Method of Silicon Application Affects Quality of Strawberry Daughter Plants during Cutting Propagation in Hydroponic Substrate System
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Silicon Treatments
2.2. Measurements of the Growth Parameters
2.3. Determination of the Si Content
2.4. High Temperature Stress Treatment
2.5. Chlorophyll Fluorescence Parameters
2.6. Activities of Antioxidant Enzymes
2.7. Statistical Analysis
3. Results
3.1. Growth and Development of Strawberry Daughter Plants as Affected by Si
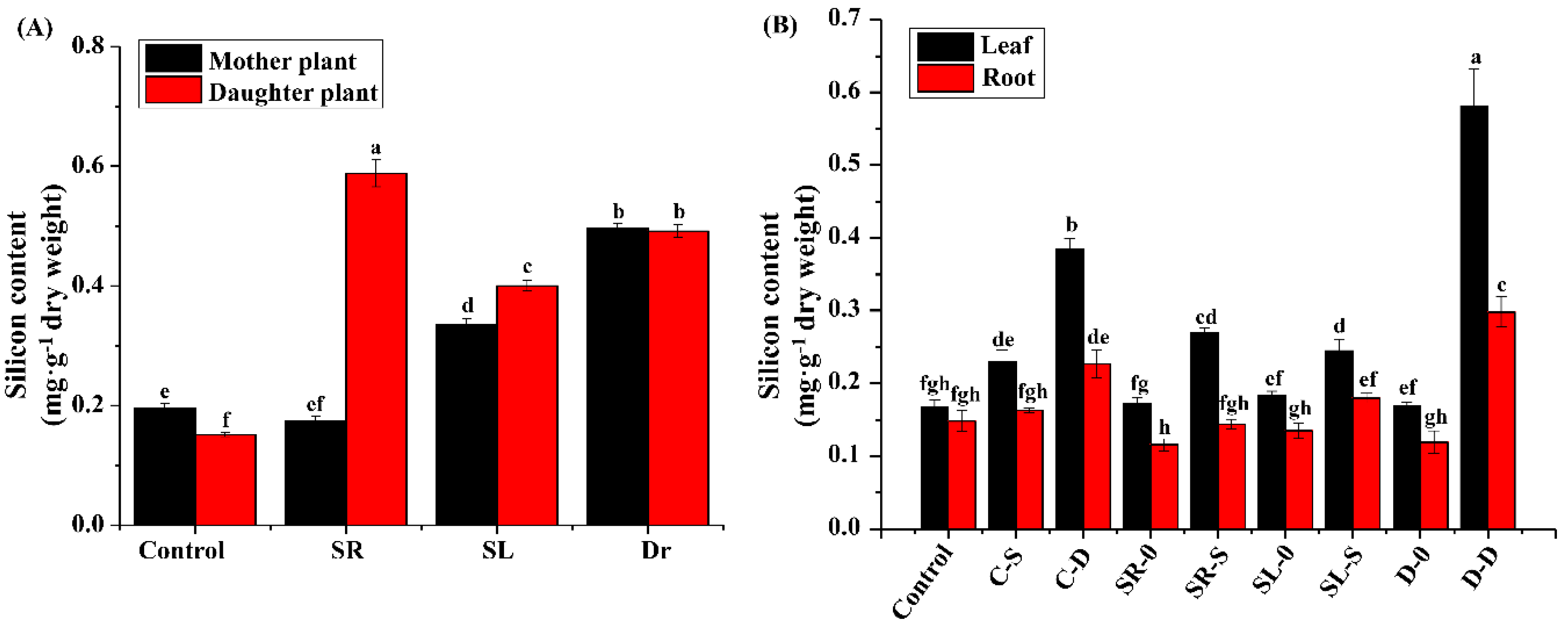
3.2. The Si Content in Strawberry
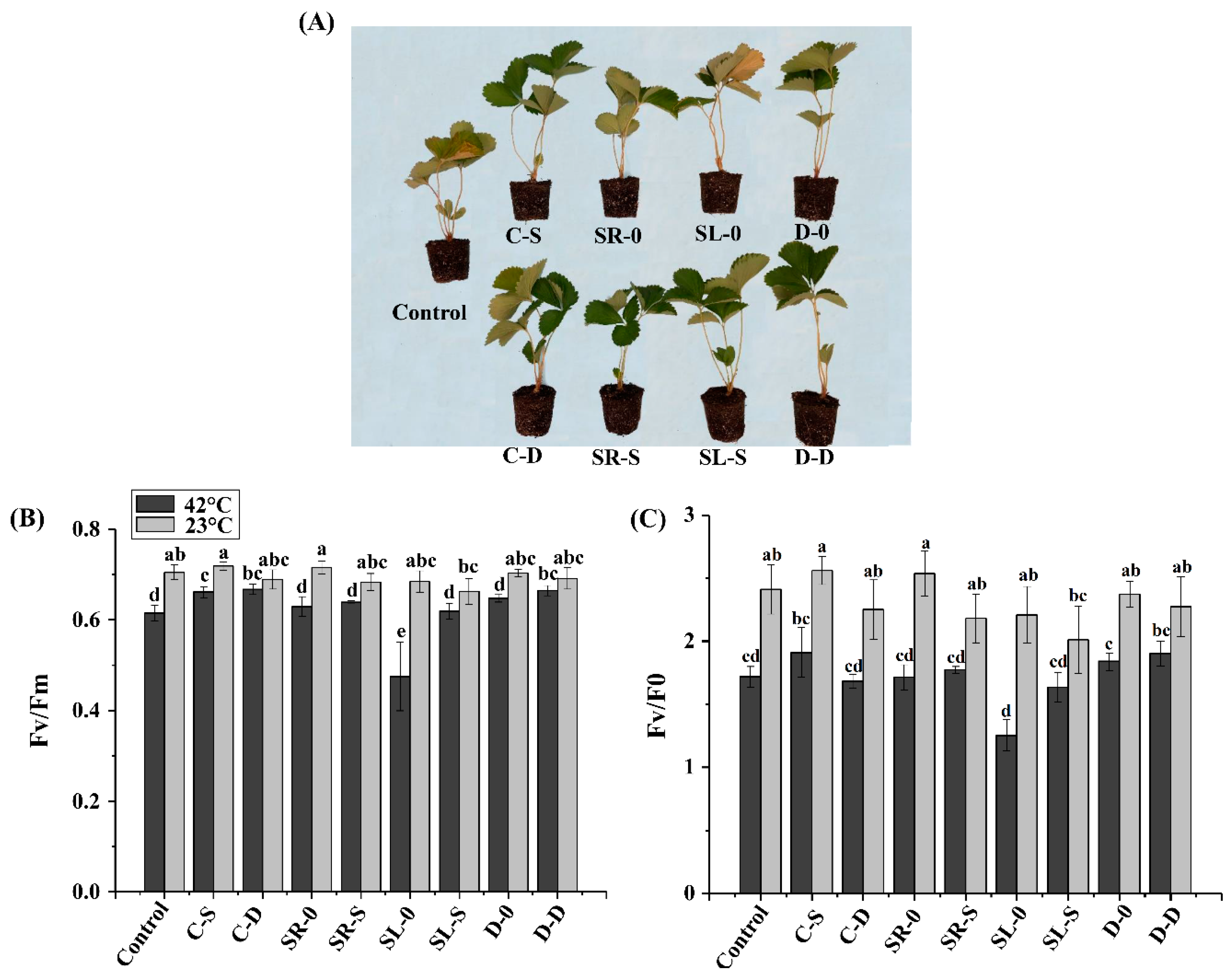
3.3. Change of the Chlorophyll Fluorescence Parameters
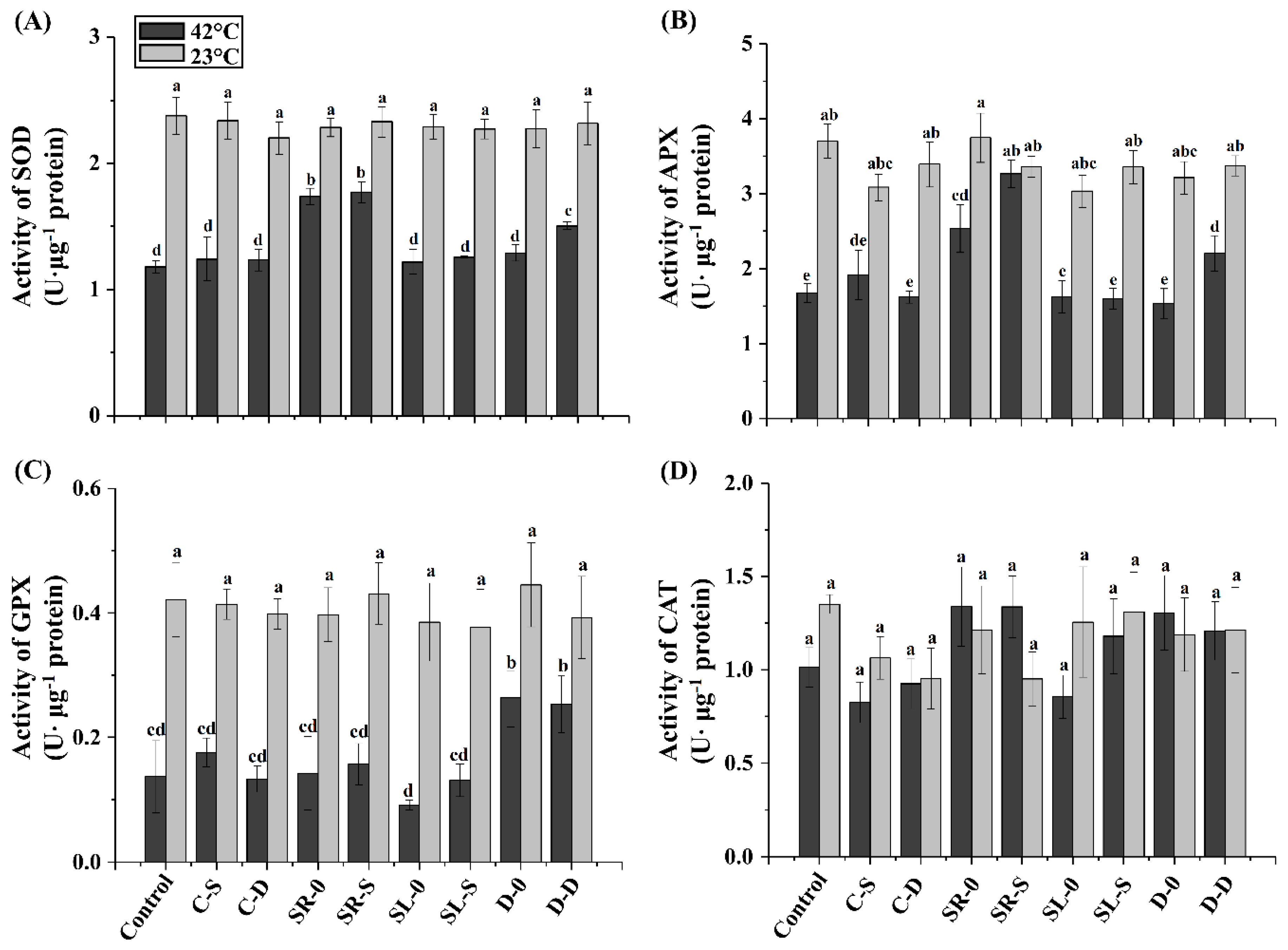
3.4. Antioxidant Enzyme Activities
3.5. Correlation Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Folta, K.M.; Gardiner, S.E. Strawberry. In Genetics and genomics of Rosaceae; Springer: New York, NY, USA, 2009; Volume 6, pp. 413–506. [Google Scholar]
- Caruana, J.C.; Sittmann, J.W.; Wang, W.; Liu, Z. Suppressor of runnerless encodes a DELLA protein that controls runner formation for asexual reproduction in strawberry. Mol. Plant 2018, 11, 230–233. [Google Scholar] [CrossRef] [PubMed]
- Muneer, S.; Park, Y.G.; Kim, S.; Jeong, B.R. Foliar or subirrigation silicon supply mitigates high temperature stress in strawberry by maintaining photosynthetic and stress-responsive proteins. J. Plant Growth Regul. 2017, 36, 836–845. [Google Scholar] [CrossRef]
- Mathur, S.; Agrawal, D.; Jajoo, A. Photosynthesis: Response to high temperature stress. J. Photochem. Photobiol. B 2014, 137, 116–126. [Google Scholar] [CrossRef] [PubMed]
- Ledesma, N.; Nakata, M.; Sugiyama, N. Effect of high temperature stress on the reproductive growth of strawberry cvs.‘Nyoho’and ‘Toyonoka’. Sci. Hortic. 2008, 116, 186–193. [Google Scholar] [CrossRef]
- Park, Y.G.; Muneer, S.; Kim, S.; Hwang, S.J.; Jeong, B.R. Silicon application during vegetative propagation affects photosynthetic protein expression in strawberry. Hortic. Environ. Technol. 2018, 59, 167–177. [Google Scholar] [CrossRef]
- Datnoff, L.E.; Rodrigues, F.A. History of silicon and plant disease. In Silicon and Plant Diseases; Springer: Cham, Switzerland, 2015; pp. 1–5. [Google Scholar]
- Coskun, D.; Britto, D.T.; Huynh, W.Q.; Kronzucker, H.J. The role of silicon in higher plants under salinity and drought stress. Front. Plant Sci. 2016, 7, 1072. [Google Scholar] [CrossRef]
- Ma, J.F.; Yamaji, N.; Mitani, N.; Tamai, K.; Konishi, S.; Fujiwara, T.; Katsuhara, M.; Yano, M. An efflux transporter of silicon in rice. Nature 2007, 448, 209. [Google Scholar] [CrossRef]
- Yamaji, N.; Ma, J.F. Further characterization of a rice silicon efflux transporter, Lsi2. Soil Sci. Plant Nutr. 2011, 57, 259–264. [Google Scholar] [CrossRef]
- Ali, S.; Farooq, M.A.; Yasmeen, T.; Hussain, S.; Arif, M.S.; Abbas, F.; Bharwana, S.A.; Zhang, G. The influence of silicon on barley growth, photosynthesis and ultra-structure under chromium stress. Ecotoxicol. Environ. Saf. 2013, 89, 66–72. [Google Scholar] [CrossRef]
- Montpetit, J.; Vivancos, J.; Mitani-Ueno, N.; Yamaji, N.; Rémus-Borel, W.; Belzile, F.; Ma, J.F.; Bélanger, R.R. Cloning, functional characterization and heterologous expression of TaLsi1, a wheat silicon transporter gene. Plant Mol. Biol. 2012, 79, 35–46. [Google Scholar] [CrossRef]
- Deshmukh, R.K.; Vivancos, J.; Guérin, V.; Sonah, H.; Labbé, C.; Belzile, F.; Bélanger, R.R. Identification and functional characterization of silicon transporters in soybean using comparative genomics of major intrinsic proteins in Arabidopsis and rice. Plant Mol. Biol. 2013, 83, 303–315. [Google Scholar] [CrossRef] [PubMed]
- Miyake, Y.; Takahashi, E. Effect of silicon on the growth and fruit production of strawberry plants in a solution culture. Soil Sci. Plant Nutr. 1986, 32, 321–326. [Google Scholar] [CrossRef]
- Hajiboland, R.; Moradtalab, N.; Eshaghi, Z.; Feizy, J. Effect of silicon supplementation on growth and metabolism of strawberry plants at three developmental stages. N. Z. J. Crop Hortic. 2018, 46, 144–161. [Google Scholar] [CrossRef]
- Peris-Felipo, F.J.; Benavent-Gil, Y.; Hernández-Apaolaza, L. Silicon beneficial effects on yield, fruit quality and shelf-life of strawberries grown in different culture substrates under different iron status. Plant Physiol. Biochem. 2020, 152, 23–31. [Google Scholar] [CrossRef] [PubMed]
- Servet, A.; Esitkin, A. Effects of silicon to salt stress on strawberry plant. Tarım Bilimleri Dergisi 2018, 22, 478–483. [Google Scholar]
- Park, Y.G.; Muneer, S.; Kim, S.; Hwang, S.J.; Jeong, B.R. Foliar or subirrigational silicon supply modulates salt stress in strawberry during vegetative propagation. Hortic. Environ. Biotechnol. 2018, 59, 11–18. [Google Scholar] [CrossRef]
- Dehghanipoodeh, S.; Ghobadi, C.; Baninasab, B.; Gheysari, M.; Shiranibidabadi, S. Effect of silicon on growth and development of strawberry under water deficit conditions. Hortic. Plant J. 2018, 4, 226–232. [Google Scholar] [CrossRef]
- Moradtalab, N.; Hajiboland, R.; Aliasgharzad, N.; Hartmann, T.E.; Neumann, G. Silicon and the association with an arbuscular-mycorrhizal fungus (Rhizophagus clarus) mitigate the adverse effects of drought stress on strawberry. Agronomy 2019, 9, 41. [Google Scholar] [CrossRef]
- Kanto, T.; Maekawa, K.; Aino, M. Suppression of conidial germination and appressorial formation by silicate treatment in powdery mildew of strawberry. J. Gen. Plant Pathol. 2007, 73, 1–7. [Google Scholar] [CrossRef]
- Fatema, K. The Effect of Silicon on Strawberry Plants and Its Role in Reducing Infection by Podosphaera aphanis. Ph.D. Thesis, University of Hertfordshire, Hatfield, UK, 2014. [Google Scholar]
- Sun, Y.P.; Hall, A.M.; Huang, Y.J.; Liu, B. Silicon location in strawberry plants to improve resistance to powdery mildew (Podosphaera aphanis). In Proceedings of the VIII International Strawberry Symposium, Québec City, QC, Canada, 13–17 August 2016; Volume 1156, pp. 821–826. [Google Scholar]
- Ouellette, S.; Goyette, M.-H.; Labbé, C.; Laur, J.; Gaudreau, L.; Gosselin, A.; Dorais, M.; Deshmukh, R.K.; Bélanger, R.R. Silicon transporters and effects of silicon amendments in strawberry under high tunnel and field conditions. Front. Plant Sci. 2017, 8, 949. [Google Scholar] [CrossRef]
- Nazarideljou, M.J.; Haghshenas, M.; Jaberian Hamedan, H.; Ferrante, A. Growth, yield and antioxidant capacity of strawberry under various K+: Ca++ ratios in hydroponic culture. Acta Agr. Scand. B-S P 2019, 69, 105–113. [Google Scholar] [CrossRef]
- Caruso, G.; Villari, G.; Melchionna, G.; Conti, S. Effects of cultural cycles and nutrient solutions on plant growth, yield and fruit quality of alpine strawberry (Fragaria vesca L.) grown in hydroponics. Sci. Hortic. 2011, 129, 479–485. [Google Scholar] [CrossRef]
- Yoon, H.-S.; Hwang, Y.-H.; An, C.-K.; Hwang, H.-J.; Rho, C.-W. Growth and fruit yield of strawberry grown in raised bed culture using growing media with lower cost. Hortic. Sci. Technol. 2004, 22, 266–269. [Google Scholar]
- Raja, W.H.; Kumawat, K.; Sharma, O.; Sharma, A.; Mir, J.; Nabi, S.U.; Lal, S.; Qureshi, I. Effect of different substrates on growth and quality of Strawberry cv. chandler in soilless culture. Pharma Innov. J. 2018, 7, 449–453. [Google Scholar]
- Zekki, H.; Gauthier, L.; Gosselin, A. Growth, productivity, and mineral composition of hydroponically cultivated greenhouse tomatoes, with or without nutrient solution recycling. J. Am. Soc. Hortic. Sci. 1996, 121, 1082–1088. [Google Scholar] [CrossRef]
- Liu, C.; Guo, Z.; Park, Y.G.; Wei, H.; Jeong, B.R. PGR and Its application method affect number and length of runners produced in ‘Maehyang’and ‘Sulhyang’strawberries. Agronomy 2019, 9, 59. [Google Scholar] [CrossRef]
- Bakhat, H.F.; Bibi, N.; Zia, Z.; Abbas, S.; Hammad, H.M.; Fahad, S.; Ashraf, M.R.; Shah, G.M.; Rabbani, F.; Saeed, S. Silicon mitigates biotic stresses in crop plants: A review. Crop Prot. 2018, 104, 21–34. [Google Scholar] [CrossRef]
- Zhang, H.; Dotson, P. The use of microwave muffle furnace for dry ashing plant tissue samples. Commun. Soil Sci. Plan. 1994, 25, 1321–1327. [Google Scholar] [CrossRef]
- Manivannan, A.; Soundararajan, P.; Arum, L.S.; Ko, C.H.; Muneer, S.; Jeong, B.R. Silicon-mediated enhancement of physiological and biochemical characteristics of Zinnia elegans ‘Dreamland Yellow’grown under salinity stress. Hortic. Environ. Biotechnol. 2015, 56, 721–731. [Google Scholar] [CrossRef]
- Mattson, N.S.; Leatherwood, W.R. Potassium silicate drenches increase leaf silicon content and affect morphological traits of several floriculture crops grown in a peat-based substrate. HortScience 2010, 45, 43–47. [Google Scholar] [CrossRef]
- Doğramaci, M.; Arthurs, S.P.; Chen, J.; Osborne, L. Silicon applications have minimal effects on Scirtothrips dorsalis (Thysanoptera: Thripidae) populations on pepper plant, Capsicum annum L. Fla. Entomol. 2013, 96, 48–54. [Google Scholar] [CrossRef]
- Guével, M.-H.; Menzies, J.G.; Bélanger, R.R. Effect of root and foliar applications of soluble silicon on powdery mildew control and growth of wheat plants. Eur. J. Plant Pathol. 2007, 119, 429–436. [Google Scholar] [CrossRef]
- Ma, J.; Nishimura, K.; Takahashi, E. Effect of silicon on the growth of rice plant at different growth stages. Soil Sci. Plant Nutr. 1989, 35, 347–356. [Google Scholar] [CrossRef]
- Lavinsky, A.O.; Detmann, K.C.; Reis, J.V.; Ávila, R.T.; Sanglard, M.L.; Pereira, L.F.; Sanglard, L.M.; Rodrigues, F.A.; Araújo, W.L.; DaMatta, F.M. Silicon improves rice grain yield and photosynthesis specifically when supplied during the reproductive growth stage. J. Plant Physiol. 2016, 206, 125–132. [Google Scholar] [CrossRef]
- Yan, G.-C.; Nikolic, M.; Ye, M.-J.; Xiao, Z.-X.; Liang, Y.-C. Silicon acquisition and accumulation in plant and its significance for agriculture. J. Integr. Agric. 2018, 17, 2138–2150. [Google Scholar] [CrossRef]
- Ma, J.F.; Takahashi, E. Silicon uptake and accumulation in plants. In Soil, Fertilizer, and Plant Silicon Research in Japan; Elsevier: Amsterdam, The Netherlands, 2002; pp. 73–106. [Google Scholar]
- Zhu, Y.-X.; Gong, H.-J.; Yin, J.-L. Role of silicon in mediating salt tolerance in plants: A review. Plants 2019, 8, 147. [Google Scholar] [CrossRef]
- Chen, W.; Yao, X.; Cai, K.; Chen, J. Silicon alleviates drought stress of rice plants by improving plant water status, photosynthesis and mineral nutrient absorption. Biol. Trace Elem. Res. 2011, 142, 67–76. [Google Scholar] [CrossRef]
- Fracheboud, Y.; Leipner, J. The application of chlorophyll fluorescence to study light, temperature, and drought stress. In Practical Applications of Chlorophyll Fluorescence in Plant Biology; Springer: Boston, MA, USA, 2003; pp. 125–150. [Google Scholar]
- Sinsawat, V.; Leipner, J.; Stamp, P.; Fracheboud, Y. Effect of heat stress on the photosynthetic apparatus in maize (Zea mays L.) grown at control or high temperature. Environ. Exp. Bot. 2004, 52, 123–129. [Google Scholar] [CrossRef]
- Kadir, S.; Sidhu, G.; Al-Khatib, K. Strawberry (Fragaria× ananassa Duch.) growth and productivity as affected by temperature. Hortscience 2006, 41, 1423–1430. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Prasad, P.V.; Seppanen, M. Selenium protects sorghum leaves from oxidative damage under high temperature stress by enhancing antioxidant defense system. Plant Physiol. Biochnol. 2010, 48, 999–1007. [Google Scholar] [CrossRef]
- Djanaguiraman, M.; Boyle, D.; Welti, R.; Jagadish, S.; Prasad, P. Decreased photosynthetic rate under high temperature in wheat is due to lipid desaturation, oxidation, acylation, and damage of organelles. BMC Plant Biol. 2018, 18, 55. [Google Scholar] [CrossRef] [PubMed]
- Ding, X.; Jiang, Y.; Hao, T.; Jin, H.; Zhang, H.; He, L.; Zhou, Q.; Huang, D.; Hui, D.; Yu, J. Effects of heat shock on photosynthetic properties, antioxidant enzyme activity, and downy mildew of cucumber (Cucumis sativus L.). PLoS ONE 2016, 11, e0152429. [Google Scholar] [CrossRef] [PubMed]
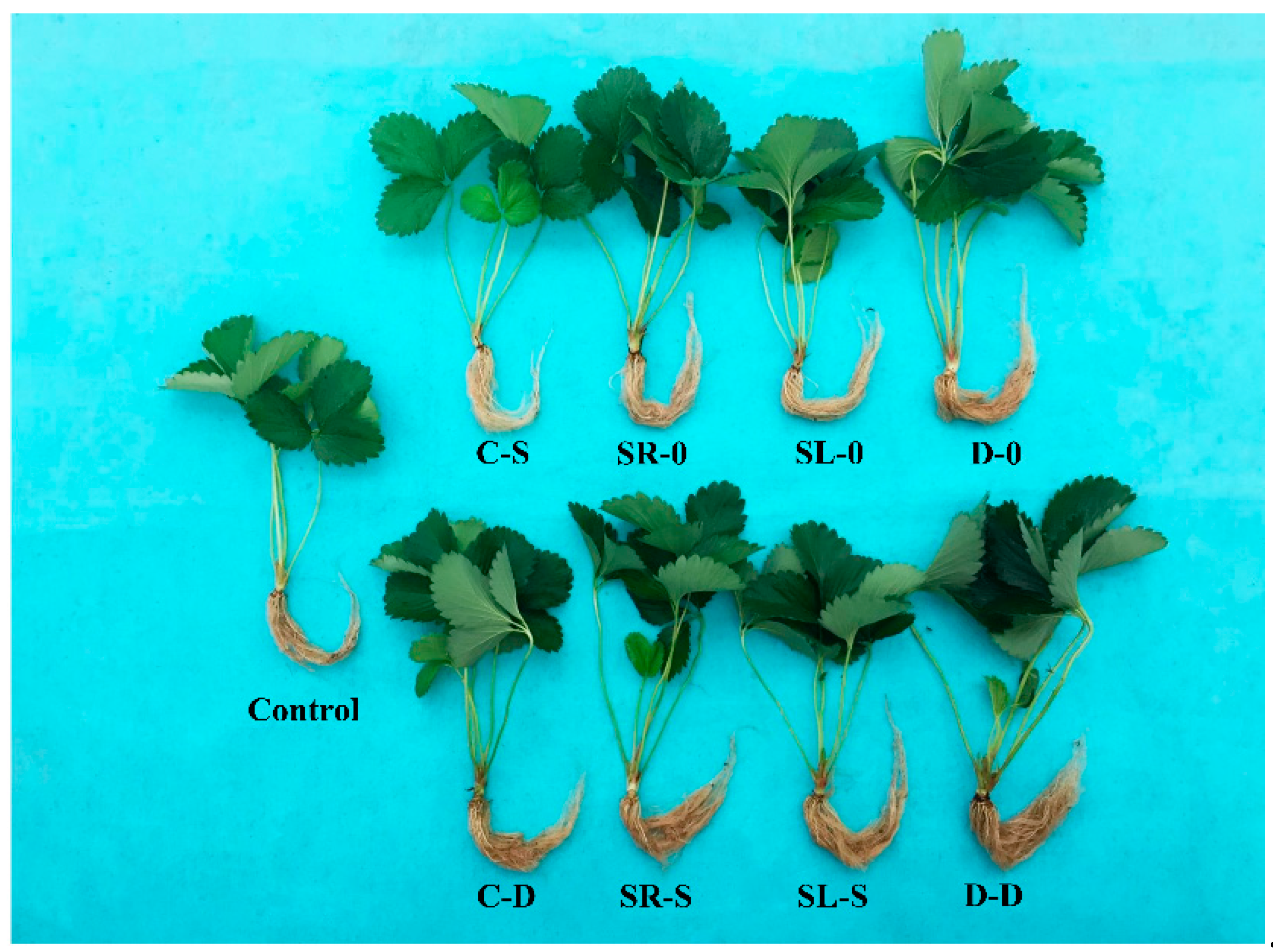
| Applications before Cutting (A) | Applications after Cutting (B) | Plant Height (cm) | Petiole | Leaf | Shoot | Root | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Length (cm) | Diameter (mm) | Length (cm) | Width (cm) | Fresh Weight (g) | Dry Weight (g) | Fresh Weight (g) | Dry Weight (g) | length (cm) | |||
| Control | 0 | 30.98 bc z | 20.83 ab | 3.31 c | 9.32 cd | 7.67 bc | 14.69 f | 2.61 d | 4.23 d | 0.37 d | 24.98 a |
| S | 30.02 c | 19.12 b | 3.41 bc | 9.65 bcd | 8.13 b | 14.69 f | 3.39 c | 5.31 bc | 0.56 bc | 25.11 a | |
| D | 34.00 ab | 22.17 ab | 3.67 ab | 10.18 bc | 8.33 b | 23.15 b | 4.29 b | 6.71 ab | 0.69 ab | 25.10 a | |
| SR | 0 | 32.77 abc | 22.55 a | 3.30 c | 9.13 d | 7.35 c | 16.64 ef | 3.38 c | 5.25 bc | 0.52 bc | 25.98 a |
| S | 34.07 ab | 22.70 a | 3.41 bc | 9.68 bcd | 7.82 b | 20.88 bc | 4.40 b | 6.25 b | 0.79 a | 25.85 a | |
| SL | 0 | 33.32 abc | 22.02 ab | 3.30 c | 9.88 bcd | 8.02 b | 19.81 cd | 3.49 c | 5.34 bc | 0.55 bc | 25.07 a |
| S | 34.58 ab | 22.70 a | 3.58 bc | 10.33 b | 8.12 b | 19.57 cd | 3.76 bc | 6.84 ab | 0.65 ab | 26.97 a | |
| Dr | 0 | 32.78 abc | 21.13 ab | 3.31 c | 10.07 bc | 8.02 b | 17.70 de | 3.38 c | 5.58 bc | 0.57 bc | 25.86 a |
| D | 36.70 a | 23.83 a | 3.89 a | 11.30 a | 9.58 a | 27.54 a | 5.41 a | 8.02 a | 0.81 a | 25.77 a | |
| F-test y | A | * | NS | NS | *** | ** | *** | ** | * | NS | NS |
| B | * | NS | *** | *** | *** | *** | *** | *** | *** | NS | |
| A×B | NS | NS | NS | NS | NS | ** | NS | NS | NS | NS | |
| Correlation Coefficient | Petiole Length | Petiole Diameter | Leaf Length | Leaf Width | Shoot Fresh Weight | Shoot Dry Weight | Root Fresh Weight | Root Dry Weight | Root Length | Si Content in Leaves | Si Content in Roots | Fv/Fm | Fv/F0 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Plant height | 0.94 ** | 0.32 * | 0.48 ** | 0.26 | 0.59 ** | 0.56 ** | 0.58 ** | 0.38 ** | 0.1 | 0.39 ** | 0.29 * | 0.04 | −0.05 |
| Petiole length | 0.19 | 0.21 | 0.04 | 0.49 ** | 0.48 ** | 0.49 ** | 0.32 * | 0.08 | 0.25 | 0.14 | −0.01 | −0.08 | |
| Petiole diameter | 0.50 ** | 0.42 ** | 0.60 ** | 0.56 ** | 0.57 ** | 0.45 ** | −0.08 | 0.61 ** | 0.56 ** | 0.24 | 0.16 | ||
| Leaf length | 0.84 ** | 0.53 ** | 0.48 ** | 0.46 ** | 0.26 | 0.09 | 0.57 ** | 0.59 ** | 0.09 | 0.08 | |||
| Leaf width | 0.43 ** | 0.45 ** | 0.36 ** | 0.26 | 0.08 | 0.60 ** | 0.60 ** | 0.07 | 0.09 | ||||
| Shoot fresh weight | 0.88 ** | 0.81 ** | 0.64 ** | 0.04 | 0.78 ** | 0.66 ** | 0.12 | 0.02 | |||||
| Shoot dry weight | 0.87 ** | 0.81 ** | 0.06 | 0.71 ** | 0.57 ** | 0.25 | 0.17 | ||||||
| Root fresh weight | 0.75 ** | 0.13 | 0.58 ** | 0.50 ** | 0.25 | 0.13 | |||||||
| Root dry weight | 0.04 | 0.45 ** | 0.31 * | 0.19 | 0.12 | ||||||||
| Root length | 0.05 | 0 | 0.25 | −0.13 | |||||||||
| Si content in leaf | 0.93 ** | 0.32 * | 0.31 * | ||||||||||
| Si content in root | 0.29 * | 0.27* | |||||||||||
| Fv/Fm | 0.6 5** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Xiao, J.; Hu, J.; Jeong, B.R. Method of Silicon Application Affects Quality of Strawberry Daughter Plants during Cutting Propagation in Hydroponic Substrate System. Agronomy 2020, 10, 1753. https://doi.org/10.3390/agronomy10111753
Li Y, Xiao J, Hu J, Jeong BR. Method of Silicon Application Affects Quality of Strawberry Daughter Plants during Cutting Propagation in Hydroponic Substrate System. Agronomy. 2020; 10(11):1753. https://doi.org/10.3390/agronomy10111753
Chicago/Turabian StyleLi, Yali, Jie Xiao, Jiangtao Hu, and Byoung Ryong Jeong. 2020. "Method of Silicon Application Affects Quality of Strawberry Daughter Plants during Cutting Propagation in Hydroponic Substrate System" Agronomy 10, no. 11: 1753. https://doi.org/10.3390/agronomy10111753
APA StyleLi, Y., Xiao, J., Hu, J., & Jeong, B. R. (2020). Method of Silicon Application Affects Quality of Strawberry Daughter Plants during Cutting Propagation in Hydroponic Substrate System. Agronomy, 10(11), 1753. https://doi.org/10.3390/agronomy10111753







