Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Materials and Cultural Conditions
2.2. Light Treatments
2.3. Measurement and Statistical Analyses
2.3.1. Morphology and Growth Characteristics
2.3.2. Physiological Characteristics
2.3.3. Photosynthetic Characteristics
2.3.4. Antioxidant Enzyme Activity
2.3.5. Microstructure and Ultrastructure Observation
2.4. Statistical Analysis
3. Results
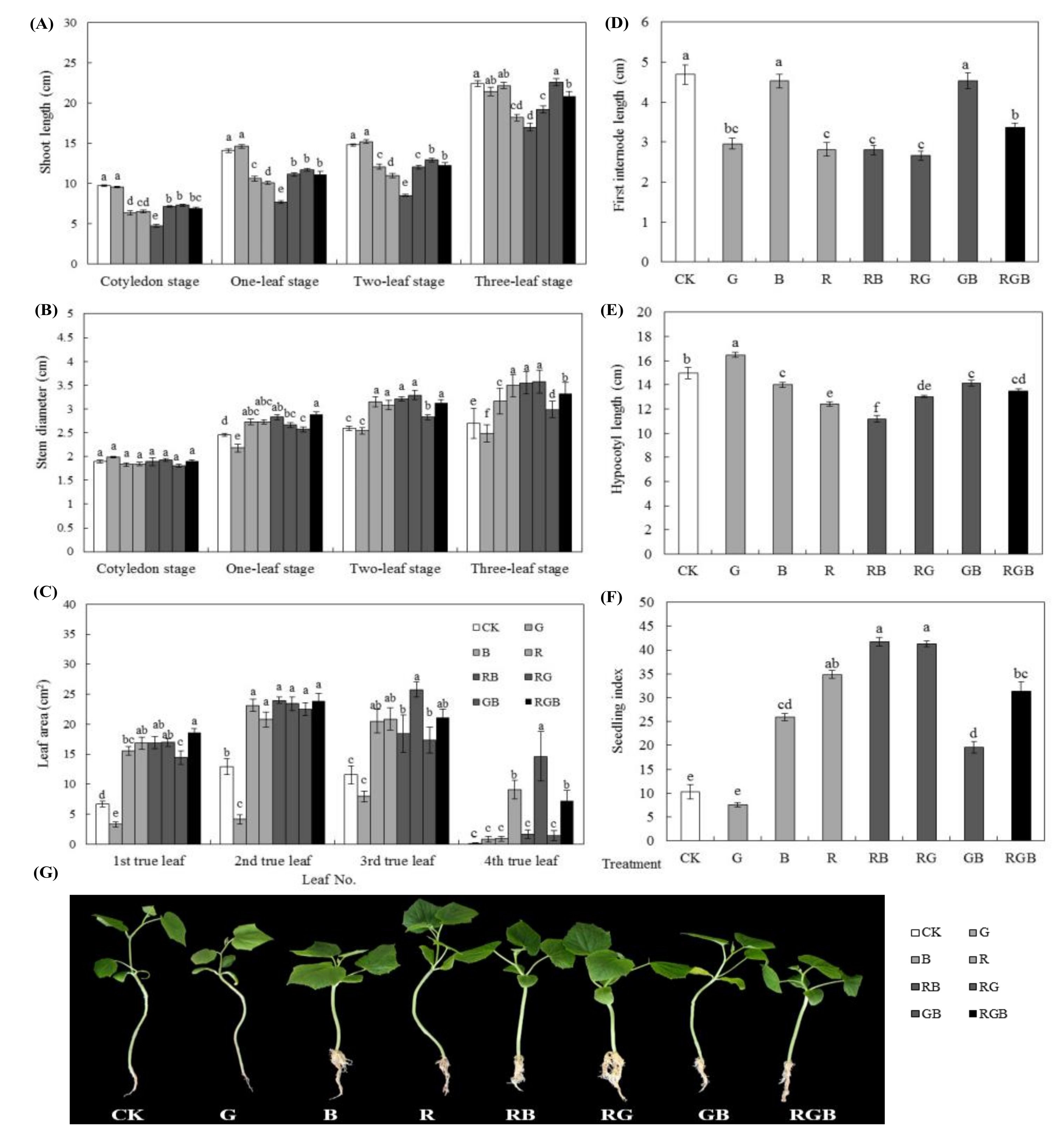
3.1. Experiment 1
3.1.1. Growth, Morphological and Physiological Parameters
3.1.2. Photosynthetic Characteristics
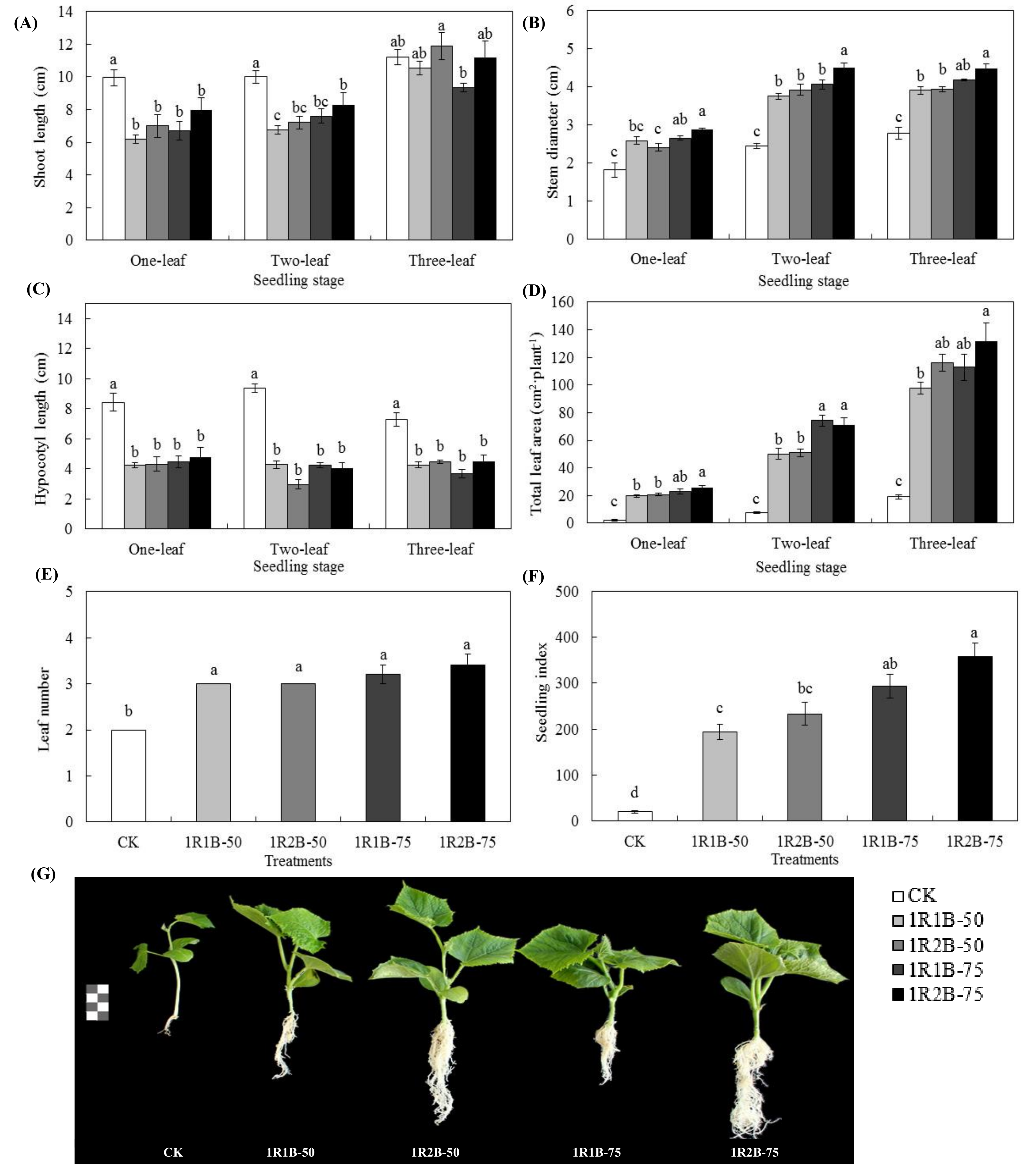
3.2. Experiment 2
3.2.1. Growth, Morphological and Physiological Parameters
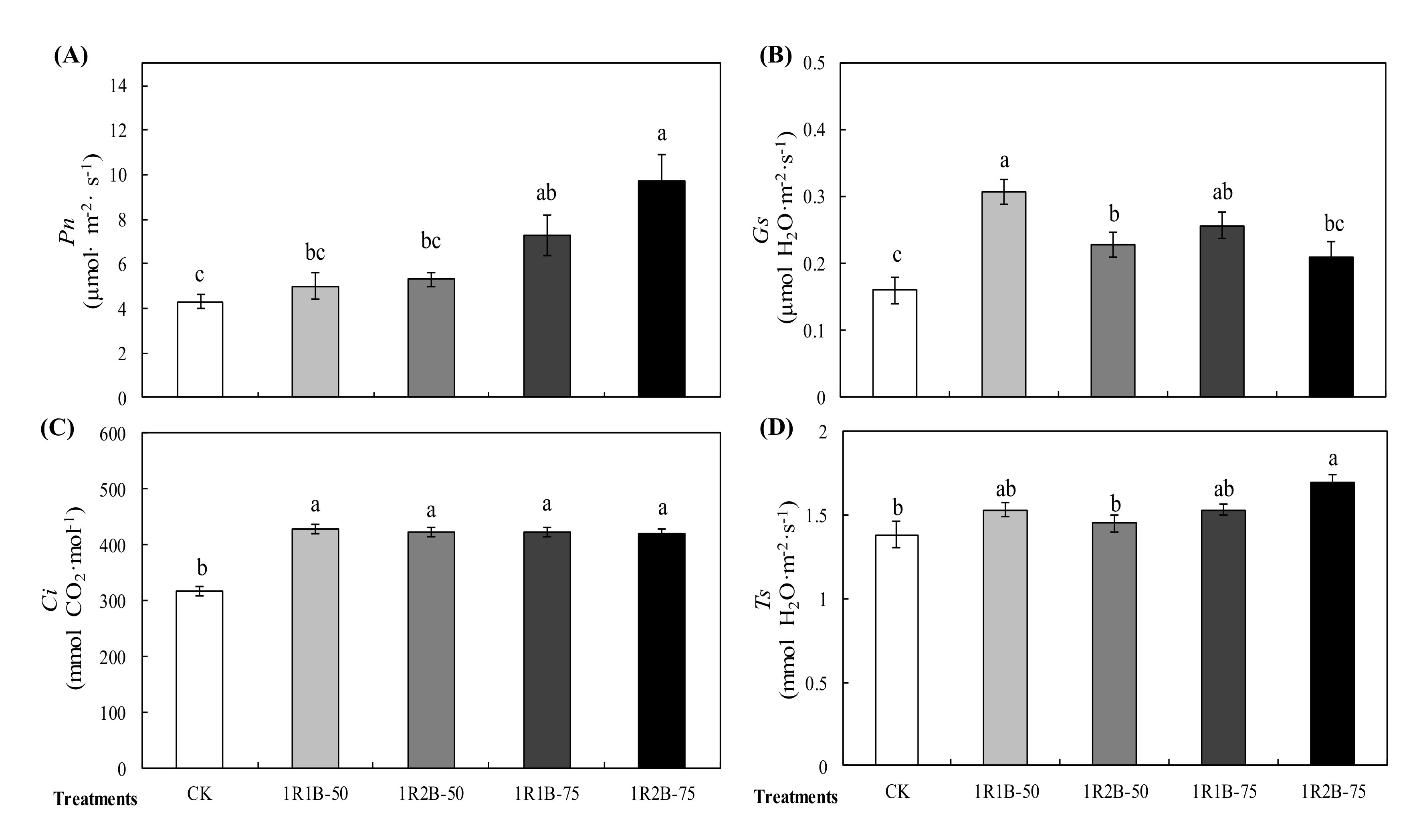
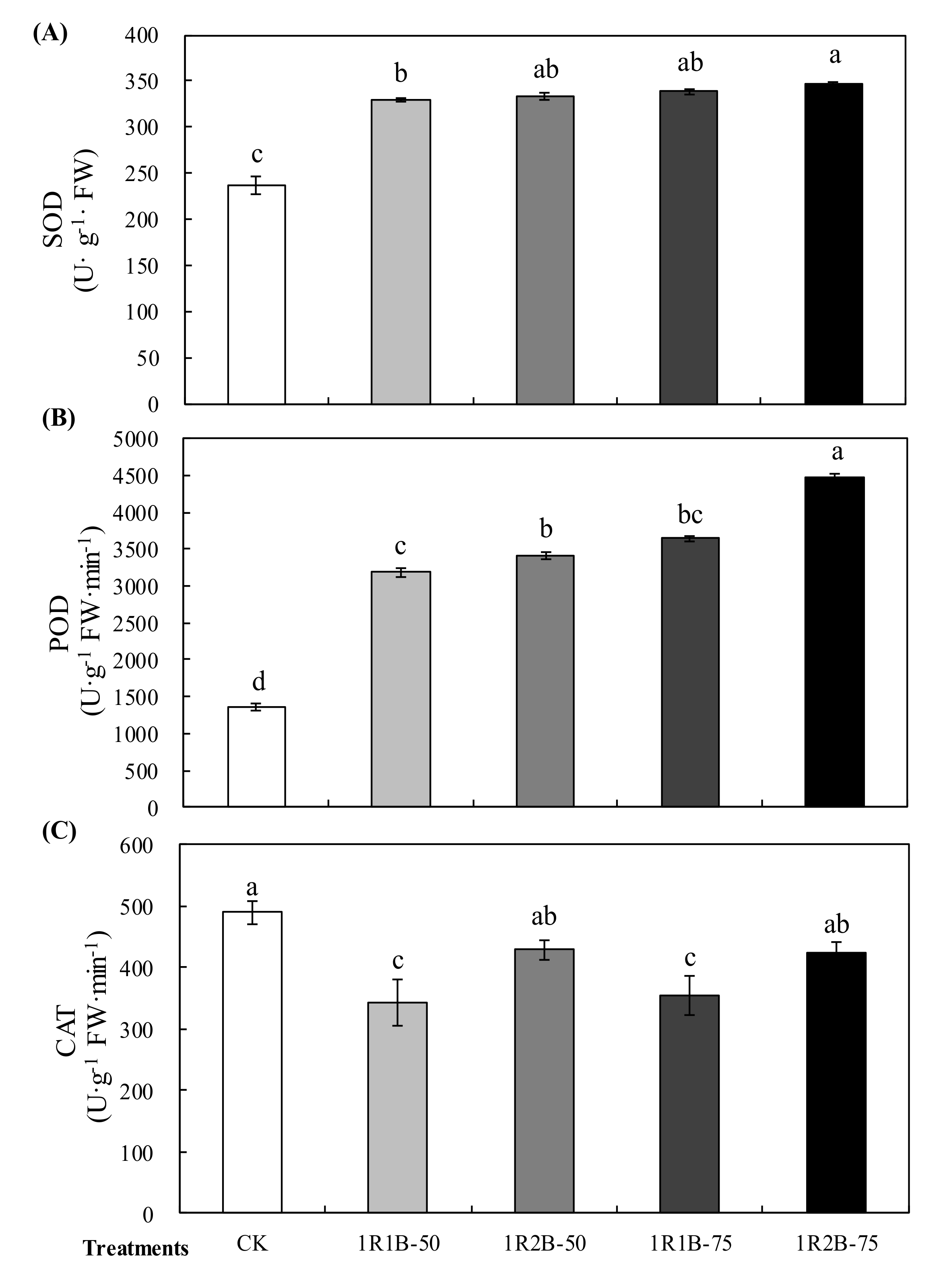
3.2.2. Photosynthetic Characteristics and Antioxidant Enzyme Activity
3.2.3. Anatomical Structure of Hypocotyl and Mesophyll
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Wei, H.; Hu, J.T.; Liu, C.; Wang, M.Z.; Zhao, J.; Kang, D.; Jeong, B.R. Effect of supplementary light source on quality of grafted tomato seedlings and expression of two photosynthetic genes. Agronomy 2018, 8, 207. [Google Scholar] [CrossRef]
- Hao, X.M.; Papadopoulos, A.P. Effects of supplemental lighting and cover materials on growth, photosynthesis, biomass partitioning, early yield and quality of greenhouse cucumber. Sci. Hortic. 1999, 80, 1–18. [Google Scholar] [CrossRef]
- Hemming, S.; van der Braak, N.; Dueck, T.; Jongschaap, R.; Marissen, N. Filtering natural light by greenhouse covering using model simulations–more production and better plant quality by diffuse light? Acta Hortic. 2006, 711, 105–110. [Google Scholar] [CrossRef]
- Hemming, S.; Mohammadkhani, V.; Dueck, T. Diffuse greenhouse covering materials–material technology, measurements and evaluation of optical properties. Acta Hortic. 2008, 797, 469–475. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Growth and morphological response of cucumber seedlings to supplemental red and blue photon flux ratios under varied solar daily light integrals. Acta Hortic. 2014, 956, 187–194. [Google Scholar] [CrossRef]
- Whitelam, G.C.; Halliday, K.J. Annual Plant Reviews, Light and Plant Development; John Wiley & Sons: Hoboken, NJ, USA, 2007; Volume 30. [Google Scholar]
- Hayashi, M.; Inoue, S.H.; Takahashi, K.; Kinoshita, T. Immunohistochemical detection of blue light-induced phosphorylation of the plasma membrane H+-ATPase in stomatal guard cells. Plant Cell Physiol. 2011, 52, 1238–1248. [Google Scholar] [CrossRef]
- McCree, K.J. The action spectrum, absorptance and quantum yield of photosynthesis in crop plants. Agric. Metereol. 1971, 9, 191–216. [Google Scholar] [CrossRef]
- Heuvelink, E. Plants in Action Adaptation in Nature, Performance in Cultivation; Atwell, B.J., Kriedmann, P.E., Turnbell, C.G.N., Eds.; Macmillan Education Australia Pty Ltd.: Melbourne, Australia, 2000. [Google Scholar]
- Folta, K.M.; Maruhnich, S.A. Green light: A signal to slow down or stop. J. Exp. Bot. 2007, 58, 3099–3111. [Google Scholar] [CrossRef]
- Savvides, A.; Fanourakis, D.; van Ieperen, W. Co-ordination of hydraulic and stomatal conductances across light qualities in cucumber leaves. J. Exp. Bot. 2012, 63, 1135–1143. [Google Scholar] [CrossRef]
- Miao, Y.X.; Wang, X.Z.; Gao, L.H.; Chen, Q.Y.; Mei, Q.U. Blue light is more essential than red light for maintaining the activities of photosystem II and I and photosynthetic electron transport capacity in cucumber leaves. J. Integr. Agric. 2016, 15, 87–100. [Google Scholar] [CrossRef]
- Hogewoning, S.W.; Trouwborst, G.; Maljaars, H.; Poorter, H.; van Leperen, W.; Harbinson, J. Blue light dose–responses of leaf photosynthesis, morphology, and chemical composition of Cucumis sativus grown under different combinations of red and blue light. J. Exp. Bot. 2010, 61, 3107–3117. [Google Scholar] [CrossRef]
- Liu, X.Y.; Chang, T.T.; Guo, S.R.; Xu, Z.G.; Li, J. Effects of different light quality of LED on growth of LED on growth and photosynthetic characters in cherry tomato seedlings. Acta Hortic. 2011, 907, 325–330. [Google Scholar] [CrossRef]
- Hernández, R.; Kubota, C. Tomato seedling growth and morphological responses to supplemental LED lighting Red:Blue rations under varied daily solar light integrals. Acta Hortic. 2012, 956, 187–194. [Google Scholar] [CrossRef]
- Nanya, K.; Ishigami, Y.; Hikosaka, S.; Goto, E. Effects of blue and red light on stem elongation and flowering of tomato seedlings. Acta Hortic. 2012, 956, 261–266. [Google Scholar] [CrossRef]
- Li, Y.M.; Zheng, Y.J.; Tan, X.; Pan, Y.; Liu, H.C. Effects of different LED supplemental lighting on growth of tomato and cucumber seedlings. China Illum. Eng. J. 2016, 27, 68–71. (In Chinese) [Google Scholar]
- Song, J.L.; Cao, K.; Hao, Y.W.; Song, S.W.; Su, W.; Liu, H.C. Hypocotyl elongation is regulated by supplemental blue and red light in cucumber seedling. Gene 2019, 707, 117–125. [Google Scholar] [CrossRef]
- Zhang, X.; Huang, G.; Bian, X.; Zhao, Q. Effects of root interaction and nitrogen fertilization on the chlorophyll content, root activity, photosynthetic characteristics of intercropped soybean and microbial quantity in the rhizosphere. Plant Soil Environ. 2013, 59, 80–88. [Google Scholar] [CrossRef]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of accurate extinction coefficients and simultaneous equations for assaying chlorophylls a and bextracted with four different solvents: Verification of the concentration of chlorophyll standards by atomic absorption spectroscopy. Biochim. Biophys. Acta (BBA) Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–250. [Google Scholar] [CrossRef]
- Yemm, E.W.; Willis, A.J. The estimation of carbohydrates in plant extracts by anthrone. Biochem. J. 1954, 57, 508–514. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Purification and quantitative relationship with water soluble protein in seedling. Plant Physiol. 1977, 59, 315–318. [Google Scholar] [CrossRef] [PubMed]
- Kochba, J.; Lavee, S.; Spiegel-Roy, P. Differences in peroxidase activity and isoenzymes in embryogenic and non-embryogenic ‘Shamouti’ orange ovular callus lines. Plant Cell Physiol. 1977, 18, 463–467. [Google Scholar] [CrossRef]
- Cakmak, I.; Marschner, H. Magnesium deficiency and high light intensity enhance activities of superoxide dismutase, ascorbate peroxidase, and glutathione reductase in bean leaves. Plant Physiol. 1992, 98, 1222–1227. [Google Scholar] [CrossRef]
- Naija, S.; Elloumi, N.; Jbir, N.; Ammar, S.; Kevers, C. Anatomical and biochemical changes during adventitious rooting of apple rootstocks MM 106 cultured in vitro. Comptes Rendus Biol. 2008, 331, 518–525. [Google Scholar] [CrossRef]
- Kim, E.Y.; Park, S.A.; Park, B.J.; Lee, Y.; Oh, M.M. Growth and antioxidant phenolic compounds in cherry tomato seedlings grown under monochromatic light-emitting diodes. Hort. Environ. Biotechnol. 2014, 55, 506–513. [Google Scholar] [CrossRef]
- Jeong, S.W.; Park, S.; Jin, J.S.; Seo, O.N.; Kim, G.S.; Kim, Y.H. Influences of four different light-emitting diode lights on flowering and polyphenol variations in the leaves of chrysanthemum. J. Agric. Food Chem. 2012, 60, 9793–9800. [Google Scholar] [CrossRef]
- Sager, J.C.; Smith, W.O.; Edwards, J.L.; Cyr, K.L. Photosynthetic efficiency and phytochrome photoequilibria determination using spectral data. Trans. ASABE 1988, 31, 1882–1889. [Google Scholar] [CrossRef]
- Bouly, J.P.; Schleicher, E.; Dionisio-Sese, M.; Vandenbussche, F.; van Der Straeten, D.; Bakrin, N. Cryptochrome blue-light photoreceptors are activated through interconversion of flavin redox states. J. Biol. Chem. 2007, 282, 9383–9391. [Google Scholar] [CrossRef]
- Wang, Y.; Folta, K.M. Contributions of green light to plant growth and development. Am. J. Bot. 2013, 100, 70–78. [Google Scholar] [CrossRef]
- Swartz, T.E.; Corchnoy, S.B.; Christie, J.M.; Lewis, J.W.; Szundi, I.; Briggs, W.R. The photocycle of a flavinbinding domain of the blue light photoreceptor phototropin. J. Biol. Chem. 2001, 276, 493–500. [Google Scholar] [CrossRef]
- Baroli, I.M.; Dean, P.G.; Badger, M.R.; Caemmerer, S.V. The contribution of photosynthesis to the red light response of stomatal conductance. Am. Soc. Plant Biol. 2008, 146, 737–747. [Google Scholar] [CrossRef] [PubMed]
- Hogewoning, S.W.; Douwstra, P.; Trouwborst, G.; van Ieperen, W.; Harbinson, J. An artificial solar spectrum substantially alters plant development compared with usual climate room irradiance spectra. J. Exp. Bot. 2010, 61, 1267–1276. [Google Scholar] [CrossRef] [PubMed]
- Johkan, M.H.; Shoji, K.; Goto, F.; Hahida, S.; Yoshihara, T. Blue light-emitting diode light irradiation of seedlings improves seedling quality and growth after transplanting in red leaf lettuce. HortScience 2010, 45, 1809–1814. [Google Scholar] [CrossRef]
- van Ieperen, W.; Savvides, A.; Fanourakis, D. Red and blue light effects during growth on hydraulic and stomatal conductance in leaves of young cucumber plants. Acta Hortic. 2012, 956, 223–230. [Google Scholar] [CrossRef]
- Farquhar, G.D.; Sharkey, T.D. Stomatal conductance and photosynthesis. Annu. Rev. Plant Physiol. 1982, 33, 317–345. [Google Scholar] [CrossRef]
- Iino, M.; Ogawa, T.; Zeiger, E. Kinetic properties of the blue-light response of stomata. Proc. Natl. Acad. Sci. USA 1985, 82, 8019–8023. [Google Scholar] [CrossRef]
- Lawson, T. Guard cell photosynthesis and stomatal function. New Phytol. 2009, 181, 13–34. [Google Scholar] [CrossRef]
- Zeiger, E.; Talbott, L.D.; Frechilla, S.; Srivastava, A.; Zhu, J. The guard cell chloroplast: A perspective for the twenty-first century. New Phytol. 2002, 153, 415–424. [Google Scholar] [CrossRef]
- Ohashi, K.K.; Matsuda, R.; Goto, E.; Fujiwara, K.; Kurata, K. Growth of rice plants under red light with or without supplemental blue light. Soil Sci. Plant Nutr. 2006, 52, 444–452. [Google Scholar] [CrossRef]
- Matsuda, R.; Ohashi-Kaneko, K.; Fujiwara, K.; Goto, E.; Kurata, K. Photosynthetic characteristics of rice leaves grown under red light with or without supplemental blue light. Plant Cell Physiol. 2004, 45, 1870–1874. [Google Scholar] [CrossRef]
- Zheng, L.; Van Labeke, M.C. Long-term effects of red- and blue-light emitting diodes on leaf anatomy and photosynthetic efficiency of three ornamental pot plants. Front. Plant Sci. 2017, 8, 917. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Lu, W.; Tong, Y.X.; Yang, Q.C. Leaf morphology, photosynthetic performance, chlorophyll fluorescence, stomatal development of lettuce (Lactuca sativa L.) exposed to different ratios of red light to blue light. Front. Plant Sci. 2016, 7, 250. [Google Scholar] [CrossRef] [PubMed]
- Hazarika, B.N. Morpho-physiological disorders in in vitro culture of plants. Sci. Hortic. 2006, 108, 105–120. [Google Scholar] [CrossRef]
- Su, N.N.; Wu, Q.; Shen, Z.G.; Xia, K.; Cui, J. Effects of light quality on the chloroplastic ultrastructure and photosynthetic characteristics of cucumber seedlings. Plant Growth Regul. 2014, 73, 227–235. [Google Scholar] [CrossRef]
- Li, C.X.; Xu, Z.G.; Dong, R.Q.; Chang, S.X.; Wang, L.Z.; Khalil, U.R.M.; Tao, J.M. An RNA-Seq analysis of grape plantlets grown in vitro reveals different responses to blue, green, red LED Light, and white fluorescent light. Front. Plant Sci. 2017, 8, 78. [Google Scholar] [CrossRef]
- Yamazaki, J.; Kamimura, Y. Relationship between photosystem stoichiometries and changes in active oxygen scavenging enzymes in natural grown rice seedlings. Plant Growth Regul. 2002, 36, 113–120. [Google Scholar] [CrossRef]

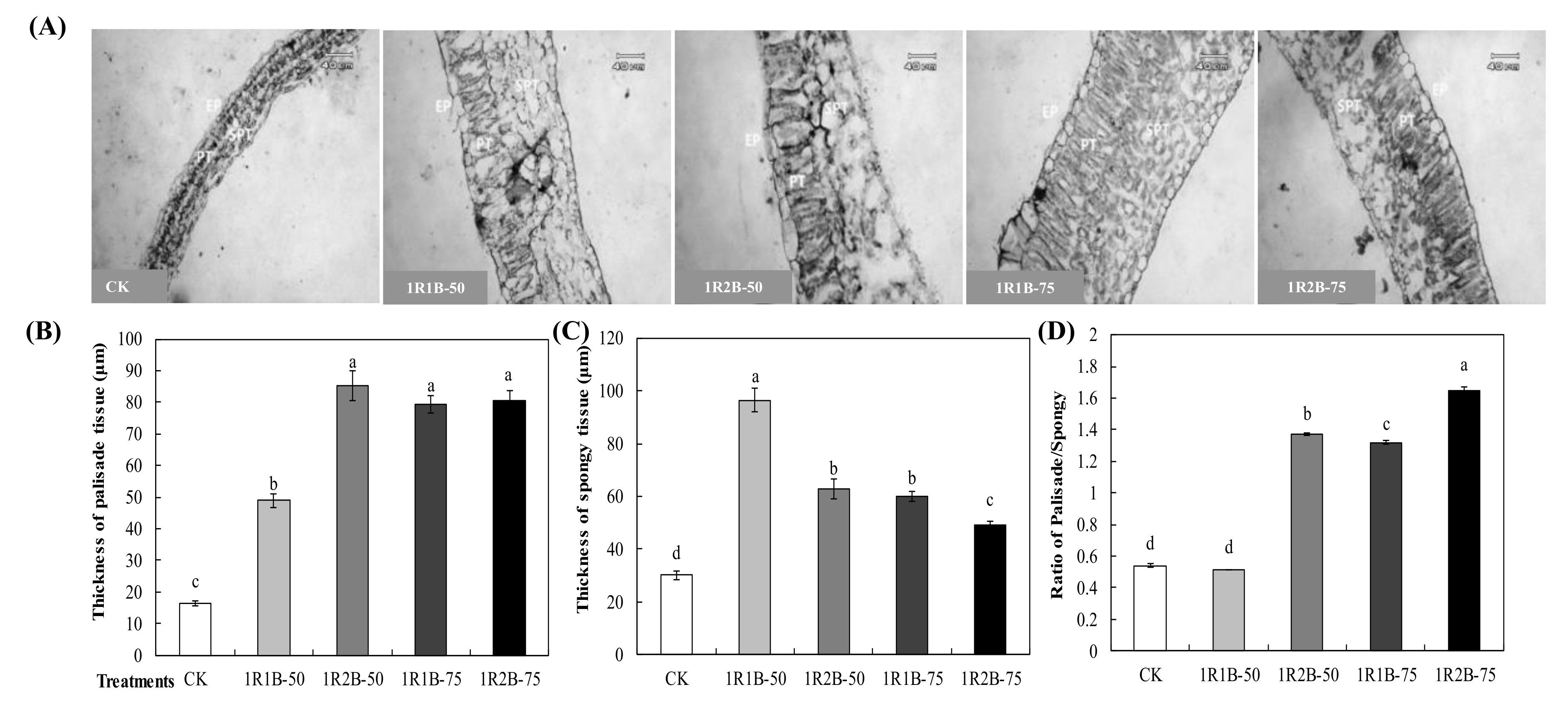
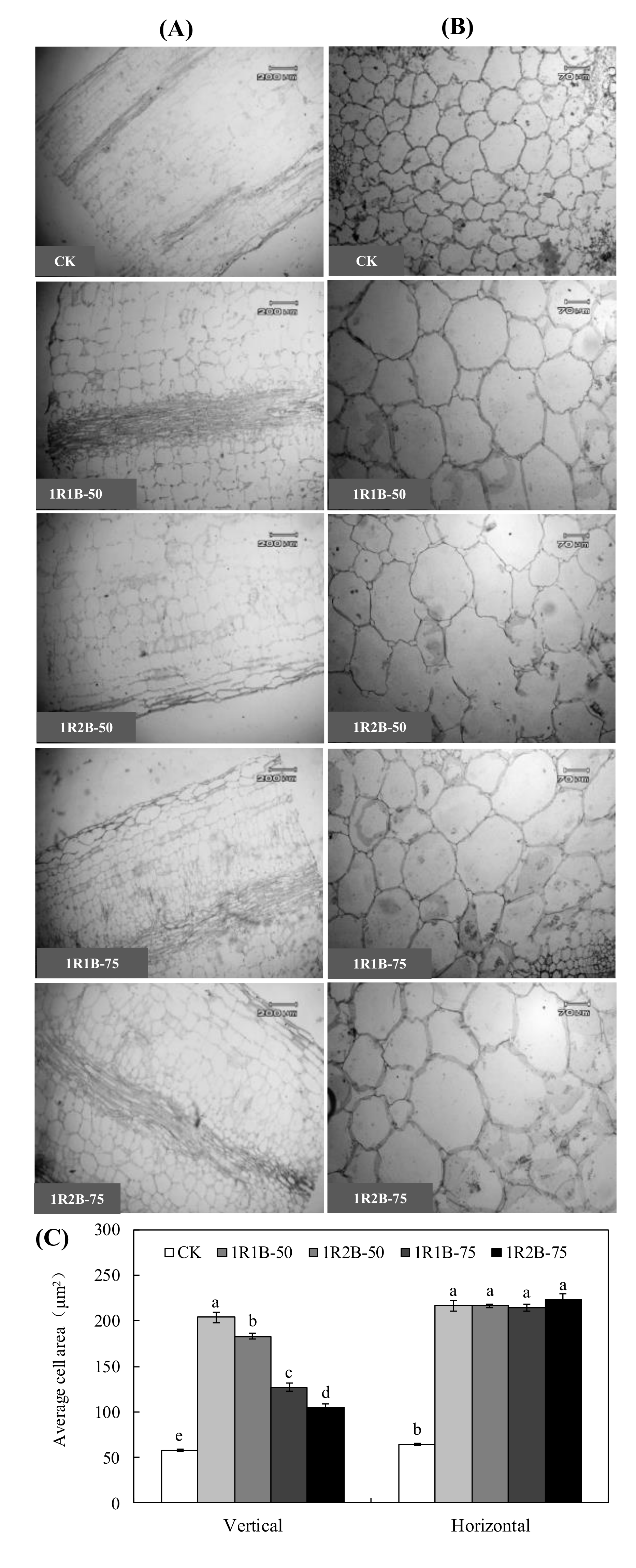
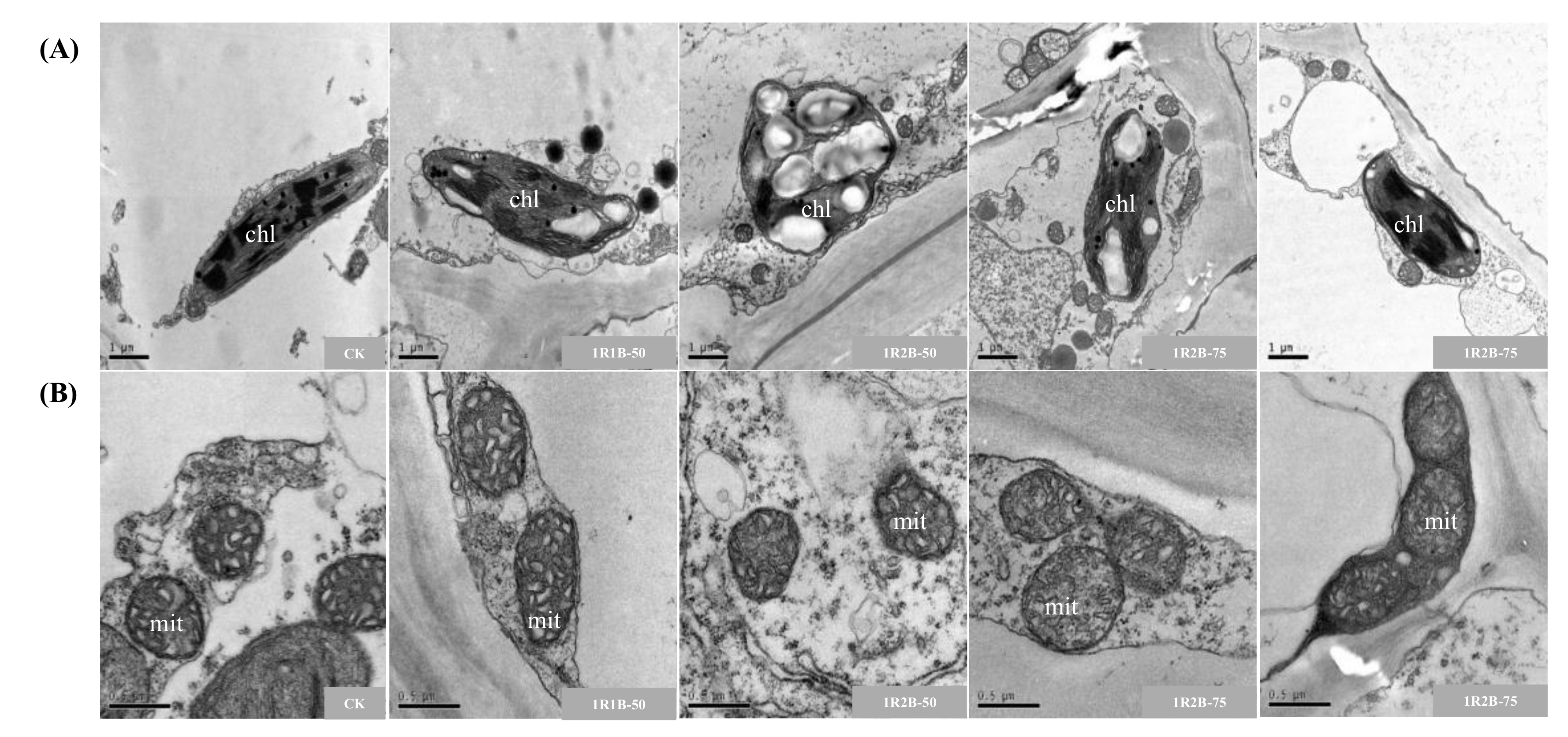
| Treatment | Fresh Weight (g·Plant−1) | Dry Weight (g·Plant−1) | Chlorophyll Content (mg·g−1) | Soluble Sugar Content (mg·g−1) | Soluble Protein Content (mg·g−1) | ||||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Total | Shoot | Root | Total | ||||
| CK | 1.85 ± 0.15 d | 0.08 ± 0.05 e | 1.94 ± 0.16 e | 0.08 ± 0.03 d | 0.003 c | 0.09 ± 0.03 d | 1.88 ± 0.01 d | 2.07 ± 0.03 d | 3.66 ± 0.16 bc |
| G | 1.49 ± 0.06 e | 0.03 ± 0.02 e | 1.52 ± 0.06 f | 0.06 ± 0.01 d | 0.003 d | 0.06 ± 0.01 d | 1.46 ± 0.03 e | 2.13 ± 0.03 d | 3.07 ± 0.09 d |
| B | 3.15 ± 0.40 b | 0.15 ± 0.01 d | 3.3 ± 0.40 cd | 0.16 ± 0.02 abc | 0.01 c | 0.17 ± 0.02 bc | 2.39 ± 0.01 a | 3.46 ± 0.15 b | 3.65 ± 0.09 bc |
| R | 3.17 ± 0.52 b | 0.26 ± 0.05 c | 3.43 ± 0.51 bc | 0.15 ± 0.04 bc | 0.017 bc | 0.17 ± 0.05 bc | 2.41 ± 0.02 a | 3.36 ± 0.04 b | 3.16 ± 0.03 d |
| RB | 3.21 ± 0.23 b | 0.48 ± 0.12 a | 3.58 ± 0.11 bc | 0.17 ± 0.01 abc | 0.027 a | 0.19 ± 0.00 ab | 2.16 ± 0.02 b | 4.16 ± 0.11 a | 3.79 ± 0.12 b |
| RG | 3.76 ± 0.28 a | 0.28 ± 0.06 c | 4.05 ± 0.23 a | 0.19 ± 0.06 a | 0.02 b | 0.21 ± 0.06 a | 1.89 ± 0.04 d | 3.37 ± 0.16 b | 3.48 ± 0.49 c |
| GB | 2.81 ± 0.19 c | 0.22 ± 0.06 c | 3.03 ± 0.22 d | 0.14 ± 0.03 c | 0.013 c | 0.15 ± 0.03 c | 2.37 ± 0.04 a | 2.93 ± 0.03 c | 4.46 ± 0.44 a |
| RGB | 3.56 ± 0.16 a | 0.38 ± 0.07 b | 3.94 ± 0.14 a | 0.17 ± 0.01 abc | 0.02 b | 0.19 ± 0.00 ab | 2.02 ± 0.02 c | 3.02 ± 0.15 c | 3.65 ± 0.67 bc |
| Treatment | Fresh Weight (g·Plant−1) | Dry Weight (g·Plant−1) | Ratio (Root/Shoot) | Root Activity (mg·g−1·h−1) | Chlorophyll Content (mg·g−1) | Soluble Sugar Content (mg·g−1) | Soluble Protein Content (mg·g−1) | ||
|---|---|---|---|---|---|---|---|---|---|
| Shoot | Root | Shoot | Root | ||||||
| CK | 1.42 ± 0.1 c | 0.10 ± 0.0 c | 0.08 ± 0.0 c | 0.01 ± 0.00 c | 0.05 ± 0.01 b | 59.67 ± 0.39 c | 2.10 ± 0.06 a | 4.03 ± 0.12 c | 5.73 ± 0.09 a |
| 1R1B-50 | 4.70 ± 0.2 b | 0.74 ± 0.3 bc | 0.47 ± 0.1 b | 0.05 ± 0.01 b | 0.10 ± 0.02 b | 60.33 ± 0.22 c | 1.70 ± 0.00 c | 7.77 ± 0.15 b | 4.98 ± 0.09 c |
| 1R2B-50 | 5.70 ± 0.3 ab | 1.42 ± 0.3 ab | 0.61 ± 0.1 ab | 0.10 ± 0.01 a | 0.16 ± 0.02 a | 107.33 ± 0.84 b | 1.85 ± 0.01 b | 7.81 ± 0.27 b | 5.08 ± 0.14 bc |
| 1R1B-75 | 5.60 ± 0.5 ab | 0.76 ± 0.1 bc | 0.60 ± 0.1 ab | 0.06 ± 0.01 b | 0.10 ± 0.01 b | 116.67 ± 0.39 b | 1.82 ± 0.04 b | 9.96 ± 0.13 a | 5.40 ± 0.09 b |
| 1R2B-75 | 6.80 ± 0.6 a | 1.82 ± 0.3 a | 0.79 ± 0.1 a | 0.12 ± 0.02 a | 0.16 ± 0.02 a | 146.67 ± 0.85 a | 1.87 ± 0.03 b | 9.81 ± 0.21 a | 5.82 ± 0.09 a |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Dong, H.; Song, S.; Su, W.; Liu, H. Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance. Agronomy 2020, 10, 1698. https://doi.org/10.3390/agronomy10111698
Zhang Y, Dong H, Song S, Su W, Liu H. Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance. Agronomy. 2020; 10(11):1698. https://doi.org/10.3390/agronomy10111698
Chicago/Turabian StyleZhang, Yiting, Hao Dong, Shiwei Song, Wei Su, and Houcheng Liu. 2020. "Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance" Agronomy 10, no. 11: 1698. https://doi.org/10.3390/agronomy10111698
APA StyleZhang, Y., Dong, H., Song, S., Su, W., & Liu, H. (2020). Morphological and Physiological Responses of Cucumber Seedlings to Supplemental LED Light under Extremely Low Irradiance. Agronomy, 10(11), 1698. https://doi.org/10.3390/agronomy10111698






