Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Site
2.2. Experimental Design
2.3. Sample Collection
2.4. Soil Properties Analysis
2.5. Microbial Analysis
2.6. Analysis of Data
3. Results
3.1. Soil Properties
3.2. Sequencing Data
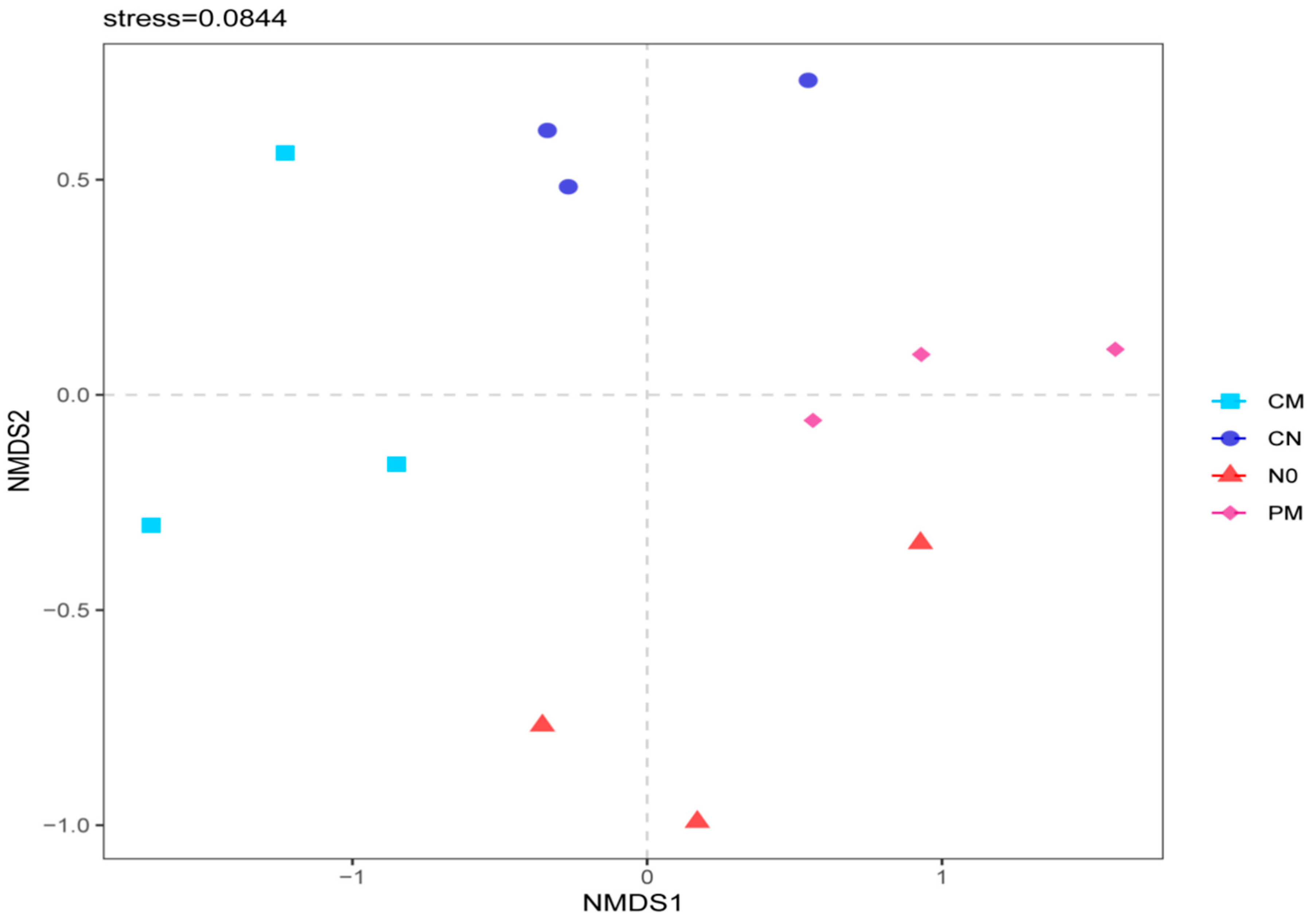
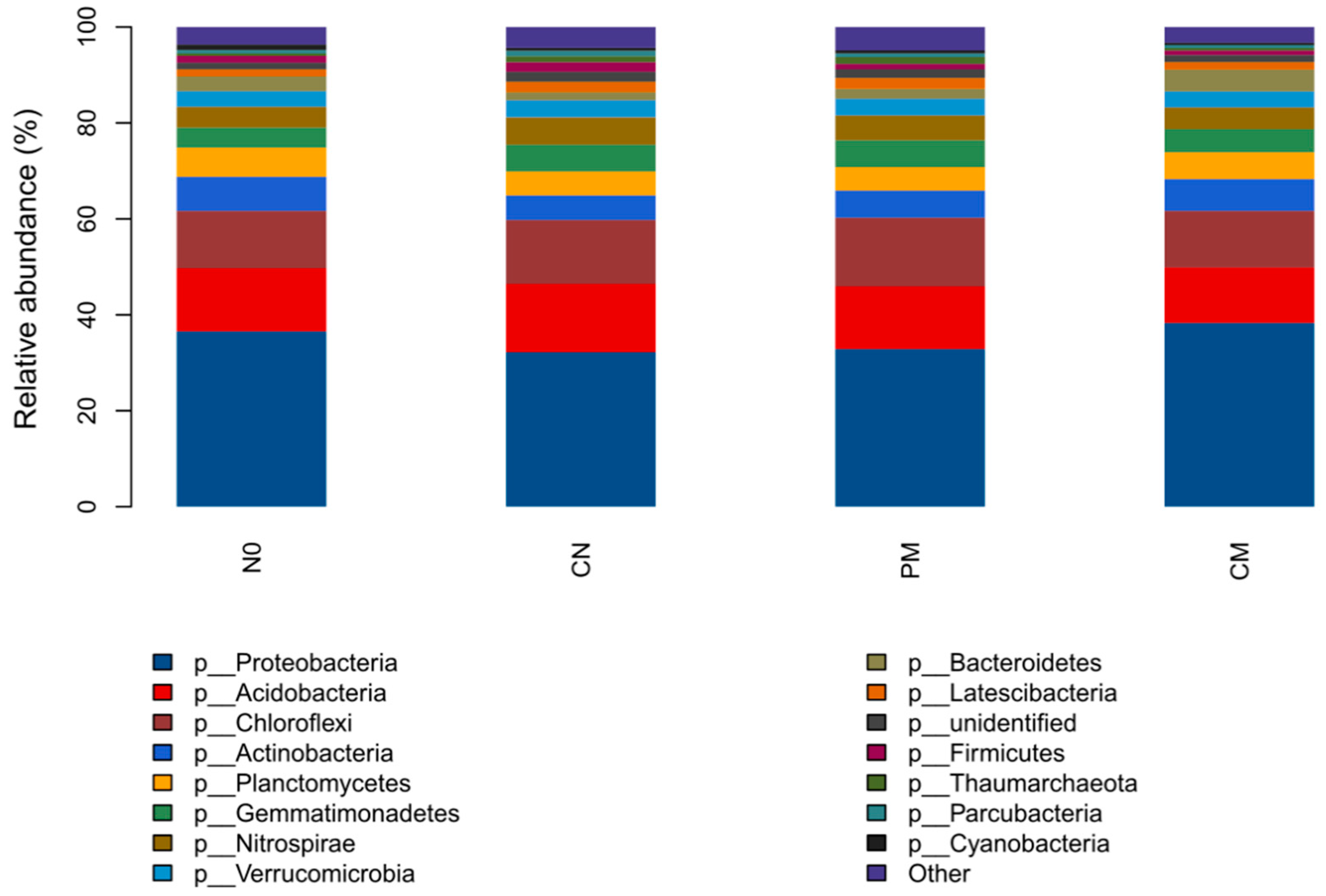
3.3. Prokaryotic Community Structures and Compositions
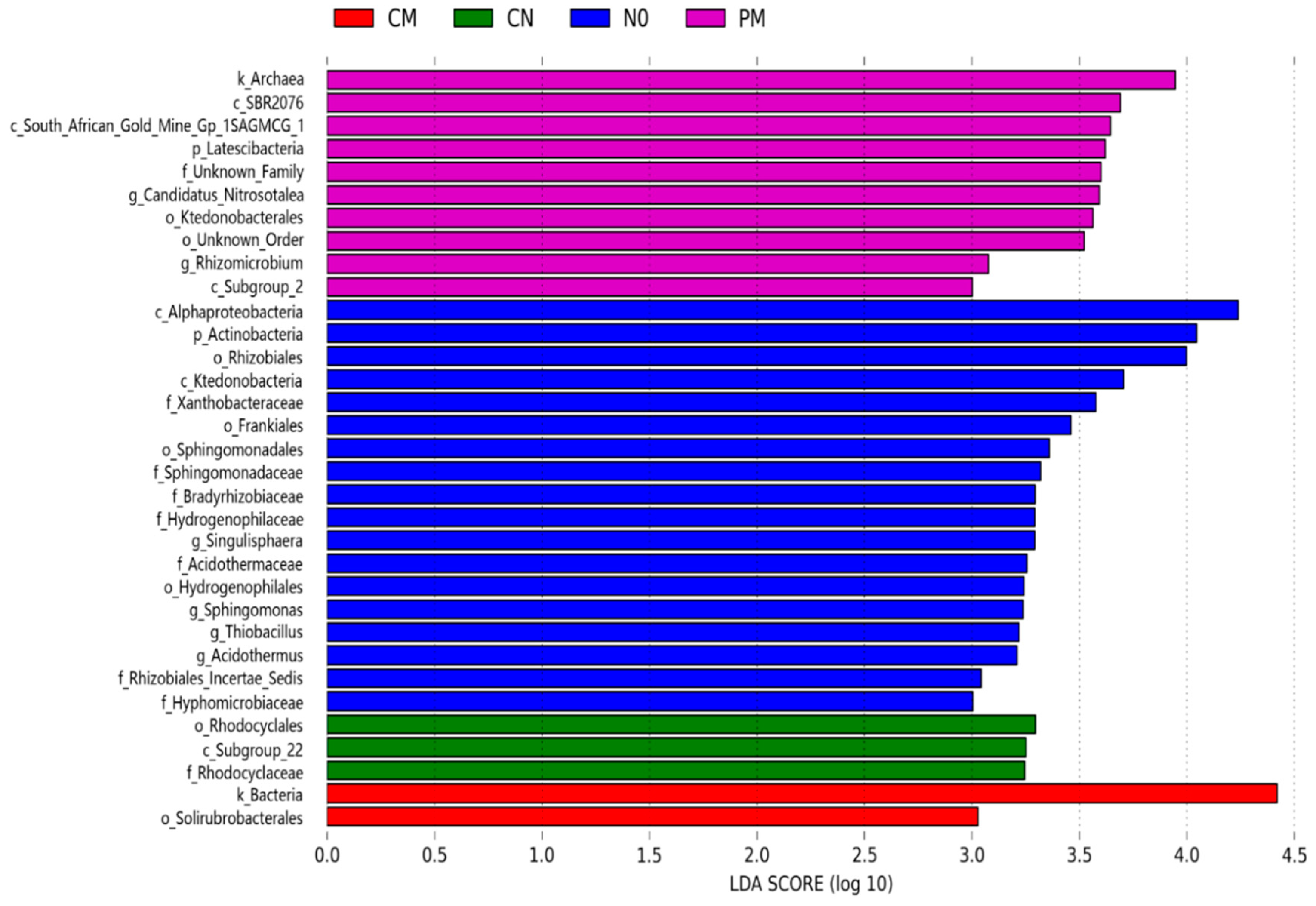
3.4. Indicator Taxa of Different Treatments Subjected to Different Long-Term Fertilization Regimes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Chen, D.; Yuan, L.; Liu, Y.; Ji, J.; Hou, H. Long-term application of manures plus chemical fertilizers sustained high rice yield and improved soil chemical and bacterial properties. Eur. J. Agron. 2017, 90, 34–42. [Google Scholar] [CrossRef]
- Cui, X.; Zhang, Y.; Gao, J.; Peng, F.; Gao, P. Long-term combined application of manure and chemical fertilizer sustained higher nutrient status and rhizospheric bacterial diversity in reddish paddy soil of Central South China. Sci. Rep. 2018, 8, 16554. [Google Scholar] [CrossRef] [PubMed]
- Chang, E.; Chung, R.; Tsai, Y.H. Effect of different application rates of organic fertilizer on soil enzyme activity and microbial population. Soil Sci. Plant Nutr. 2007, 53, 132–140. [Google Scholar] [CrossRef]
- Gong, W.; Yan, X.; Wang, J.; Hu, T.; Gong, Y. Long-term manuring and fertilization effects on soil organic carbon pools under a wheat–maize cropping system in North China Plain. Plant Soil 2008, 314, 67–76. [Google Scholar] [CrossRef]
- Ning, C.; Gao, P.; Wang, B.; Lin, W.; Jiang, N.; Cai, K. Impacts of chemical fertilizer reduction and organic amendments supplementation on soil nutrient, enzyme activity and heavy metal content. J. Integr. Agric. 2017, 16, 1819–1831. [Google Scholar] [CrossRef]
- Bakht, J.; Shafi, M.; Jan, M.T.; Shah, Z. Influence of crop residue management, cropping system and N fertilizer on soil N and C dynamics and sustainable wheat (Triticum aestivum L.) production. Soil Tillage Res. 2009, 104, 233–240. [Google Scholar] [CrossRef]
- Sun, R.; Zhang, X.; Guo, X.; Wang, D.; Chu, H. Bacterial diversity in soils subjected to long-term chemical fertilization can be more stably maintained with the addition of livestock manure than wheat straw. Soil Biol. Biochem. 2015, 88, 9–18. [Google Scholar] [CrossRef]
- Li, X.; Guo, J.; Dong, R.; Ahring, B.K.; Zhang, W. Properties of plant nutrient: Comparison of two nutrient recovery techniques using liquid fraction of digestate from anaerobic digester treating pig manure. Sci. Total Environ. 2016, 544, 774–781. [Google Scholar] [CrossRef]
- Zhang, P.; Chen, X.; Wei, T.; Yang, Z.; Jia, Z.; Yang, B. Effects of straw incorporation on the soil nutrient contents, enzyme activities, and crop yield in a semiarid region of China. Soil Tillage Res. 2016, 160, 65–72. [Google Scholar] [CrossRef]
- Hati, K.M.; Mandal, K.G.; Misra, A.K.; Ghosh, P.K.; Bandyopadhyay, K.K. Effect of inorganic fertilizer and farmyard manure on soil physical properties, root distribution, and water-use efficiency of soybean in Vertisols of central India. Bioresour. Technol. 2006, 97, 2182–2188. [Google Scholar] [CrossRef]
- Bhattacharyya, R.; Chandra, S.; Singh, R.; Kundu, S.; Srivastva, A.; Gupta, H. Long-term farmyard manure application effects on properties of a silty clay loam soil under irrigated wheat-soybean rotation. Soil Tillage Res. 2007, 94, 386–396. [Google Scholar] [CrossRef]
- Amusan, A.O.; Adetunji, M.; Azeez, J.O.; Bodunde, J.G. Effect of the integrated use of legume residue, poultry manure and inorganic fertilizers on maize yield, nutrient uptake and soil properties. Nutr. Cycl. Agroecosyst. 2011, 90, 321–330. [Google Scholar] [CrossRef]
- Ge, G.; Li, Z.; Fan, F.; Chu, G.; Hou, Z.; Liang, Y. Soil biological activity and their seasonal variations in response to long-term application of organic and inorganic fertilizers. Plant Soil. 2009, 326, 31–44. [Google Scholar] [CrossRef]
- Bandyopadhyay, K.K.; Misra, A.K.; Ghosh, P.K.; Hati, K.M. Effect of integrated use of farmyard manure and chemical fertilizers on soil physical properties and productivity of soybean. Soil Tillage Res. 2010, 110, 115–125. [Google Scholar] [CrossRef]
- Chen, M.; Cui, Y.; Bai, F.; Wang, J. Effect of two biogas residues’ application on copper and zinc fractionation and release in different soils. J. Environ. Sci. 2013, 25, 1865–1873. [Google Scholar] [CrossRef]
- Zhang, H.; Xu, M.; Zhang, F. Long-term effects of manure application on grain yield under different cropping systems and ecological conditions in China. J. Agric. Sci. 2008, 147, 31–42. [Google Scholar] [CrossRef]
- Yanardağ, İ.H.; Zornoza, R.; Cano, A.F.; Yanardağ, A.B.; Mermut, A.R. Evaluation of carbon and nitrogen dynamics in different soil types amended with pig slurry, pig manure and its biochar by chemical and thermogravimetric analysis. Biol. Fertil. Soils. 2014, 51, 183–196. [Google Scholar] [CrossRef]
- Calderon, F.J.; Benjamin, J.; Vigil, M.F. A comparison of corn (Zea mays L.) residue and its biochar on soil C and plant growth. PLoS ONE 2015, 10, 0121006. [Google Scholar] [CrossRef][Green Version]
- Chu, H.; Lin, X.; Fujii, T.; Morimoto, S.; Yagi, K.; Hu, J. Soil microbial biomass, dehydrogenase activity, bacterial community structure in response to long-term fertilizer management. Soil Biol. Biochem. 2007, 39, 2971–2976. [Google Scholar] [CrossRef]
- Islam, M.R.; Chauhan, P.S.; Kim, Y.; Kim, M.; Sa, T. Community level functional diversity and enzyme activities in paddy soils under different long-term fertilizer management practices. Biol. Fertil. Soils. 2010, 47, 599–604. [Google Scholar] [CrossRef]
- Li, C.; Yan, K.; Tang, L.; Jia, Z.; Li, Y. Change in deep soil microbial communities due to long-term fertilization. Soil Biol. Biochem. 2014, 75, 264–272. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, Q.; Liang, B.; Li, J. Changes in the abundance and structure of bacterial communities in the greenhouse tomato cultivation system under long-term fertilization treatments. Appl. Soil. Ecol. 2017, 121, 82–89. [Google Scholar] [CrossRef]
- Chen, M.; Chen, Y.; Dong, S.; Lan, S.; Zhou, H.; Tan, Z. Mixed nitrifying bacteria culture under different temperature dropping strategies: Nitrification performance, activity, and community. Chemosphere 2018, 195, 800–809. [Google Scholar] [CrossRef] [PubMed]
- Naghdi, M.; Cledon, M.; Brar, S.K.; Ramirez, A.A. Nitrification of vegetable waste using nitrifying bacteria. Ecol. Eng. 2018, 121, 83–88. [Google Scholar] [CrossRef]
- King, K.W.; Smith, P.H. Comparisons of two media proposed for the isolation of bacteria from the rumen. J. Bacteriol. 1955, 70, 726. [Google Scholar] [CrossRef]
- Seki, H. Microbial biomass on particulate organic matter in seawater of the euphotic zone. Appl. Microb. 1970, 19, 960–962. [Google Scholar] [CrossRef]
- Bossio, D.A.; Scow, K.M. Impacts of carbon and flooding on soil microbial communities: Phospholipid fatty acid profiles and substrate utilization patterns. Appl. Environ. Microb. 1995, 61, 4043–4050. [Google Scholar] [CrossRef]
- Joseph, S.J.; Hugenholtz, P.; Sangwan, P.; Osborne, C.A.; Janssen, P.H. Laboratory cultivation of widespread and previously uncultured soil bacteria. Appl. Environ. Microb. 2003, 69, 7210–7215. [Google Scholar] [CrossRef]
- Smalla, K.; Wachtendorf, U.; Heuer, H.; Liu, W.T.; Forney, L. Analysis of BIOLOG GN substrate utilization patterns by microbial communities. Appl. Environ. Microb. 1998, 64, 1220–1225. [Google Scholar] [CrossRef]
- Shendure, J.; Ji, H. Next-generation DNA sequencing. Nat. Biotechnol. 2008, 26, 1135–1145. [Google Scholar] [CrossRef]
- Smets, W.; Leff, J.W.; Bradford, M.A.; McCulley, R.L.; Lebeer, S.; Fierer, N. A method for simultaneous measurement of soil bacterial abundances and community composition via 16S rRNA gene sequencing. Soil Biol. Biochem. 2016, 96, 145–151. [Google Scholar] [CrossRef]
- Chávez-Romero, Y.; Navarro-Noya, Y.E.; Reynoso-Martínez, S.C.; Sarria-Guzmán, Y.; Govaerts, B.; Verhulst, N. 16S metagenomics reveals changes in the soil bacterial community driven by soil organic C, N-fertilizer and tillage-crop residue management. Soil Tillage Res. 2016, 159, 1–8. [Google Scholar] [CrossRef]
- Ansola, G.; Arroyo, P.; de Miera, L.E.S. Characterisation of the soil bacterial community structure and composition of natural and constructed wetlands. Sci. Total Environ. 2014, 473–474, 63–71. [Google Scholar] [CrossRef] [PubMed]
- Daquiado, A.R.; Kuppusamy, S.; Kim, S.Y.; Kim, J.H.; Yoon, Y.E.; Kim, P.J. Pyrosequencing analysis of bacterial community diversity in long-term fertilized paddy field soil. Appl. Soil Ecol. 2016, 108, 84–91. [Google Scholar] [CrossRef]
- Dai, H.; Chen, Y.; Yang, X.; Cui, J.; Sui, P. The effect of different organic materials amendment on soil bacteria communities in barren sandy loam soil. Environ. Sci. Pollut. Res. 2017, 24, 24019–24028. [Google Scholar] [CrossRef]
- Bao, S.D. Analytical Methods of Soil Agrochemistry, 3rd ed.; China Agricultural Press: Beijing, China, 1999. (In Chinese) [Google Scholar]
- Edgar, R.C. UPARSE: Highly accurate OTU sequences from microbial amplicon reads. Nat. Methods 2013, 10, 996–998. [Google Scholar] [CrossRef]
- Cole, J.R.; Wang, Q.; Cardenas, E.; Fish, J.; Chai, B.; Farris, R.J. The Ribosomal Database Project: Improved alignments and new tools for rRNA analysis. Nucleic Acids Res. 2009, 37, D141–D145. [Google Scholar] [CrossRef]
- Wang, Q.; Garrity, G.M.; Tiedje, J.M.; Cole, J.R. Naive Bayesian Classifier for Rapid Assignment of rRNA Sequences into the New Bacterial Taxonomy. Appl. Environ. Microb. 2007, 73, 5261–5267. [Google Scholar] [CrossRef]
- Chen, C.; Zhang, J.; Lu, M.; Qin, C.; Chen, Y.; Yang, L. Microbial communities of an arable soil treated for 8 years with organic and inorganic fertilizers. Biol. Fertil. Soils 2016, 52, 455–467. [Google Scholar] [CrossRef]
- Cooper, J.M.; Warman, P.R. Efects of three fertility amendments on soil dehydrogenase activity, organic C and pH. Can. J. Soil Sci. 1997, 77, 281–283. [Google Scholar] [CrossRef]
- Li, J.; Cooper, J.M.; Lin, Z.; Li, Y.; Yang, X.; Zhao, B. Soil microbial community structure and function are significantly affected by long-term organic and mineral fertilization regimes in the North China Plain. Appl. Soil Ecol. 2015, 96, 75–87. [Google Scholar] [CrossRef]
- Li, X.L.; George, E.; Marschner, H. Phosphorus depletion and pH decrease at the root-soil and hyphae-soil interfaces of VA mycorrhizal white clover fertilized with ammonium. New Phytol. 1991, 119, 397–404. [Google Scholar] [CrossRef]
- Wei, M.; Hu, G.; Wang, H.; Bai, E.; Lou, Y.; Zhang, A. 35 years of manure and chemical fertilizer application alters soil microbial community composition in a Fluvo-aquic soil in Northern China. Eur. J. Soil Biol. 2017, 82, 27–34. [Google Scholar] [CrossRef]
- Ding, X.; Han, X.; Liang, Y.; Qiao, Y.; Li, L.; Li, N. Changes in soil organic carbon pools after 10 years of continuous manuring combined with chemical fertilizer in a Mollisol in China. Soil Tillage Res. 2012, 122, 36–41. [Google Scholar] [CrossRef]
- Zhong, W.; Gu, T.; Wang, W.; Zhang, B.; Lin, X.; Huang, Q. The effects of mineral fertilizer and organic manure on soil microbial community and diversity. Plant Soil 2009, 326, 511–522. [Google Scholar] [CrossRef]
- Ahn, J.H.; Song, J.; Kim, B.Y.; Kim, M.S.; Joa, J.H.; Weon, H.Y. Characterization of the bacterial and archaeal communities in rice field soils subjected to long-term fertilization practices. J. Microbiol. 2012, 50, 754–765. [Google Scholar] [CrossRef]
- Yuan, H.; Ge, T.; Zhou, P.; Liu, S.; Roberts, P.; Zhu, H. Soil microbial biomass and bacterial and fungal community structures responses to long-term fertilization in paddy soils. J. Soil Sediments 2013, 13, 877–886. [Google Scholar] [CrossRef]
- Zhao, Z.B.; He, J.Z.; Geisen, S.; Han, L.L.; Wang, J.T.; Shen, J.P. Protist communities are more sensitive to nitrogen fertilization than other microorganisms in diverse agricultural soils. Microbiome 2019, 7, 33. [Google Scholar] [CrossRef]
- Byss, M.; Elhottova, D.; Triska, J.; Baldrian, P. Fungal bioremediation of the creosote-contaminated soil: Influence of Pleurotus ostreatus and Irpex lacteus on polycyclic aromatic hydrocarbons removal and soil microbial community composition in the laboratory-scale study. Chemosphere 2008, 73, 1518–1523. [Google Scholar] [CrossRef]
- Lazcano, C.; Gómez-Brandón, M.; Revilla, P.; Domínguez, J. Short-term effects of organic and inorganic fertilizers on soil microbial community structure and function. Biol. Fertil. Soils 2012, 49, 723–733. [Google Scholar] [CrossRef]
- Lauber, C.L.; Hamady, M.; Knight, R.; Fierer, N. Pyrosequencing-based assessment of soil pH as a predictor of soil bacterial community structure at the continental scale. Appl. Environ. Microbiol. 2009, 75, 5111–5120. [Google Scholar] [CrossRef] [PubMed]
- Rousk, J.; Baath, E.; Brookes, P.C.; Lauber, C.L.; Lozupone, C.; Caporaso, J.G. Soil bacterial and fungal communities across a pH gradient in an arable soil. ISME J. 2010, 4, 1340–1351. [Google Scholar] [CrossRef] [PubMed]

| pH | Soil Organic Carbon (SOC) (g/kg) | Total N (TN) (g/kg) | Alkali-Hydrolyzable Nitrogen (AN) (mg/kg) | Total P (TP) (g/kg) | Available P (AP) (mg/kg) | Total K (TK) (g/kg) | Available K (AK) (mg/kg) |
|---|---|---|---|---|---|---|---|
| 5.61 | 16.62 | 1.21 | 48.93 | 0.54 | 21.25 | 11.51 | 155.7 |
| Organic Material | pH | N (%) | P2O5 (%) | K2O (%) | C (%) | Moisture Content (%) |
|---|---|---|---|---|---|---|
| PM | 8.38 | 0.23 | 0.23 | 0.13 | 13.49 | 68.7 |
| CM | 8.28 | 0.83 | 1.07 | 0.75 | 30.05 | 32.1 |
| Treatment | pH | SOC (g/kg) | TN (g/kg) | TP (mg/kg) | SOC/TN |
|---|---|---|---|---|---|
| NO | 6.61 ± 0.01 b | 19.97 ± 0.39 c | 1.39 ± 0.06 b | 360.64 ± 16.03 b | 14.38 ± 0.56 a |
| CN | 6.44 ± 0.03 c | 20.91 ± 1.13 bc | 1.46 ± 0.07 b | 348.20 ± 14.51 c | 14.29 ± 0.58 a |
| PM | 6.64 ± 0.04 ab | 23.25 ± 1.58 ab | 1.73 ± 0.05 a | 397.63 ± 26.38 a | 13.41 ± 1.18 a |
| CM | 6.69 ± 0.03 a | 24.38 ± 1.64 a | 1.68 ± 0.05 a | 374.12 ± 12.71 ab | 14.54 ± 0.59 a |
| Treatment | Observed OTUs | Shannon | Chao1 | Coverage |
|---|---|---|---|---|
| NO | 6014.07 ± 360.68 a | 10.76 ± 0.1542 a | 8281.18 ± 410.84 a | 0.9642 ± 0.0016 a |
| CN | 6065.17 ± 82.41 a | 10.74 ± 0.13424 a | 8276.39 ± 166.56 a | 0.9643 ± 0.0010 a |
| PM | 5891.23 ± 244.51 a | 10.66 ± 0.12511 a | 8263.72 ± 501.33 a | 0.9645 ± 0.0023 a |
| CM | 6125.60 ± 280.43 a | 10.80 ± 0.07543 a | 8278.63 ± 421.21 a | 0.9644 ± 0.0024 a |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, L.; Li, C.; Zhu, S.; Xu, Y.; Li, H.; Zheng, X.; Shi, R. Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions. Agronomy 2020, 10, 132. https://doi.org/10.3390/agronomy10010132
Liu L, Li C, Zhu S, Xu Y, Li H, Zheng X, Shi R. Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions. Agronomy. 2020; 10(1):132. https://doi.org/10.3390/agronomy10010132
Chicago/Turabian StyleLiu, Liyuan, Chuanzong Li, Shuhao Zhu, Yan Xu, Houyu Li, Xiangqun Zheng, and Rongguang Shi. 2020. "Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions" Agronomy 10, no. 1: 132. https://doi.org/10.3390/agronomy10010132
APA StyleLiu, L., Li, C., Zhu, S., Xu, Y., Li, H., Zheng, X., & Shi, R. (2020). Combined Application of Organic and Inorganic Nitrogen Fertilizers Affects Soil Prokaryotic Communities Compositions. Agronomy, 10(1), 132. https://doi.org/10.3390/agronomy10010132





