Gene Expression and Metabolomics Profiling of the Common Wheat Obtaining Leaf Rust Resistance by Salicylic or Jasmonic Acid through a Novel Detached Leaf Rust Assay
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Senescence Retardant Chemicals and Preparation of the Medium
2.3. Detached Leaf Assay Preparation
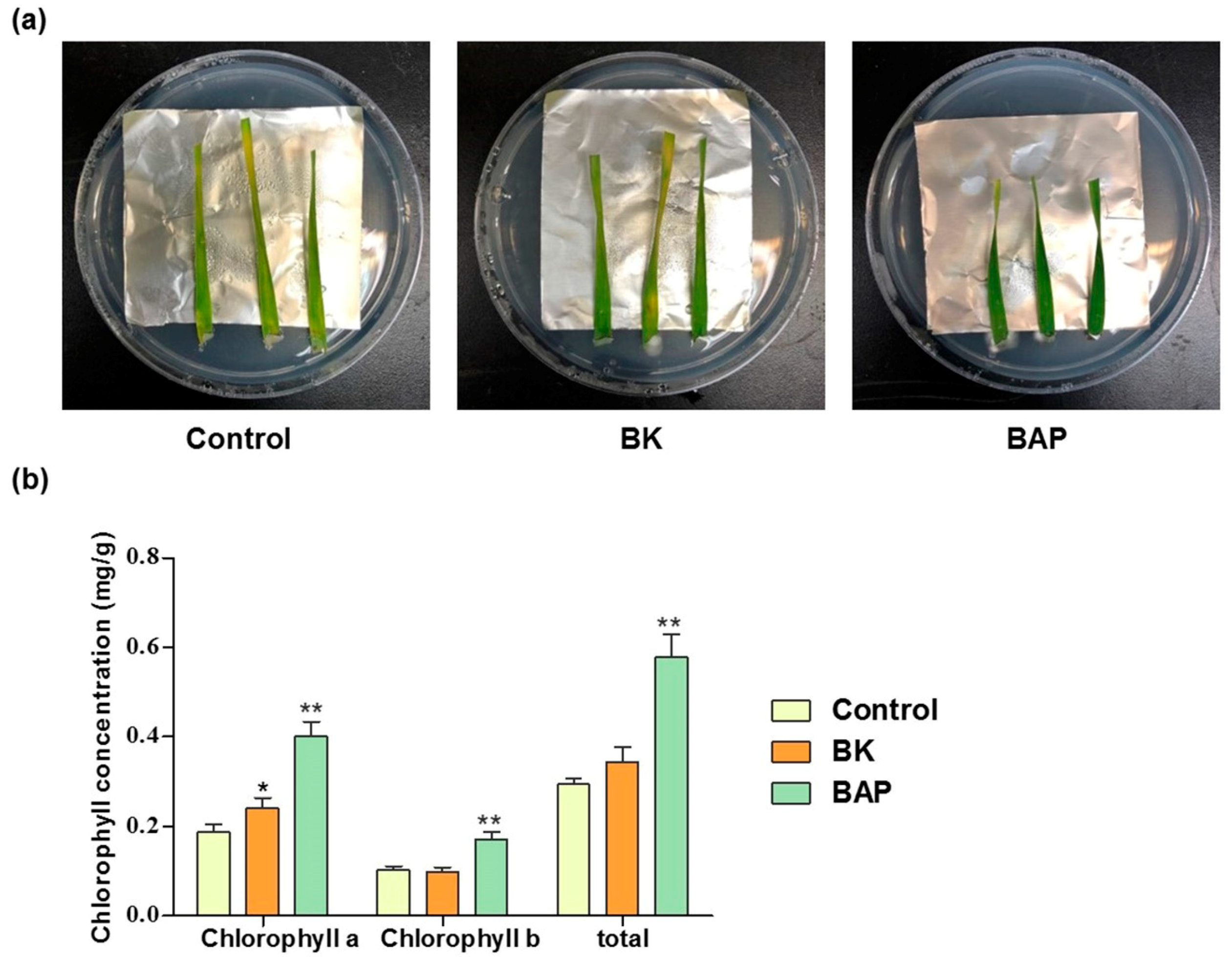
2.4. Chlorophyll Assay
2.5. Leaf Rust Inoculation
2.6. Fluorescence Microscopy
2.7. Real-Time Quantitative Reverse Transcription PCR Analysis for Gene Expression
2.8. Chitinase Assay
2.9. Peroxidase Activity
2.10. Gas Chromatography–Mass Spectrometry for Metabolite Analysis
3. Results and Discussion
3.1. Development of Defined Media for Detached Leaves with Retarded Senescence
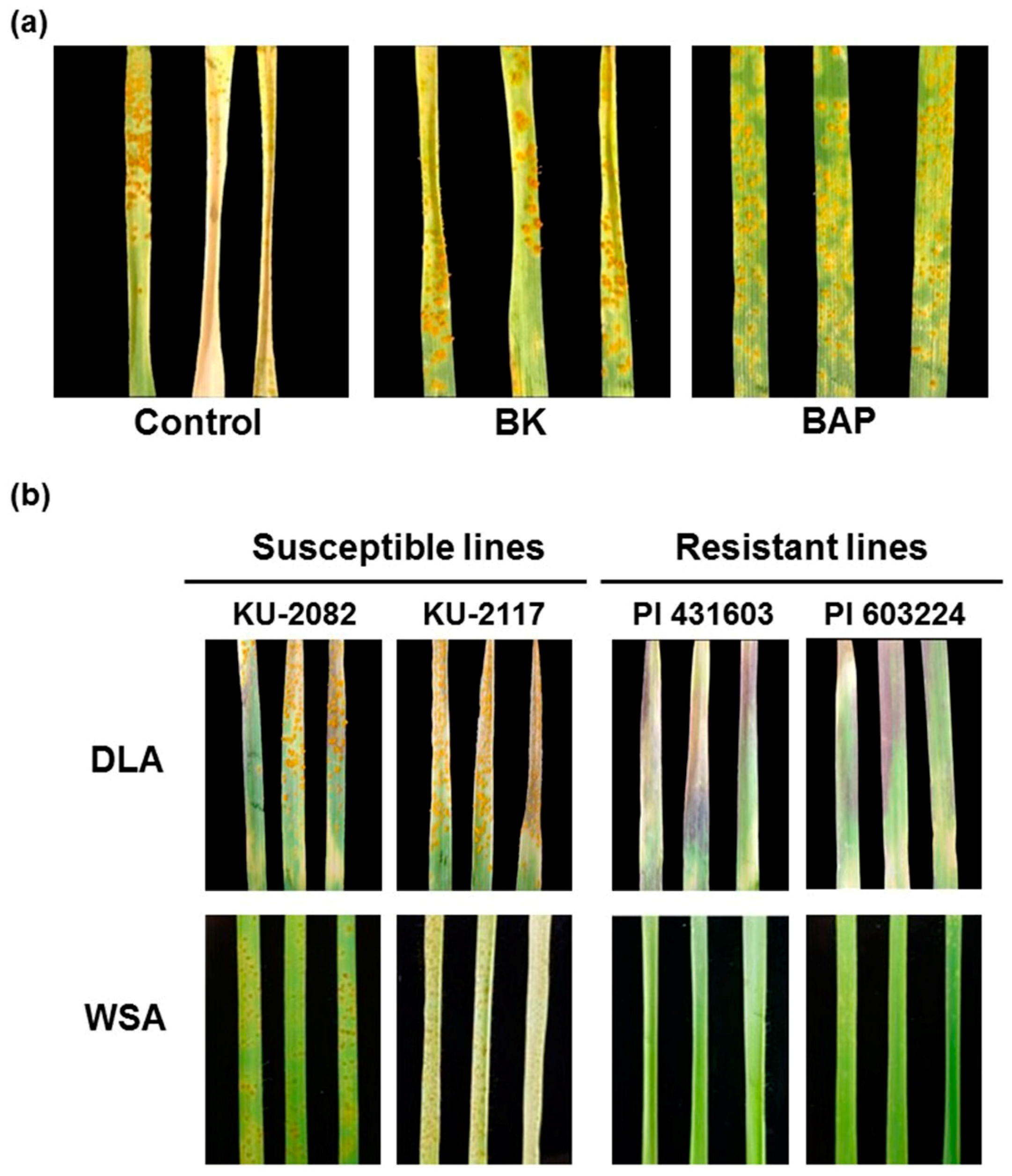
3.2. Evaluating Leaf Rust Resistance of Wheat Varieties Using the Detached Leaf Rust Assay in a Controlled Environment
3.3. Salicylic Acid and Jasmonic Acid Confer Leaf Rust Resistance to Common Wheat
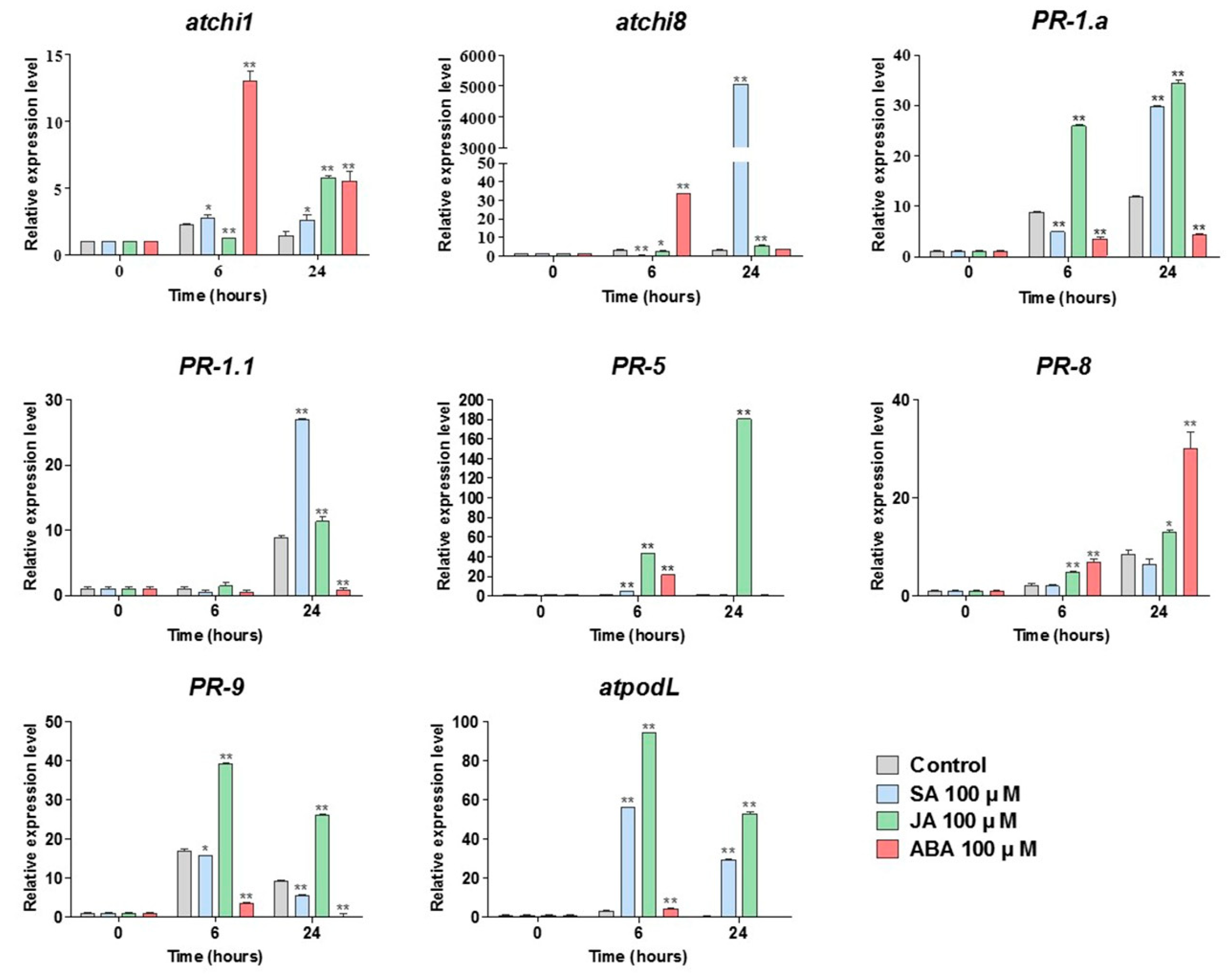
3.4. Salicylic Acid Increases the Expression and Activity of Chitinase in Wheat Leaves Inoculated with Leaf Rust
3.5. Exogenous SA Application Alters Metabolite Profiles Related to the Defense Biosynthesis Pathway
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Yang, W.; Liu, D.; Li, J.; Zhang, L.; Wei, H.; Hu, X.; Zheng, Y.; He, Z.; Zou, Y. Synthetic hexaploid wheat and its utilization for wheat genetic improvement in China. J. Genet. Genom. 2009, 36, 539–546. [Google Scholar] [CrossRef]
- Jafarzadeh, J.; Bonnett, D.; Jannink, J.-L.; Akdemir, D.; Dreisigacker, S.; Sorrells, M.E. Breeding Value of Primary Synthetic Wheat Genotypes for Grain Yield. PLoS ONE 2016, 11, e0162860. [Google Scholar] [CrossRef] [PubMed]
- Bolton, M.D.; Kolmer, J.A.; Garvin, D.F. Wheat leaf rust caused byPuccinia triticina. Mol. Plant Pathol. 2008, 9, 563–575. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.K.M. Aerial Dispersal of Pathogens on the Global and Continental Scales and Its Impact on Plant Disease. Science 2002, 297, 537–541. [Google Scholar] [CrossRef]
- Grassini, P.; Eskridge, K.M.; Cassman, K.G. Distinguishing between yield advances and yield plateaus in historical crop production trends. Nat. Commun. 2013, 4, 2918. [Google Scholar] [CrossRef] [PubMed]
- Xu, X.; Yuan, D.; Li, D.; Gao, Y.; Wang, Z.; Liu, Y.; Wang, S.; Xuan, Y.H.; Zhao, H.; Li, T.Y.; et al. Identification of stem rust resistance genes in wheat cultivars in China using molecular markers. PeerJ 2018, 6, e4882. [Google Scholar] [CrossRef]
- Huerta-Espino, J.; Singh, R.P.; Germán, S.; McCallum, B.D.; Park, R.F.; Chen, W.Q.; Bhardwaj, S.C.; Goyeau, H. Global status of wheat leaf rust caused by Puccinia triticina. Euphytica 2011, 179, 143–160. [Google Scholar] [CrossRef]
- Halim, V.A.; Vess, A.; Scheel, D.; Rosahl, S. The Role of Salicylic Acid and Jasmonic Acid in Pathogen Defence. Plant Biol. 2006, 8, 307–313. [Google Scholar] [CrossRef]
- Chanclud, E.; Morel, J. Plant hormones: A fungal point of view. Mol. Plant Pathol. 2016, 17, 1289–1297. [Google Scholar] [CrossRef]
- Davis, J.M.; Wu, H.; Cooke, J.E.K.; Reed, J.M.; Luce, K.S.; Michler, C.H. Pathogen Challenge, Salicylic Acid, and Jasmonic Acid Regulate Expression of Chitinase Gene Homologs in Pine. Mol. Plant-Microbe Interact. 2002, 15, 380–387. [Google Scholar] [CrossRef]
- Crampton, B.G.; Hein, I.; Berger, D.K. Salicylic acid confers resistance to a biotrophic rust pathogen, Puccinia substriata, in pearl millet (Pennisetum glaucum). Mol. Plant Pathol. 2009, 10, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Sairam, R.; Srivastava, G.; Tyagi, A.; Meena, R. Role of ABA, salicylic acid, calcium and hydrogen peroxide on antioxidant enzymes induction in wheat seedlings. Plant Sci. 2005, 169, 559–570. [Google Scholar] [CrossRef]
- Faize, L.; Faize, M. Functional Analogues of Salicylic Acid and Their Use in Crop Protection. Agronomy 2018, 8, 5. [Google Scholar] [CrossRef]
- Jayaraj, J.; Muthukrishnan, S.; Liang, G.; Velazhahan, R. Jasmonic Acid and Salicylic Acid Induce Accumulation of β-1,3-Glucanase and Thaumatin-Like Proteins in Wheat and Enhance Resistance Against Stagonospora nodorum. Biol. Plant. 2004, 48, 425–430. [Google Scholar] [CrossRef]
- Rauscher, M.; Adam, A.L.; Wirtz, S.; Guggenheim, R.; Mendgen, K.; Deising, H.B. PR-1 protein inhibits the differentiation of rust infection hyphae in leaves of acquired resistant broad bean. Plant J. 1999, 19, 625–633. [Google Scholar] [CrossRef] [PubMed]
- Makandar, R.; Nalam, V.; Lee, H.; Trick, H.N.; Dong, Y.; Shah, J. Salicylic Acid Regulates Basal Resistance to Fusarium Head Blight in Wheat. Mol. Plant-Microbe Interact. 2012, 25, 431–439. [Google Scholar] [CrossRef]
- Duan, X.; Wang, X.; Fu, Y.; Tang, C.; Li, X.; Cheng, Y.; Feng, H.; Huang, L.; Kang, Z. TaEIL1, a wheat homologue of AtEIN3, acts as a negative regulator in the wheat–stripe rust fungus interaction. Mol. Plant Pathol. 2013, 14, 728–739. [Google Scholar] [CrossRef]
- Plotnikova, L.I.; Shtubeĭ, T.I. Influence of salicylic and succinic acids on the cytophysiological reactions of wheat infected by brown rust. Tsitologiya 2009, 51, 43–52. [Google Scholar]
- Studham, M.E.; MacIntosh, G.C. Phytohormone signaling pathway analysis method for comparing hormone responses in plant-pest interactions. BMC Res. Notes 2012, 5, 392. [Google Scholar] [CrossRef]
- Truong, H.A.; Jeong, C.Y.; Lee, W.J.; Lee, B.C.; Chung, N.; Kang, C.-S.; Cheong, Y.-K.; Hong, S.-W.; Lee, H. Evaluation of a Rapid Method for Screening Heat Stress Tolerance Using Three Korean Wheat (Triticum aestivum L.) Cultivars. J. Agric. Food Chem. 2017, 65, 5589–5597. [Google Scholar] [CrossRef]
- Ko, D.K.; Rohozinski, D.; Song, Q.; Taylor, S.H.; Juenger, T.E.; Harmon, F.G.; Chen, Z.J. Temporal Shift of Circadian-Mediated Gene Expression and Carbon Fixation Contributes to Biomass Heterosis in Maize Hybrids. PLoS Genet. 2016, 12, e1006197. [Google Scholar] [CrossRef] [PubMed]
- Goutam, U.; Kukreja, S.; Yadav, R.; Salaria, N.; Thakur, K.; Goyal, A.K. Recent trends and perspectives of molecular markers against fungal diseases in wheat. Front. Microbiol. 2015, 6, 861. [Google Scholar] [CrossRef] [PubMed]
- Ayliffe, M.; Periyannan, S.K.; Feechan, A.; Dry, I.; Schumann, U.; Wang, M.-B.; Pryor, A.; Lagudah, E. A Simple Method for Comparing Fungal Biomass in Infected Plant Tissues. Mol. Plant-Microbe Interact. 2013, 26, 658–667. [Google Scholar] [CrossRef]
- Nguyen, N.H.; Lee, H. MYB-related transcription factors function as regulators of the circadian clock and anthocyanin biosynthesis in Arabidopsis. Plant Signal. Behav. 2016, 11, e1139278. [Google Scholar] [CrossRef] [PubMed]
- Jeong, C.Y.; Lee, W.J.; Truong, H.A.; Trịnh, C.S.; Hong, S.-W.; Lee, H. AtMybL-O modulates abscisic acid biosynthesis to optimize plant growth and ABA signaling in response to drought stress. Appl. Biol. Chem. 2018, 61, 473–477. [Google Scholar] [CrossRef]
- Lee, J.; Tan, W.; Ting, A.S.Y. Revealing the antimicrobial and enzymatic potentials of culturable fungal endophytes from tropical pitcher plants (Nepenthes spp.). Mycosphere 2014, 5, 364–377. [Google Scholar] [CrossRef]
- Miller, G.L. Use of Dinitrosalicylic Acid Reagent for Determination of Reducing Sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Ambatkar, M.; Mukundan, U. Calcium Salts Enhance Activity and Azo Dye Decolourisation Capacity of Crude Peroxidase from Armoracia rusticana. Am. J. Plant Sci. 2014, 5, 212–218. [Google Scholar] [CrossRef][Green Version]
- Blättel, V.; Larisika, M.; Pfeiffer, P.; Nowak, C.; Eich, A.; Eckelt, J.; König, H. β-1,3-Glucanase fromDelftia tsuruhatensisStrain MV01 and Its Potential Application in Vinification. Appl. Environ. Microbiol. 2010, 77, 983–990. [Google Scholar] [CrossRef]
- Kwon, M.C.; Kim, Y.X.; Lee, S.; Jung, E.S.; Singh, D.; Sung, J.; Lee, C.H. Comparative Metabolomics Unravel the Effect of Magnesium Oversupply on Tomato Fruit Quality and Associated Plant Metabolism. Metabolites 2019, 9, 231. [Google Scholar] [CrossRef]
- Boydom, A.; Dawit, W.; W/ab, G. Evaluation of Detached Leaf Assay for Assessing Leaf Rust (Puccinia triticina Eriks.) Resistance in Wheat. J. Plant Pathol. Microbiol. 2015, 4, 1–5. [Google Scholar] [CrossRef]
- Jackson, E.W.; Obert, D.E.; Chong, J.; Avant, J.B.; Bonman, J.M. Detached-Leaf Method for Propagating Puccinia coronata and Assessing Crown Rust Resistance in Oat. Plant Dis. 2008, 92, 1400–1406. [Google Scholar] [CrossRef]
- Lee, A.; Trinh, C.S.; Lee, W.J.; Kim, M.; Lee, H.; Pathiraja, D.; Choi, I.-G.; Chung, N.; Choi, C.; Lee, B.C.; et al. Characterization of two leaf rust-resistant Aegilops tauschii accessions for the synthetic wheat development. Appl. Biol. Chem. 2020, 63, 1–14. [Google Scholar] [CrossRef]
- Ku, Y.-S.; Sintaha, M.; Cheung, M.-Y.; Lam, H.-M. Plant Hormone Signaling Crosstalks between Biotic and Abiotic Stress Responses. Int. J. Mol. Sci. 2018, 19, 3206. [Google Scholar] [CrossRef] [PubMed]
- Verma, V.; Ravindran, P.; Kumar, P.P. Plant hormone-mediated regulation of stress responses. BMC Plant Biol. 2016, 16, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Bari, R.; Jones, J.D.G. Role of plant hormones in plant defence responses. Plant Mol. Biol. 2008, 69, 473–488. [Google Scholar] [CrossRef]
- Crocoll, C.; Kettner, J.; Dörffling, K. Abscisic acid in saprophytic and parasitic species of fungi. Phytochemistry 1991, 30, 1059–1060. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Yang, Y.; Cruz, C.V.; Höfte, M. Abscisic Acid-Induced Resistance against the Brown Spot Pathogen Cochliobolus miyabeanus in Rice Involves MAP Kinase-Mediated Repression of Ethylene Signaling. Plant Physiol. 2010, 152, 2036–2052. [Google Scholar] [CrossRef]
- De Vleesschauwer, D.; Gheysen, G.; Höfte, M. Hormone defense networking in rice: Tales from a different world. Trends Plant Sci. 2013, 18, 555–565. [Google Scholar] [CrossRef]
- Devos, S.; Laukens, K.; Deckers, P.; Van Der Straeten, D.; Beeckman, T.; Inzé, D.; Van Onckelen, H.; Witters, E.; Prinsen, E. A Hormone and Proteome Approach to Picturing the Initial Metabolic Events during Plasmodiophora brassicae Infection on Arabidopsis. Mol. Plant-Microbe Interact. 2006, 19, 1431–1443. [Google Scholar] [CrossRef]
- Truong, H.A.; Lee, W.J.; Kishii, M.; Hong, S.-W.; Kang, C.-S.; Lee, B.C.; Lee, H. Assessment of synthetic hexaploid wheats in response to heat stress and leaf rust infection for the improvement of wheat production. Crop. Pasture Sci. 2019, 70, 837–848. [Google Scholar] [CrossRef]
- Seevers, P.M.; Daly, J.M.; Catedral, F.F. The Role of Peroxidase Isozymes in Resistance to Wheat Stem Rust Disease. Plant Physiol. 1971, 48, 353–360. [Google Scholar] [CrossRef]
- Flott, B.E.; Moerschbacher, B.M.; Reisener, H.-J. Peroxidase isoenzyme patterns of resistant and susceptible wheat leaves following stem rust infection. New Phytol. 1989, 111, 413–421. [Google Scholar] [CrossRef]
- Anguelova-Merhar, V.S.; Westhuizen, A.J.; Pretorius, Z.A. β-1,3-Glucanase and Chitinase Activities and the Resistance Response of Wheat to Leaf Rust. J. Phytopathol. 2008, 149, 381–384. [Google Scholar] [CrossRef]
- Thomason, K.; Babar, A.; Erickson, J.E.; Mulvaney, M.; Beecher, C.; Macdonald, G. Comparative physiological and metabolomics analysis of wheat (Triticum aestivum L.) following post-anthesis heat stress. PLoS ONE 2018, 13, e0197919. [Google Scholar] [CrossRef] [PubMed]
- Baba, V.Y.; Braghini, M.T.; Dos Santos, T.B.; De Carvalho, K.; Soares, J.D.M.; Ivamoto-Suzuki, S.T.; Maluf, M.P.; Padilha, L.; Paccola-Meirelles, L.D.; Pereira, L.F.; et al. Transcriptional patterns of Coffea arabica L. nitrate reductase, glutamine and asparagine synthetase genes are modulated under nitrogen suppression and coffee leaf rust. PeerJ 2020, 8, e8320. [Google Scholar] [CrossRef] [PubMed]
- Zaynab, M.; Fatima, M.; Abbas, S.; Sharif, Y.; Umair, M.; Zafar, M.H.; Bahadar, K. Role of secondary metabolites in plant defense against pathogens. Microb. Pathog. 2018, 124, 198–202. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Wang, Z.; Wei, C.; Amo, A.; Ahmad, B.; Yang, X.; Zhang, X. Phenylpropanoid Pathway Engineering: An Emerging Approach towards Plant Defense. Pathogens 2020, 9, 312. [Google Scholar] [CrossRef]
- Lu, Y.; Chen, Q.; Bu, Y.; Luo, R.; Hao, S.; Zhang, J.; Tian, J.; Yao, Y. Flavonoid Accumulation Plays an Important Role in the Rust Resistance of Malus Plant Leaves. Front. Plant Sci. 2017, 8, 1286. [Google Scholar] [CrossRef]
- Shi, L.; Tan, Y.; Sun, Z.; Ren, A.; Zhu, J.; Zhao, M.-W. Exogenous Salicylic Acid (SA) Promotes the Accumulation of Biomass and Flavonoid Content in Phellinus igniarius (Agaricomycetes). Int. J. Med. Mushrooms 2019, 21, 955–963. [Google Scholar] [CrossRef]
- Mithen, R. Leaf glucosinolate profiles and their relationship to pest and disease resistance in oilseed rape. Euphytica 1992, 63, 71–83. [Google Scholar] [CrossRef]
- Hildebrandt, T.M.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P. Amino Acid Catabolism in Plants. Mol. Plant 2015, 8, 1563–1579. [Google Scholar] [CrossRef]
- Forde, B.G.; Lea, P.J. Glutamate in plants: Metabolism, regulation, and signalling. J. Exp. Bot. 2007, 58, 2339–2358. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.-M.; Sun, Y.-Y.; Ye, X.-Y.; Li, Z.-G. Signaling Role of Glutamate in Plants. Front. Plant Sci. 2020, 10, 1743. [Google Scholar] [CrossRef] [PubMed]
- Tauzin, A.S.; Egiardina, T. Sucrose and invertases, a part of the plant defense response to the biotic stresses. Front. Plant Sci. 2014, 5, 293. [Google Scholar] [CrossRef] [PubMed]




| Media | Components | Amount |
|---|---|---|
| Control | Phytoagar | 12 g/L |
| Sucrose | 20 g/L | |
| Murashige and skoog | 2 g/L | |
| Streptomycin | 100 μg/L | |
| BK medium | Phytoagar | 12 g/L |
| (Benzimidazole + kinetin) | Sucrose | 20 g/L |
| Murashige and skoog | 2 g/L | |
| Benzimidazole | 30 mg/L | |
| Kinetin | 10 mg/L | |
| Streptomycin | 100 μg/L | |
| BAP medium | Phytoagar | 12 g/L |
| (6-Benzylaminopurine) | Sucrose | 20 g/L |
| Murashige and skoog | 2 g/L | |
| 6-Benzylaminopurine | 100 mg/L | |
| Streptomycin | 100 μg/L |
| Phenotypic Reaction * | |||||
|---|---|---|---|---|---|
| Accession Name (Triticum aestivum L.) | DLA | WSA | Accession Name (Triticum aestivum L.) | DLA | WSA |
| Alchan | 3 | 3 | Jojoong | 3 | 3 |
| Anbaek | 3 | 3 | Jokyung | 3 | 3 |
| Baekchal | 3 | 3 | Jonong | 1 | 1 |
| Baekjoong | 2 | 2 | Jopum | 3 | 3 |
| Baek-kang | 2 | 2 | Keumkang | 3 | 3 |
| Chung-kye | 3 | 3 | Milseoung | 2 | 2 |
| Dabun | 3 | 3 | Namhae | 3 | 3 |
| Dahong | 3 | 3 | Ol | 1 | 1 |
| Dajung | 3 | 3 | Olgeru | 2 | 2 |
| Eunpa | 2 | 2 | SaeKeumKang | 3 | 3 |
| Geuru | 3 | 3 | Saeol | 3 | 3 |
| Gobun | 3 | 3 | Seodun | 2 | 2 |
| Goso | 3 | 3 | Shinmichal | 3 | 3 |
| Hanbaek | 3 | 3 | Shinmichal No.1 | 2 | 2 |
| HoJoong | 2 | 2 | Suan | 3 | 3 |
| Jeokjoong | 3 | 3 | Sukang | 3 | 3 |
| Jinpum | 3 | 3 | Taejoong | 2 | 2 |
| Joa | 3 | 3 | Tapdong | 2 | 2 |
| Jo-eun | 2 | 2 | Uri | 2 | 2 |
| Johan | 2 | 2 | Younbaeck | 2 | 2 |
| Accession Name (Aegilops tauschii) | DLA | WSA | Accession Name (Aegilops tauschii) | DLA | WSA |
| 16 | 0 | 1 | PI 330489 | 3 | 3 |
| 79TK057-324 | 1 | 1 | PI 349037 | 1 | 1 |
| KU-2019 | 3 | 4 | PI 431603 | 0 | 0 |
| KU-2082 | 3 | 4 | PI 603221 | 1 | 0 |
| KU-2117 | 3 | 3 | PI 603224 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Lee, A.; Roh, Y.J.; Lee, H.M.; Jo, Y.; Cho, H.; Choi, D.W.; Choi, M.; Eyun, S.-i.; Choi, C.; et al. Gene Expression and Metabolomics Profiling of the Common Wheat Obtaining Leaf Rust Resistance by Salicylic or Jasmonic Acid through a Novel Detached Leaf Rust Assay. Agronomy 2020, 10, 1668. https://doi.org/10.3390/agronomy10111668
Kim M, Lee A, Roh YJ, Lee HM, Jo Y, Cho H, Choi DW, Choi M, Eyun S-i, Choi C, et al. Gene Expression and Metabolomics Profiling of the Common Wheat Obtaining Leaf Rust Resistance by Salicylic or Jasmonic Acid through a Novel Detached Leaf Rust Assay. Agronomy. 2020; 10(11):1668. https://doi.org/10.3390/agronomy10111668
Chicago/Turabian StyleKim, Minseo, Aro Lee, Yeon Jin Roh, Hae Min Lee, Youngho Jo, Hwayeon Cho, Dong Wook Choi, Meena Choi, Seong-il Eyun, Changhyun Choi, and et al. 2020. "Gene Expression and Metabolomics Profiling of the Common Wheat Obtaining Leaf Rust Resistance by Salicylic or Jasmonic Acid through a Novel Detached Leaf Rust Assay" Agronomy 10, no. 11: 1668. https://doi.org/10.3390/agronomy10111668
APA StyleKim, M., Lee, A., Roh, Y. J., Lee, H. M., Jo, Y., Cho, H., Choi, D. W., Choi, M., Eyun, S.-i., Choi, C., Chung, N., Lee, H., & Lee, B. C. (2020). Gene Expression and Metabolomics Profiling of the Common Wheat Obtaining Leaf Rust Resistance by Salicylic or Jasmonic Acid through a Novel Detached Leaf Rust Assay. Agronomy, 10(11), 1668. https://doi.org/10.3390/agronomy10111668








