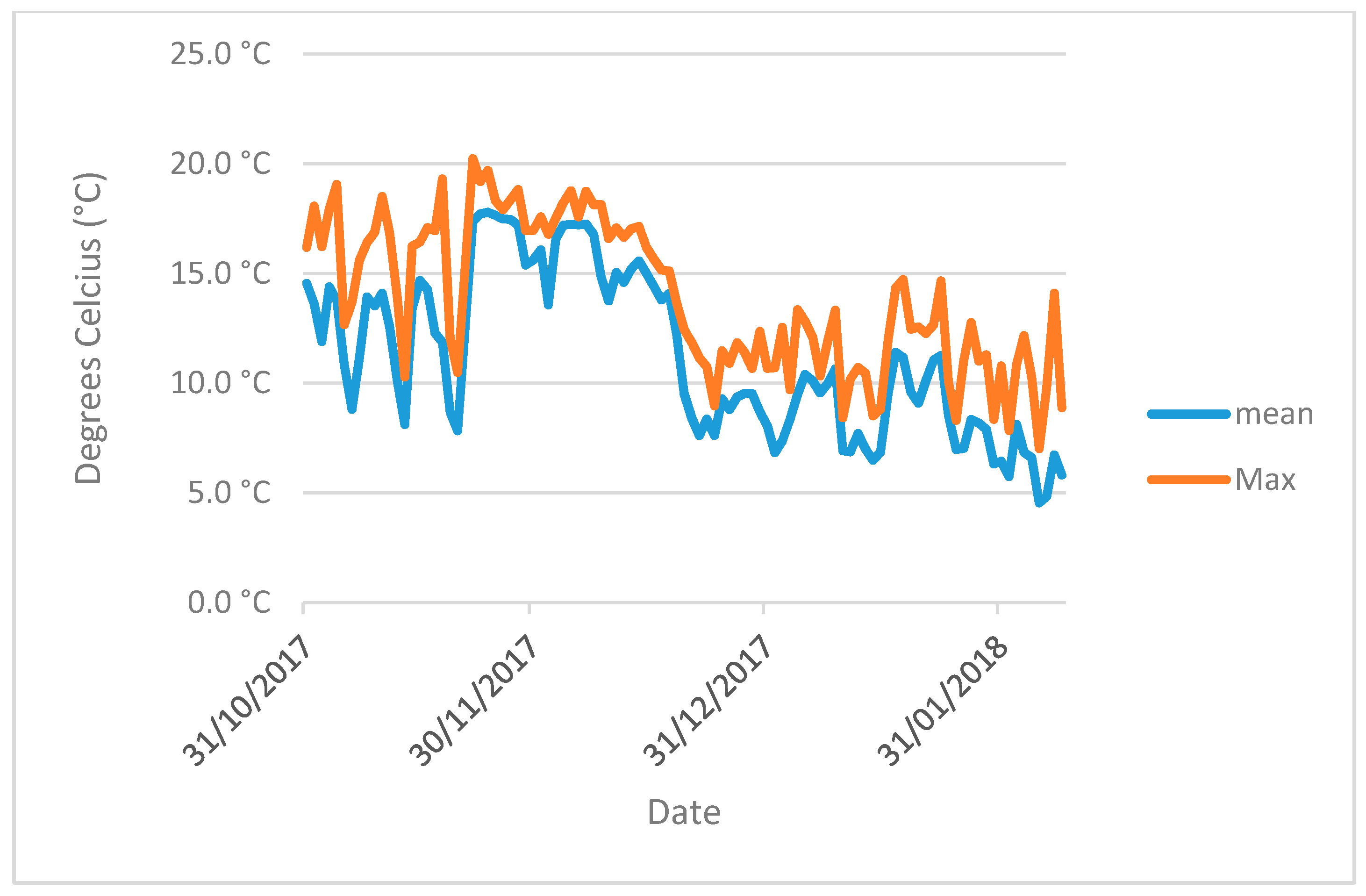
The pots were kept in glass greenhouses for 102 days from 31 October 2017 (
Figure A3). Soil and ambient air temperature (
Figure A1 and
Figure A2) were logged using a Tinytag Plus 2 TGP-4510 datalogger and a soil probe (PB-5001) measuring to a soil depth of 15 cm. Artificial lights provided an additional PAR (photosynthetically active radiation) of 175 µmol/m
−2 s
−1 above natural daylight during the hours of 4.30 pm to 8.30 am. The artificial light and heat provided were designed to represent a growing season of cover crops sown in early August and harvested/returned to soil in February–April and to prevent freezing.
2.4. Cover Crop Sampling
After 100 days of growth, the cover crops were cut at ground level and the biomass divided by hand into cover crops and weeds. The cover crop biomass was chopped to a maximum length of 3 cm, with a 50 g representative subsample removed and then dried at 60 °C for 48 h (i.e., until there was no further weight decrease). The remaining biomass was added back to its original pot and incorporated into the soil to a depth of 10 cm; the weeds were not reincorporated. The control, with its weeds, was treated as a cover crop hence it does not appear under weed biomass production in the tables/figures. Brassica cover crops had the taproots removed and weighed separately. These roots from the brassica species were not reincorporated into the soil and consequently would have removed minor amounts of N. The rationale was that brassicas produce larger root biomass, which is a considerable proportion of their overall biomass [
3] compared to other cover crop species.
2.5. C and N Determination of Cover Crop Biomass
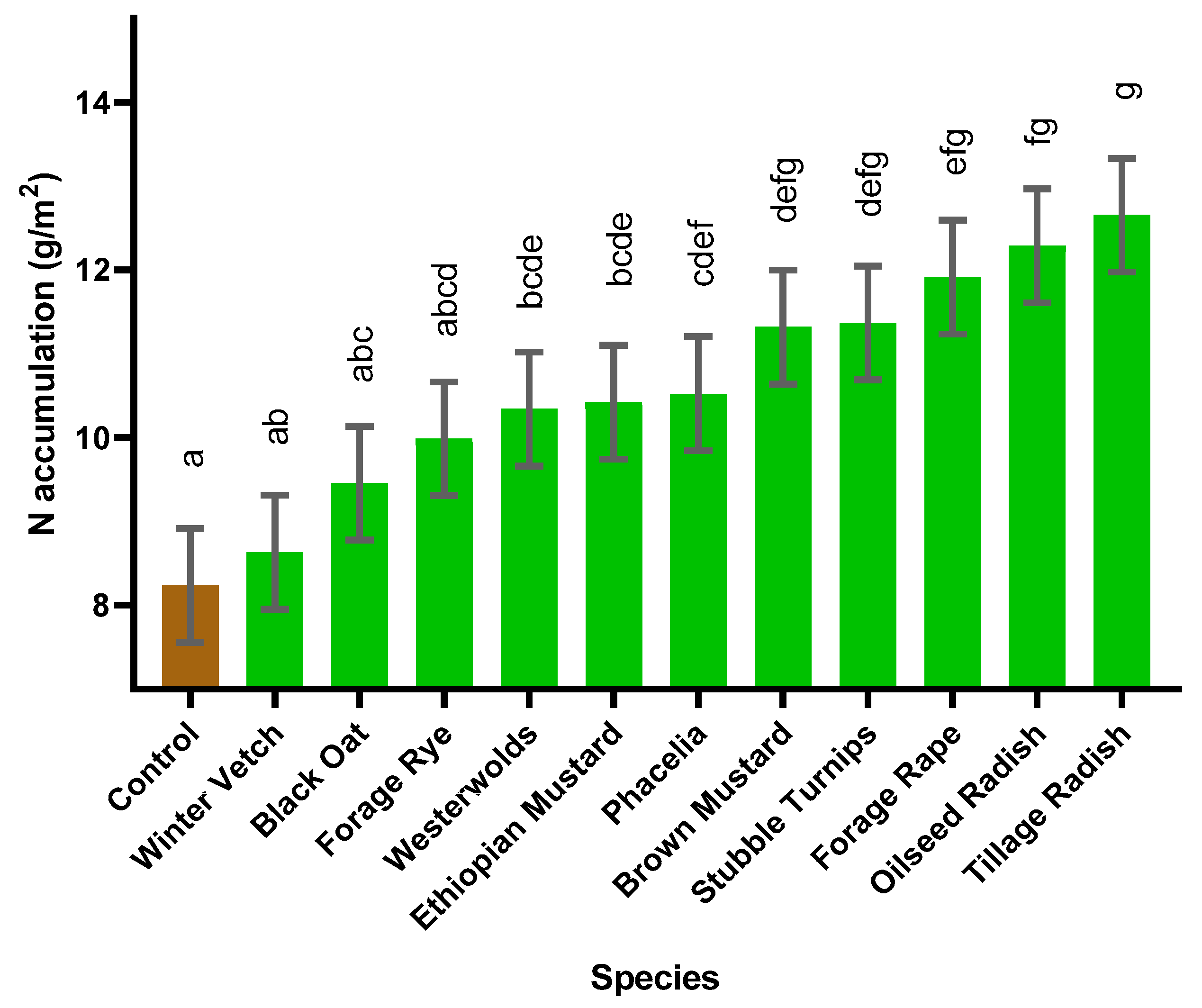
The dried biomass samples of aboveground shoots were milled to 1.0 mm by a Cyclotec 293 mill (FOSS, Warrington, Britain). N and C content were analysed using the Dumas dry combustion method with a Trumac CN analyser (Leco Corporation, St. Joseph, MI, USA) with a furnace temperature of 1350 °C. Quality controls included an in-house verified reference material run with every 20 samples. Accumulated N offtake was calculated as percentage N (% N) multiplied by cover crop biomass. Roots were not analysed for N accumulation due to many species not producing a sufficient sample for analysis after milling losses.
2.6. Energy Dispersive X-ray Fluorescence (EDXRF) Analysis
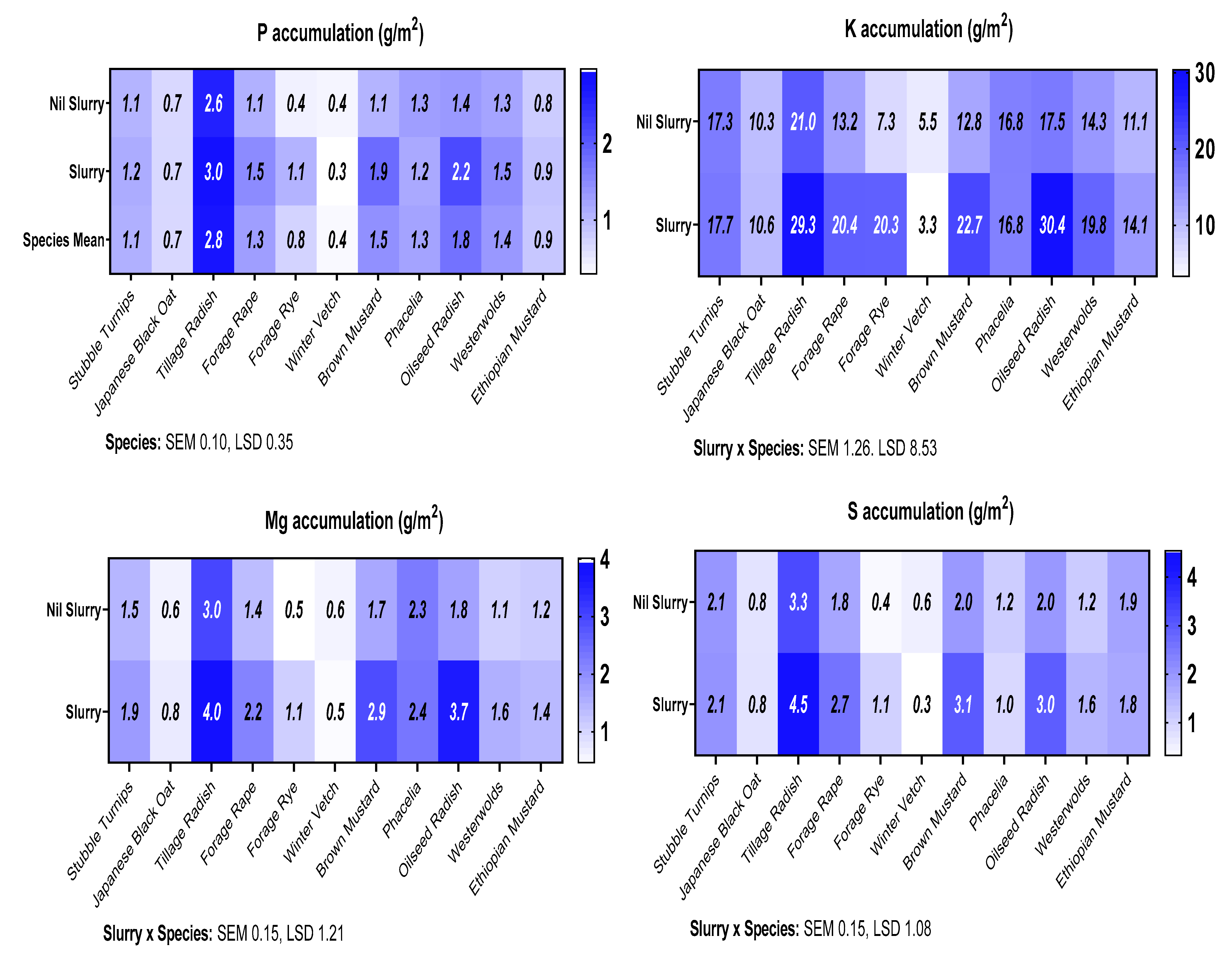
EDXRF was used to measure a broad-spectrum nutrient profile in a select list of the best performing cover crops. Quantities of 2.5–3.5 g (species depending) of milled biomass samples were loaded into sample-cups to a depth >4 mm. To create a pellet, 300 pounds per square inch (PSI) was applied for 20 s. A suitable certified reference material (mixed Polish herbs INCT-MPH-2) was used in each batch of sample allowing recoveries to be detected and coefficient of variation (CV) to be gauged. Only recoveries of 100 ± 20% with a maximum CV of 10% were used as parameters to accept the specific nutrients from the profile measured (
Table 3.). Nutrient uptakes were calculated by multiplying cover crop biomass by production by its relative concentration. The nutrient accumulation of the weeds was not added onto the results shown.
2.7. Sowing of Spring Barley
The pots were moved out of the greenhouse on 16th February 2018 and left outside thereafter. They were sown on 15 April 2018 with spring barley cv. KWS Irina, a widely-used variety in the UK (
Figure A4). Spring barley was planted at 2.5 cm soil depth at 15 seeds/pot (equivalent to 375 seeds/m
2 assuming an establishment rate of 99%, and a thousand grain weight (TGW) of 44 g). A spray programme (
Table A2) was used to keep weeds, aphids and disease to a minimum, with no additional inorganic fertiliser applied.
2.8. Spring Barley Harvest and N Offtake
The spring barley was harvested when ripe by cutting stems at 2–3 cm above the soil surface. Samples were dried at 60 °C for 48 h, then weighed. Ears were counted and separated from the straw and a Wintersteiger LD350 thresher was used to separate the grain from the chaff. The straw and chaff fractions were bulked together, and all samples were re-dried at 60 °C for 48 h and reweighed. Samples, grain and non-grain (straw + chaff), were milled to 1mm using a Foss Clyclotec CT293 mill and analysed for % N (using the same method described in 2.5 above). N uptake by the spring barley was calculated through multiplying dried grain yield by its relative % N and by adding the dried straw + chaff weight multiplied by its relative % N.
2.9. Soil Mineral Nitrogen (SMN)
Ten days post-harvest (13 September 2018), 60 g of fresh soil was sampled on three replicates of each treatment with a soil corer to a depth of 30 cm. A total of 40 g of soil were incubated at 20 °C for 24 h, sieved to 2 mm and extracted with 80 mL of 2M KCl. The mix of soil and KCl was shaken in an orbital shaker for 1 h at 200 RPM, then centrifuged at 2970× g for 4 min and the liquid fraction filtered through No. 40 Whatman filter paper. Two “blanks” were run with the extractions to determine and adjust for any contamination. Extracts were analysed using a calorimetric Skalar San Plus Auto Analyser (Skalar Analytical B.V., Breda, Netherlands) based on the cadmium reduction method for nitrate and nitrite and the Berthelot reaction for ammonia. Banked samples of soil taken prior to planting the cover crop were frozen and stored at −18 °C. Three subsamples were defrosted in a fridge for 16 h and analysed for SMN as described above. The result was quantified through multiplying the concentrations of nitrate, nitrite and ammonium by the relative dry weight of the soil in the pots then transformed to g/m2. SMN, prior to planting the cover crops, is estimated to be 6.46 g/m2.
2.10. Total Detectable N
Evaluating spring barley N uptake combined with SMN post-harvest allows between species evaluation to identify if the legumes fixed additional N and if the cover crop residue either mineralised or immobilised nitrogen. The SMN g/m
2 was calculated by multiplying the total nitrate + nitrite + ammonium by the weight of the soil in the 30 cm profile sampled using the dry bulk density. Total detectable N was assumed to be the sum of the spring barley crop N added to SMN (30 cm soil depth) to provide an uptake per pot, which was then converted to g/m
2. This is shown in Equation (1).
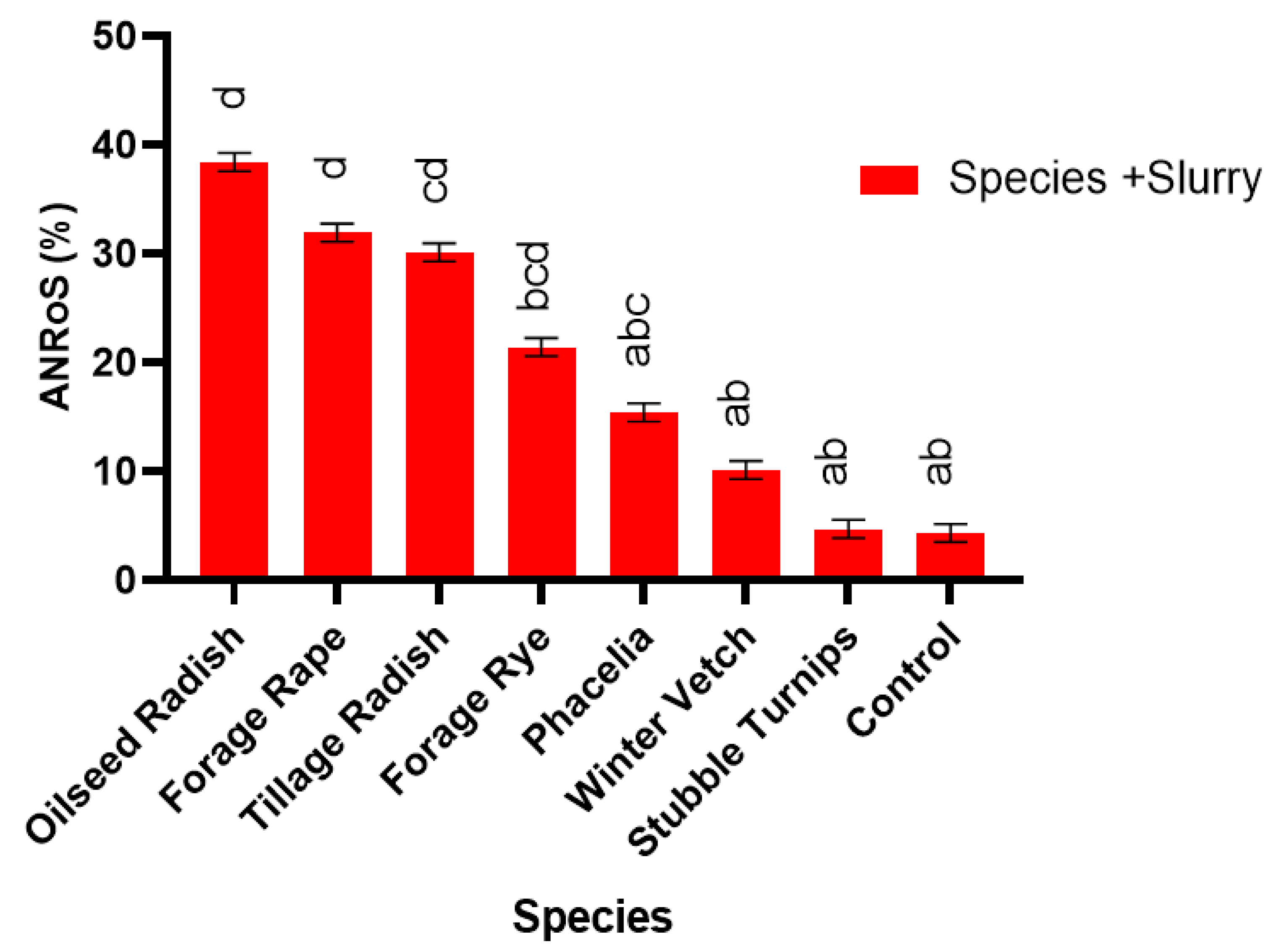
2.11. Apparent N Recovery
Total apparent N recovery (TANR) of the N applied was calculated in Equation (2) as described by Liu et al. [
46]. The apparent N recovery in response to the treatment of slurry was calculated in Equation (3) as described by Cavalli et al. [
47], for each species. This is denoted by ANRoS
. Both equations assume slurry to have an availability of 50 % as directed by the AHDB RB209 [
34] and the Nitrates Action Programme [
32].
Total N applied = Initial SMN + N applied in slurry.
2.12. Statistical Analysis
Two-way analysis of variance (ANOVA) was used to identify significant differences between treatments in the response variables of crop growth, water usage, weed production and all aspects of spring barley yield, using Genstat (V18) [
48]. Restricted maximum likelihood (REML) was applied to analyse the N accumulation due to the unequal number of observations. Fisher’s post-hoc analysis was applied to discriminate differences between means. Results are deemed significantly different at
p < 0.05 and tendencies are regarded as less than 10% (
p = 0.10).
5. Conclusions
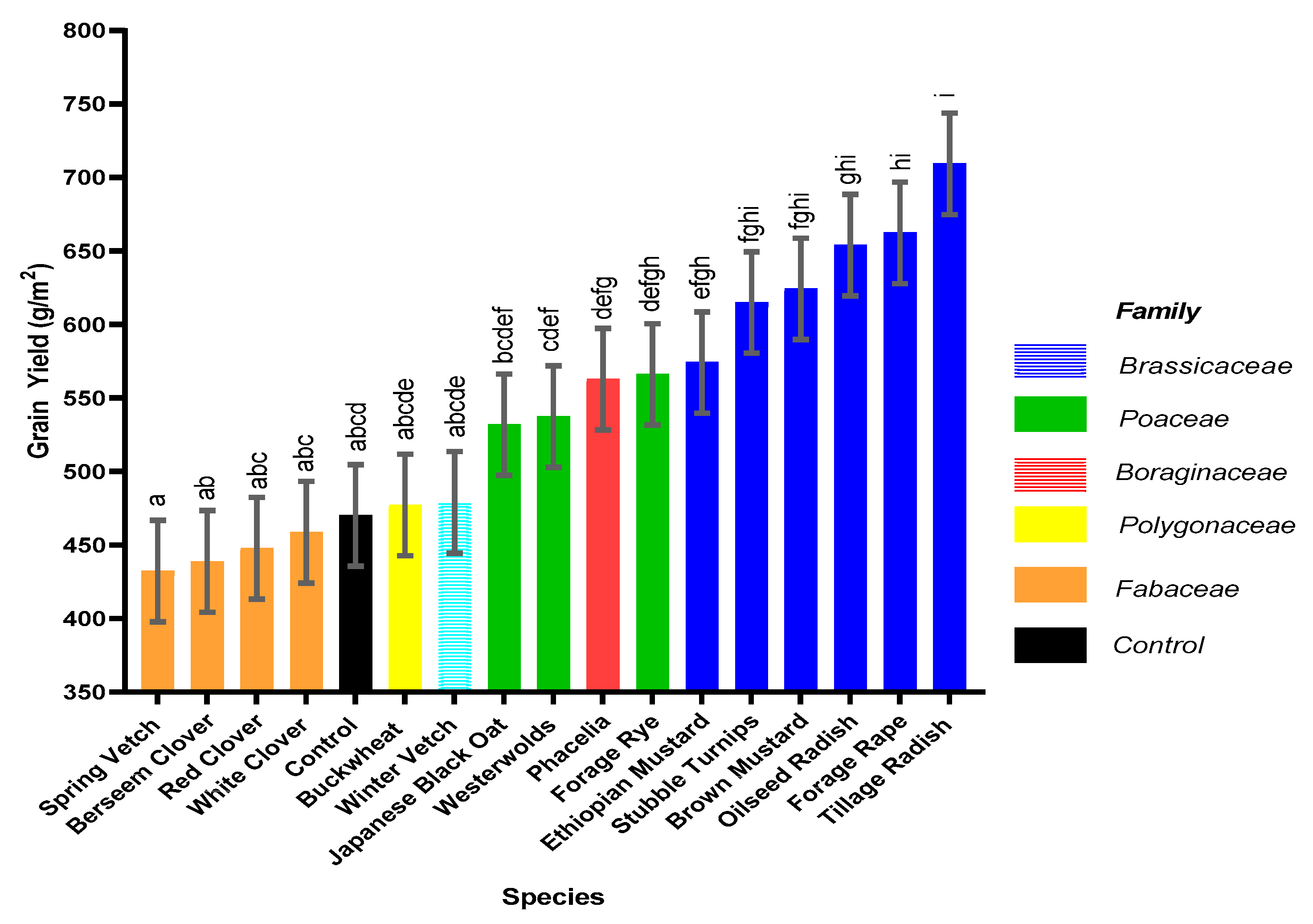
This pot experiment shows that replacing fallow land with cover crops offers a multitude of benefits for sustainable development when the best species are used. Benefits include weed suppression, increased water usage and addition of organic matter to the soil, increased N accumulation in the cover crop and increased N uptake in the subsequent spring barley crop with the potential to increase commercial crop yields. Furthermore, certain cover crop species, forage rye, brown mustard, oilseed radish and tillage radish, can respond synergistically to slurry addition, which acts as an effective sink for N. Thus, N could be sequestered during high risk leaching periods and be released to the growing crop through production of a higher quality residue (lower C:N ratio). In addition, greater mineralisation could occur when the cover crop is incorporated, thus increasing commercial crop yield, in comparison to leaving ground fallow. An increased grain yield was observed following Ethiopian mustard, stubble turnips, brown mustard, oilseed radish, forage rape and tillage radish, in comparison to the control.
This study demonstrates for the first time that applications of slurry to selected species of responsive cover crops can simultaneously produce a greater cover crop biomass of increased quality that is more beneficial for both spring barley yield and environmental benefits compared to applications of organic manures to fallow land, fostering sustainable intensification.