Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response
Abstract
1. Introduction
2. Materials and Methods
2.1. Site Description
2.2. Experimental Treatments, Layout and Plot Management
2.3. Data Collection and Statistical Analysis
2.3.1. Plant and Soil Sample Preparation
2.3.2. Bioassay for Acid and Alkaline Phosphatase Activity in Rhizosphere Soil
2.3.3. Determination of Soil-P in the Rhizosphere Soils
2.3.4. Measurement of P Concentration in Plant Tissues
2.3.5. Yield
2.3.6. Land Equivalent Ratio (LER)
2.3.7. Statistical Analysis
3. Results
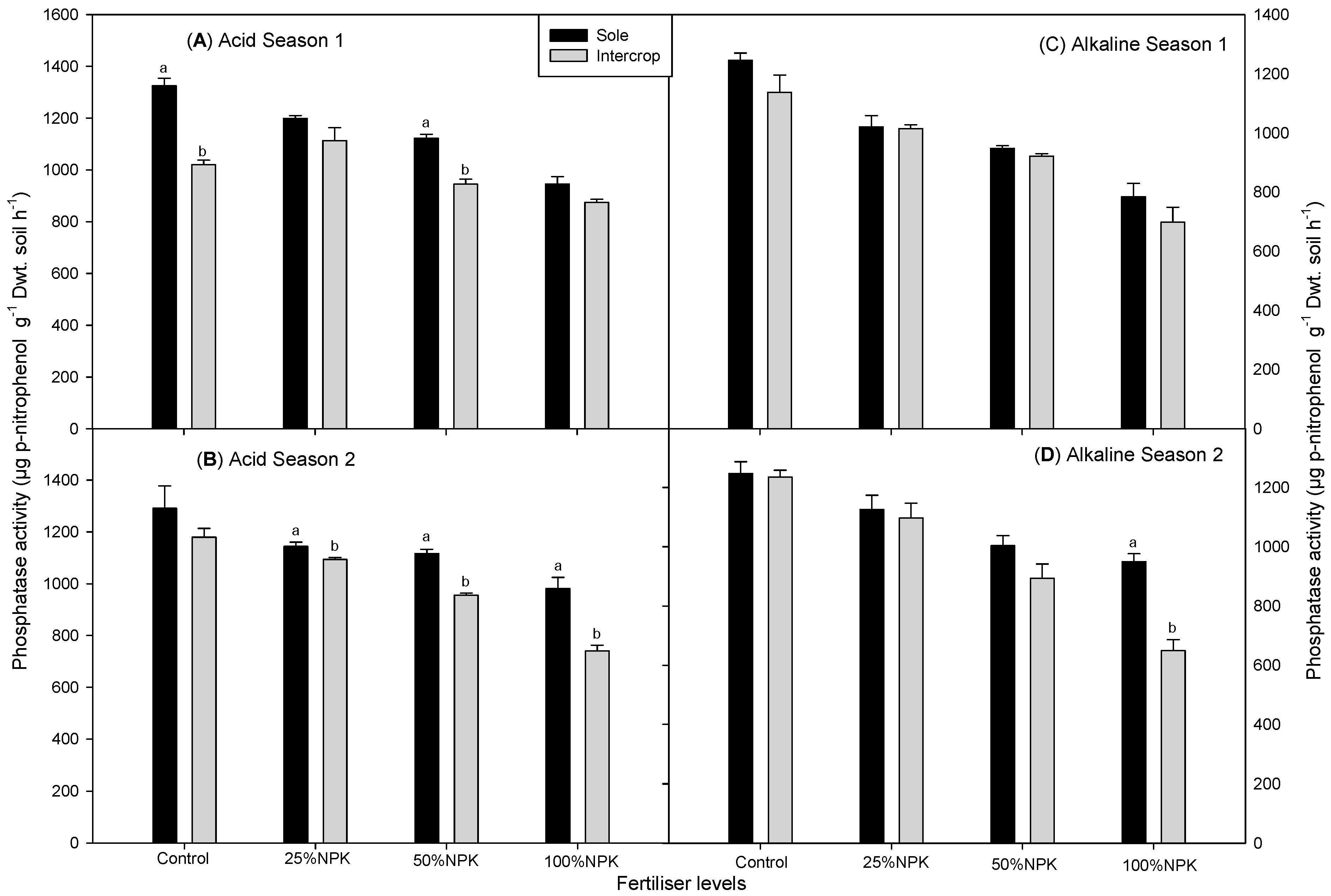
3.1. Phosphatase Activity in the Rhizosphere of Cowpea and Amaranth
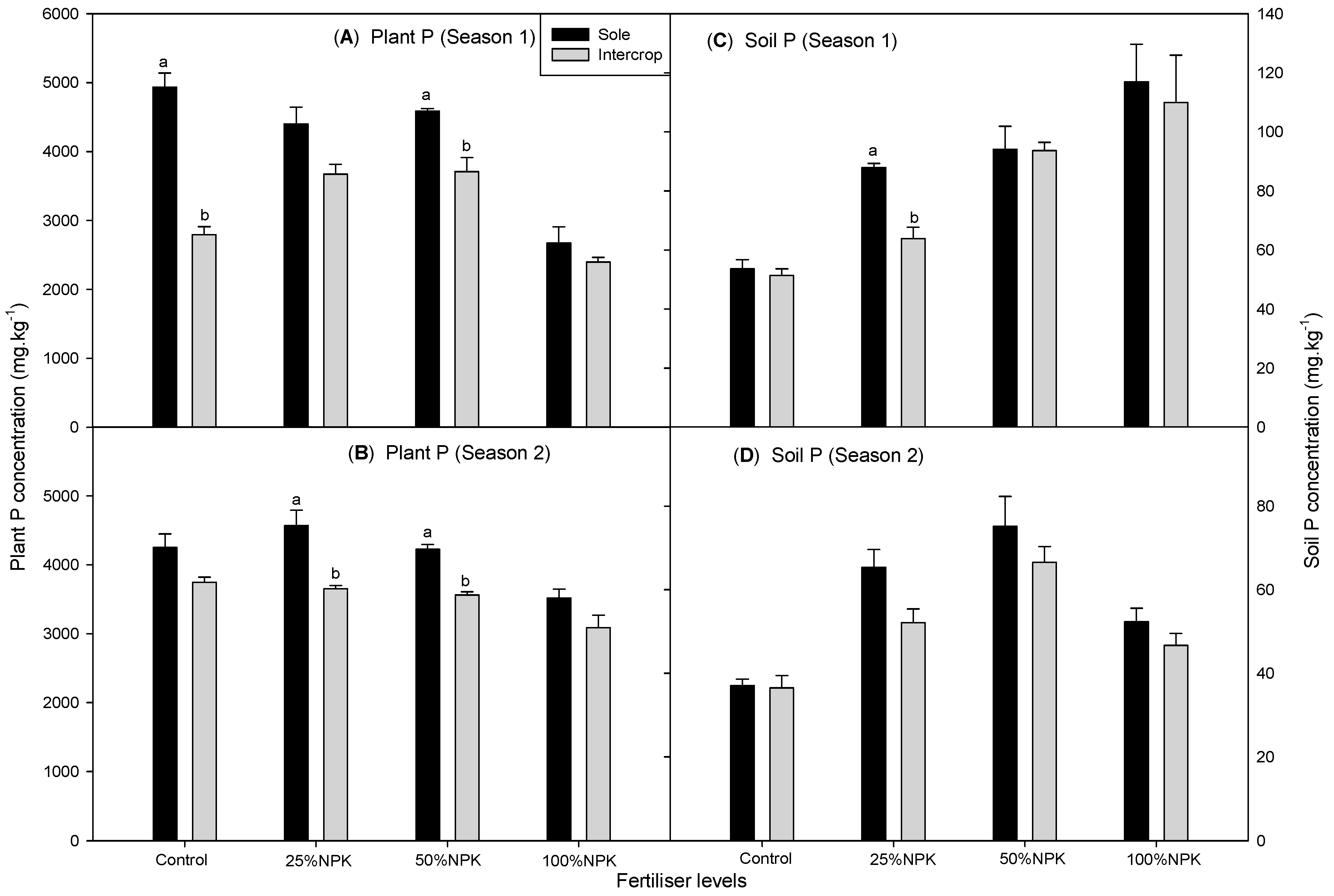
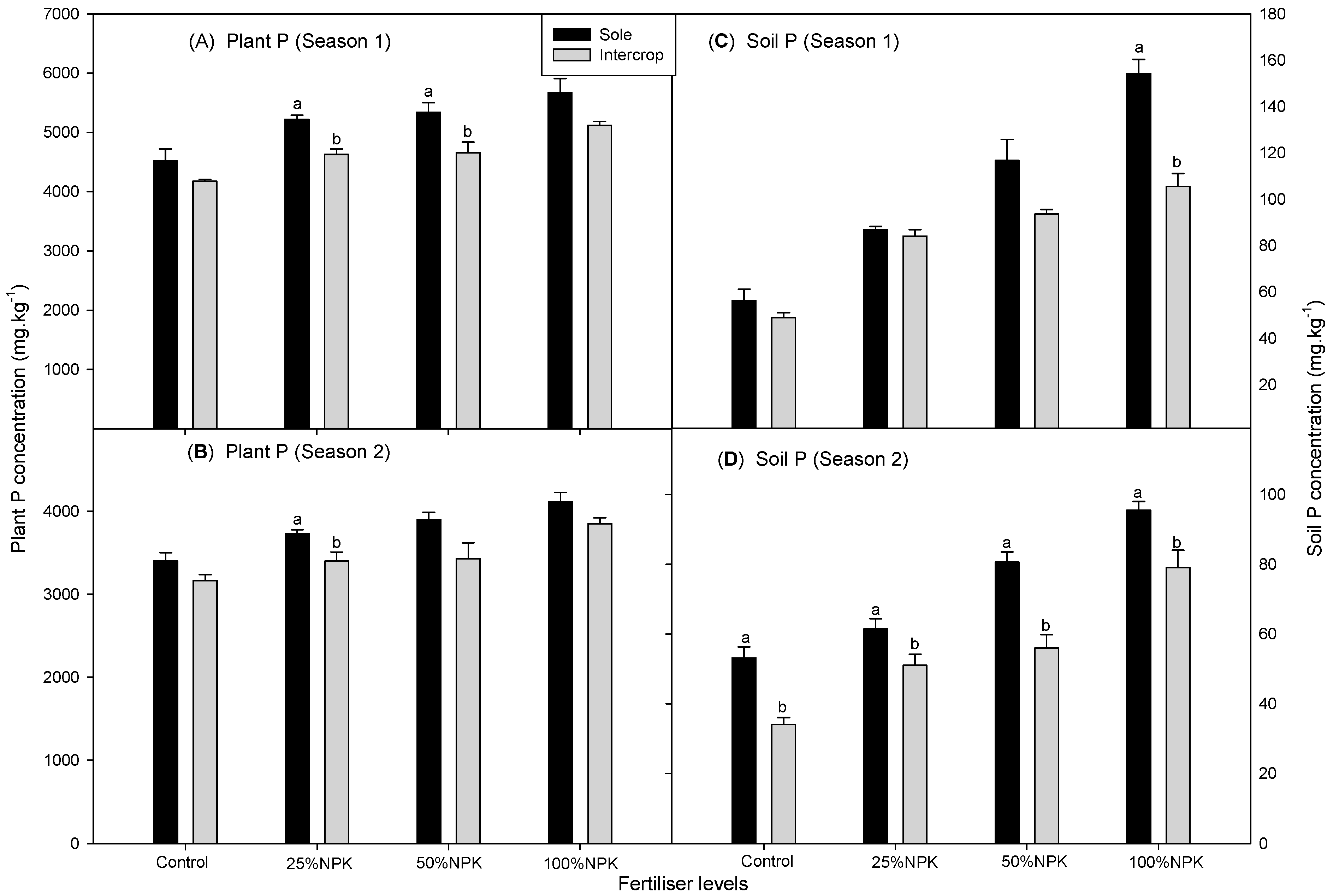
3.2. Soil P Concentration in Cowpea and Amaranth
3.3. Plant P Concentration in Cowpea and Amaranth
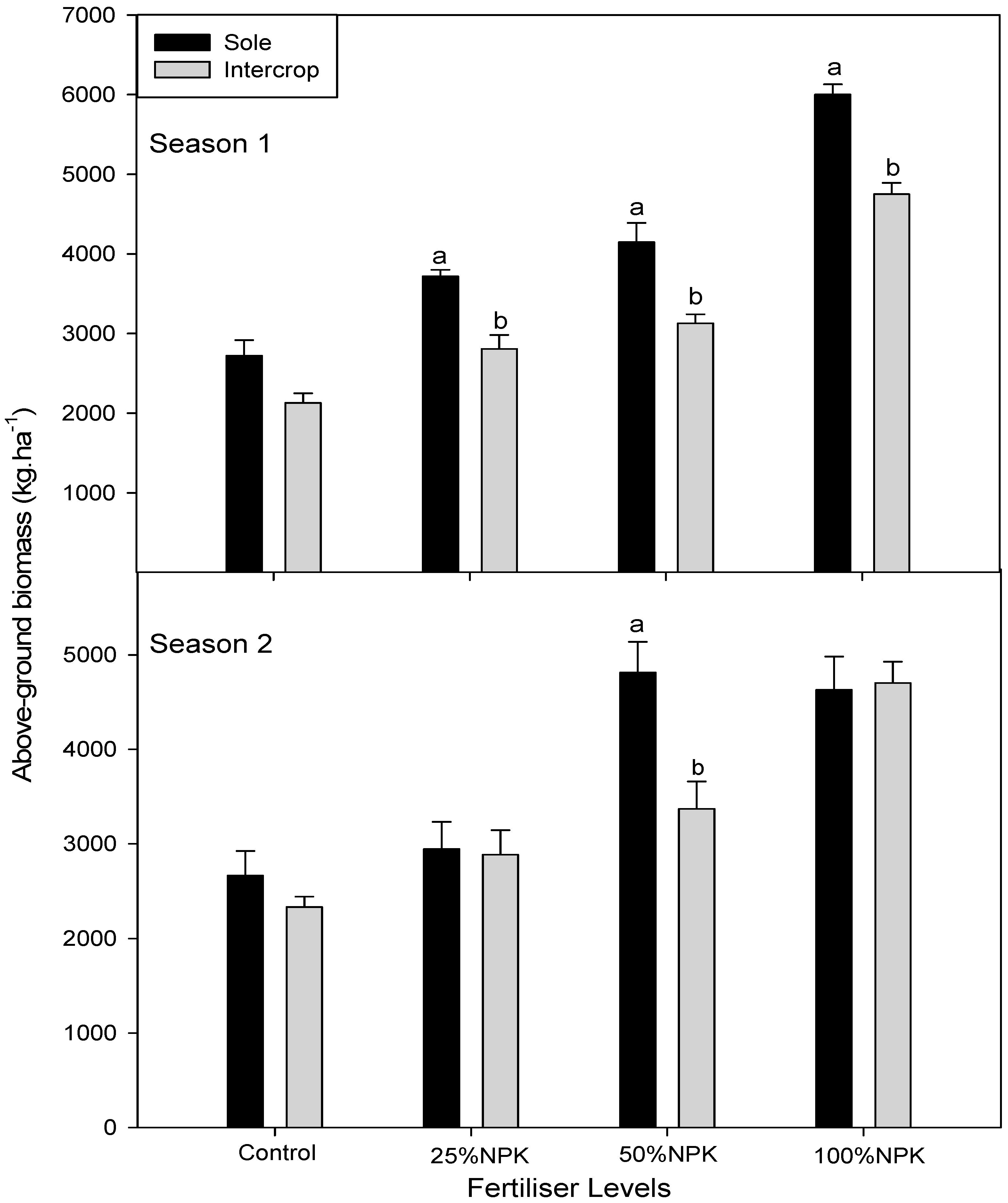
3.4. Above Ground, above Ground Edible on Dry Weight in Cowpea and Amaranth
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Tumushabe, J.T. Climate Change, Food Security and Sustainable Development in Africa. In The Palgrave Handbook of African Politics, Governance and Development; Palgrave Macmillan: New York, NY, USA, 2018; pp. 853–868. [Google Scholar]
- Ochieng, J.; Afari-Sefa, V.; Karanja, D.; Kessy, R.; Rajendran, S.; Samali, S. How promoting consumption of traditional African vegetables affects household nutrition security in Tanzania. Renew. Agric. Food Syst. 2018, 33, 105–115. [Google Scholar] [CrossRef]
- Masso, C.; Nziguheba, G.; Mutegi, J.; Galy-Lacaux, C.; Wendt, J.; Butterbach-Bahl, K.; Wairegi, L.; Datta, A. Soil Fertility Management in Sub-Saharan Africa. In Sustainable Agriculture Reviews; Springer: Berlin/Heidelberg, Germany, 2017; pp. 205–231. [Google Scholar]
- Materechera, S.A. Soil properties and subsoil constraints of urban and peri-urban agriculture within Mahikeng city in the North West Province (South Africa). J. Soils Sediments. 2018, 18, 494–505. [Google Scholar] [CrossRef]
- Power, S.C.; Cramer, M.D.; Verboom, G.A.; Chimphango, S.B. Does phosphate acquisition constrain legume persistence in the fynbos of the Cape Floristic Region? Plant Soil. 2010, 334, 33–46. [Google Scholar] [CrossRef]
- Nongqwenga, N.; Modi, A.T. Phosphorus and Potassium Quantity/Intensity Properties of Selected South African Soils (Kwa-Zulu-Natal) and Their Correlation with Selected Soil Parameters. Appl. Ecol. Environ. Res. 2017, 15, 1–14. [Google Scholar] [CrossRef]
- Nziguheba, G.; Zingore, S.; Kihara, J.; Merckx, R.; Njoroge, S.; Otinga, A.; Vanlauwe, B. Phosphorus in smallholder farming systems of sub-Saharan Africa: Implications for agricultural intensification. Nutr. Cycl. Agroecosyst. 2016, 104, 321–340. [Google Scholar] [CrossRef]
- Malepfane, N.M.; Muchaonyerwa, P. Pre-incubation in soil improves the nitrogen fertiliser value of hair waste. Environ. Monit. Assess. 2018, 190, 94. [Google Scholar] [CrossRef]
- Bulmer, D.; Kar, G.; Hamilton, J.; Siciliano, S.; Peak, D. Extent and mechanism of interaction between phosphate and citrate in a calcareous soil. Soil Sci. Soc. Am. J. 2018, 82, 315–322. [Google Scholar] [CrossRef]
- Bargaz, A.; Noyce, G.L.; Fulthorpe, R.; Carlsson, G.; Furze, J.R.; Jensen, E.S.; Isaac, M.E. Species interactions enhance root allocation, microbial diversity and P acquisition in intercropped wheat and soybean under P deficiency. Appl. Soil Ecol. 2017, 120, 179–188. [Google Scholar] [CrossRef]
- Adigbo, S.O. Performance of cowpea intercropped with amaranth in an inland valley of a derived savanna. Agric. Trop. Subtrop. 2009, 42, 59–64. [Google Scholar]
- Aworh, O.C. From lesser-known to super vegetables: The growing profile of African traditional leafy vegetables in promoting food security and wellness. J. Sci. Food Agric. 2018, 98, 3609–3613. [Google Scholar] [CrossRef]
- Singh, R.; Singh, A.P. Effect of phosphorus, sulphur and biofertilisers on yield, quality of uptake of nutrients in cowpea (Vigna unguiculata). Ann. Plant Soil Res. 2017, 19, 175–179. [Google Scholar]
- Margalef, O.; Sardans, J.; Fernández-Martínez, M.; Molowny-Horas, R.; Janssens, I.A.; Ciais, P.; Peñuelas, J. Global patterns of phosphatase activity in natural soils. Sci. Rep. 2017, 7, 1337. [Google Scholar] [CrossRef] [PubMed]
- Luciano, C.S.; Newell, S.J. Effects of prey, pitcher age, and microbes on acid phosphatase activity in fluid from pitchers of Sarracenia purpurea (Sarraceniaceae). PLoS ONE 2017, 12, e0181252. [Google Scholar] [CrossRef] [PubMed]
- Shahzad, S.M.; Arif, M.S.; Riaz, M.; Ashraf, M.; Yasmeen, T.; Zaheer, A.; Robroek, B.J. Interaction of compost additives with phosphate solubilizing rhizobacteria improved maize production and soil biochemical properties under dryland agriculture. Soil Tillage Res. 2017, 174, 70–80. [Google Scholar] [CrossRef]
- Thabet, O.B.D.; Gtari, M.; Sghaier, H. Microbial diversity in phosphate rock and phosphogypsum. Waste Biomass Valoriz. 2017, 8, 2473–2483. [Google Scholar] [CrossRef]
- Satyaprakash, M.; Nikitha, T.; Reddi, E.U.B.; Sadhana, B.; Vani, S.S. Phosphorous and phosphate solubilising bacteria and their role in plant nutrition. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 2133–2144. [Google Scholar]
- Nesme, T.; Colomb, B.; Hinsinger, P.; Watson, C.A. Soil phosphorus management in organic cropping systems: From current practices to avenues for a more efficient use of P resources. In Organic Farming, Prototype for Sustainable Agricultures; Springer: Berlin/Heidelberg, Germany, 2014; pp. 23–45. [Google Scholar]
- Tang, X.; Placella, S.A.; Daydé, F.; Bernard, L.; Robin, A.; Journet, E.P.; Hinsinger, P. Phosphorus availability and microbial community in the rhizosphere of intercropped cereal and legume along a P-fertiliser gradient. Plant Soil 2016, 407, 119–134. [Google Scholar] [CrossRef]
- Maseko, S.T.; Dakora, F.D. Accumulation of mineral elements in the rhizosphere and shoots of Cyclopia and Aspalathus species under different settings of the Cape fynbos. S. Afr. J. Bot. 2017, 110, 103–109. [Google Scholar] [CrossRef]
- Fraser, T.D.; Lynch, D.H.; Gaiero, J.; Khosla, K.; Dunfield, K.E. Quantification of bacterial non-specific acid (phoC) and alkaline (phoD) phosphatase genes in bulk and rhizosphere soil from organically managed soybean fields. Appl. Soil Ecol. 2017, 111, 48–56. [Google Scholar] [CrossRef]
- Makoi, J.H.; Chimphango, S.B.; Dakora, F.D. Elevated levels of acid and alkaline phosphatase activity in roots and rhizosphere of cowpea (Vigna unguiculata L. Walp.) genotypes grown in mixed culture and at different densities with sorghum (Sorghum bicolor L.). Crop Pasture Sci. 2010, 61, 279–286. [Google Scholar] [CrossRef]
- Bhavya, G.; Shaker, K.C.; Jayasree, G.; Reddy, M.M. Effect of Integrate Use of Phosphorus, PSB and Vermicompost on Acid and Alkaline Phosphatase Activity and Yield of Green Gram (Vigna radiata L.). Int. J. Curr. Microbiol. Appl. Sci. 2018, 7, 1465–1468. [Google Scholar] [CrossRef]
- Li, S.M.; Li, L.; Zhang, F.S.; Tang, C. Acid phosphatase role in chickpea/maize intercropping. Ann. Bot. 2004, 94, 297–303. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Mu, Y.; Cheng, Y.; Liu, X.; Nina, H. Effects of intercropping sugarcane and soybean on growth, rhizosphere soil microbes, nitrogen and phosphorus availability. Acta Physiol. Plant. 2013, 35, 1113–1119. [Google Scholar] [CrossRef]
- Shata, S.M.; Mahmoud, A.; Siam, S. Improving calcareous soil productivity by integrated effect of intercropping and fertilizer. Eur. J. Anaesthesiol. 2007, 3, 733–739. [Google Scholar]
- Eivazi, F.; Tabatabai, M.A. Phosphatases in soils. Soil Biol. Biochem. 1977, 9, 167–172. [Google Scholar] [CrossRef]
- Hedley, M.J.; Stewart, J.W.B.; Chauhan, B.S. Changes in inorganic and organic soil phosphorus fractions induced by cultivation practices and by laboratory incubations. Soil Sci. Soc. Am. J. 1982, 46, 970–976. [Google Scholar] [CrossRef]
- Dyer, B. On the analytical determination of probable available mineral plant food in soils. Trans. Chem. Soc. 1894, 65, 115–167. [Google Scholar] [CrossRef]
- Division of Chemical Services. Analytical Methods; Division of Chemical Services, Department of Agriculture: Pretoria, South Africa, 1956. [Google Scholar]
- Du Plessis, S.F.; Burger, R.D.T. A comparison of Chemical extraction methods for the evaluation of phosphate availability of top soils. S. Afr. J. Agric. Sci. 1964, 8, 11–13. [Google Scholar]
- Giron, H.C. Comparison between Dry Ashing and Wet Digestion in the Preparation of Plant Material for Atomic Absorption Analysis. 1973. Available online: https://scholar.google.com/scholar_lookup?title=Comparison%20between%20dry%20ashing%20and%20wet%20digestion%20in%20the%20preparation%20of%20plant%20material%20for%20atomic%20absorption&author=HC.%20Giron&journal=Atomic%20Absorption%20Newsletter&volume=12&pages=28-29&publication_year=1973 (accessed on 4 November 2019).
- Mitran, T.; Meena, R.S.; Lal, R.; Layek, J.; Kumar, S.; Datta, R. Role of Soil Phosphorus on Legume Production. In Legumes for Soil Health and Sustainable Management; Springer: Singapore, 2018; pp. 487–510. [Google Scholar]
- Dakora, F.D.; Phillips, D.A. Root exudates as mediators as mineral acquisition in low-nutrient environments. Plant Soil. 2002, 245, 35–47. [Google Scholar] [CrossRef]
- Duff, S.M.; Sarath, G.; Plaxton, W.C. The role of acid phosphatase in plant phosphorus metabolism. Phys. Plant 1994, 90, 791–800. [Google Scholar] [CrossRef]
- Tabatabai, M.A. Soil enzymes. In Methods of Soil Analysis. Part 2—Microbiological and Biochemical Properties; SSSA Book Series; Soil Science Society of America: Madison, WI, USA, 1994; pp. 775–859. [Google Scholar]
- Ndungu, S.M.; Messmer, M.M.; Ziegler, D.; Thuita, M.; Vanlauwe, B.; Frossard, E.; Thonar, C. Evaluation of MALDI-TOF mass spectrometry for the competitiveness analysis of selected indigenous cowpea (Vigna unguiculata L. Walp.) Bradyrhizobium strains from Kenya. Appl. Microbiol. Biotechnol. 2018, 102, 5265–5278. [Google Scholar] [CrossRef] [PubMed]
- Nuruzzaman, M.; Lambers, H.; Bolland, M.D.A.; Veneklaas, E.J. Distribution of carboxylates and acid phosphatase and depletion of different phosphorus fractions in the rhizosphere of a cereal and three grain legumes. Plant Soil. 2006, 281, 109–120. [Google Scholar] [CrossRef]
- Eivazi, F.; Bayan, M.R.; Schmidt, K. Select soil enzyme activities in the Historic Sanborn Field as affected by long-term cropping systems. Commun. Soil Sci. Plant Anal. 2003, 34, 2259–2275. [Google Scholar] [CrossRef]
- Maseko, S.T.; Dakora, F.D. Relationship between acid phosphatase activity and P concentration in organs of Cyclopia and Aspalathus species, and a non-legume of the Cape Floristic Region. J. Plant Ecol. 2018, 12, 387–392. [Google Scholar] [CrossRef]
- Koffi, G.A.; Ndoye, F.; Dabonne, S.; Bakhoum, N.; Faye, M.N.; Fall, D.; Diouf, D. Effect of maize and peanut crops on Ivory Coast northern soil biological activities and their response to arbuscular mycorrhizal fungi inoculation. Afr. J. Microbiol. Res. 2018, 12, 171–180. [Google Scholar]
- Maseko, S.T.; Dakora, F.D. Plant enzymes, root exudates, cluster roots and mycorrhizal symbiosis are the drivers of P nutrition in native legumes growing in P deficient soil of the Cape fynbos in South Africa. J. Agric. Sci. Technol. A. 2013, 3, 331. [Google Scholar]
- Ndakidemi, P.A. Manipulating legume/cereal mixtures to optimize the above and belowground interactions in the traditional African cropping systems. Afr. J. Biotechnol. 2006, 5, 2526–2533. [Google Scholar]
- Vardien, W.; Mesjasz-Przybylowicz, J.; Przybylowicz, W.J.; Wang, Y.; Steenkamp, E.T.; Valentine, A.J. Nodules from Fynbos legume Virgilia divaricata have high functional plasticity under variable P supply levels. Plant Physiol. 2014, 171, 1732–1739. [Google Scholar] [CrossRef]
- Maseko, S.; Dakora, F. Rhizosphere acid and alkaline phosphatase activity as a marker of p nutrition in nodulated Cyclopia and Aspalathus species in the cape fynbos of South Africa. S. Afr. J. Bot. 2013, 89, 289–295. [Google Scholar] [CrossRef]
- Makoi, J.H.; Ndakidemi, P.A. Selected soil enzymes: Examples of their potential roles in the ecosystem. Afr. J. Biotechnol. 2008, 7, 3. [Google Scholar]
- Mouradi, M.; Farissi, M.; Makoudi, B.; Bouizgaren, A.; Ghoulam, C. Effect of faba bean (Vicia faba L.)–rhizobia symbiosis on barley’s growth, phosphorus uptake and acid phosphatase activity in the intercropping system. Ann. Agrar. Sci. 2018, 16, 297–303. [Google Scholar] [CrossRef]
- Hauggaard-Nielsen, H.; Jensen, E.S. Facilitative root interactions in intercrops. Plant Soil. 2005, 274, 237–250. [Google Scholar] [CrossRef]
- Wieder, W.R.; Cleveland, C.C.; Smith, W.K.; Todd-Brown, K. Future productivity and carbon storage limited by terrestrial nutrient availability. Nat. Geosci. 2015, 8, 441. [Google Scholar] [CrossRef]
- Kalembasa, S.; Symanowicz, B. Enzymatic activity of soil after applying various waste organic materials, ash, and mineral fertilisers. Pol. J. Environ. Stud. 2012, 21, 1635–1641. [Google Scholar]
- Wang, L.; Liu, D. Functions and regulation of phosphate starvation-induced secreted acid phosphatases in higher plants. Plant Sci. J. 2018, 271, 108–116. [Google Scholar] [CrossRef]
- Giacometti, C.; Cavani, L.; Baldoni, G.; Ciavatta, C.; Marzadori, C.; Kandeler, E. Microplate-scale fluorometric soil enzyme assays as tools to assess soil quality in a long-term agricultural field experiment. Appl. Soil Ecol. 2014, 75, 80–85. [Google Scholar] [CrossRef]
- Betencourt, E.; Duputel, M.; Colomb, B.; Desclaux, D.; Hinsinger, P. Intercropping promotes the ability of durum wheat and chickpea to increase rhizosphere phosphorus availability in a low P soil. Soil Biol. Biochem. 2012, 46, 181–190. [Google Scholar] [CrossRef]
- Sun, J.; Zou, L.; Li, W.; Yang, J.; Wang, Y.; Xia, Q.; Peng, M. Rhizosphere soil properties and banana Fusarium wilt suppression influenced by combined chemical and organic fertilizations. Agric. Ecosyst. Environ. 2018, 254, 60–68. [Google Scholar] [CrossRef]
- Latati, M.; Blavet, D.; Alkama, N.; Laoufi, H.; Drevon, J.J.; Gerard, F.; Ounane, S.M. The intercropping cowpea-maize improves soil phosphorus availability and maize yields in an alkaline soil. Plant Soil. 2014, 385, 181–191. [Google Scholar] [CrossRef]
- Eskandari, H.; Ghanbari, A. Intercropping of maize (Zea mays) and cowpea (Vigna sinensis) as whole-crop forage: Effect of different planting pattern on total dry matter production and maize forage quality. Not. Bot. Hort. Agrobot. Cluj Nacopa. 2009, 37, 152–155. [Google Scholar]
- Manasa, P.; Maitra, S.; Reddy, M.D. Effect of Summer Maize-Legume Intercropping System on Growth, Productivity and Competitive Ability of Crops. Int. J. Eng. Res. Manag. Technol. 2018, 8, 2871–2875. [Google Scholar]
- Mahallati, M.; Koocheki, A.; Mondani, F.; Feizi, H.; Amirmoradi, S. Determination of optimal strip width in strip intercropping of maize (Zea mays L.) and bean (Phaseolus vulgaris L.). J. Clean. Prod. 2015, 106, 343–350. [Google Scholar] [CrossRef]
- Ajala, R.O.; Awodun, M.A.; Adeyemo, A.J.; Dada, B.F. Assessment of Wood Ash Application on Yield Advantage Indices of Maize and Lima Beans in an Intercrop. J. Exp. Agric. Int. 2019, 34, 1–11. [Google Scholar] [CrossRef][Green Version]
- Hauggaard-Nielsen, H.; Ambus, P.; Jensen, E.S. Interspecific competition, N use and interference with weeds in pea–barley intercropping. Field Crop. Res. 2001, 70, 101–109. [Google Scholar] [CrossRef]
- Li, Q.Z.; Sun, J.H.; Wei, X.J.; Christie, P.; Zhang, F.S.; Li, L. Overyielding and interspecific interactions mediated by nitrogen fertilization in strip intercropping of maize with faba bean, wheat and barley. Plant Soil. 2011, 339, 147–161. [Google Scholar] [CrossRef]
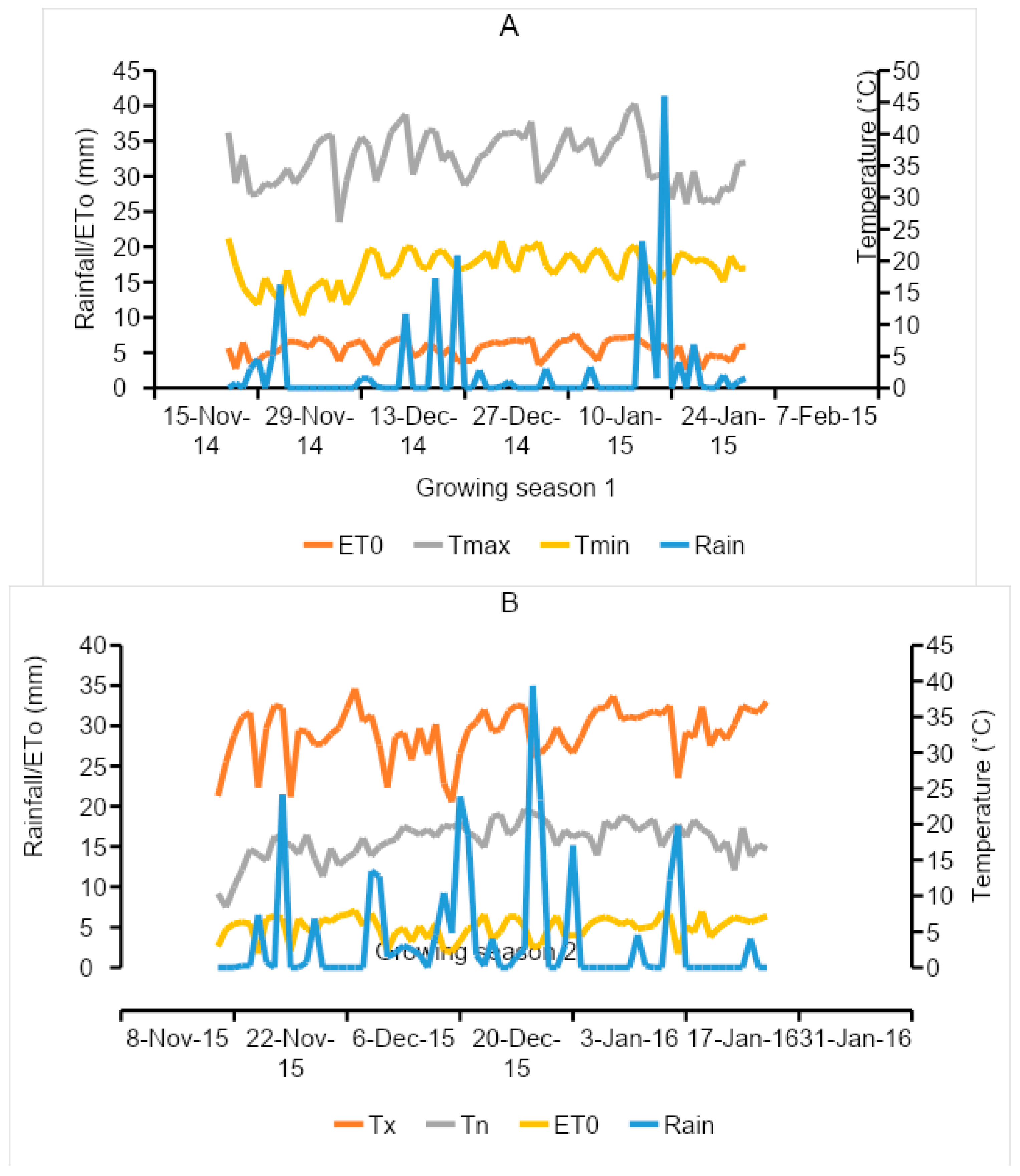


| Chemical Properties | 2014/15 | 2015/16 | ||
|---|---|---|---|---|
| Before | After | Before | After | |
| pH (H2O) | 6.2 ± 0.4 | 6.2 ± 0.2 | 7.3 ± 0.4 | 7.2 ± 0.5 |
| P(Bray 1) (mg kg−1) | 20 ± 0.6 | 18 ± 1.6 | 57 ± 3 | 55 ± 2 |
| K (mg kg−1) | 218 ± 3.9 | 203 ± 14 | 158 ± 14 | 155 ± 14 |
| Na (mg kg−1) | 18 ± 0.9 | 15 ± 1 | 56 ± 1.2 | 56 ± 1 |
| Ca (mg kg−1) | 635 ± 3.3 | 602 ± 40 | 857 ± 49 | 847 ± 5 |
| Mg (mg kg−1) | 198 ± 1.3 | 190 ± 13 | 174 ± 11 | 170 ± 1.5 |
| NO3-N (mg kg−1) | 7.8 ± 0.6 | 7.7 ± 0.6 | 2.6 ± 0.3 | 2.4 ± 0.8 |
| NH4-N (mg kg−1) | 2.4 ± 0.2 | 2.2 ± 0.1 | 3.6 ± 0.4 | 3.9 ± 0.1 |
| Clay % | 25 ± 2 | 25 ± 2 | 32 ± 2 | 32 ± 2 |
| Cropping System | Cowpea | Amaranth | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Fertiliser Levels | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | Season 1 | Season 2 | |
| Acid Phosphatase Activity | Alkaline Phosphatase Activity | Acid Phosphatase Activity | Alkaline Phosphatase Activity | ||||||
| (µg p-nitrophenol g−1 DWt. soil h−1) | |||||||||
| Sole | CONTROL | 1394 ± 7a1 | 1246 ± 9ab1 | 950 ± 4a1 | 888 ± 6a1 | 1325 ± 8a1 | 1292 ± 8a1 | 1246 ± 8a1 | 1471 ± 4a1 |
| 25%NPK | 1325 ± 4ab1,2 | 1292 ± 6a1 | 766 ± 4b2 | 868 ± 7a1 | 1199 ± 8b2 | 1144 ± 7b12 | 1021 ± 7c2 | 1126 ± 4bc12 | |
| 50%NPK | 1244 ± 8bc2 | 1144 ± 7bc12 | 667 ± 3bc2 | 630 ± 4b2 | 1122 ± 9bc2 | 1116 ± 7b2 | 948 ± 7c2 | 1004 ± 8bc2 | |
| 100%NPK | 978 ± 7d3 | 998 ± 6cde2 | 575 ± 4cd2 | 501 ± 3cd2 | 946 ± 7de3 | 982 ± 8c3 | 784 ± 5d3 | 949 ± 7c2 | |
| Intercrop | CONTROL | 1144 ± 5c1 | 1099 ± 7bcd1 | 892 ± 5a1 | 597 ± 4bc2 | 1021 ± 8d1 | 1179 ± 7b1 | 1137 ± 8b1 | 1236 ± 9ab1 |
| 25%NPK | 1177 ± 6c1 | 1119 ± 8bcd1 | 626 ± 3cd2 | 712 ± 5b1 | 1113 ± 6c12 | 1094 ± 8b2 | 1015 ± 8c2 | 1098 ± 6bc1 | |
| 50%NPK | 1150 ± 7c1 | 977 ± 8de2 | 542 ± 3d23 | 610 ± 4bc12 | 945 ± 9de23 | 956 ± 6c3 | 922 ± 7c2 | 894 ± 5cd2 | |
| 100%NPK | 946 ± 6d2 | 879 ± 6e2 | 395 ± 2e3 | 468 ± 2d3 | 875 ± 6e3 | 740 ± 4d4 | 698 ± 5d3 | 649 ± 5d3 | |
| LSD (p-value) | Cropping | 56 (<0.001) | 69 (<0.001) | 53 (<0.001) | 55 (<0.001) | 39 (<0.001) | 55 (<0.001) | 49 (0.025) | 124 (0.011) |
| LSD (p-value) | Fertiliser Level | 79 (<0.001) | 97 (<0.001) | 75 (<0.001) | 78 (<0.001) | 55 (<0.001) | 78 (<0.001) | 69 (<0.001) | 176 (<0.001) |
| LSD (p-value) | Cropping X Fertiliser Level | 111 (0.017) | 138 (0.935) | 107 (0.404) | 110 (0.007) | 77 (0.001) | 110 (0.101) | 97 (0.366) | 248 (0.375) |
| Cropping System | Fertiliser Levels | Cowpea | Amaranth | ||
|---|---|---|---|---|---|
| Season 1 | Season 2 | Season 1 | Season 2 | ||
| mg.kg−1 | |||||
| Sole | CONTROL | 53.7 ± 2.5d3 | 37.0 ± 3.2c3 | 78.7 ± 2.2bc2 | 50.1 ± 3.0c3 |
| 25%NPK | 84.8 ± 1.9c2 | 65.4 ± 3.6a12 | 97.9 ± 3.1abc12 | 64.1 ± 4.2bc23 | |
| 50%NPK | 97.2 ± 2.9bc12 | 75.1 ± 5.4a1 | 102.1 ± 2.4ab12 | 81.4 ± 4.7a12 | |
| 100%NPK | 131.2 ± 9.5a1 | 52.3 ± b23 | 142.4 ± 10.8a1 | 92.0 ± 8a1 | |
| Intercrop | CONTROL | 51.4 ± 2.8d2 | 36.5 ± 2.9c3 | 48.9 ± 3c2 | 34.1 ± 2.1d2 |
| 25%NPK | 63.9 ± 3.9d2 | 52.1 ± 2.6b2 | 77.7 ± 4.7bc12 | 51.0 ± 3.4c12 | |
| 50%NPK | 88.0 ± 5.3c1 | 66.6 ± 3.9a1 | 97.2 ± 3.5abc1 | 56.0 ± 4.5c12 | |
| 100%NPK | 101.4 ± 9.1b1 | 46.7 ± 2.8bc23 | 104.1 ± 9.8ab1 | 79.0 ± 6.9ab1 | |
| LSD (p-value) | Cropping | 6.1 (<0.001) | 6.2 (0.030) | 23.3 (0.050) | 7.9 (<0.001) |
| LSD (p-value) | Fertiliser Level | 8.7 (<0.001) | 8.8 (<0.001) | 33.0 (0.013) | 11.2 (<0.001) |
| LSD (p-value) | Cropping X Fertiliser Level | 12.2 (0.019) | 12.5 (0.492) | 46.6 (0.732) | 15.9 (0.609) |
| Cropping System | Fertiliser Levels | Cowpea | Amaranth | ||
|---|---|---|---|---|---|
| Season 1 | Season 2 | Season 1 | Season 2 | ||
| mg kg−1 | |||||
| Sole | CONTROL | 4935 ± 23a1 | 4249 ± 22a1 | 4514 ± 14cd2 | 3401 ± 15cd3 |
| 25%NPK | 4586 ± 34a1 | 4569 ± 24a1 | 5220 ± 9b1 | 3732 ± 18bc23 | |
| 50%NPK | 4401 ± 35a1 | 4224 ± 21a1 | 5340 ± 37ab1 | 3896 ± 19ab12 | |
| 100%NPK | 2675 ± 16c2 | 3517 ± 19b2 | 5671 ± 32a1 | 4114 ± 3a1 | |
| Intercrop | CONTROL | 2793 ± 22c2 | 3744 ± 20b1 | 4174 ± 28d3 | 3166 ± 19d2 |
| 25%NPK | 3673 ± 27b1 | 3651 ± 18b1 | 4629 ± 31c2 | 3399 ± 22cd12 | |
| 50%NPK | 3710 ± 22b1 | 3560 ± 19b1 | 4653 ± 38c2 | 3427 ± 28cd12 | |
| 100%NPK | 2396 ± 24c2 | 3086 ± 21c2 | 5120 ± 48b1 | 3851 ± 26ab1 | |
| LSD (p-value) | Cropping | 256 (<0.001) | 215 (<0.001) | 184 (<0.001) | 163.2 (<0.001) |
| LSD (p-value) | Fertiliser Level | 363 (<0.001) | 304 (<0.001) | 260 (<0.001) | 230.9 (<0.001) |
| LSD (p-value) | Cropping X Fertiliser Level | 513 (<0.001) | 430 (0.362) | 368 (0.552) | 326.5 (0.707) |
| 2014/15 | F. Level | Cowpea | Amaranth | |||||
|---|---|---|---|---|---|---|---|---|
| AGB | AGEB | GRAIN YIELD | AGB | AGEB | F. level | LER | ||
| kg.ha−1 | kg.ha−1 | kg.ha−1 | kg.ha−1 | kg.ha−1 | ||||
| Sole | CONTROL | 2731 ± 137c3 | 573 ± 29b2 | 3577 ± 179b2 | 2389 ± 119c1 | 116 ± 6de2 | ||
| 25%NPK | 4126 ± 165b2 | 760 ± 30a1 | 5678 ± 227a1 | 3519 ± 141abc12 | 177 ± 7bcd2 | |||
| 50%NPK | 5034 ± 151a1 | 869 ± 26a1 | 6016 ± 180a1 | 4370 ± 131ab23 | 206 ± 6bc2 | |||
| 100%NPK | 4238 ± 254b2 | 816 ± 49a1 | 3259 ± 196bc2 | 5278 ± 317a3 | 388 ± 23a1 | |||
| Intercrop | CONTROL | 2605 ± 130c2 | 507 ± 25bc1 | 2415 ± 121bc23 | 2056 ± 103c1 | 103 ± 5e3 | CONTROL | 1.81 |
| 25%NPK | 4009 ± 160b1 | 546 ± 22bc1 | 5709 ± 228a1 | 2481 ± 99bc1 | 126 ± 5de23 | 25%NPK | 1.68 | |
| 50%NPK | 2108 ± 63d3 | 406 ± 12bc1 | 3642 ± 109b2 | 3074 ± 92bc1 | 146 ± 4cde2 | 50%NPK | 1.12 | |
| 100%NPK | 1485 ± 89e4 | 393 ± 24c2 | 1989 ± 119c3 | 3870 ± 232abc1 | 211 ± 13b1 | 100%NPK | 1.08 | |
| LSD (p-value) | Cropping | 230 (<0.001) | 106 (0.157) | 578 (<0.001) | 862 (0.024) | 29 (<0.001) | ||
| LSD (p-value) | Fertiliser Level | 325 (<0.001) | 75 (<0.001) | 817 (<0.001) | 1220 (0.007) | 41 (<0.001) | ||
| LSD (p-value) | Cropping X Fertiliser Level | 460 (<0.001) | 151 (0.003) | 1156 (0.050) | 1725 (0.783) | 58 (0.004) | ||
| 2015/16 | ||||||||
| Sole | CONTROL | 3996 ± 216ab1 | 1796 ± 90bc2 | 3430 ± 172c2 | 2667 ± 133b2 | 107 ± 5c3 | ||
| 25%NPK | 4319 ± 177a1 | 2749 ± 110a1 | 5230 ± 209a1 | 2944 ± 118b2 | 118 ± 5c3 | |||
| 50%NPK | 4419 ± 88a1 | 1945 ± 58b2 | 4275 ± 128 bc12 | 4815 ± 144a1 | 187 ± 6b2 | |||
| 100%NPK | 2922 ± 222c2 | 1060 ± 64b2 | 2035 ± 122d3 | 4630 ± 278a1 | 270 ± 16a1 | |||
| Intercrop | CONTROL | 3702 ± 179b2 | 1574 ± 79d3 | 1993 ± 100d2 | 2333 ± 117b1 | 117 ± 6c2 | CONTROL | 1.80 |
| 25%NPK | 3576 ± 174b2 | 1710 ± 68c1 | 4843 ± 194ab1 | 2889 ± 116b1 | 126 ± 5c2 | 25%NPK | 1.81 | |
| 50%NPK | 4354 ± 85a1 | 1555 ± 47bc1 | 4226 ± 127bc1 | 2889 ± 87 b1 | 141 ± 4bc12 | 50%NPK | 1.59 | |
| 100%NPK | 2835 ± 85c3 | 1134 ± 60cd2 | 1553 ± 93d2 | 3407 ± 204ab1 | 190 ± 11b1 | 100%NPK | 1.71 | |
| LSD (p-value) | Cropping | 205 (0.008) | 155 (<0.001) | 429 (0.011) | 684 (0.015) | 25 (0.033) | ||
| LSD (p-value) | Fertiliser Level | 290 (<0.001) | 219 (<0.001) | 607 (<0.001) | 967 (0.012) | 35 (<0.001) | ||
| LSD (p-value) | Cropping X Fertiliser Level | 410 (0.085) | 309 (<0.001) | 859 (0.132) | 1368 (0.194) | 49 (0.042) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mndzebele, B.; Ncube, B.; Fessehazion, M.; Mabhaudhi, T.; Amoo, S.; du Plooy, C.; Venter, S.; Modi, A. Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response. Agronomy 2020, 10, 79. https://doi.org/10.3390/agronomy10010079
Mndzebele B, Ncube B, Fessehazion M, Mabhaudhi T, Amoo S, du Plooy C, Venter S, Modi A. Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response. Agronomy. 2020; 10(1):79. https://doi.org/10.3390/agronomy10010079
Chicago/Turabian StyleMndzebele, Buhlebelive, Bhekumthetho Ncube, Melake Fessehazion, Tafadzwanashe Mabhaudhi, Stephen Amoo, Christian du Plooy, Sonja Venter, and Albert Modi. 2020. "Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response" Agronomy 10, no. 1: 79. https://doi.org/10.3390/agronomy10010079
APA StyleMndzebele, B., Ncube, B., Fessehazion, M., Mabhaudhi, T., Amoo, S., du Plooy, C., Venter, S., & Modi, A. (2020). Effects of Cowpea-Amaranth Intercropping and Fertiliser Application on Soil Phosphatase Activities, Available Soil Phosphorus, and Crop Growth Response. Agronomy, 10(1), 79. https://doi.org/10.3390/agronomy10010079








