Combining Ability of Early-Maturing Yellow Maize Inbreds under Combined Drought and Heat Stress and Well-Watered Environments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Genetic Materials and Generation of Diallel Crosses
2.2. Field Evaluations
2.3. Data Collection
2.4. Statistical Analysis
3. Results
3.1. Analyses of Variance and Combining Ability Estimates of Grain Yield and Other Traits
3.2. Performance and Stability of the Single Cross Hybrids across Different Environments
3.3. Selection of the Best Hybrids under Drought Using the Base Index
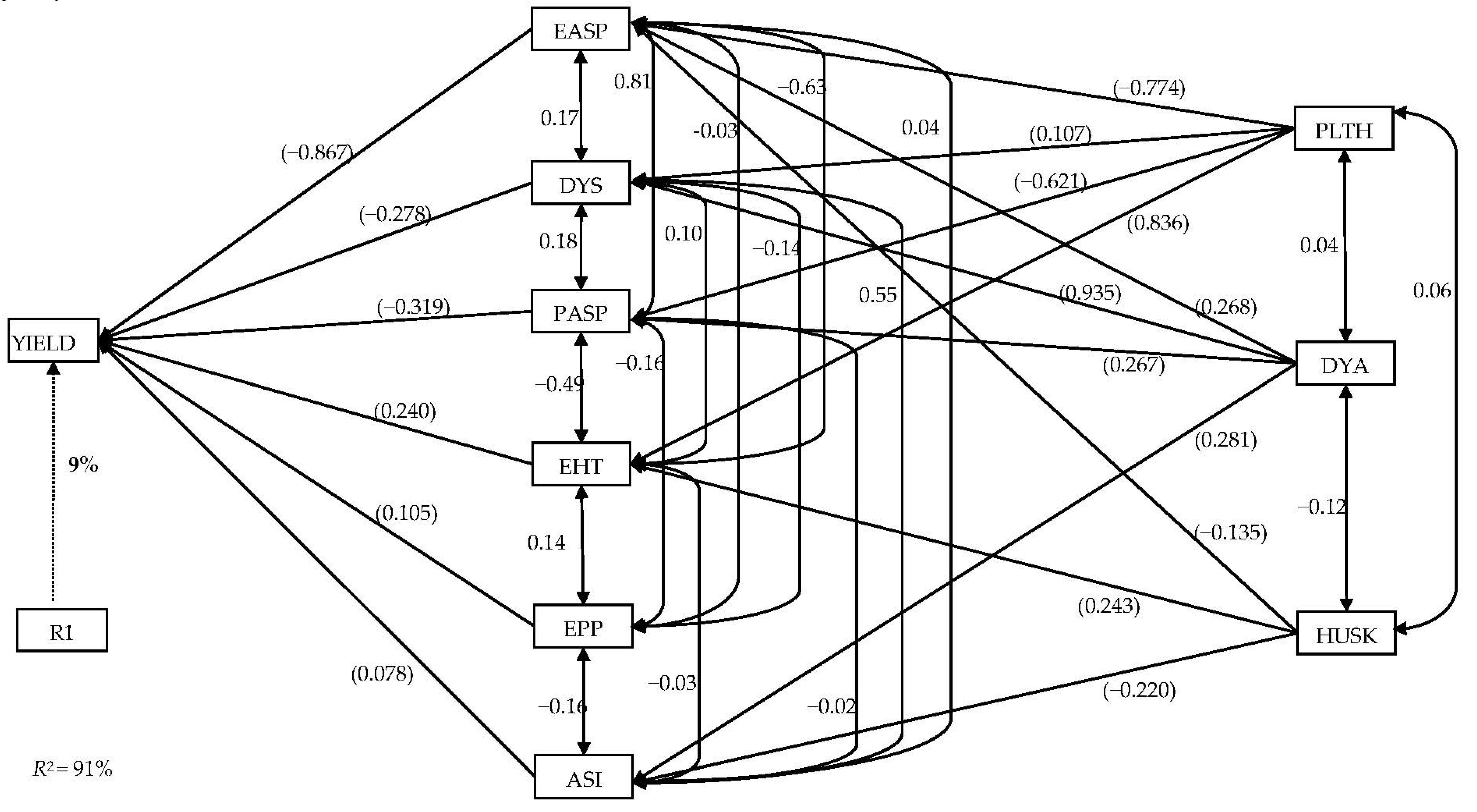
3.4. Stepwise Multiple Regression and Sequential Path Analyses
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Gibbon, D.; Dixon, J.; Flores, D. Beyond Drought Tolerant Maize: Study of Additional Priorities in Maize; Report to Generation Challenge Program; CIMMYT Impacts, Targeting and Assessment Unit, CIMMYT: Ciudad de México, México, 2007. [Google Scholar]
- Ribaut, J.; Betran, J.; Monneveux, P.; Setter, T. Drought Tolerance in Maize. In Handbook of Maize: Its Biology; Bennetzen, J.L., Hake, S.C., Eds.; Springer: New York, NY, USA, 2009; pp. 311–344. [Google Scholar]
- Badu-Apraku, B.; Menkir, A.; Fakorede, M.A.B.; Kamara, A.Y.; Adam, A. Effects of Drought Screening Methodology on Genetic Variances and Covariances in Pool 16 DT Maize Population. J. Agric. Sci. 2004, 142, 445–452. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Lum, A.F. The Pattern of Grain Yield Response of Normal and Quality Protein Maize Cultivars in Stress and Non-stress Environments. Agron. J. 2010, 102, 381–394. [Google Scholar] [CrossRef] [Green Version]
- Fischer, K.S.; Johnson, E.C.; Edmeades, G.O. Breeding and Selection for Drought Resistance in Tropical Maize. In Proceedings of the Symposium on Principles and Methods in Crop Improvement for Drought Resistance: With Emphasis on Rice, Mexico City, Mexico, 4–8 May 1982. [Google Scholar]
- NeSmith, D.S.; Ritchie, J.T. Effects of Soil Water-deficit during Tassel Emergence on Development and Yield Components of Maize (Zea mays L.). Field Crop. Res. 1992, 28, 251–256. [Google Scholar] [CrossRef]
- Pswarayi, A.; Vivek, B. Combining Ability Amongst CIMMYT’s Early Maturing Maize (Zea mays L.) Germplasm under Stress and Non-stress Conditions and Identification of Testers. Euphytica 2008, 162, 353–362. [Google Scholar] [CrossRef]
- Edmeades, G.O.; Chapman, S.C.; Bolaños, J.; Bänziger, M.; Lafitte, H.R. Recent Evaluations of Progress in Selection for Drought Tolerance in Tropical Maize. In Proceedings of the Fourth Eastern, Central and Southern African Regional Maize Conference, Harare, Zimbabwe, 28 March–1 April 1994; p. 89. [Google Scholar]
- Fakorede, M.A.B.; Akinyemiju, O.A. Climatic Change: Effects of Maize Production in a Tropical Rainforest Location. In Maize Revolution in West and Central Africa, Proceedings of a Regional Maize Workshop, IITA—Cotonou, Benin, 14–8 May 2003; Badu-Apraku, B., Fakorede, M.A.B., Ouedraogo, M., Carsky, R.J., Menkir, A., Eds.; IITA: Cotonou, Benin Republic, 2003; pp. 272–282. [Google Scholar]
- Adewale, S.A.; Akinwale, R.O.; Fakorede, M.A.B.; Badu-Apraku, B. Genetic Analysis of Drought-Adaptive Traits at Seedling Stage in Early-Maturing Maize Inbred Lines and Field Performance under Stress Conditions. Euphytica 2018, 214, 145. [Google Scholar] [CrossRef]
- Grant, R.F.; Jackson, B.S.; Kiniry, J.R.; Arkin, G.F. Water Deficit Timing Effects on Yield Components in Maize. Agron. J. 1989, 81, 61–65. [Google Scholar] [CrossRef]
- Monneveux, P.; Sanchez, C.; Beck, D.; Edmeades, G.O. Drought Tolerance Improvement in Tropical Maize Source Populations. Evidence of Progress. Crop Sci. 2006, 46, 180–191. [Google Scholar] [CrossRef]
- Neilson, R.L. Assessing Effects of Drought on Corn Grain Yield; Purdue University: West Lafayette, IN, USA, 2007; Available online: http://www.kingcorn.org/news/article.07/drought-0705.html (accessed on 22 November 2019).
- Meseka, S.; Menkir, A.; Bossey, B.; Mengesha, W. Performance Assessment of Drought Tolerant Maize Hybrids under Combined Drought and Heat Stress. Agronomy 2018, 8, 274. [Google Scholar] [CrossRef] [Green Version]
- Cairns, J.E.; Crossa, J.; Zaidi, P.; Grudloyma, P.; Sanchez, C.; Araus, J.L.; Thaitad, S.; Makumbi, D.; Magorokosho, C.; Bänziger, M.; et al. Identification of Drought, Heat, and Combined Drought and Heat Tolerant Donors in Maize (Zea mays L.). Crop Sci. 2013, 53, 1335–1346. [Google Scholar] [CrossRef] [Green Version]
- Lobell, D.B.; Burke, M.B. On the use of statistical models to predict crop yield responses to climate change. Agric. For. Meteorol. 2010, 150, 1443–1452. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B. Advances in Genetic Enhancement of Early and Extra-Early Maize for Sub-Saharan Africa; Springer International Publishing AG: Cham, Switzerland, 2017. [Google Scholar]
- Thompson, L.M. Weather variability, climatic change, and grain production. Science 1975, 188, 535–541. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Hunter, R.B.; Tollenaar, M. Effect of during grain filling on whole plant and grain yield in maize (Zea mays L.). Can. J. Plant Sci. 1983, 63, 357–363. [Google Scholar] [CrossRef] [Green Version]
- Muchow, R.C.; Sinclair, T.R.; Bennett, J.M. Temperature and solar radiation effects on potential maize yield across locations. Agron. J. 1990, 82, 338–343. [Google Scholar] [CrossRef]
- Stone, P. The Effects of Heat Stress on Cereal Yield and Quality. In Crop Responses and Adaptations to Temperature Stress; Basara, A.S., Ed.; Food Products Press: Binghamton, NY, USA, 2001; pp. 243–291. [Google Scholar]
- Crafts-Brander, S.J.; Salvucci, M.E. Sensitivity of photosynthesis in a C4 plant, maize, to heat stress. Plant Physiol. 2002, 129, 1773–1780. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schoper, J.B.; Lambert, R.J.; Vasilas, B.L. Pollen viability, pollen shedding and combining ability for tassel heat tolerance in maize. Crop Sci. 1987, 27, 27–31. [Google Scholar] [CrossRef]
- Schoper, J.B.; Lambert, R.J.; Vasilas, B.L.; Westgate, M.E. Plant factors controlling seed set in maize: The influence of silk, pollen and ear-leaf water status and tassel heat treatment at pollination. Plant Physiol. 1987, 83, 121–125. [Google Scholar] [CrossRef] [Green Version]
- Dupuis, I.; Dumas, C. Influence of temperature stress on in vitro fertilization and heat shock protein synthesis in maize (Zea mays L.) reproductive tissues. Plant Physiol. 1990, 94, 665–670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cicchino, M.; Edreria, J.I.R.; Uribelarrea, M.; Otegui, M.E. Heat Stress in Field-Grown Maize: Response of Physiological Determinants of Grain Yield. Crop Sci. 2011, 50, 1438–1448. [Google Scholar] [CrossRef]
- Jagadish, K.S.V.; Cairn, J.E.; Kumar, A.; Somayanda, I.M.; Craufurd, P.Q. Does Susceptibility to Heat Stress Confound Screening for Drought Tolerance in Rice? Funct. Plant Biol. 2011, 38, 261–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vani, B.; Saradhi, P.P.; Mohanty, P. Alteration in Chloroplast Structure and Thylakoid Membrane Composition due to In-vitro Heat Treatment of Rice Seedlings: Correlation with the Functional Changes. J. Plant Physiol. 2001, 158, 583–592. [Google Scholar] [CrossRef]
- Contarero, M.G.; Cirilo, A.G.; Andrade, F.H. Night Temperature at Silking affects Kernel Set in Maize. Crop Sci. 1999, 39, 701–710. [Google Scholar]
- Pradhan, G.P.; Prasad, P.W.; Fritz, A.K.; Kirkham, M.B.; Gill, B.S. Effects of Drought and High Temperature Stress on Synthetic Hexaploid Wheat. Funct. Plant Biol. 2012, 39, 190–198. [Google Scholar] [CrossRef] [PubMed]
- Lobell, D.B.; Bänziger, M.; Magorokosho, C.; Vivek, B. Nonlinear Heat Effects on African Maize as Evidenced by Historical Yield Trials. Nat. Clim. Chang. 2011, 1, 42–45. [Google Scholar] [CrossRef]
- Niang, I.; Ruppel, O.C.; Abdrabo, M.A.; Essel, A.; Lennard, C.; Padgham, J.; Urquhart, P. Africa. In Climate Change 2014: Impacts, Adaptation, and Vulnerability. Part B: Regional Aspects. Contribution of Working Group II to the Fifth Assessment Report of the Intergovernmental Panel on Climate Change; Barros, V.R., Field, C.B., Dokken, D.J., Mastrandrea, M.D., Mach, K.J., Bilir, T.E., Chatterjee, M., Ebi, K.L., Estrada, Y.O., Genova, R.C., Eds.; Cambridge University Press: Cambridge, UK; New York, NY, USA, 2014. [Google Scholar]
- Edmeades, G.O. Drought Tolerance in Maize: An Emerging Reality. A Feature in James, Clive. Global Status of Commercialized Biotech/GM Crops; International Service for the Acquisition of Agri-Biotech Applications (ISAAA) Brief No. 39; ISAAA: Ithaca, NY, USA, 2008; p. 8. [Google Scholar]
- Campos, H.; Cooper, M.J.; Habben, E.; Edmeades, G.O.; Schussler, J.R. Improving Drought Tolerance in Maize: A View from Industry. Field Crop. Res. 2004, 90, 19–34. [Google Scholar] [CrossRef]
- Nelimor, C.; Badu-Apraku, B.; Tetteh, A.Y.; N’guetta, A.S.P. Assessment of Genetic Diversity for Drought, Heat and Combined Drought and Heat Stress Tolerance in Early Maturing Maize Landraces. Plants 2019, 8, 518. [Google Scholar] [CrossRef] [Green Version]
- Panhwar, S.A.; Baloch, M.J.; Jatoi, W.A.; Veesar, N.F.; Majeedano, M.S. Combining ability estimates from line x tester mating design in upland cotton. Pak. Acad. Sci. 2008, 45, 69–74. [Google Scholar]
- Elmyhun, M.; Liyew, C.; Shita, A.; Andualem, M. Combining ability performance and heterotic grouping of maize (Zea mays) inbred lines in testcross formation in Western Amhara, North West Ethiopia. Cogent Food Agric. 2020, 6, 1727625. [Google Scholar] [CrossRef]
- Oyekunle, M.; Badu-Apraku, B.; Hearne, S.; Franco, J. Genetic diversity of tropical early-maturing maize inbreds and their performance in hybrid combinations under drought and optimum growing conditions. Field Crop. Res. 2015, 170, 55–65. [Google Scholar] [CrossRef] [Green Version]
- Akinwale, R.O.; Badu-Apraku, B.; Fakorede, M.A.B.; Vroh-Bi, I. Heterotic grouping of tropical early-maturing maize inbred lines based on combining ability in Striga-infested and Striga-free environments and the use of SSR markers for genotyping. Field Crop. Res. 2014, 156, 48–62. [Google Scholar] [CrossRef]
- Oyekunle, M.; Badu-Apraku, B. Genetic analysis of grain yield and other traits of early-maturing maize inbreds under drought and well-watered conditions. J. Agron. Crop Sci. 2014. [Google Scholar] [CrossRef]
- Derera, J.; Tongoona, P.; Vivek, B.S.; Laing, M.D. Gene Action Controlling Grain Yield and Secondary Traits in Southern African Maize Hybrids under Drought and Non-drought Environments. Euphytica 2008, 162, 411–422. [Google Scholar] [CrossRef]
- Librando, R.P.; Magulama, E.E. Classifying White Inbred Lines into Heterotic Groups Using Yield Combining Ability Effects. USMR DJ 2008, 16, 99–103. [Google Scholar]
- Kanyamasoro, M.G.; Karungi, J.; Asea, G.; Gibson, P. Determination of the Heterotic Groups of Maize Inbred Lines and the Inheritance of their Resistance to the Maize Weevil. Afr. Crop Sci. J. 2012, 20, 99–104. [Google Scholar]
- Badu-Apraku, B.; Fontem, L.A.; Akinwale, R.O.; Oyekunle, M. Biplot Analysis of Diallel Crosses of Early Maturing Tropical Yellow Maize Inbreds in Stress and Non-stress Environments. Crop Sci. 2011, 51, 173–188. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M.; Akinwale, R.O.; Aderounmu, M. Combining Ability and Genetic Diversity of Extra-early White Maize Inbreds under Stress and Non-stress environments. Crop Sci. 2013, 53, 9–26. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. The Importance of the Anthesis-silking Interval in Breeding for Drought Tolerance in Tropical Maize. Field Crop. Res. 1996, 48, 65–80. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Fakorede, M.A.B.; Oyekunle, M.; Yallou, G.C.; Obeng-Antwi, K.; Haruna, A.; Usman, I.S.; Akinwale, R.O. Gains in Grain Yield of Early Maize Cultivars Developed during Three Breeding Eras under multiple environments. Crop Sci. 2015, 55, 527–539. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Annor, B.; Oyekunle, M.; Akinwale, R.O.; Fakorede, M.A.B.; Talabi, A.O.; Akaogu, I.C.; Melaku, G.; Fasanmade, Y. Grouping of early maturing quality protein maize inbreds based on SNP markers and combining ability under multiple environments. Field Crop. Res. 2015, 183, 169–183. [Google Scholar] [CrossRef] [Green Version]
- Snedecor, G.W.; Cochran, W.G. Statistical Methods, 8th ed.; Iowa State Univ Press: Ames, IA, USA, 1989. [Google Scholar]
- Griffing, B. Concept of General and Specific Combining Ability in Relation to Diallel Crossing Systems. Aust. J. Biol. Sci. 1956, 9, 463–493. [Google Scholar] [CrossRef]
- Zhang, Y.; Kang, M.S.; Lamkey, K.R. DIALLEL-SAS05: A Comprehensive Program for Griffing’s and Gardner-Eberhart Analyses. Agron. J. 2005, 97, 1097–1106. [Google Scholar] [CrossRef]
- Hung, H.Y.; Holland, J.B. Diallel Analysis of Resistance to Fusarium Ear Rot and Fumonisin Contamination in Maize. Crop Sci. 2012, 52, 2173–2181. [Google Scholar] [CrossRef] [Green Version]
- Yan, W.; Tinker, N.A. Biplot Analysis of Multi-Environment Trial Data: Principles and Applications. Can. J. Plant Sci. 2006, 86, 623–645. [Google Scholar] [CrossRef] [Green Version]
- Mohammadi, S.A.; Prasanna, B.M.; Singh, N.N. Sequential Path Model for Determining Interrelationships among Grain Yield and Related Characters in Maize. Crop Sci. 2003, 43, 1690–1697. [Google Scholar] [CrossRef]
- SPSS Inc. SPSS Base 17.0 for Windows User’s Guide; SPSS Inc.: Chicago, IL, USA, 2007. [Google Scholar]
- Badu-Apraku, B.; Akinwale, R.O.; Oyekunle, M. Efficiency of Secondary Traits in Selecting for Improved Grain Yield in Extra-early Maize under Striga-infested and Striga-free Environments. Plant Breed. 2014, 133, 373–380. [Google Scholar] [CrossRef]
- Talabi, A.O.; Badu-Apraku, B.; Fakorede, M.A.B. Genetic Variances and Relationship among Traits of an Early Maturing Maize Population under Drought-stress and Low Nitrogen Environments. Crop Sci. 2017, 57, 681–692. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Akinwale, R.O.; Menkir, A.; Coulibaly, N.; Onyibe, J.E.; Yallou, G.C.; Abdullai, M.S.; Didjera, A. Use of GGE biplot for targeting early maturing maize cultivars to mega-environment in West Africa. Afr. Crop Sci. J. 2011, 19, 79–96. [Google Scholar] [CrossRef] [Green Version]
- Betràn, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Genetic Analysis of Inbred and Hybrid Grain Yield under Stress and Non-stress Environments in Tropical Maize. Crop Sci. 2003, 43, 807–817. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M.; Akinwale, R.O.; Lum, A.F. Combining Ability of Early-maturing White Maize Inbreds under Stress and Non-stress Environments. Agron. J. 2011, 103, 544–557. [Google Scholar] [CrossRef]
- Melani, M.D.; Carena, M.J. Alternative maize heterotic patterns for the northern corn belt. Crop Sci. 2005, 45, 2186–2194. [Google Scholar] [CrossRef]
- Bolaños, J.; Edmeades, G.O. Eight Cycles of Selection for Drought Tolerance in Lowland Tropical Maize. II. Responses in Reproductive Behavior. Field Crop. Res. 1993, 31, 253–268. [Google Scholar] [CrossRef]
- Obeng-Bio, E. Genetic Analysis of Grain Yield and Other Traits of Early Maturing Provitamin A-Quality Maize Inbred Lines under Drought and Low Soil Nitrogen Conditions. Ph.D. Thesis, University of Ghana, Accra, Ghana, 2018. [Google Scholar]
- Lawson, D.J.; Falush, D. Population identification using genetic data. Annu. Rev. Genom. Hum. Genet. 2012, 13, 337–361. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Yallou, C.; Obeng-Antwi, K.; Alidu, H.; Talabi, A.O.; Annor, B.; Oyekunle, M.; Akaogu, I.C.; Aderounmu, M. Yield gains in extra-early maize cultivars of three breeding eras under multiple environments. Agron. J. 2017, 109, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Makumbi, D.; Betrán, J.F.; Bänziger, M.; Ribaut, J. Combining Ability, Heterosis and Genetic Diversity in Tropical Maize (Zea mays L.) Under Stress and Non-stress Conditions. Euphytica 2011, 180, 143–162. [Google Scholar] [CrossRef]
- Badu-Apraku, B.; Oyekunle, M. Genetic Analysis of Grain Yield and other Traits of Extra-early Yellow Maize Inbreds and Hybrid Performance under Contrasting Environments. Field Crop. Res. 2012, 129, 99–110. [Google Scholar] [CrossRef]
- Betràn, F.J.; Beck, D.; Bänziger, M.; Edmeades, G.O. Secondary Traits in Parental Inbreds and Hybrids under Stress and Non-stress Environments in Tropical Maize. Field Crop. Res. 2003, 83, 51–65. [Google Scholar] [CrossRef]

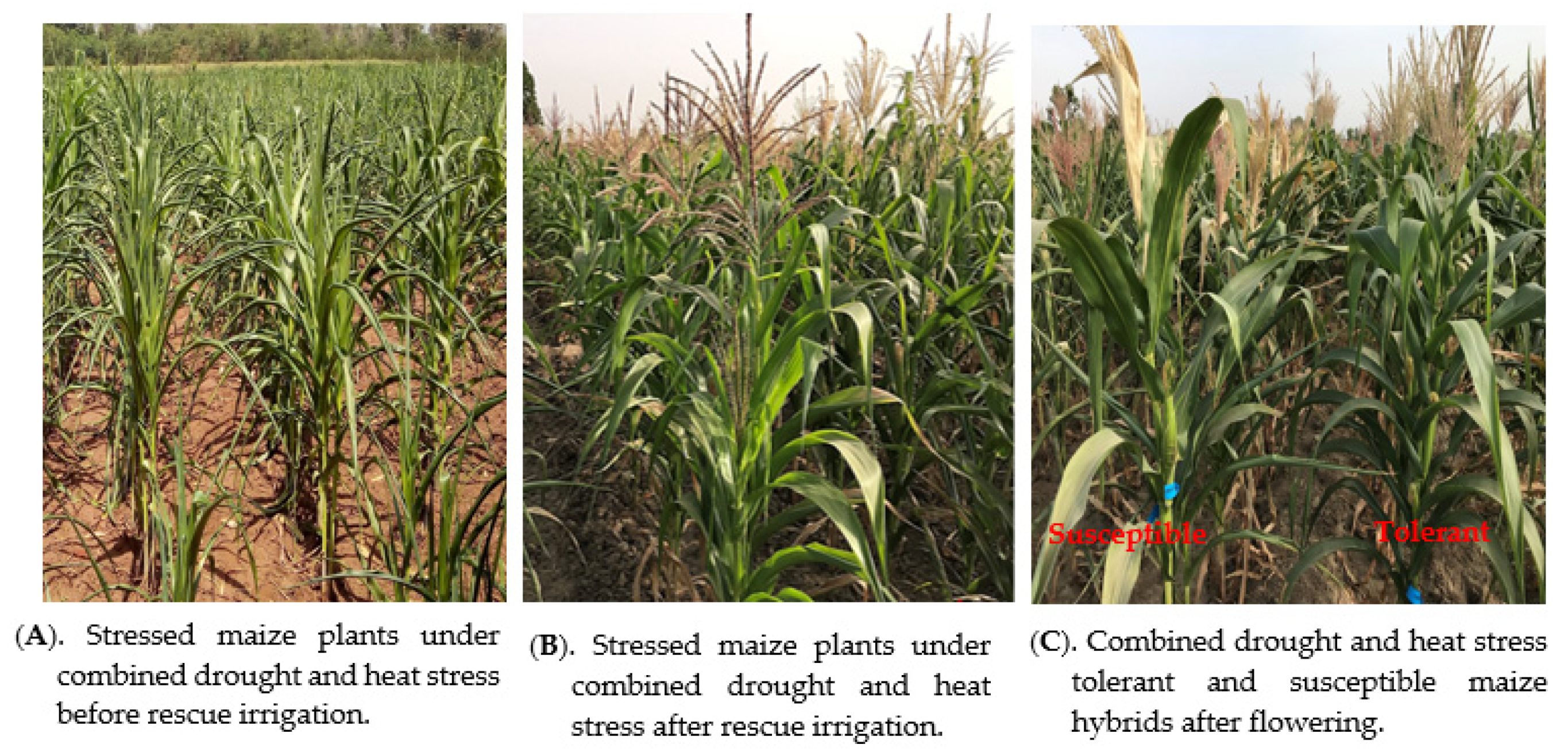
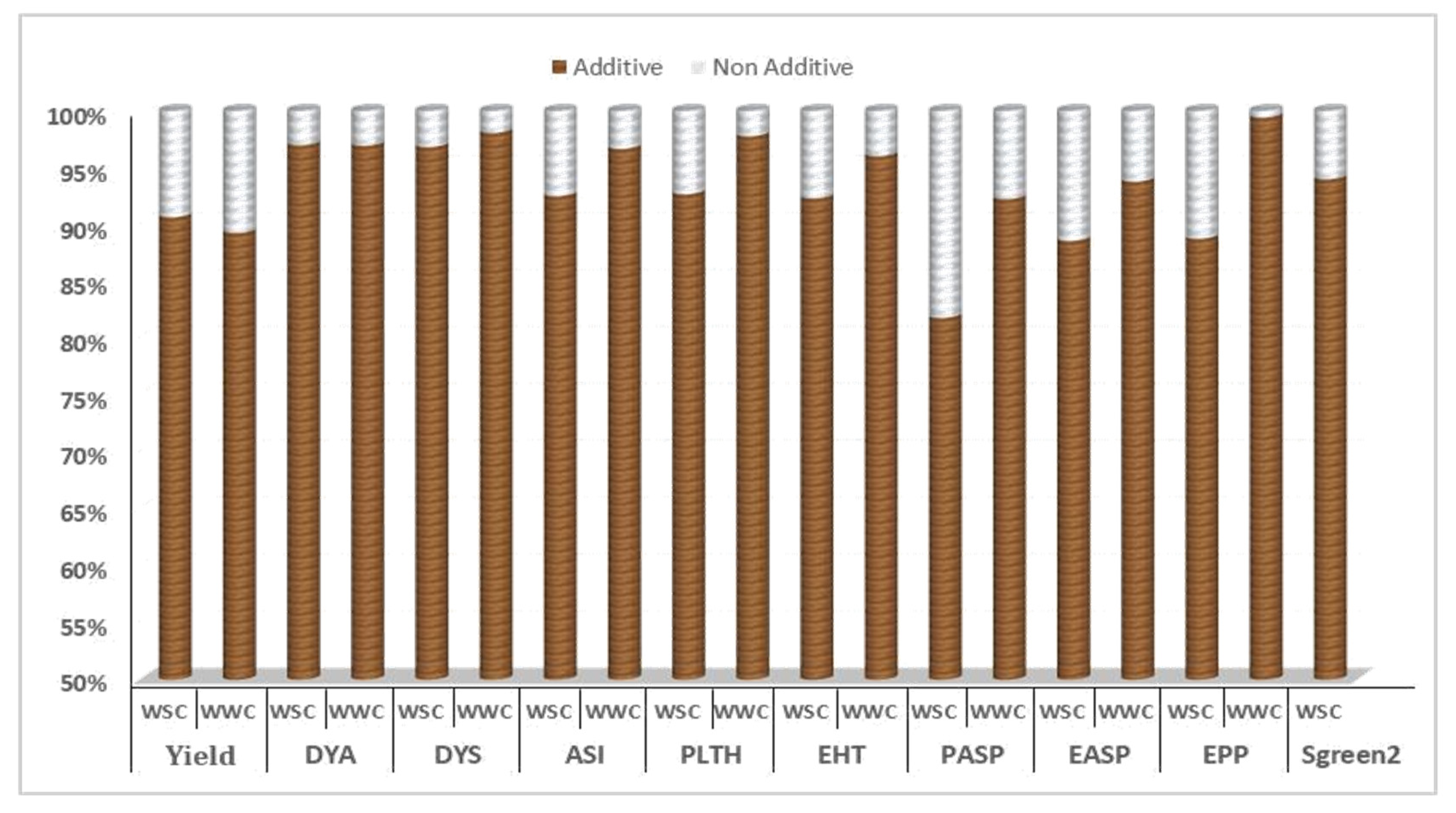

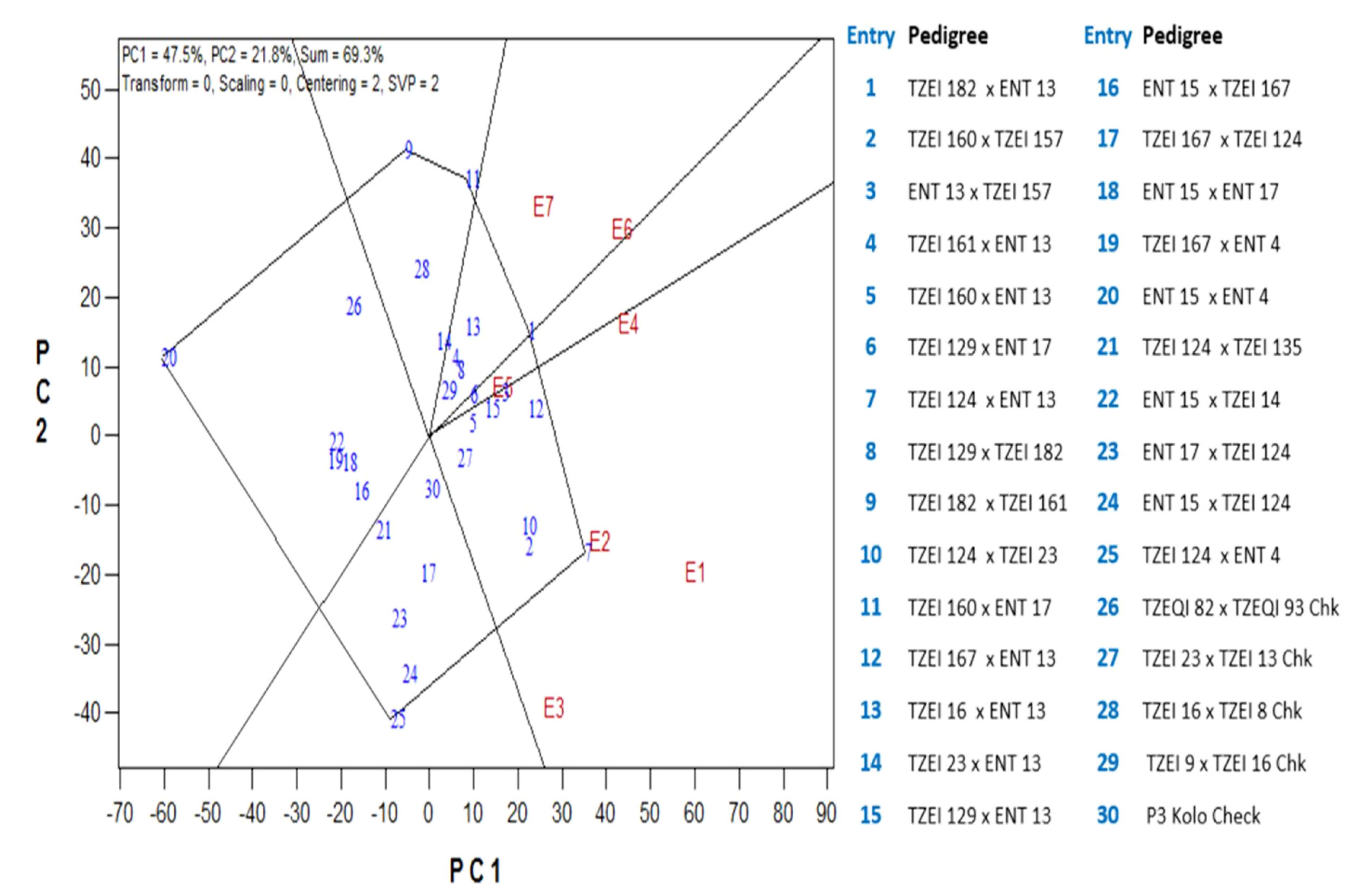
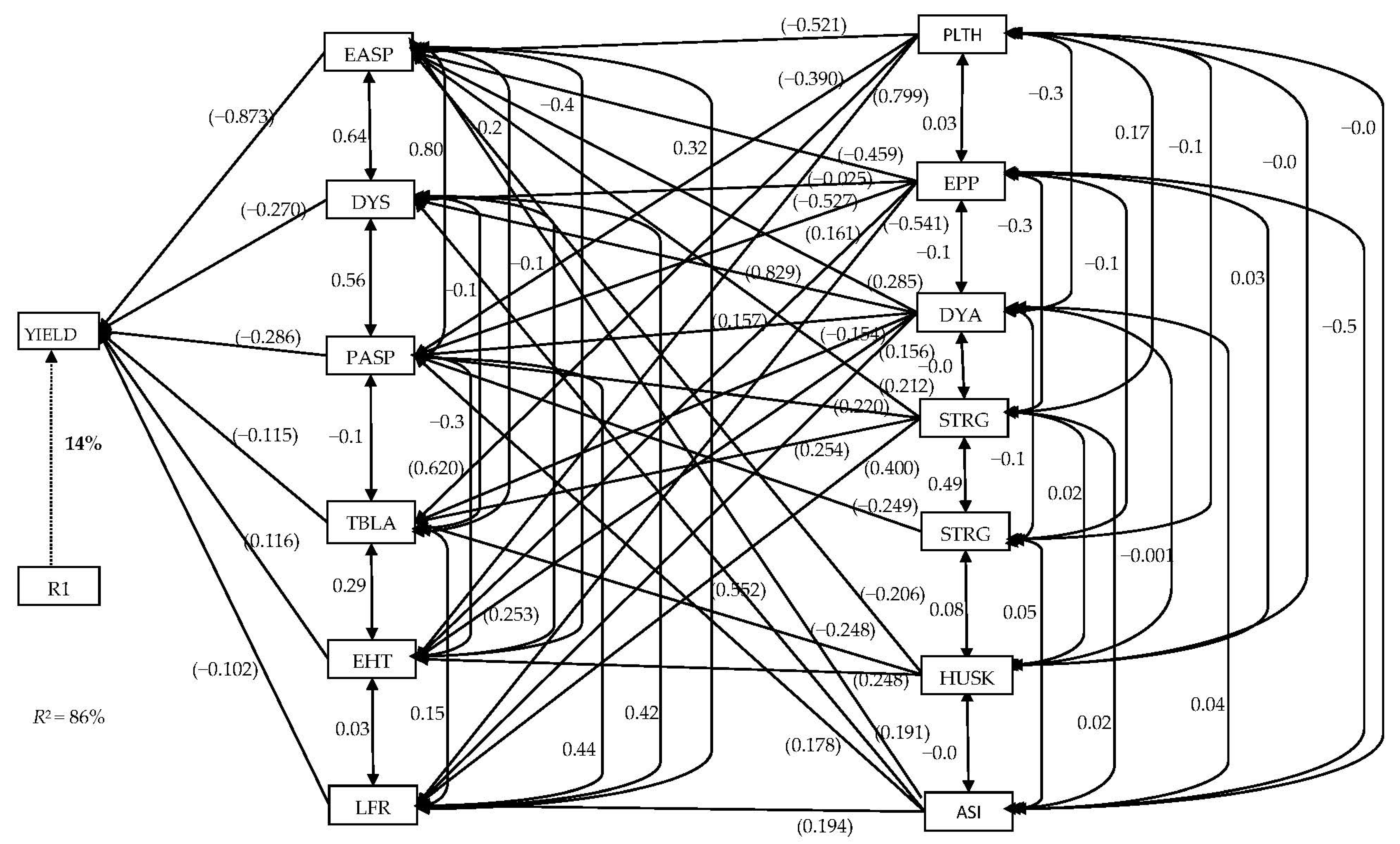

| No | Entries | Origin | Pedigree | Reaction to Drought Stress |
|---|---|---|---|---|
| 1 | ENT 4 | CIMMYT | [[KILIMA ST94A]-30/MSV-03-2-10-B-1-B-B-xP84c1 F26-2-2-6-B-3-B] F17-1-2-1-1 × P43C9-1-1-1-1-1-BBBB-1-Xp | Tolerant |
| 2 | ENT 13 | CIMMYT | [M37W/ZM607#bF37sr-2-3sr-6- … 1-B × CML486]-1 | Tolerant |
| 3 | ENT 15 | CIMMYT | CLA149 | Tolerant |
| 4 | ENT 17 | CIMMYT | [(87036/87923)-X-800-3-1-X-1-B-B-1-1-1 | Tolerant |
| 5 | TZEI 14 | IITA | TZE Comp5-Y C6 S6 Inbred 21 | Tolerant |
| 6 | TZEI 16 | IITA | TZE Comp5-Y C6 S6 Inbred 31 | Tolerant |
| 7 | TZEI 129 | IITA | TZE-Y Pop STR Co S6 Inbred 16-1-3 | Susceptible |
| 8 | TZEI 23 | IITA | TZE-Y Pop STR C0 S6 Inbred 62-2-3 | Tolerant |
| 9 | TZEI 124 | IITA | TZE-Y Pop STR Co S6 Inbred 3-1-3 | Susceptible |
| 10 | TZEI 135 | IITA | TZE-Y Pop STR Co S6 Inbred 17-2-3 | Tolerant |
| 11 | TZEI 157 | IITA | TZE-Y Pop STR Co S6 Inbred 102-1-2 | Tolerant |
| 12 | TZEI 161 | IITA | TZE-Y Pop STR Co S6 Inbred 103-2-3 | Tolerant |
| 13 | TZEI 167 | IITA | TZE Comp5-Y C6 S6 Inbred 13 | Susceptible |
| 14 | TZEI 160 | IITA | TZE-Y Pop STR Co S6 Inbred 102-2-3 | Tolerant |
| 15 | TZEI 182 | IITA | TZE-Y Pop STR Co S6 Inbred 152-2-2 | Tolerant |
| Source of Variation | DF | Grain Yield (kg ha−1) | Days to Anthesis | Days to Silking | Anthesis-Silking Interval | Plant Height (cm) | Ear Height (cm) | Plant Aspect | Ear Aspect | Ears per Plant | Husk Cover | Stay Green Characteristic (100 DAP) |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Combined drought and heat stress environments | ||||||||||||
| Env † | 2 | 33,974,256 ** | 1732.5 ** | 4016.7 ** | 675.5 ** | 4418 ** | 376 * | 2.5 * | 0.7 ns | 4.80 ** | 18.3 ** | 106.3 ** |
| Rep (Env) | 3 | 6,374,226 ** | 17.1 * | 153.9 ** | 169.9 ** | 1633 ** | 591 ** | 3.8 ** | 6.8 ** | 0.09 ns | 0.8 ** | 4.3 ** |
| Block (Rep × Env) | 60 | 1,415,603 ** | 8.8 * | 22.5 ** | 14.5 * | 572.9 ** | 254 ** | 1.4 ** | 1.1 ** | 0.06 ns | 0.2 ns | 3.2 ** |
| Hybrids | 104 | 1,388,241 ** | 46.4 ** | 65.4 ** | 21.7 ** | 594 ** | 352 ** | 1.4 ** | 0.9 ** | 0.09 ** | 0.7 ** | 2.8 ** |
| GCA | 14 | 4,439,140 ** | 246.3 ** | 342.4 ** | 79.0 ** | 2192 ** | 1262 ** | 2.7 ** | 2.5 ** | 0.26 ** | 3.0 ** | 11.4 ** |
| SCA | 90 | 913,656 ** | 15.3 ** | 22.3 ** | 12.8 ** | 346 ** | 210 ** | 1.2 ** | 0.6 ** | 0.07 ns | 0.3 ** | 1.5 * |
| Env × Hybrids | 208 | 620,636 ns | 6.9 ** | 14.4 ns | 11.3 * | 207 ns | 87 ns | 0.7 ns | 0.5 * | 0.07 ** | 0.3 ** | 1.4 * |
| Env × GCA | 28 | 1,456,065 ** | 12.6 ** | 26.8 ** | 23.3 ** | 323 * | 160 * | 0.9 ns | 0.9 ** | 0.10 ** | 0.5 ** | 2.4 ** |
| Env × SCA | 180 | 490,680 ns | 6.0 ns | 12.5 ns | 9.5 ns | 189 ns | 76 ns | 0.6 ns | 0.4 ns | 0.07 ns | 0.2 * | 1.3 ns |
| Residual | 312 | 531,587 | 5.0 | 12.7 | 8.6 | 206 | 91 | 0.6 | 0.4 | 0.05 | 0.2 | 1.1 |
| Well-watered environments | ||||||||||||
| Env | 3 | 120,650,005 ** | 31,206.0 ** | 29,741.4 ** | 76.5 ** | 49,811 ** | 74,953 ** | 8.2 ** | 20.4 ** | 6.26 ** | 9.2 ** | |
| Rep (Env) | 4 | 7,391,247 ** | 24.8 ** | 34.4 ** | 3.1 ns | 1325 ** | 674 ** | 0.7 ns | 1.4 ** | 0.07 * | 0.4 * | |
| Block (Rep × Env) | 80 | 1,880,461 ** | 3.3 ns | 4.1 ns | 1.7 ns | 137 ns | 83 ns | 0.5 * | 0.02 ns | 0.30 * | 0.2 ns | |
| Hybrids | 104 | 5,555,332 ** | 28.1 ** | 40.7 ** | 5.4 ** | 1570 ** | 490 ** | 1.7 ** | 1.6 ** | 0.07 ** | 0.9 ** | |
| GCA | 14 | 16,217,668 ** | 148.9 ** | 241.1 ** | 27.9 ** | 9041 ** | 2379 ** | 6.1 ** | 6.6 ** | 5.25 ** | 3.2 ** | |
| SCA | 90 | 3,896,747 ** | 9.4 ** | 9.5 ** | 1.9 ** | 408 ** | 196 ** | 1.0 ** | 0.9 ** | 0.06 ** | 0.5 ** | |
| Env × Hybrids | 312 | 1,296,562 ** | 4.0 ** | 5.9 ** | 3.0 ** | 183 ** | 95 ** | 0.5 ** | 0.3 ** | 0.04 ** | 0.2 ** | |
| Env × GCA | 42 | 3,049,903 ** | 10.9 ** | 17.6 ** | 8.2 ** | 273 ** | 254 ** | 1.1 ** | 0.6 ** | 0.04 ** | 0.5 ** | |
| Env × SCA | 270 | 1,023,820 * | 2.9 ns | 4.0 * | 2.2 * | 169 * | 70 ns | 0.4 ns | 0.2 * | 0.02 ** | 0.1 ns | |
| Residual | 416 | 804,728 | 2.5 | 3.2 | 1.7 | 139 | 64 | 0.3 | 0.2 | 0.01 | 0.1 | |
| Inbreds | Grain Yield (kg/ha) | Days to Silking | Plant Height | Plant Aspect | Ears per Plant | Stay Green Characteristic (100 DAP) | |||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | CDHS | |
| TZEI 160 | −49 ns | 114 ns | −1.9 ns | −2.4 ** | −12 ** | −4.4 ** | 0.2 * | −0.07 ns | 0.04 ns | 0.04 ns | −0.05 ns |
| TZEI 129 | 517 ** | 30 ns | −0.4 ns | −0.1 ns | 14 ** | 7.5 ** | −0.1 * | 0.03 ns | −0.01 ns | 0.08 * | −0.14 ns |
| ENT 15 | −109 ns | −286 ** | 1.6 ns | 2.2 ** | 5 * | 0.1 ns | 0.1 ns | 0.24 ** | −0.03 ns | −0.07 * | 1.15 ** |
| ENT 17 | 37 ns | −65 ns | 0.7 ns | 1.3 * | 4 ns | −2.5 ns | 0.1 ns | −0.02 ns | 0.06 ** | −0.02 ns | 0.23 ns |
| TZEI 182 | −446 ** | 333 ** | −1.2 ns | −2.6 ** | −8 ** | 0.3 ns | 0.2 ** | −0.20 * | −0.01 ns | 0.07 * | −0.86 ** |
| TZEI 167 | −270 * | −122 ns | 1.4 ns | 1.3 * | −5 ** | −4.4 ** | −0.1 ns | 0.06 ns | −0.05 * | 0.00 ns | 0.03 ns |
| TZEI 14 | −830 ** | −210 * | 2.6 * | 3.0 ** | −9 ** | −8.3 ** | 0.2 ** | 0.16 ns | −0.02 ns | −0.06 * | −0.15 ns |
| TZEI 124 | 612 ** | −320 ** | −0.3 ns | 1.4 * | 11 ** | 9.7 ** | −0.6 ** | 0.04 ns | −0.02 ns | −0.12 ** | 0.92 ** |
| TZEI 16 | −121 ns | −202 * | 2.0 ns | 2.0 ** | 3 ns | −0.7 ns | 0.1 ns | −0.03 ns | 0.08 ** | 0.03 ns | −0.04 ns |
| ENT 4 | −96 ns | −253 ** | 1.0 ns | 2.1 ** | 3 ns | −0.4 ns | 0.1 * | 0.31 ** | −0.01 ns | −0.04 ns | 0.63 ** |
| TZEI 161 | 59 ns | 222 * | −1.8 ns | −2.4 ** | −7 ** | −0.4 ns | 0.1 * | −0.01 ns | 0.00 ns | −0.02 ns | −0.38 * |
| TZEI 23 | −168 ns | 151 ns | −2.5 * | −3.3 ** | −18 ** | −8.4 ** | 0.2 ** | −0.06 ns | 0.01 ns | 0.04 ns | −0.26 ns |
| ENT 13 | 693 ** | 472 ** | 0.0 ns | −0.8 ns | 13 ** | 6.2 ** | −0.4 ** | −0.47 ** | −0.01 ns | 0.07 * | −0.74 ** |
| TZEI 135 | −0.1 ns | −20 ns | −0.5 ns | 0.1 ns | 5 ** | 2.2 ns | 0.1 ns | 0.13 ns | −0.01 ns | 0.00 ns | −0.19 ns |
| TZEI 157 | 172 ns | 157 ns | −0.8 ns | −1.9 ** | 3 ns | 3.7 * | −0.2 * | −0.10 ns | −0.02 ns | 0.03 ns | −0.14 ns |
| SE ± | 43 | 34 | 0.4 | 0.3 | 0.7 | 0.6 | 0.02 | 0.03 | 0.03 | 0.01 | 0.06 |
| Hybrids | Grain Yield (Kg/ha) | Days to Silking | Anthesis-Silkiing Interval | Plant Height (cm) | Plant Aspect | Ears per Plant | Ear Aspect | Stay Green Characteristic (100 DAP) | Base Index | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | WW | CDHS | CDHS | ||
| TZEI 182 × ENT 13 | 5416 | 2753 | 58 | 72 | 1 | 5 | 189 | 158 | 2.6 | 2.7 | 1.1 | 1.0 | 2.2 | 2.9 | 6.0 | 5.1 |
| TZEI 160 × TZEI 157 | 5708 | 2570 | 57 | 72 | 1 | 5 | 182 | 157 | 2.8 | 3.0 | 1.2 | 1.1 | 2.5 | 3.0 | 6.7 | 4.4 |
| ENT 13 × TZEI 157 | 5454 | 2560 | 60 | 75 | 1 | 6 | 195 | 161 | 2.1 | 2.7 | 1.2 | 1.0 | 2.0 | 2.9 | 5.5 | 4.4 |
| TZEI 161 × ENT 13 | 4854 | 2383 | 57 | 73 | 0 | 4 | 182 | 150 | 2.8 | 2.8 | 1.2 | 1.0 | 2.5 | 3.1 | 6.5 | 3.9 |
| TZEQI 82 × TZEQI 93 (Chk) | 3792 | 2309 | 65 | 78 | 2 | 4 | 175 | 147 | 2.9 | 2.3 | 1.0 | 1.0 | 3.3 | 3.5 | 5.2 | 3.8 |
| TZEI 160 × ENT 13 | 5190 | 2222 | 57 | 73 | −1 | 5 | 172 | 149 | 2.9 | 2.8 | 1.2 | 1.2 | 2.3 | 3.3 | 7.7 | 3.8 |
| TZEI 129 × ENT 17 | 5141 | 2399 | 60 | 75 | 1 | 4 | 191 | 156 | 3.0 | 3.0 | 1.2 | 1.0 | 2.2 | 3.1 | 6.2 | 3.8 |
| TZEI 124 × ENT 13 | 6584 | 1993 | 58 | 77 | 1 | 5 | 194 | 164 | 1.1 | 2.7 | 1.1 | 1.0 | 1.4 | 2.8 | 6.0 | 3.5 |
| TZEI 129 × TZEI 182 | 4915 | 2216 | 58 | 77 | 1 | 6 | 176 | 144 | 3.3 | 3.2 | 1.1 | 1.2 | 2.4 | 3.1 | 6.0 | 3.5 |
| TZEI 182 × TZEI 161 | 4045 | 2401 | 58 | 71 | 2 | 4 | 166 | 145 | 3.4 | 3.2 | 1.1 | 1.1 | 2.8 | 3.4 | 4.7 | 3.5 |
| TZEI 124 × TZEI 23 | 5806 | 1897 | 56 | 70 | 1 | 5 | 170 | 151 | 2.5 | 2.8 | 1.1 | 1.1 | 2.2 | 2.9 | 6.3 | 3.4 |
| TZEI 23 × TZEI 13 (Chk) | 5063 | 1992 | 59 | 76 | 1 | 4 | 168 | 134 | 2.9 | 3.3 | 1.3 | 1.1 | 2.7 | 3.5 | 6.5 | 2.3 |
| TZEI 16 × TZEI 8 (Chk) | 4423 | 2008 | 59 | 76 | 1 | 7 | 156 | 135 | 2.8 | 3.2 | 1.2 | 1.0 | 2.8 | 3.4 | 7.3 | 1.4 |
| TZEI 9 × TZEI 16 (Chk) | 4981 | 1835 | 59 | 76 | 1 | 7 | 164 | 144 | 2.5 | 3.5 | 1.1 | 1.0 | 2.4 | 3.6 | 7.0 | 0.2 |
| P3 Kolo (Chk) | 4690 | 1439 | 55 | 70 | 1 | 6 | 172 | 156 | 3.0 | 3.8 | 1.3 | 0.9 | 2.9 | 4.1 | 6.8 | −1.7 |
| ENT 15 × TZEI 167 | 4354 | 1069 | 64 | 82 | 3 | 9 | 175 | 137 | 3.0 | 4.3 | 1.1 | 0.9 | 2.7 | 4.2 | 7.3 | −3.8 |
| TZEI 167 × TZEI 124 | 5149 | 1200 | 61 | 79 | 1 | 11 | 193 | 153 | 2.1 | 4.0 | 1.1 | 0.7 | 2.1 | 4.0 | 7.8 | −4.0 |
| ENT 15 × ENT 17 | 4043 | 984 | 61 | 82 | 1 | 8 | 183 | 139 | 3.1 | 4.3 | 1.1 | 0.7 | 2.6 | 4.1 | 7.5 | −4.4 |
| TZEI 167 × ENT 4 | 3996 | 952 | 82 | 3 | 8 | 174 | 138 | 3.0 | 4.3 | 1.0 | 0.8 | 2.5 | 4.4 | 7.7 | −4.5 | |
| ENT 15 × ENT 4 | 2021 | 594 | 66 | 84 | 2 | 7 | 167 | 131 | 4.4 | 4.3 | 1.0 | 0.8 | 3.4 | 4.2 | 7.2 | −4.8 |
| TZEI 124 × TZEI 135 | 4634 | 949 | 60 | 81 | 2 | 10 | 187 | 154 | 2.8 | 4.2 | 1.1 | 0.7 | 2.3 | 4.2 | 7.2 | −5.0 |
| ENT 15 × TZEI 14 | 4020 | 849 | 63 | 83 | 3 | 9 | 177 | 137 | 3.4 | 4.3 | 1.1 | 0.7 | 2.6 | 4.3 | 7.2 | −5.1 |
| ENT 17 × TZEI 124 | 4859 | 721 | 60 | 84 | 1 | 10 | 190 | 142 | 2.8 | 4.5 | 1.2 | 0.7 | 2.2 | 4.6 | 7.8 | −6.1 |
| ENT 15 × TZEI 124 | 5290 | 736 | 61 | 83 | 3 | 12 | 189 | 156 | 2.5 | 4.2 | 1.2 | 0.5 | 2.1 | 4.2 | 7.7 | −6.5 |
| TZEI 124 × ENT 4 | 5097 | 649 | 60 | 82 | 2 | 14 | 182 | 154 | 2.5 | 4.2 | 1.1 | 0.7 | 2.1 | 4.3 | 7.3 | −6.7 |
| Grand mean | 4571 | 1684 | 60 | 76 | 1 | 7 | 174 | 145 | 3.0 | 3.5 | 1.1 | 0.9 | 2.6 | 3.7 | 6.5 | |
| * LSD | 865 | 775 | 1.7 | 3.9 | 1.3 | 3.2 | 12 | 15 | 0.50 | 0.90 | 0.10 | 0.30 | 0.40 | 0.70 | 1.10 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nasser, L.M.; Badu-Apraku, B.; Gracen, V.E.; Mafouasson, H.N.A. Combining Ability of Early-Maturing Yellow Maize Inbreds under Combined Drought and Heat Stress and Well-Watered Environments. Agronomy 2020, 10, 1585. https://doi.org/10.3390/agronomy10101585
Nasser LM, Badu-Apraku B, Gracen VE, Mafouasson HNA. Combining Ability of Early-Maturing Yellow Maize Inbreds under Combined Drought and Heat Stress and Well-Watered Environments. Agronomy. 2020; 10(10):1585. https://doi.org/10.3390/agronomy10101585
Chicago/Turabian StyleNasser, Laouali M., Baffour Badu-Apraku, Vernon E. Gracen, and Hortense N. A. Mafouasson. 2020. "Combining Ability of Early-Maturing Yellow Maize Inbreds under Combined Drought and Heat Stress and Well-Watered Environments" Agronomy 10, no. 10: 1585. https://doi.org/10.3390/agronomy10101585






