Photochromic Polyurethanes Showing a Strong Change of Transparency and Refractive Index
Abstract
:1. Introduction
2. Materials and Methods
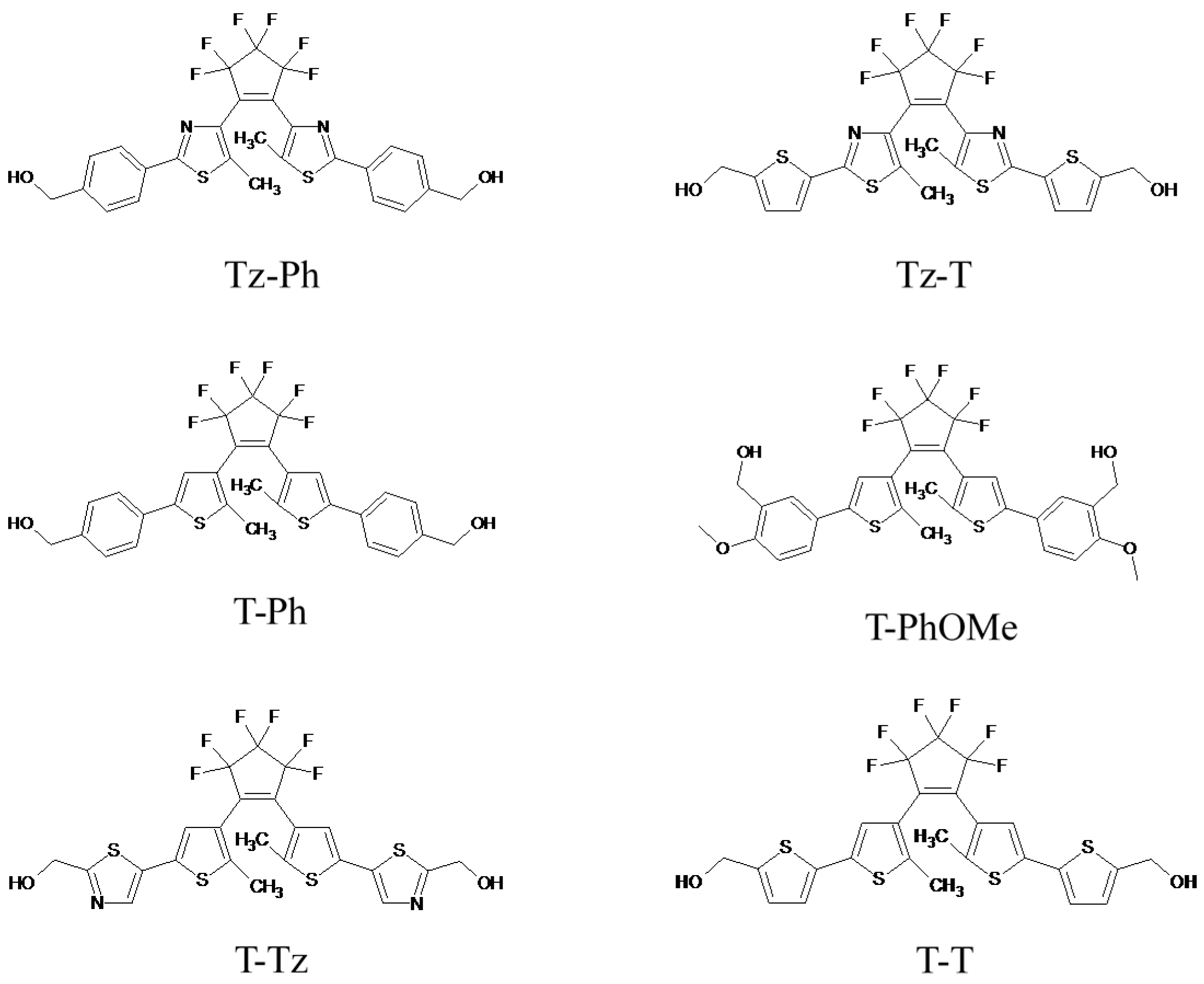
2.1. Synthesis of the Monomers
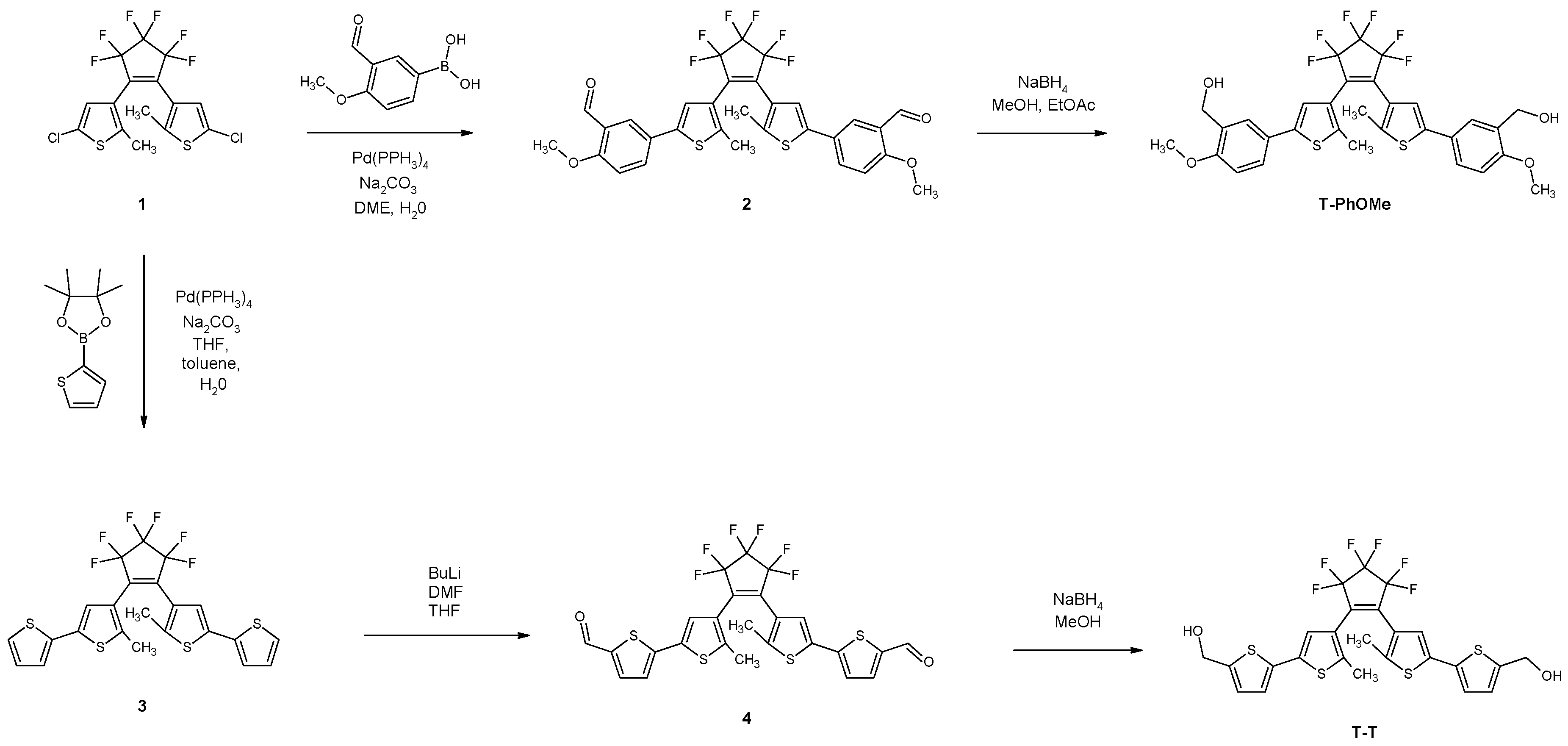
1,2-Bis-[5-p-methoxy-m-formylphenyl-3-thienyl]hexafluorocyclopentene (2)
1,2-Bis-[5-p-methoxy-m-hydroxymethylphenyl-3-thienyl]hexafluorocyclopentene (T-PhOMe)
1,2-Bis-[2-methyl-5-(2′-thienyl)-3-thienyl]hexafluorocyclopentene (3)
1,2-Bis-[2-methyl-5-(5′-formyl-2′-thienyl)-3-thienyl]hexafluorocyclopentene (4)
1,2-Bis-[2-methyl-5-(5′-hydroxylmethyl-2′-thienyl)-3-thienyl]hexafluorocyclopentene (T-T)
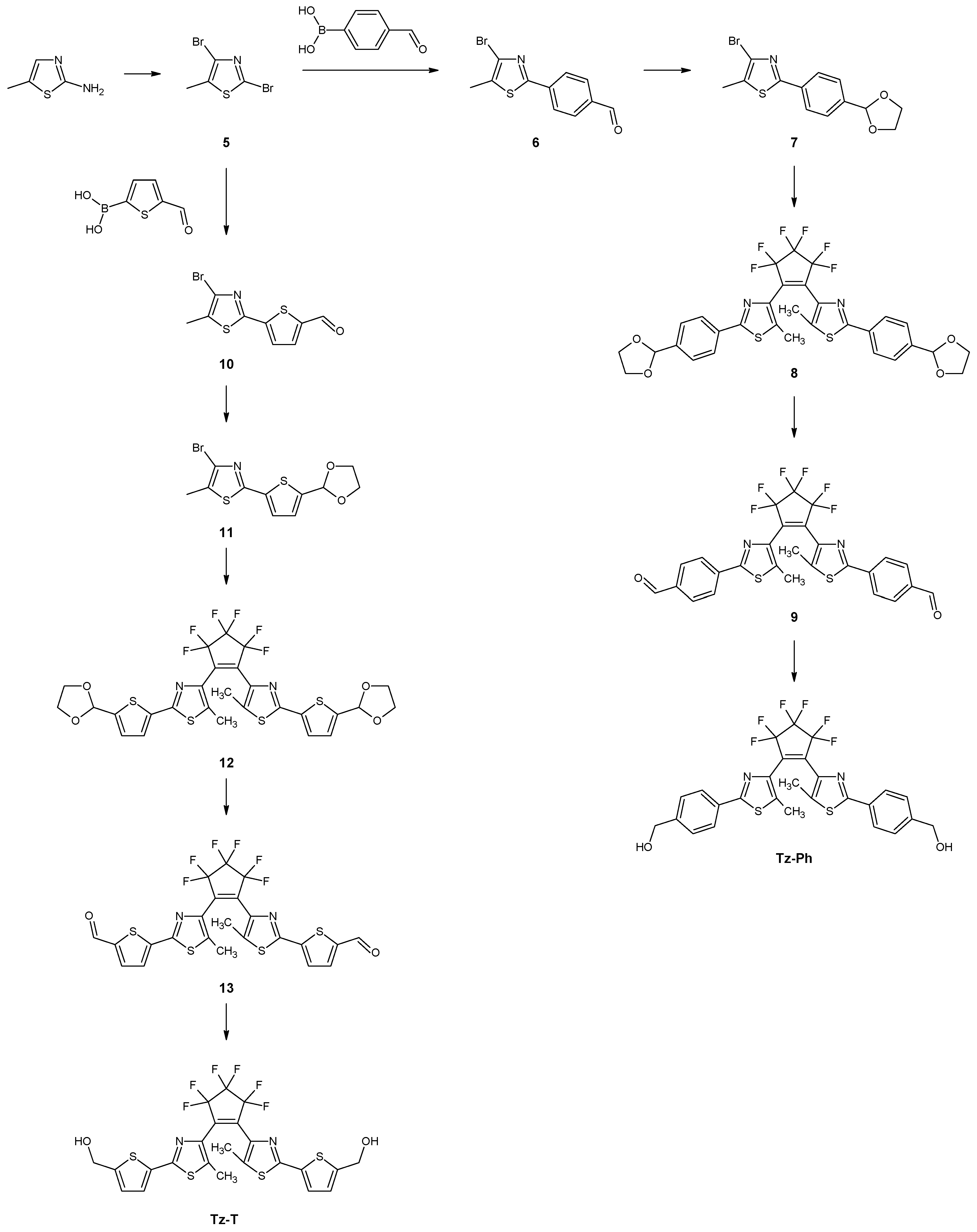
2,4-Dibromo-5-methylthiazole (5)
4-Bromo-5-methyl-2-p-formylphenylthiazole (6)
4-Bromo-5-methyl-2-[p-(1,3-dioxolane)phenyl]thiazole (7)
1,2-Bis-[2-methyl-5-(p-(1,3-dioxolane)phenyl)-thiazol-3-yl]perfluorocyclopentene (8)
1,2-Bis-[2-methyl-5-(p-formyl)-phenyl-thiazol-3-yl]perfluorocyclopentene (9)
1,2-Bis-[2-methyl-5-(p-hydroxymethyl)-phenyl-3-thiazolyl]perfluorocyclopentene (Tz-Ph)
4-Bromo-5-methyl-2-p-formylthienylthiazole (10)
4-Bromo-5-methyl-[5-(1,3-dioxolane)thien-2-yl]thiazole (11)
1,2-Bis-[2-methyl-(5-(1,3-dioxolane)thien-2-yl)-3-thiazolyl]perfluorocyclopentene (12)
1,2-Bis-[2-methyl-(5-formylthien-2-yl)-3-thiazolyl]perfluorocyclopentene (13)
1,2-Bis-[2-methyl-(5-hydroxymethyl-thien-2-yl)-3-thiazolyl]perfluorocyclopentene (Tz-T)
2-Thiazolyl-1,3-dioxolane (14)
Tributyl-5-(1,3-dioxolane)-thiazol-2-yl stannate (15)
1,2-Bis-[2-methyl-5-((1,3-dioxolane)-thiazol-2-yl)-3-thienyl]perfluorocyclopentene (16)
1,2-Bis-[2-methyl-5-(4-formyl-thiazol-2-yl)-3-thienyl]perfluorocyclopentene (17)
1,2-Bis-[2-methyl-5-(4-hydroxymethyl-thiazol-2-yl)-3-thienyl]perfluorocyclopentene (T-Tz)
2.2. Polyurethane Production
2.3. UV–Vis Absorption Spectrsocopy
2.4. Spectroscopic Ellipsometry
2.5. Microscopy
2.6. Optical Tests
3. Results
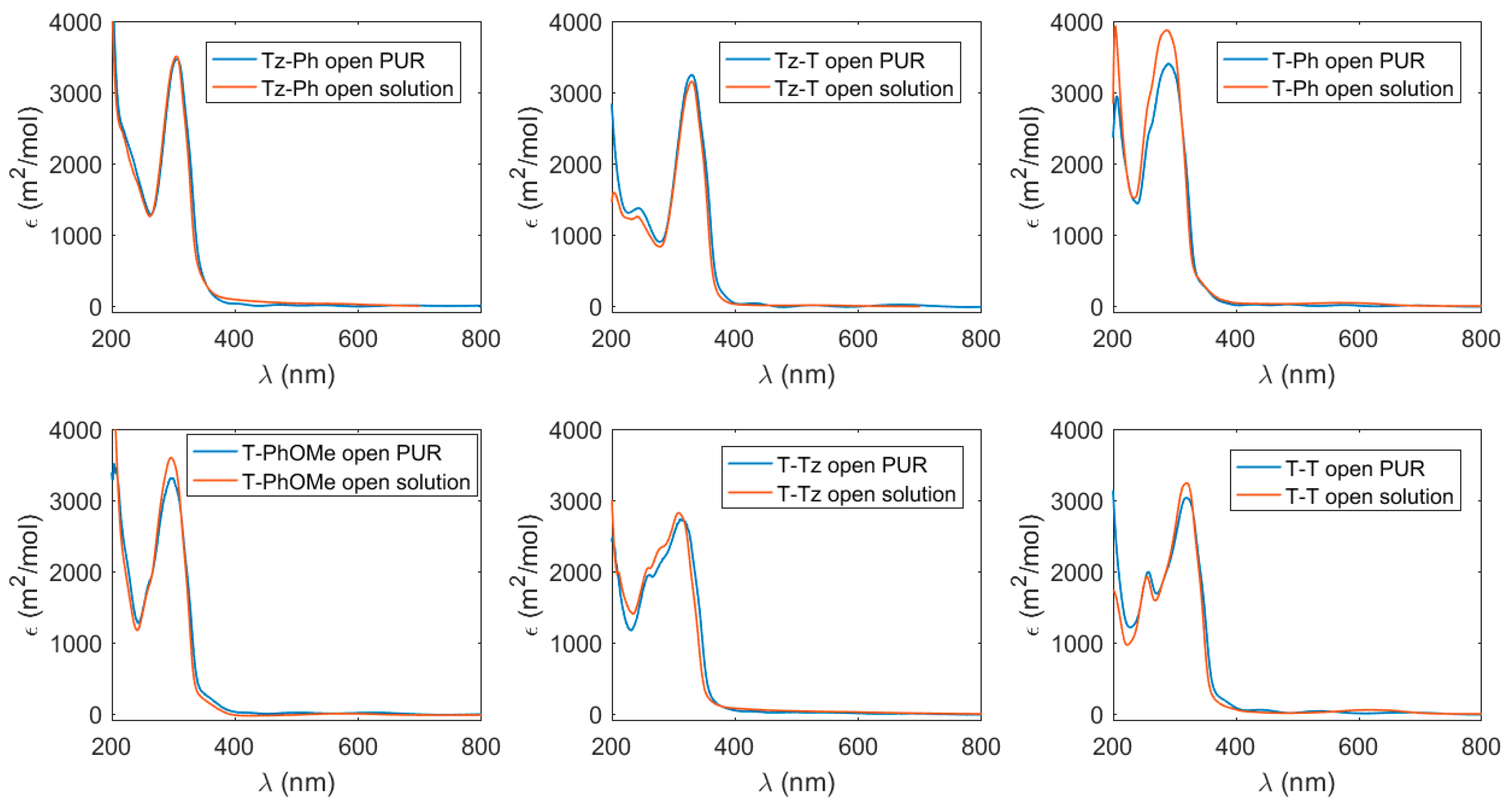
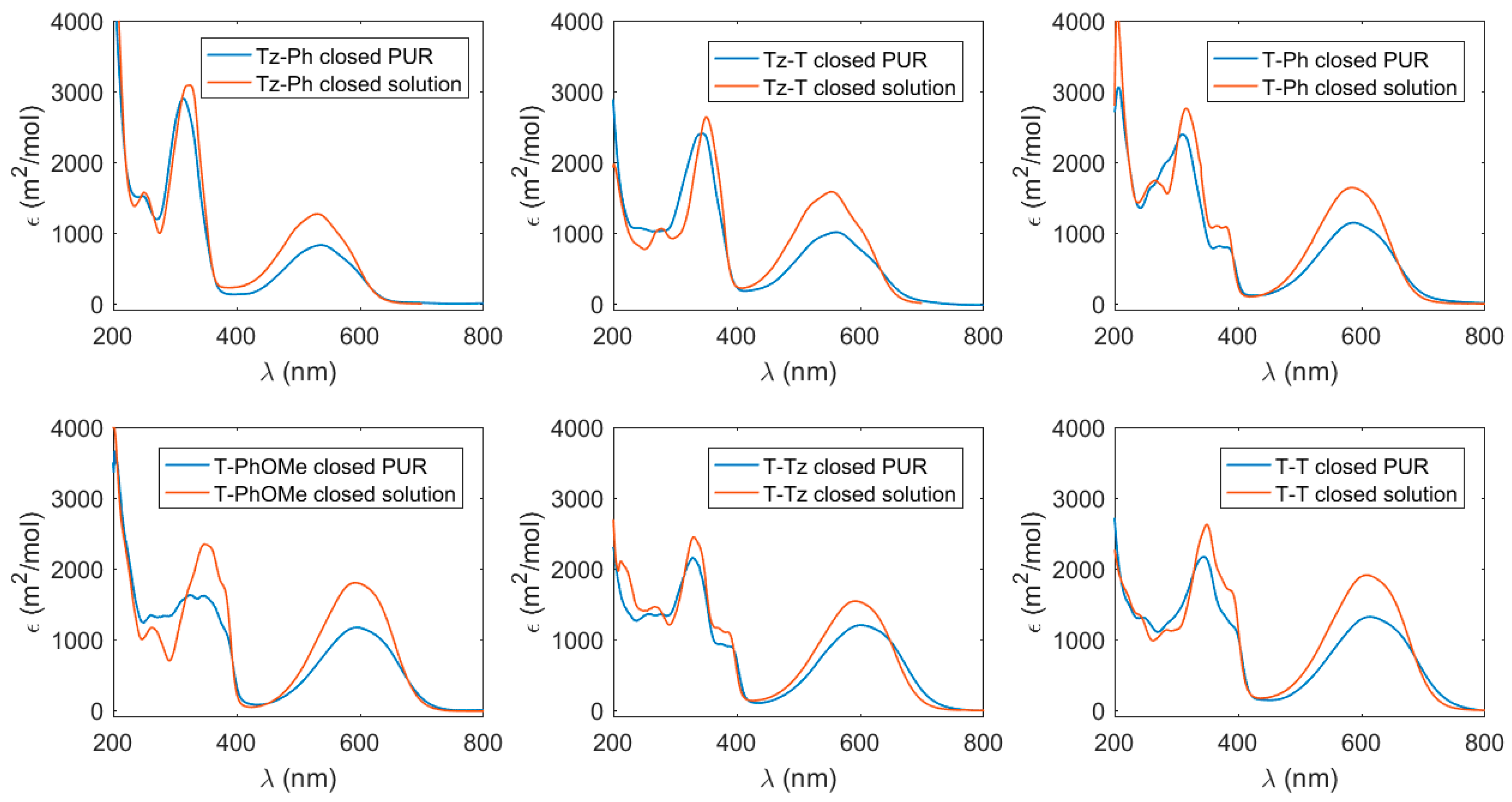
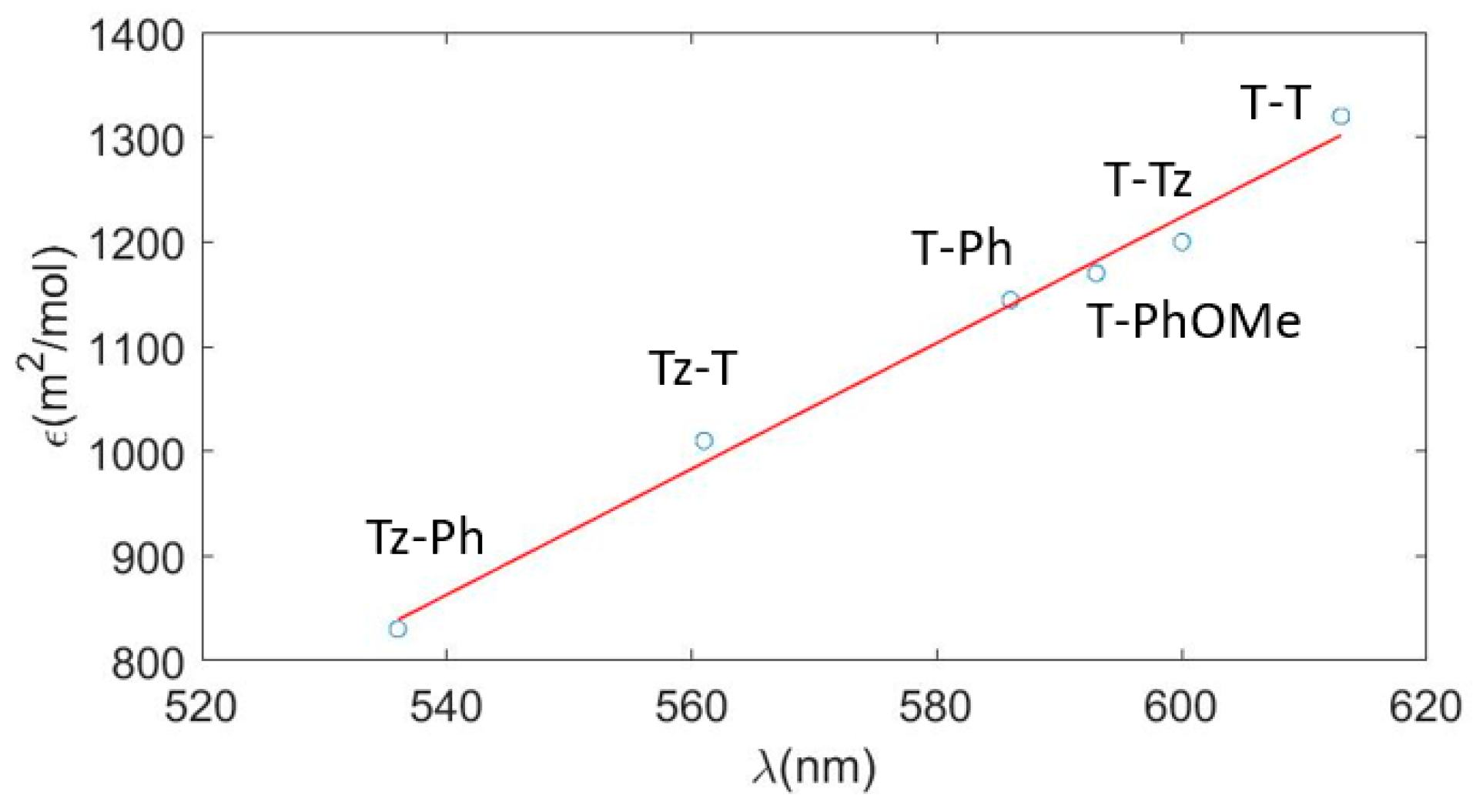
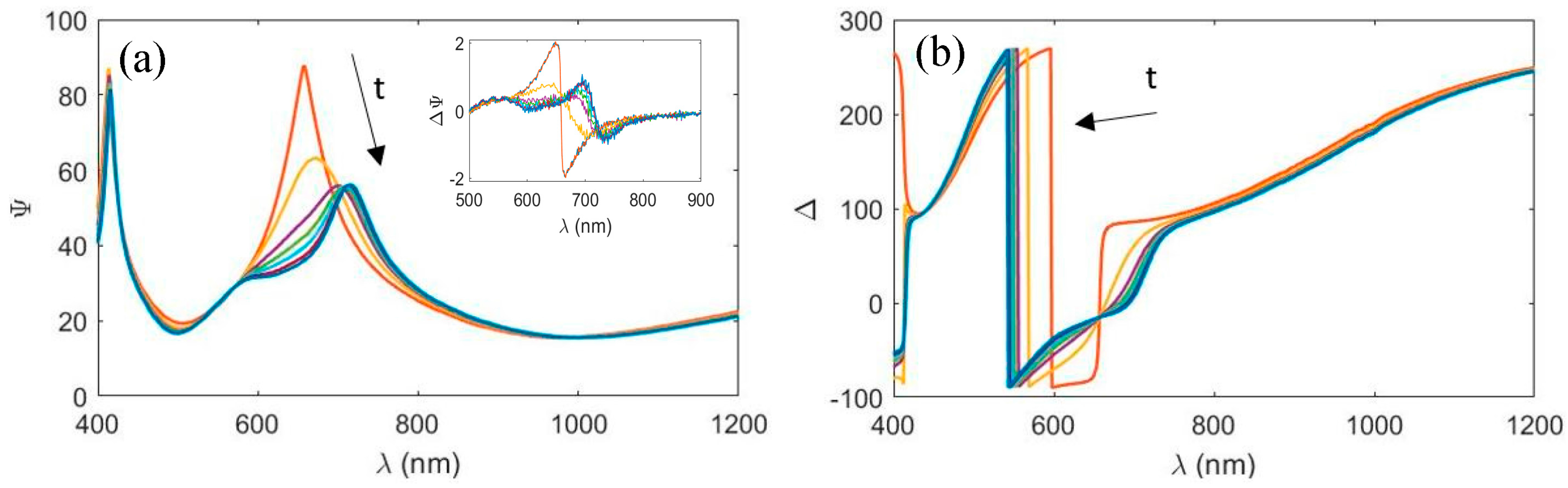
3.1. Absorption Properties in the UV–Vis Spectral Range
3.2. Refractive Index Change in the NIR Spectral Range
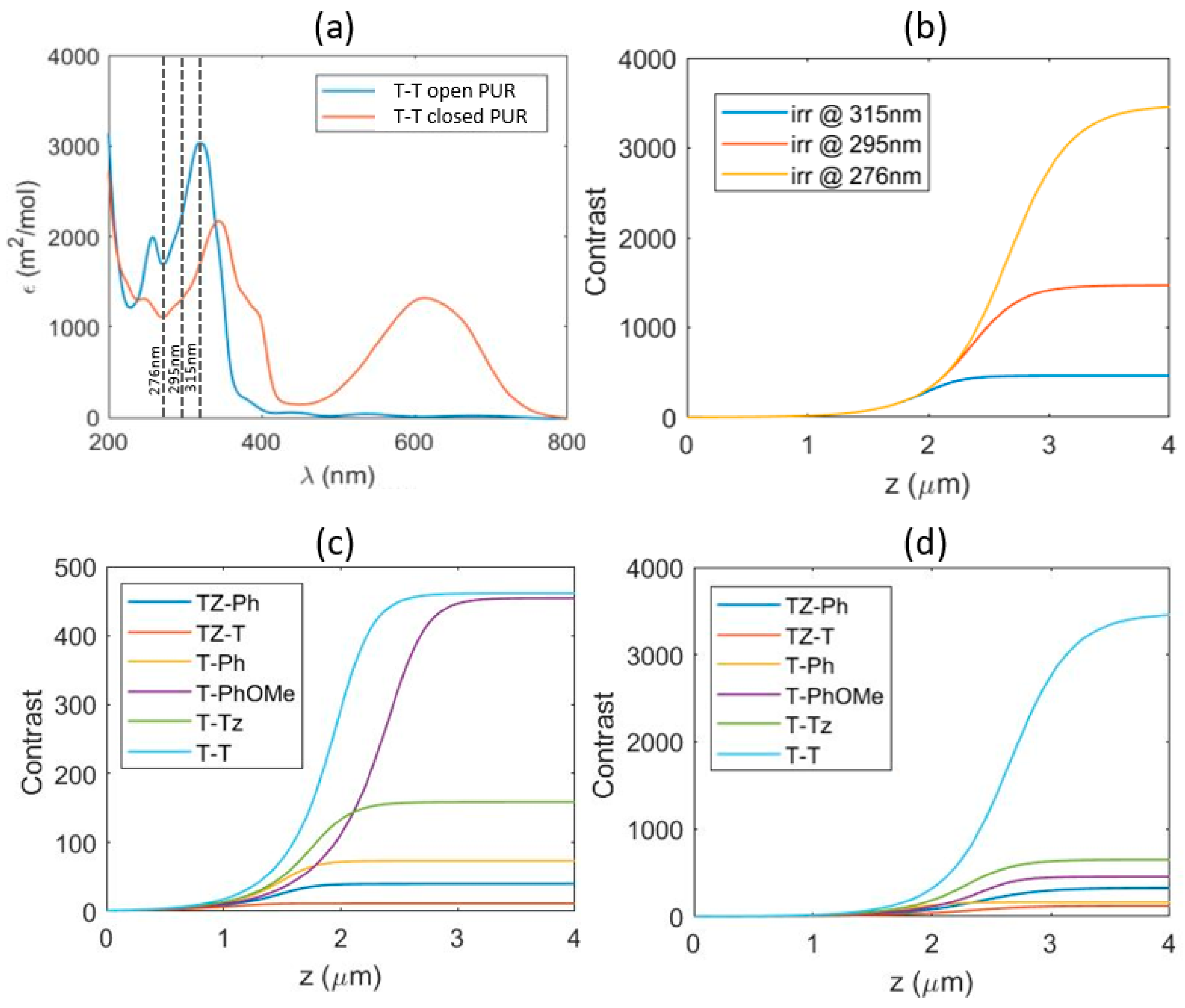
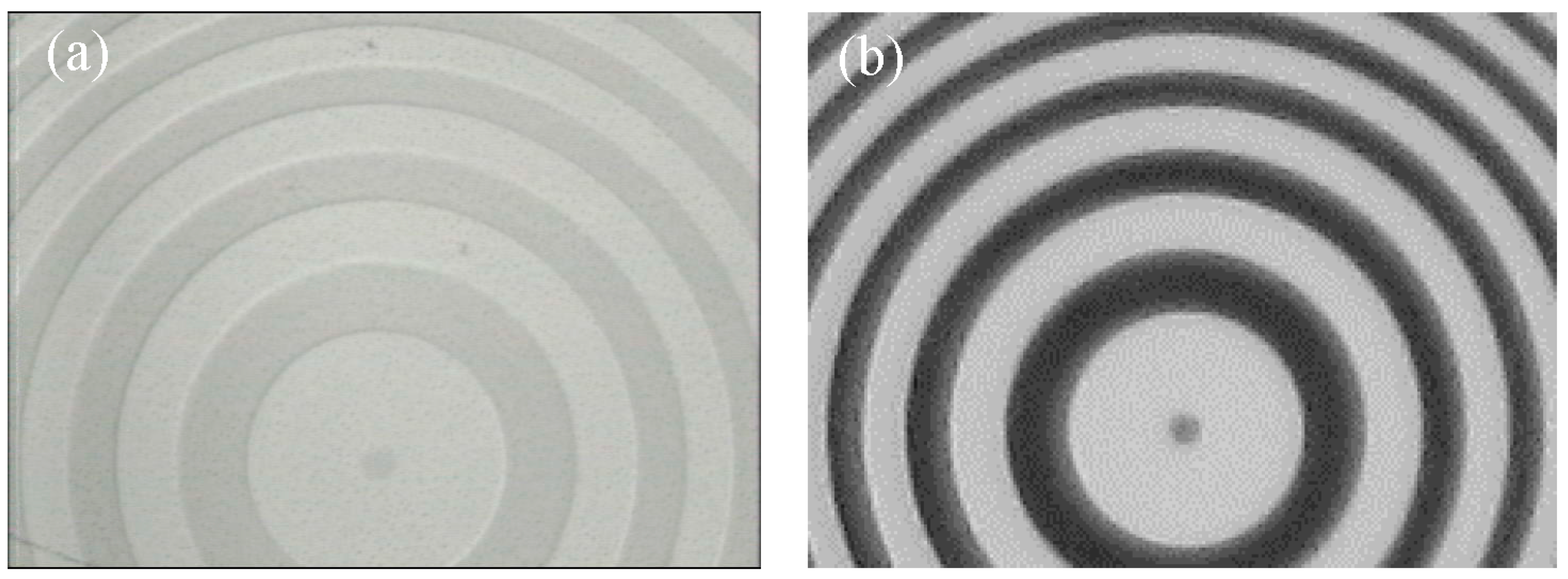
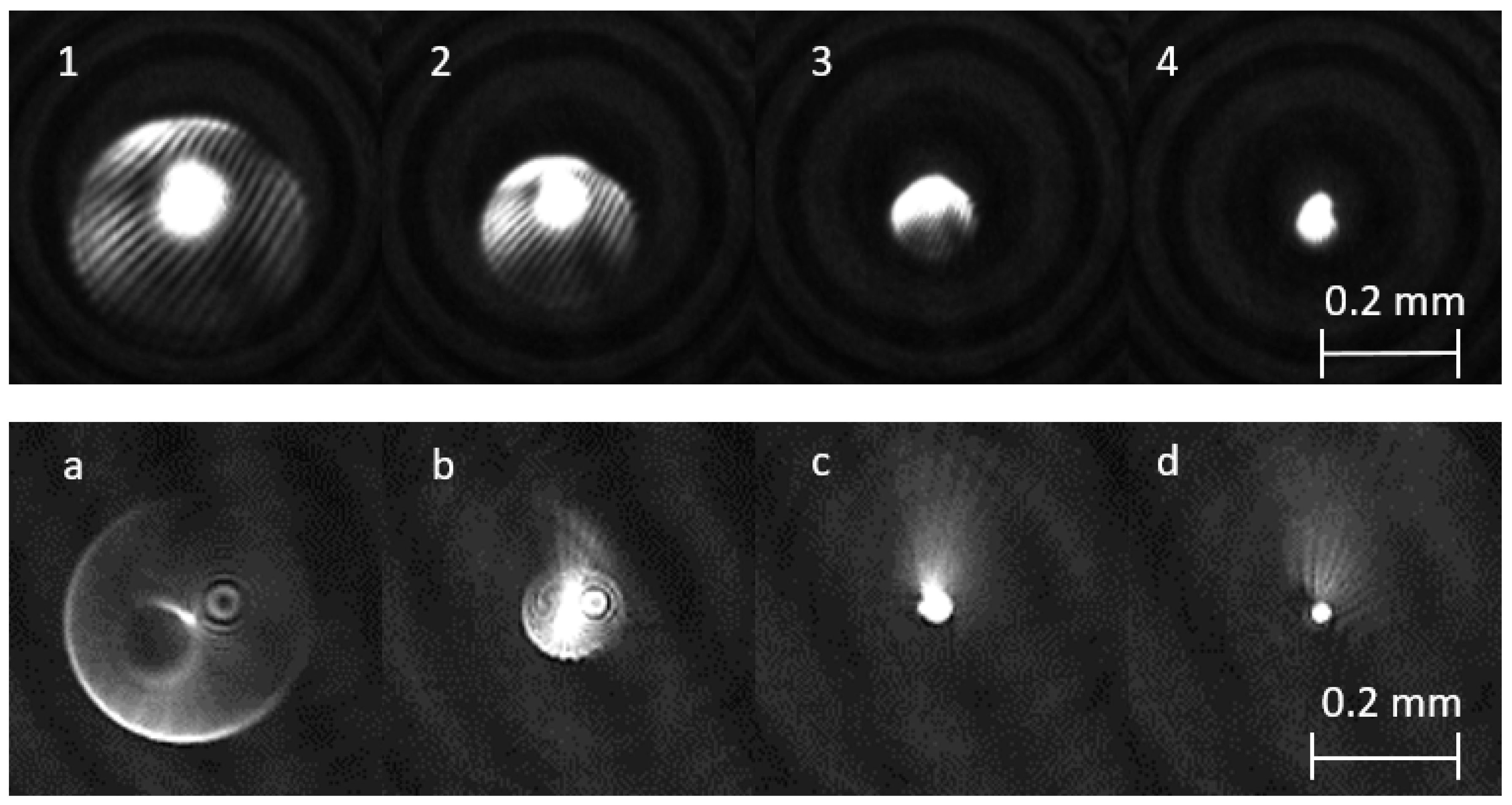
3.3. Phase and Amplitude Patterns on PU Films
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Crano, J.C.; Flood, T.; Knowles, D.; Kumar, A.; Van Gemert, B. Photochromic compounds: Chemistry and application in ophthalmic lenses. Pure Appl. Chem. 1996, 68, 1395–1398. [Google Scholar] [CrossRef]
- Irie, M.; Fukaminato, T.; Matsuda, K.; Kobatake, S. Photochromism of diarylethene molecules and crystals: Memories, switches, and actuators. Chem. Rev. 2014, 114, 12174–12277. [Google Scholar] [CrossRef] [PubMed]
- Spanò, P.; Zerbi, F.M.; Norrie, C.J.; Cunningham, C.R.; Strassmeier, K.G.; Bianco, A.; Blanche, P.A.; Bougoin, M.; Ghigo, M.; Hartmann, P.; et al. Challenges in optics for Extremely Large Telescope instrumentation. Astron. Nachr. 2006, 327, 649–673. [Google Scholar] [CrossRef]
- Bertarelli, C.; Bianco, A.; Castagna, R.; Pariani, G. Photochromism into optics: Opportunities to develop light-triggered optical elements. J. Photochem. Photobiol. C 2011, 12, 106–125. [Google Scholar] [CrossRef]
- Alata, R.; Pariani, G.; Zamkotsian, F.; Lanzoni, P.; Bianco, A.; Bertarelli, C. Programmable CGH on photochromic plates coded with DMD generated masks. Opt. Express 2017, 25, 6945–6953. [Google Scholar] [CrossRef] [PubMed]
- Pariani, G.; Bertarelli, C.; Dassa, G.; Bianco, A.; Zerbi, G. Photochromic polyurethanes for rewritable CGHs in optical testing. Opt. Express 2011, 19, 4536–4541. [Google Scholar] [CrossRef] [PubMed]
- Andrew, T.L.; Tsai, H.Y.; Menon, R. Confining Light to Deep Subwavelength Dimensions to Enable Optical Nanopatterning. Science 2009, 324, 917–921. [Google Scholar] [CrossRef] [PubMed]
- Menon, R.; Smith, H.I. Absorbance-modulation optical lithography. J. Opt. Soc. Am. A 2006, 23, 2290–2294. [Google Scholar] [CrossRef]
- Pariani, G.; Castagna, R.; Menon, R.; Bertarelli, C.; Bianco, A. Modeling absorbance-modulation optical lithography in photochromic films. Opt. Lett. 2013, 38, 3024–3027. [Google Scholar] [CrossRef] [PubMed]
- Bianco, A.; Bertarelli, C.; Gallazzi, M.C.; Zerbi, G.; Giro, E.; Molinari, E. Smart focal plane masks: Rewritable photochromic films for astronomical multi-object spectroscopy. Astron. Nachr. 2005, 326, 370–374. [Google Scholar] [CrossRef]
- Stellacci, F.; Bertarelli, C.; Toscano, F.; Gallazzi, M.C.; Zotti, G.; Zerbi, G. A high quantum yield diarylethene-backbone photochromic polymer. Adv. Mater. 1999, 11, 292–295. [Google Scholar] [CrossRef]
- Bens, A.T.; Comanici, R.; Gabel, B.; Kryschi, C.; Martin, H.D.; Ritter, H. Novel photosensitive polyurethanes based on photochromic dithienylethene monomers: Synthesis, characterization and photophysical properties of a new film-building material for photonic applications. e-Polymers 2003, 3, 1–13. [Google Scholar] [CrossRef]
- Wigglesworth, T.J.; Myles, A.J.; Branda, N.R. High-content photochromic polymers based on dithienylethenes. Eur. J. Org. Chem. 2005, 2005, 1233–1238. [Google Scholar] [CrossRef]
- Pariani, G.; Castagna, R.; Dassa, G.; Hermes, S.; Vailati, C.; Bianco, A.; Bertarelli, C. Diarylethene-based photochromic polyurethanes for multistate optical memories. J. Mater. Chem. 2011, 21, 13223–13231. [Google Scholar] [CrossRef]
- Hermes, S.; Dassa, G.; Toso, G.; Bianco, A.; Bertarelli, C.; Zerbi, G. New fast synthesis route for symmetric and asymmetric phenyl-substituted photochromic dithienylethenes bearing functional groups such as alcohols, carboxylic acids, or amines. Tetrahedron Lett. 2009, 50, 1614–1617. [Google Scholar] [CrossRef]
- Sevez, G.; Gan, J.A.; Pan, J.F.; Sallenave, X.; Colin, A.; Saadoui, H.; Saleh, A.; Vogtle, F.; Pozzo, J.L. Multi-addressable supramolecular gels based on linear amino acid and bisthienylcyclopentene. J. Phys. Org. Chem. 2007, 20, 888–893. [Google Scholar] [CrossRef]
- Toccafondi, C.; Occhi, L.; Cavalleri, O.; Penco, A.; Castagna, R.; Bianco, A.; Bertarelli, C.; Comoretto, D.; Canepa, M. Photochromic and photomechanical responses of an amorphous diarylethene-based polymer: A spectroscopic ellipsometry investigation of ultrathin films. J. Mater. Chem. C 2014, 2, 4692–4698. [Google Scholar] [CrossRef]
- Pariani, G.; Bianco, A.; Castagna, R.; Bertarelli, C. Kinetics of Photochromic Conversion at the Solid State: Quantum Yield of Dithienylethene-Based Films. J. Phys. Chem. A 2011, 115, 12184–12193. [Google Scholar] [CrossRef] [PubMed]
- Pariani, G.; Bertarelli, C.; Bianco, A.; Schaal, F.; Pruss, C. Characterization of photochromic computer-generated holograms for optical testing. Proc. SPIE 2012, 8450, 845010–845018. [Google Scholar]

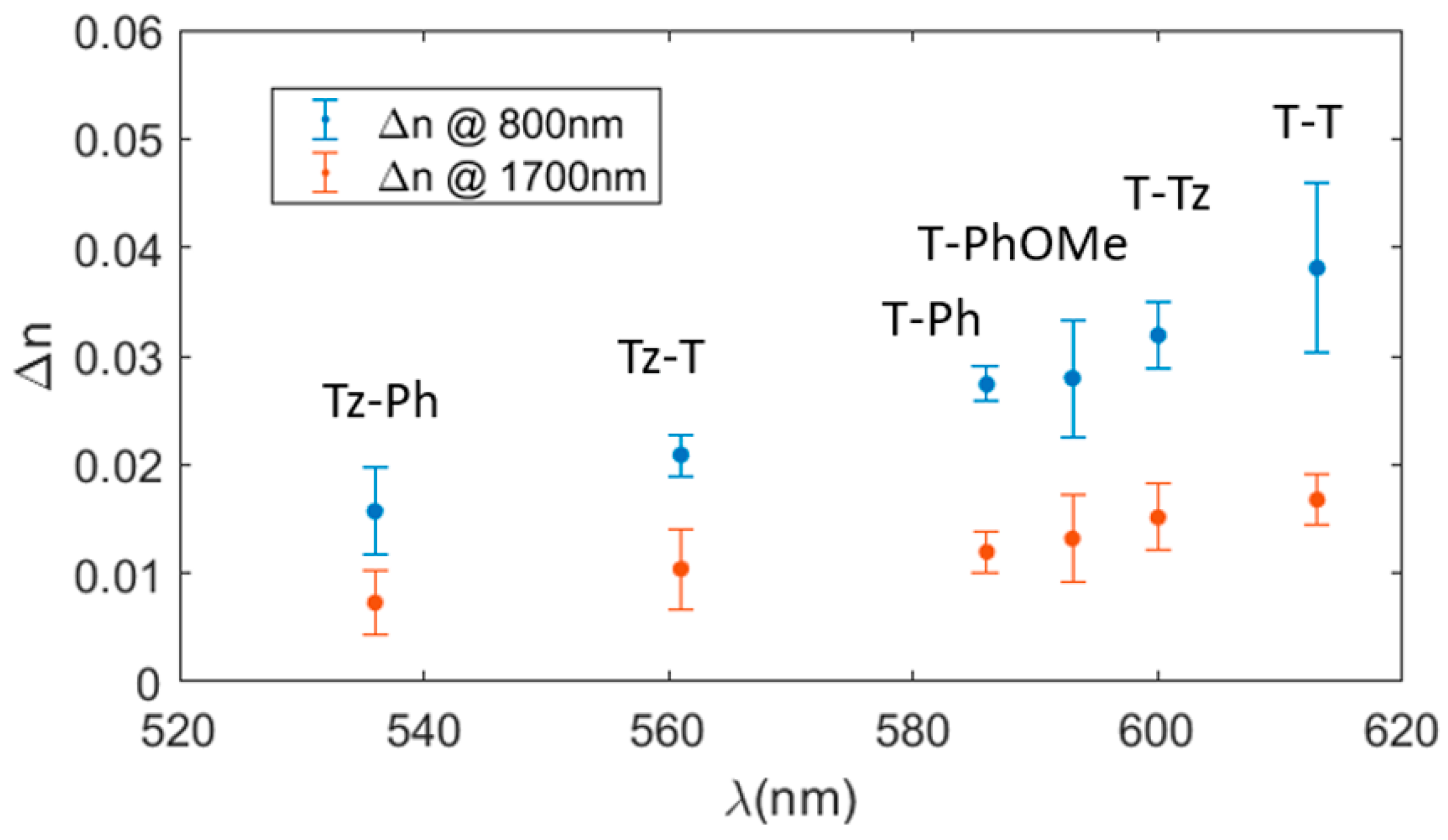
| PU | Thickness (nm) | Δn at 800 nm × 10−2 | Δn at 1700 nm × 10−2 |
|---|---|---|---|
| Tz-Ph | 480 | 1.6 | 0.7 |
| Tz-T | 370,410,500 | 2.1 | 1.0 |
| T-Ph | 420,470,510 | 2.7 | 1.2 |
| T-PhOMe | 270,420 | 2.8 | 1.3 |
| T-Tz | 360,340 | 3.2 | 1.5 |
| T-T | 360,380,510 | 3.8 | 1.7 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oggioni, L.; Toccafondi, C.; Pariani, G.; Colella, L.; Canepa, M.; Bertarelli, C.; Bianco, A. Photochromic Polyurethanes Showing a Strong Change of Transparency and Refractive Index. Polymers 2017, 9, 462. https://doi.org/10.3390/polym9090462
Oggioni L, Toccafondi C, Pariani G, Colella L, Canepa M, Bertarelli C, Bianco A. Photochromic Polyurethanes Showing a Strong Change of Transparency and Refractive Index. Polymers. 2017; 9(9):462. https://doi.org/10.3390/polym9090462
Chicago/Turabian StyleOggioni, Luca, Chiara Toccafondi, Giorgio Pariani, Letizia Colella, Maurizio Canepa, Chiara Bertarelli, and Andrea Bianco. 2017. "Photochromic Polyurethanes Showing a Strong Change of Transparency and Refractive Index" Polymers 9, no. 9: 462. https://doi.org/10.3390/polym9090462
APA StyleOggioni, L., Toccafondi, C., Pariani, G., Colella, L., Canepa, M., Bertarelli, C., & Bianco, A. (2017). Photochromic Polyurethanes Showing a Strong Change of Transparency and Refractive Index. Polymers, 9(9), 462. https://doi.org/10.3390/polym9090462