Yield and Quality in Purple-Grained Wheat Isogenic Lines
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Multilocational Experiement in 2017
2.3. Experiement in Turkey in 2018
3. Results
3.1. Genotype by Environment Interaction for Agronomic and Quality Traits in the 2017 Experiments
3.2. Effects of Purple Grain Genes on Agronomic Traits, Grain, Flour, and Bread Quality in the 2018 Experiment
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Lia, D.; Wanga, P.; Luob, Y.; Zhaoa, M.; Chena, F. Health benefits of anthocyanins and molecular mechanisms: Update from recent decade. Crit. Rev. Food Sci. Nutr. 2017, 57, 1729–1741. [Google Scholar] [CrossRef]
- Grausgruber, H.; Atzgersdorfer, K.; Bohmdorefr, S. Purple and blue wheat—health-promoting grains with increased antioxidant activity. Cereal Foods World 2018, 63, 217–220. [Google Scholar]
- Sytar, O.; Bosko, P.; Zivcak, M.; Brestic, M.; Smetanska, I. Bioactive phytochemicals and antioxidant properties of the grain and sprouts of colored wheat genotypes. Molecules 2018, 23, 2282. [Google Scholar] [CrossRef] [PubMed]
- Zeven, A.C. Wheats with purple and blue grain. Euphytica 1991, 56, 243–258. [Google Scholar] [CrossRef]
- Kassegn, H.H. Determination of proximate composition and bioactive compounds of the Abyssinian purple wheat. Cogent Food Agric. 2018, 4, 1421415. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Pestsova, E.G.; Röder, M.S.; Börner, A. Molecular mapping, phenotypic expression and geographical distribution of genes determining anthocyanin pigmentation of coleoptiles in wheat (Triticum aestivum L.). Theor. Appl. Genet. 2002, 104, 632–637. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Pshenichnikova, T.A.; Röder, M.S.; Börner, A. Clustering anthocyanin pigmentation genes in wheat group 7 chromosomes. Cereal Res. Commun. 2009, 37, 391–398. [Google Scholar] [CrossRef]
- Khlestkina, E.K.; Gordeeva, E.I.; Arbuzova, V.S. Molecular and functional characterization of wheat near-isogenic line ‘i:S29Ra’ having intensive anthocyanin pigmentation of the coleoptile, culm, leaves and auricles. Plant Breed. 2014, 133, 454–458. [Google Scholar] [CrossRef]
- Tereshchenko, O.Y.; Gordeeva, E.I.; Arbuzova, V.S.; Börner, A.; Khlestkina, E.K. The D genome carries a gene determining purple grain colour in wheat. Cereal Res. Commun. 2012, 40, 334–341. [Google Scholar] [CrossRef]
- Tereshchenko, O.Y.; Arbuzova, V.S.; Khlestkina, E.K. Allelic state of the genes conferring purple pigmentation of different wheat organs predetermines transcriptional activity of the anthocyanin biosynthesis structural genes. J. Cereal Sci. 2013, 57, 10–13. [Google Scholar] [CrossRef]
- Gordeeva, E.I.; Shoyeva, O.Y.; Khlestkina, E.K. Marker-assisted development of bread wheat near-isogenic lines carrying various combinations of purple pericarp (Pp) alleles. Euphytica 2015, 203, 469–476. [Google Scholar] [CrossRef]
- Shoeva, O.Y.; Gordeeva, E.I.; Khlestkina, E.K. The regulation of anthocyanin synthesis in the wheat pericarp. Molecules 2014, 19, 20266–20279. [Google Scholar] [CrossRef] [PubMed]
- Martinek, P.; Jirsa, O.; Vaculova, K.; Chrpova, J.; Watanabe, N.; Buresova, V.; Kopecky, D.; Stiasna, K.; Vyhnanek, T.; Trojan, V. Use of wheat gene resources with different colour in breeding. In Proceedings of the 64 Tagung der Vereiningung der pflanzenzuchter und saatgutkaufleute Osterreichs, Raumberg-Gumpenstein, Austria, 25–26 November 2013; pp. 1–4. [Google Scholar]
- Hong, M.J.; Kim, D.Y.; Nam, B.M.; Ahn, J.-W.; Kwon, S.-J.; Seo, Y.W.; Kim, J.-B. Characterization of novel mutants of hexaploid wheat (Triticum aestivum) with various depths of purple grain color and antioxidant capacity. J. Sci. Food Agric. 2019, 99, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Bohmdorfer, S.; Oberlerchner, J.T.; Fuchs, C.; Rosenau, T.; Graugsgruber, H. Profiling and quantification of grain anthocyanins in purple pericarp x blue aleurone wheat crosses by high-performance thin-layer chromatography and densitometry. Plant Methods 2018, 14, 29. [Google Scholar] [CrossRef]
- Kryger, S.; Morlock, G.E. Fingerprinting and characterization of anthocyanins in 94 colored wheat varieties and blue aleurone and purple pericarp wheat crosses. J. Chromatogr. A 2018, 1538, 75–85. [Google Scholar] [CrossRef]
- Syed Jafar, S.; Baron, J.; Siebenhandl-Ehn, S.; Rosenau, T.; Bohmdorfer, S.; Graugsgruber, H. Increased anthocyanin content in purple pericarp x blue aleurone wheat crosses. Plant Breed. 2013, 132, 546–552. [Google Scholar] [CrossRef]
- Knievel, D.C.; Abdel-Aal, E.-S.; Rabalski, I.; Nakamura, T.; Hucl, P. Grain color development and the inheritance of high anthocyanin blue aleurone and purple pericarp in spring wheat. J. Cereal Sci. 2009, 50, 113–120. [Google Scholar] [CrossRef]
- Abdel-Aal, E.-S.; Hucl, P.; Shipp, J.; Rabalski, I. Compositional differences in anthocyanins from blue- and purple- grained spring wheat grown jn four environments in central Saskatchewan. Cereal Chem. 2016, 93, 32–38. [Google Scholar] [CrossRef]
- Bustos, D.V.; Riegel, R.; Calderini, D.F. Anthocyanin content of grain in purple wheat is affected by grain position, assimilate availability and agronomic management. J. Cereal Sci. 2011, 55, 257–264. [Google Scholar] [CrossRef]
- Hosseinian, F.S.; Li, W.; Beta, T. Measurement of anthocyanins and other phytochemicals in purple wheat. Food Chem. 2008, 109, 916–924. [Google Scholar] [CrossRef]
- Li, X.; Lv, X.; Wang, X.; Zhang, M.; Ren, M. Effects of abiotic stress on anthocyanin accumulation and grain weight in purple wheat. Crop Pasture Sci. 2018, 69, 1208–1214. [Google Scholar] [CrossRef]
- Ma, D.; Zhang, J.; Hou, J.; Li, Y.; Huang, X.; Wang, C.; Lu, H.; Zhu, Y.; Guo, T. Evaluation of yield, processing quality and nutritional quality in different-colored wheat grains under nitrogen and phosphorus application. Crop Sci. 2018, 58, 402–415. [Google Scholar] [CrossRef]
- Shoeva, O.Yu.; Gordeeva, E.I.; Arbuzova, V.S.; Khlestkina, E.K. Anthocyanins participate in protection of wheat seedlings from osmotic stress. Cereal Res. Commun. 2017, 45, 47–56. [Google Scholar] [CrossRef]
- Shoeva, O.Yu.; Khlestkina, E.K. Anthocyanins participate in the protection of wheat seedlings against cadmium stress. Cereal Res. Commun. 2018, 46, 242–252. [Google Scholar] [CrossRef]
- Gordeeva, E.I.; Shoeva, O.Yu.; Yudina, R.S.; Kukoeva, T.V.; Khlestkina, E.K. Effect of seed pre-sowing gamma-irradiation treatment in bread wheat lines differing by anthocyanin pigmentation. Cereal Res. Commun. 2018, 46, 41–53. [Google Scholar] [CrossRef]
- Zrckova, M.; Capuchova, I.; Eliasova, M.; Paznoch, L.; Pasderu, K.; Dvorak, P.; Konvalina, P.; Orsak, M.; Sterba, Z. The effect of genotypes, weather conditions and cropping system on antioxidant activity and content of selected antioxidant compunds in wheat with colored grain. Plant Soil Eviron. 2018, 64, 530–538. [Google Scholar] [CrossRef]
- Eticha, F.; Grausgruber, H.; Siebenhandl-Ehn, S.; Berghofer, E. Some agronomic and chemical traits of blue aleurone and purple pericarp wheat (Triticum L.). J. Agric. Sci. Technol. 2011, 1, 48–58. [Google Scholar]
- Li, W.; Pickard, M.D.; Beta, T. Evaluation of antioxidant activity and electronic taste and aroma properties of antho-beers from purple grain. J. Agric. Food Chem. 2007, 55, 8958–8966. [Google Scholar] [CrossRef]
- Pasqualone, A.; Bianco, A.M.; Paradiso, V.M.; Summo, C.; Gabarcorta, G.; Caponio, F.; Blanco, A. Production and characterization of functional biscuits obtained from purple wheat. Food Chem. 2015, 180, 64–70. [Google Scholar] [CrossRef]
- Usenko, N.I.; Khlestkina, E.К.; Asavasanti, S.; Gordeeva, E.I.; Yudina, R.S.; Otmakhova, Y.S. Possibilities of enriching food products with anthocyanins by using new forms of cereals. Foods Raw Mater. 2018, 6, 128–135. [Google Scholar] [CrossRef]
- Fiocco, D.B.M.; De Simone, V.; De Leonardis, A.M.; Giovaniello, V.; Del Nobile, M.A.; Padalino, L.; Lecce, L.; Borelli, G.M.; De Vita, P. Use of purple durum wheat to produce naturally functional fresh and dry pasta. Food Chem. 2016, 205, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Beta, T. Flour and bread from black-, purple-, and blue-colored wheats. In Flour and Breads and Their Fortification in Health and Disease Prevention; Preedy, V.R., Watson, R.R., Patel, V.B., Eds.; Academic Press: Cambridge, MA, USA, 2011; pp. 59–67. [Google Scholar]
- Khlestkina, E.К.; Usenko, N.I.; Gordeeva, E.I.; Stabrovskaya, O.I.; Sharfunova, I.B.; Otmakhova, Y.S. Evaluation of wheat products with high flavonoid content: Justification of importance of marker-assisted development and production of flavonoid-rich wheat cultivars. Vavilovskii Zhurnal Genetiki i Selektsii 2017, 21, 545–553. [Google Scholar] [CrossRef]
- Paznocht, L.; Kotilkova, Z.; Orsak, M.; Lachman, J.; Martinek, P. Carotenoid changes of colored-grain wheat flours during bun-making. Food Chem. 2019, 277, 727–734. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aal, E.-S.; Hucl, P.; Rabalski, I. Composition and antioxidant properties of anthocyanin-rich products prepared from purple wheat. Food Chem. 2018, 254, 13–19. [Google Scholar] [CrossRef] [PubMed]
- Ma, D.; Zhang, J.; Li, Y.; Wang, C. Quality of noodles made from colour-grained wheat. Czech J. Food Sci. 2018, 36, 314–320. [Google Scholar]
- Tian, S.-Q.; Chen, Z.; Wei, Y. Measurement of colour-grained wheat nutrient compounds and the application of combination technology in dough. J. Cereal Sci. 2018, 83, 63–67. [Google Scholar] [CrossRef]
- Hrivna, L.; Zigmundova, V.; Buresova, I.; Maco, R.; Vyhnanek, T.; Trojan, V. Pheological properties of dough and baking quality of products using coloured wheat. Plant Soil Environ. 2018, 64, 203–208. [Google Scholar]
- Genetic Resources Information System for Wheat and Triticale. SARATOVSKAYA-29. Available online: http://wheatpedigree.net/sort/show/56274 (accessed on 5 October 2019).
- Genetic Resources Information System for Wheat and Triticale. PURPLE-GRAIN. Available online: http://wheatpedigree.net/sort/show/50574 (accessed on 5 October 2019).
- Genetic Resources Information System for Wheat and Triticale. PURPLE-FEED. Available online: http://wheatpedigree.net/sort/show/50573 (accessed on 5 October 2019).
- Buresova, I.; Trojan, V.; Helis, M. Characteristics of flour and dough from purple and blue wheat grain. Potravin. Slovak J. Food Sci. 2019, 13, 163–166. [Google Scholar]
- Arbuzova, V.S.; Maystrenko, O.I.; Popova, O.M. Development of near-isogenic lines of the common wheat cultivar ‘Saratovskaya 29’. Cereal Res. Commun. 1998, 26, 39–46. [Google Scholar]
- Pask, A.J.D.; Pietragalla, J.; Mullan, D.M.; Reynolds, M.P. Physiological Breeding II: A Field Guide to Wheat Phenotyping; CIMMYT: El Batán, Mexico, 2012; pp. 72–130. [Google Scholar]
- Shao, Y.; Xu, F.; Sun, X.; Bao, J.; Beta, T. Phenolic acids, anthocyanins, and antioxidant capacity in rice (Oryza sativa L.) grains at four stages of development after flowering. Food Chem. 2014, 143, 90–96. [Google Scholar] [CrossRef]
- Zhu, K.X.; Lian, C.X.; Guo, X.N.; Peng, W.; Zhou, H.M. Antioxidant activities and total phenolic contents of various extracts from defatted wheat germ. Food Chem. 2011, 126, 1122–1126. [Google Scholar] [CrossRef]
- Anonymous. Approved Method; American Association of Cereal Chemists International: St. Paul, MN, USA, 2000. [Google Scholar]
- Anonymous. Approved Method; American Association of Cereal Chemists International: St. Paul, MN, USA, 2010. [Google Scholar]
- Singleton, V.L.; Rossi, J.A. Colorimetry of total phenolics with phosphomolybdic-phosphotungistic acid reagent. Am. J. Enol. Viticul. 1965, 16, 144–158. [Google Scholar]
- Gao, L.; Wang, S.; Oomah, B.D.; Mazza, G. Wheat quality: Antioxidant activity of wheat millstreams. In Wheat Quality Education; Ng, P., Wrigley, C.W., Eds.; AACC International: St Paul, MN, USA, 1995; pp. 219–233. [Google Scholar]
- Brand-Williams, W.; Cuvelier, M.E.; Berset, C. Use of a radical method to evaluate antioxidant activity. Food Sci. Technol. 1995, 28, 25–30. [Google Scholar] [CrossRef]
- Kaçar, B.; Inal, A. Bitki Analizleri; Nobel: Ankara, Turkey, 2008; 912p. [Google Scholar]
- Henderson, J.W.; Ricker, R.D.; Bidlingmeyer, B.A.; Woodward, C. Rapid, Accurate, Sensitive, and Reproducible HPLC Analysis of Amino Acids; Agilent technologies: Santa Clara, CA, USA, 2000. [Google Scholar]
- Chandi, G.K.; Seetharaman, K. Optimization of gluten peak tester: A statistical approach. J. Food Qual. 2012, 35, 69–75. [Google Scholar] [CrossRef]
- Garg, M.; Meenakshi, C.; Venkatesh, R.K.; Saloni, S.; Nand, S.; Aman, K.; Jaspreet, J.; Manpreet, S.; Sukhvinder, S. Transfer of grain colors to elite wheat cultivars and their characterization. J. Cereal Sci. 2016, 71, 138–144. [Google Scholar] [CrossRef]
- Shin, O.H.; Kim, D.Y.; Seo, Y.W. Effects of different depth of grain colour on antioxidant capacity during water imbibition in wheat (Triticum aestivum L.). J. Sci. Food Agric. 2017, 97, 2750–2758. [Google Scholar] [CrossRef]
- Erayman, M.; Sandhu, D.; Sidhu, D.; Dilbirligi, M.; Baenziger, P.S.; Gill, K.S. Demarcating the gene-rich regions of the wheat genome. Nucleic Acids Res. 2004, 32, 3546–3565. [Google Scholar] [CrossRef]
- Röder, M.S.; Huang, X.Q.; Börner, A. Fine mapping of the region on wheat chromosome 7D controlling grain weight. Funct. Integr. Genom. 2008, 8, 79–86. [Google Scholar] [CrossRef]
- Listman, M.; Guzman, C.; Palacios-Rojas, N.; Pfeiffer, W.H.; San Vicente, F.; Govindan, V. Improving nutrition through biofortification: Preharvest and postharvest technologies. Cereal Foods World 2019, 64. [Google Scholar] [CrossRef]

| No. | Genotype Name | Abbreviation | Genotype 1 | Pp3 Gene Source | Grain Color |
|---|---|---|---|---|---|
| 1 | Seri-82 (Check) | Seri | - | - | White |
| 2 | Saratovskaya 29 (Recurrent parent) | S29 | pp-D1; pp3 | - | Red |
| 3 | Saratovskaya 29-Purple PF | S29-Purple (PF) | Pp-D1; Pp3PF | Purple Feed | Purple |
| 4 | Saratovskaya 29-Red PF | S29-Red (PF) | Pp-D1; pp3PF | Purple Feed | Red |
| 5 | Saratovskaya 29-Purple P | S29-Purple (P) | Pp-D1; Pp3P | Purple | Purple |
| 6 | Saratovskaya 29-Red P | S29-Red (P) | Pp-D1; pp3P | Purple | Red |
| Site | Latitude (°N) | Longitude (°E) | Season | Seasonal Rainfall (mm) | Plots (m2) | Reps | Yield (kg/ha) | Protein (%) |
|---|---|---|---|---|---|---|---|---|
| Konya, TR 1 | 37.53 | 32.29 | Nov–Jun | 189 | 5 | 2 | 2317 | 11.9 |
| Cumra, TR | 37.38 | 32.44 | 182 | 5 | 2 | 4016 | 12.5 | |
| Ankara, TR | 39.36 | 32.40 | 224 | 1 | 1 | - | 13.7 | |
| Izmir, TR | 38.33 | 27.02 | 504 | 1 | 1 | - | 13.5 | |
| Omsk, RU | 55.01 | 73.18 | May–Sep | 170 | 5 | 3 | 3141 | 14.4 |
| Astana, KZ | 51.38 | 71.01 | 103 | 1 | 2 | 1666 | 12.0 | |
| Kostanay, KZ | 53.51 | 62.08 | 218 | 1 | 1 | 1838 | 14.1 |
| Trait | Number of Sites Trait Recorded | Trait Mean Value | Significance 1 of Main Effects and Their Interactions | CV (%) | ||
|---|---|---|---|---|---|---|
| Sites | Genotypes | Genotypes × Sites | ||||
| Grain yield, kg/ha | 5 | 2841 | *** | * | * | 12.9 |
| Spike length, cm | 4 | 8.53 | *** | ns | ns | 6.7 |
| Spikelets/spike | 4 | 16.5 | *** | *** | ns | 5.1 |
| Grains/spike | 4 | 37.0 | * | ns | ns | 8.4 |
| 1000-kernel weight, g | 7 | 32.5 | *** | *** | * | 2.8 |
| Grain weight/spike, g | 4 | 1.43 | *** | ns | ns | 13.9 |
| Test weight, kg/hl | 7 | 74.9 | *** | *** | *** | 0.7 |
| Grain brightness (L) (UNL) | 7 | 83.7 | *** | *** | ** | 0.5 |
| Grain redness (a) (UNL) | 7 | 1.32 | *** | *** | * | 8.3 |
| Grain yellowness (b) (UNL) | 7 | 11.0 | *** | *** | *** | 2.1 |
| Zn content, mg/kg (Mex) | 7 | 25.1 | *** | ns | * | 9.3 |
| Fe content, mg/kg (Mex) | 7 | 34.7 | *** | *** | * | 5.5 |
| Anthocyanin content, mM/g | 7 | 130.9 | ** | ** | ns | 12.6 |
| Antioxidants content, mM/g | 7 | 0.216 | * | ns | ns | 6.0 |
| Grain hardness (ESK) | 6 | 78.7 | *** | *** | *** | 1.7 |
| Grain protein content, % (Mex) | 7 | 13.2 | *** | ** | *** | 3.2 |
| SDS sedimentation, mL (Mex) | 3 | 20.5 | *** | *** | * | 2.6 |
| Alveograph, W (Mex) | 3 | 489 | *** | *** | ns | 7.6 |
| Alveograph, P/L (Mex) | 3 | 3.14 | *** | ns | ns | 25.8 |
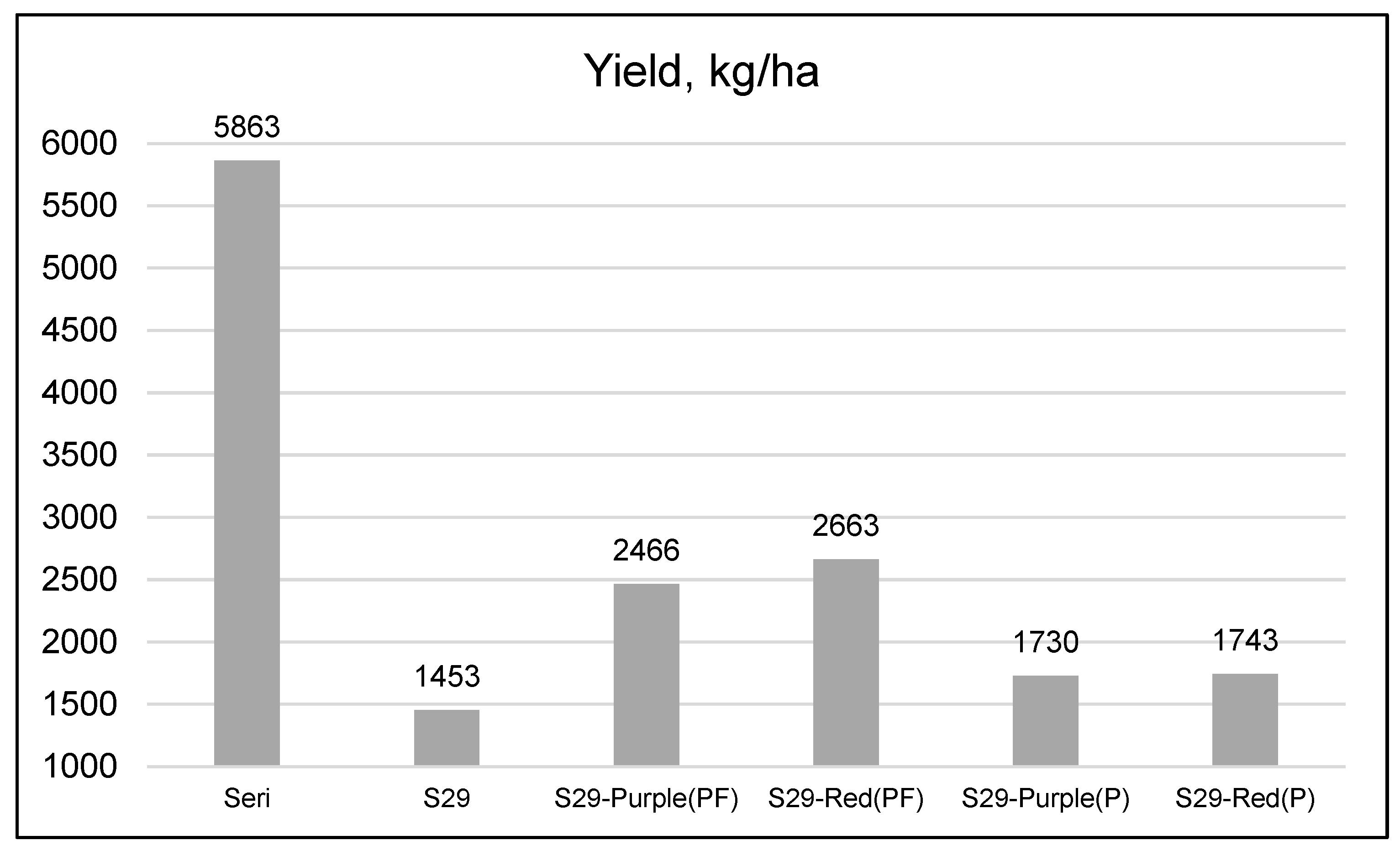
| Genotype | Spike Length (cm) | Spikelets/Spike | Grains/Spike | 1000-Kernal Weight (g) | Grain Weight/Spike (g) | Test Weight (kg/m3) | Yield (kg/ha) |
|---|---|---|---|---|---|---|---|
| Seri | 8.94 | 19.2a 1 | 46.2 | 34.2a | 1.77 | 75.6 | 3197a |
| S29 | 8.31 | 16.1b | 36.3 | 32.1b | 1.41 | 75.6 | 2752bc |
| S29-Purple(PF) | 8.33 | 15.4b | 31.8 | 34.0a | 1.30 | 74.9 | 3010ab |
| S29-Red(PF) | 8.92 | 16.2b | 35.3 | 32.7b | 1.40 | 74.4 | 2801bc |
| S29-Purple(P) | 8.14 | 16.1b | 36.4 | 31.7b | 1.37 | 74.0 | 2769bc |
| S29-Red(P) | 8.50 | 16.0b | 36.2 | 30.2c | 1.35 | 75.1 | 2518c |
| Genotype | L | a | b | Anthocyan in (mM/g) | Fe (mg/kg) | Protein Content, (%) | SDS Sedimen-Tation (mL) | Alveograph W |
|---|---|---|---|---|---|---|---|---|
| Seri | 86.6a 1 | 1.00d | 11.5c | 112b | 38.3a | 13.2ab | 12.3b | 188c |
| S29 | 84.8b | 1.22c | 11.8b | 125b | 33.7bc | 12.9b | 21.9a | 506b |
| S29-Purple(PF) | 80.5c | 1.56b | 9.2e | 151a | 35.5b | 13.6a | 22.3a | 565ab |
| S29-Red(PF) | 84.6b | 1.17c | 12.0a | 132b | 33.8bc | 13.3b | 22.0a | 543ab |
| S29-Purple(P) | 80.5c | 1.78a | 9.4d | 140ab | 33.1c | 13.0b | 22.6a | 579a |
| S29-Red(P) | 84.9b | 1.18c | 12.1a | 124b | 33.5bc | 13.3b | 22.1a | 550ab |
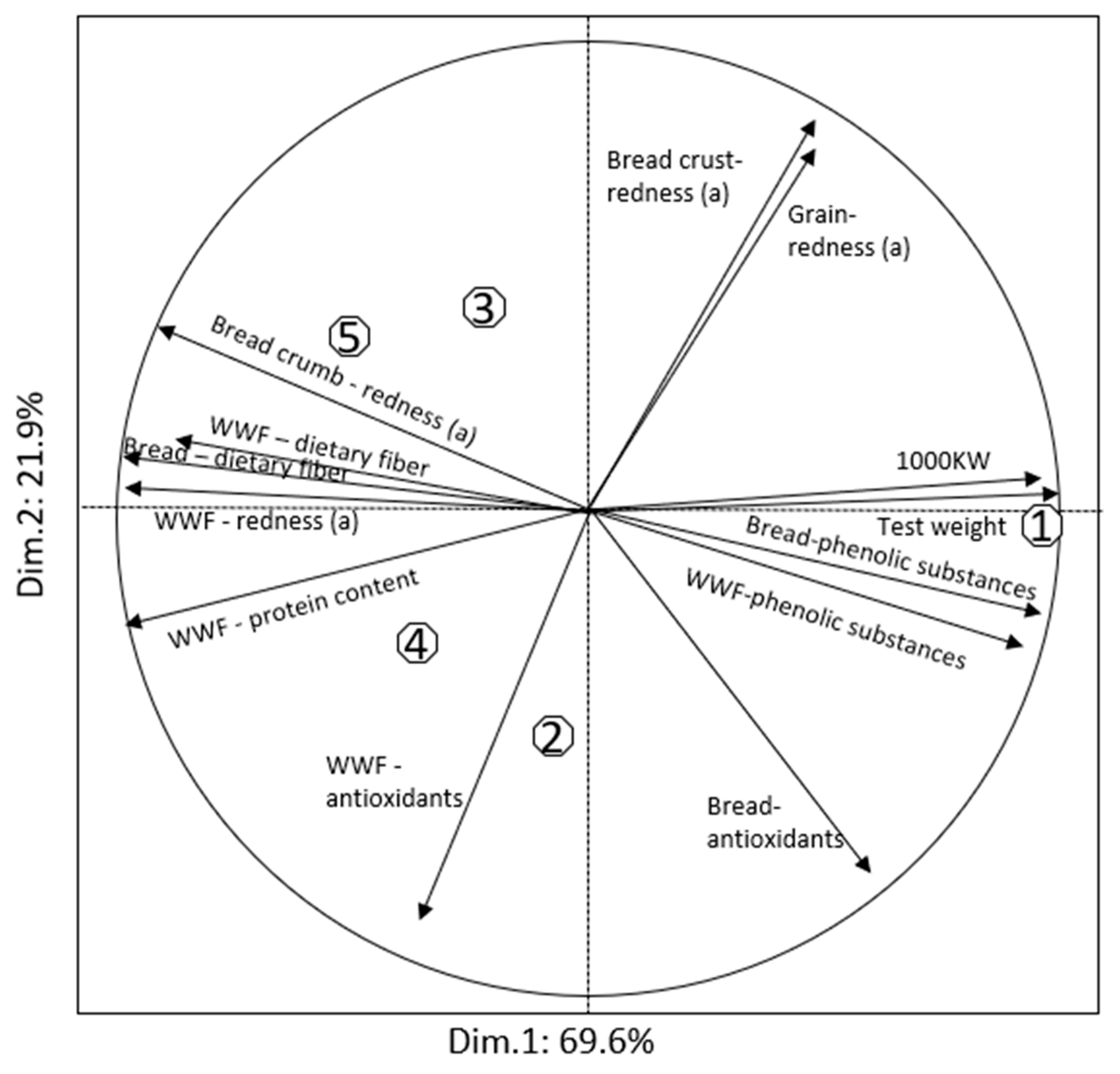
| Trait | Product | Seri | S29-Purple (PF) | S29-Red (PF) | S29-Purple (P) | S29-Red (P) |
|---|---|---|---|---|---|---|
| Test weight, kg/m3 | Grain | 79.7a 1 | 78.0b | 77.5b | 77.5b | 78.1b |
| 1000-kernel weight, g | Grain | 35.6a | 31.9b | 32.0b | 29.9b | 29.7b |
| Protein content, % | Flour | 11.1b | 13.2a | 12.3a | 12.7a | 12.4a |
| WWF 2 | 13.5b | 15.6a | 15.2a | 15.5a | 15.4a | |
| Zeleny sediment., mL | Flour | 30.0b | 54.2a | 53.5a | 59.5a | 57.0a |
| Color: brightness (L) | Grain | 56.1a | 41.9c | 50.9b | 44.5c | 51.0b |
| WWF | 82.8a | 75.1c | 79.0b | 75.3c | 78.9b | |
| B. Crust | 46.2a | 35.8b | 43.0a | 37.6b | 42.3a | |
| B. Crumb | 56.5a | 38.9c | 46.7b | 41.0c | 46.9b | |
| Color: redness (a) | Grain | 8.99 | 8.19 | 8.81 | 8.58 | 8.71 |
| WWF | 3.12b | 3.74a | 3.89a | 4.04a | 3.98a | |
| B. Crust | 8.08a | 6.49b | 7.72a | 7.04ab | 7.60a | |
| B. Crumb | 4.70c | 5.04b | 5.35a | 5.22ab | 5.44a | |
| Color: yellowness (b) | Grain | 27.9a | 17.2e | 24.1b | 19.2d | 23.2c |
| WWF | 16.2 | 13.7 | 15.0 | 14.1 | 17.2 | |
| B. Crust | 15.2a | 10.6c | 13.8b | 11.4c | 13.5b | |
| B. Crumb | 16.9a | 10.2c | 13.4b | 10.9c | 13.8b | |
| Total dietary fiber, % | WWF | 14.8c | 16.1b | 16.5ab | 16.8a | 16.9a |
| Bread | 15.4c | 16.4b | 16.9b | 18.2a | 18.3a | |
| Total phenolic content, mg GAE/kg | WWF | 1104a | 908bc | 819c | 931b | 854bc |
| Bread | 1077a | 909b | 838c | 896b | 867bc | |
| Antioxidant activity, µmol TE/g | WWF | 2.20 | 2.56 | 2.08 | 2.65 | 2.34 |
| Bread | 3.80 | 3.75 | 3.61 | 3.76 | 3.57 | |
| Development time, min | Flour | 2.72c | 3.33b | 3.43ab | 3.51ab | 3.83a |
| BEM (Max. Torque) | WWF | 36.0b | 60.7a | 54.2a | 63.0a | 63.0a |
| Flour | 39.5d | 44.0b | 42.7c | 46.5a | 44.7b | |
| Volume, mL | Bread | 296 | 311 | 283 | 312 | 302 |
| Specific volume | Bread | 2.14 | 2.24 | 2.06 | 2.22 | 2.13 |
| Springiness | Bread | 0.88 | 0.92 | 0.90 | 0.90 | 0.87 |
| Cohesiveness | Bread | 0.61b | 0.66a | 0.65a | 0.68a | 0.66a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morgounov, A.; Karaduman, Y.; Akin, B.; Aydogan, S.; Baenziger, P.S.; Bhatta, M.; Chudinov, V.; Dreisigacker, S.; Govindan, V.; Güler, S.; et al. Yield and Quality in Purple-Grained Wheat Isogenic Lines. Agronomy 2020, 10, 86. https://doi.org/10.3390/agronomy10010086
Morgounov A, Karaduman Y, Akin B, Aydogan S, Baenziger PS, Bhatta M, Chudinov V, Dreisigacker S, Govindan V, Güler S, et al. Yield and Quality in Purple-Grained Wheat Isogenic Lines. Agronomy. 2020; 10(1):86. https://doi.org/10.3390/agronomy10010086
Chicago/Turabian StyleMorgounov, Alexey, Yaşar Karaduman, Beyhan Akin, Sinan Aydogan, Peter Stephen Baenziger, Madhav Bhatta, Vladimir Chudinov, Susanne Dreisigacker, Velu Govindan, Safure Güler, and et al. 2020. "Yield and Quality in Purple-Grained Wheat Isogenic Lines" Agronomy 10, no. 1: 86. https://doi.org/10.3390/agronomy10010086
APA StyleMorgounov, A., Karaduman, Y., Akin, B., Aydogan, S., Baenziger, P. S., Bhatta, M., Chudinov, V., Dreisigacker, S., Govindan, V., Güler, S., Guzman, C., Nehe, A., Poudel, R., Rose, D., Gordeeva, E., Shamanin, V., Subasi, K., Zelenskiy, Y., & Khlestkina, E. (2020). Yield and Quality in Purple-Grained Wheat Isogenic Lines. Agronomy, 10(1), 86. https://doi.org/10.3390/agronomy10010086







