Effect of the Grafting Reaction of Aluminum Nitride on the Multi-Walled Carbon Nanotubes on the Thermal Properties of the Poly(phenylene sulfide) Composites
Abstract
:1. Introduction
2. Experimental
2.1. Materials
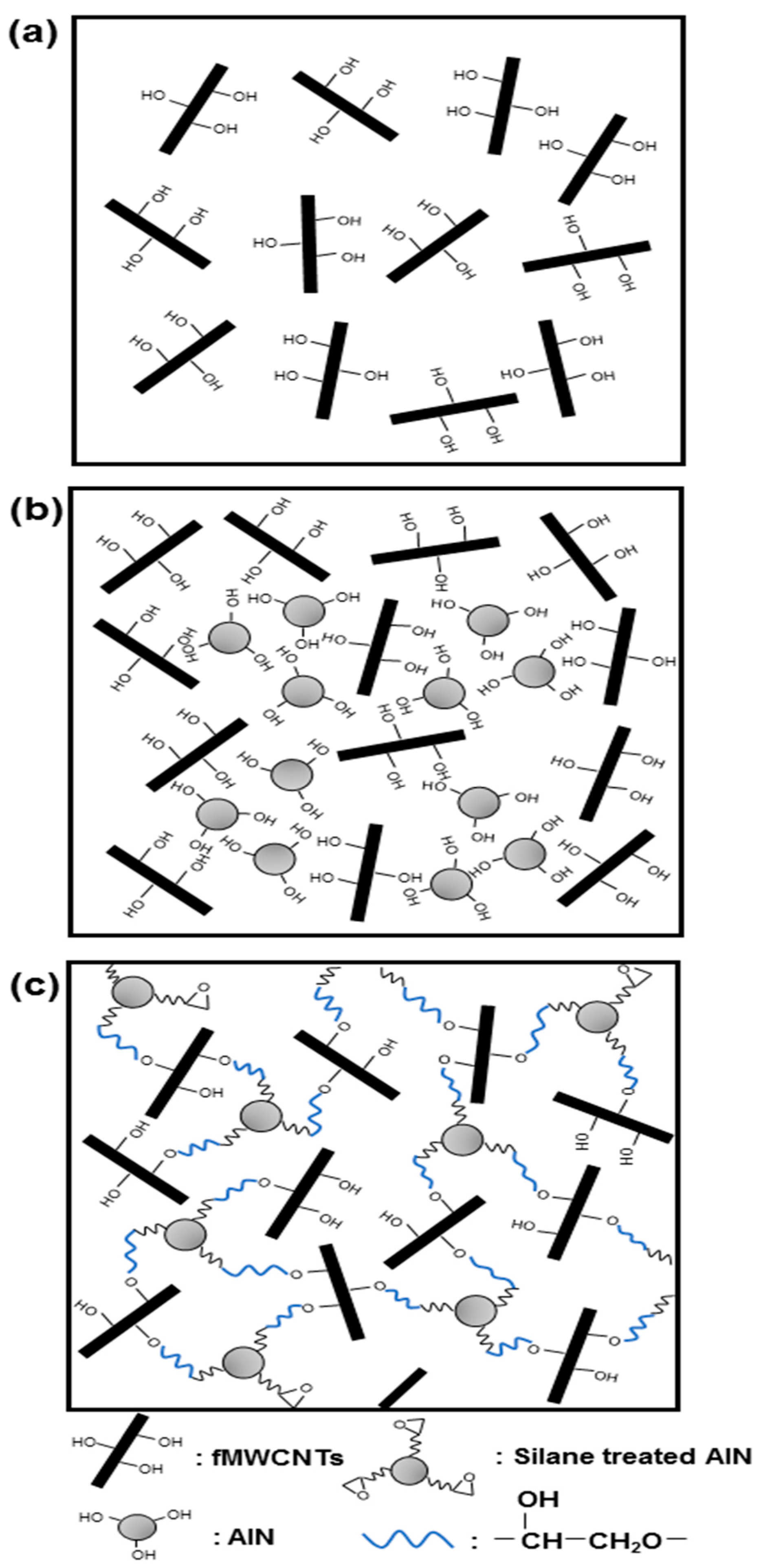
2.2. Covalent Functionalization of the MWCNTs
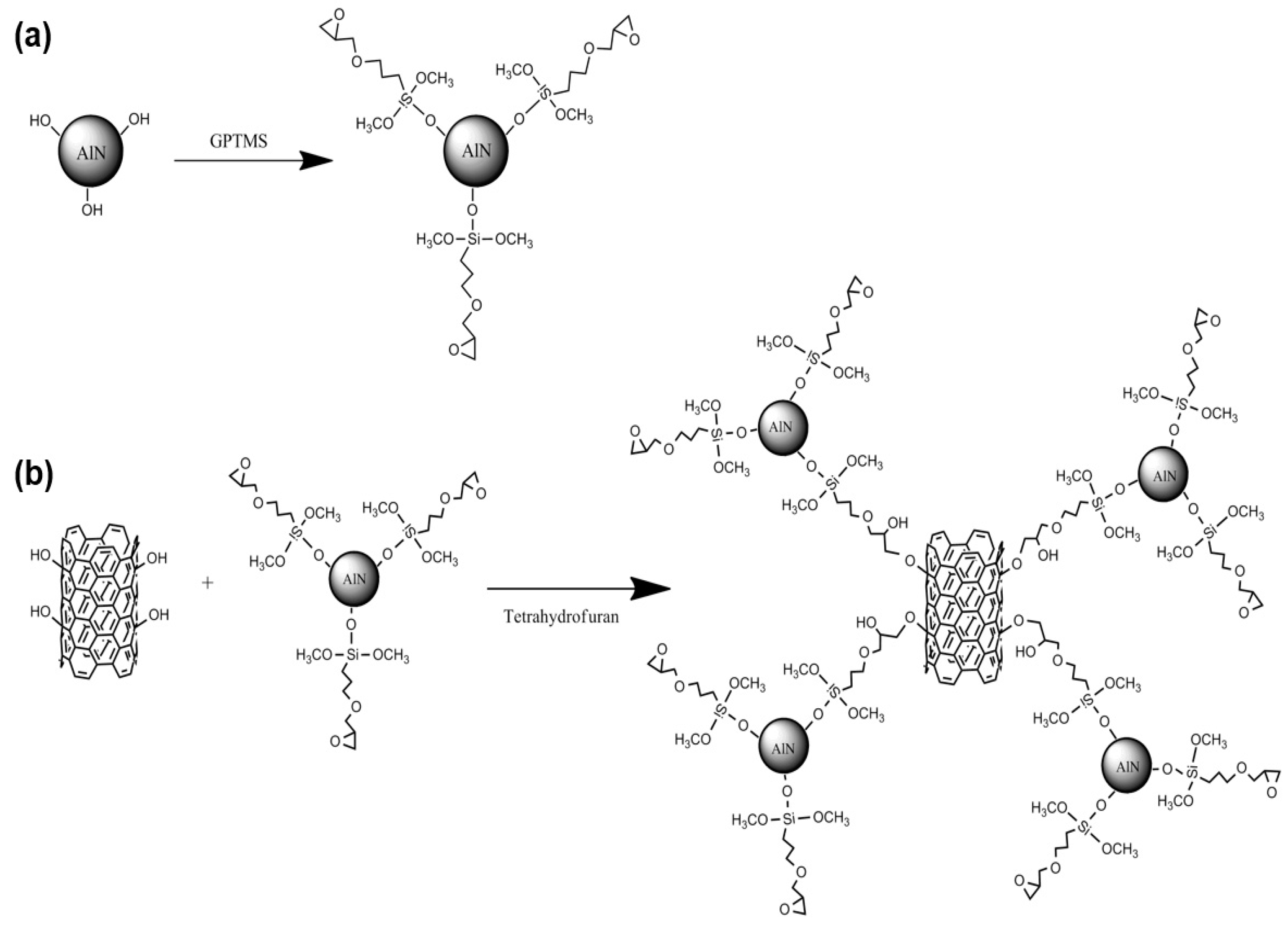
2.3. Surface Modification of the AlN Particles
2.4. The Grafting Reaction of the AlN Particles on the MWCNTs
2.5. Preparation of the PPS/MWCNTs/AlN Composites
2.6. Characterization
3. Results and Discussion
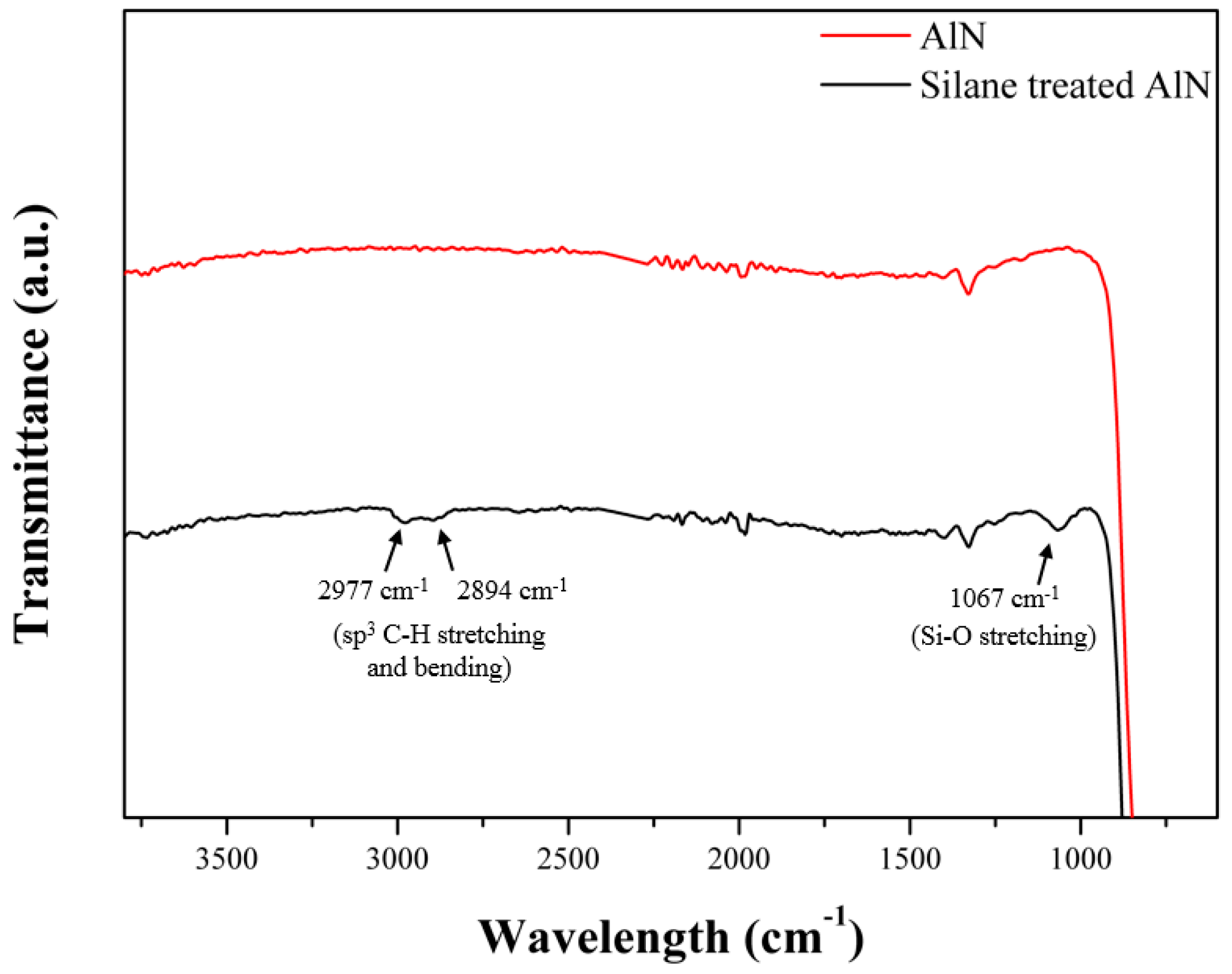
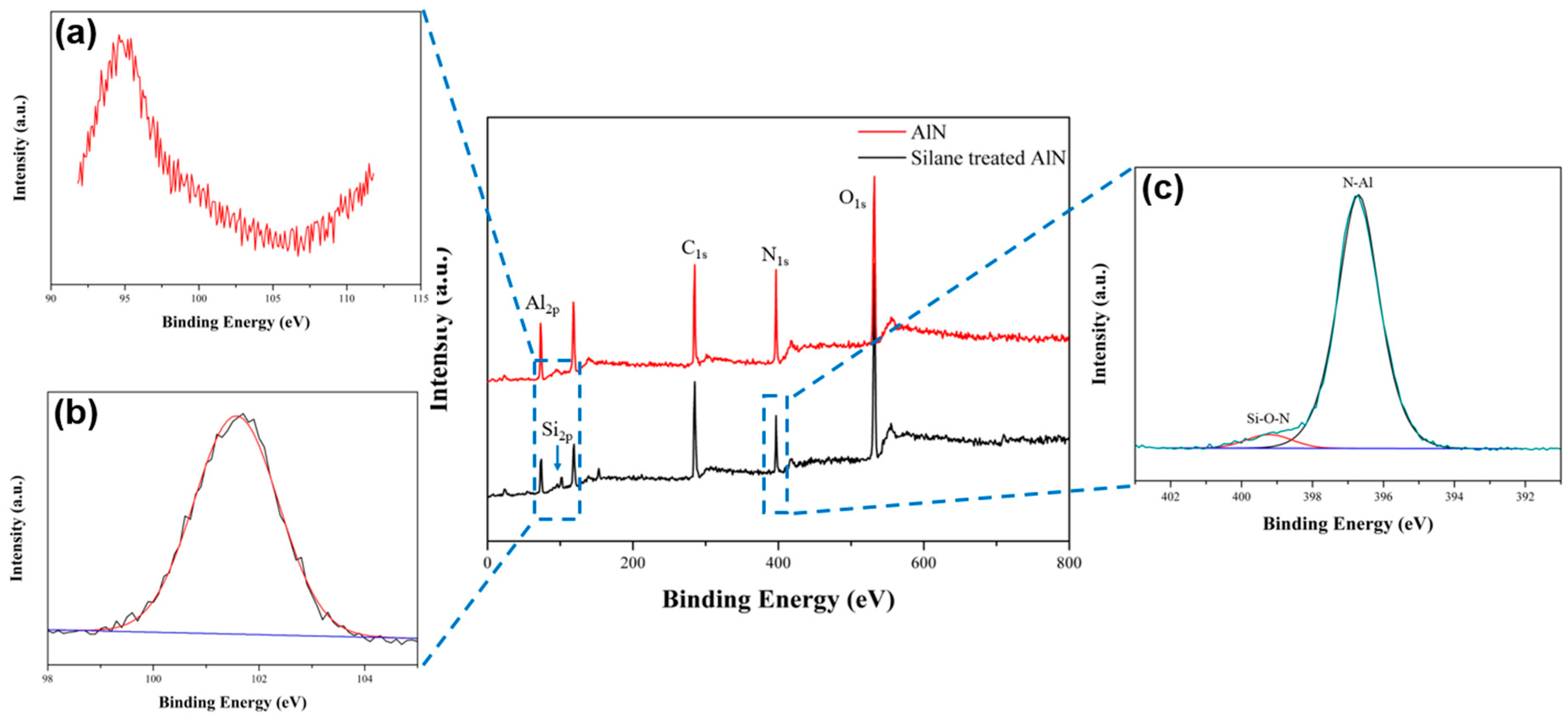
3.1. Characterization of the Surface Modification of the AlN Particles
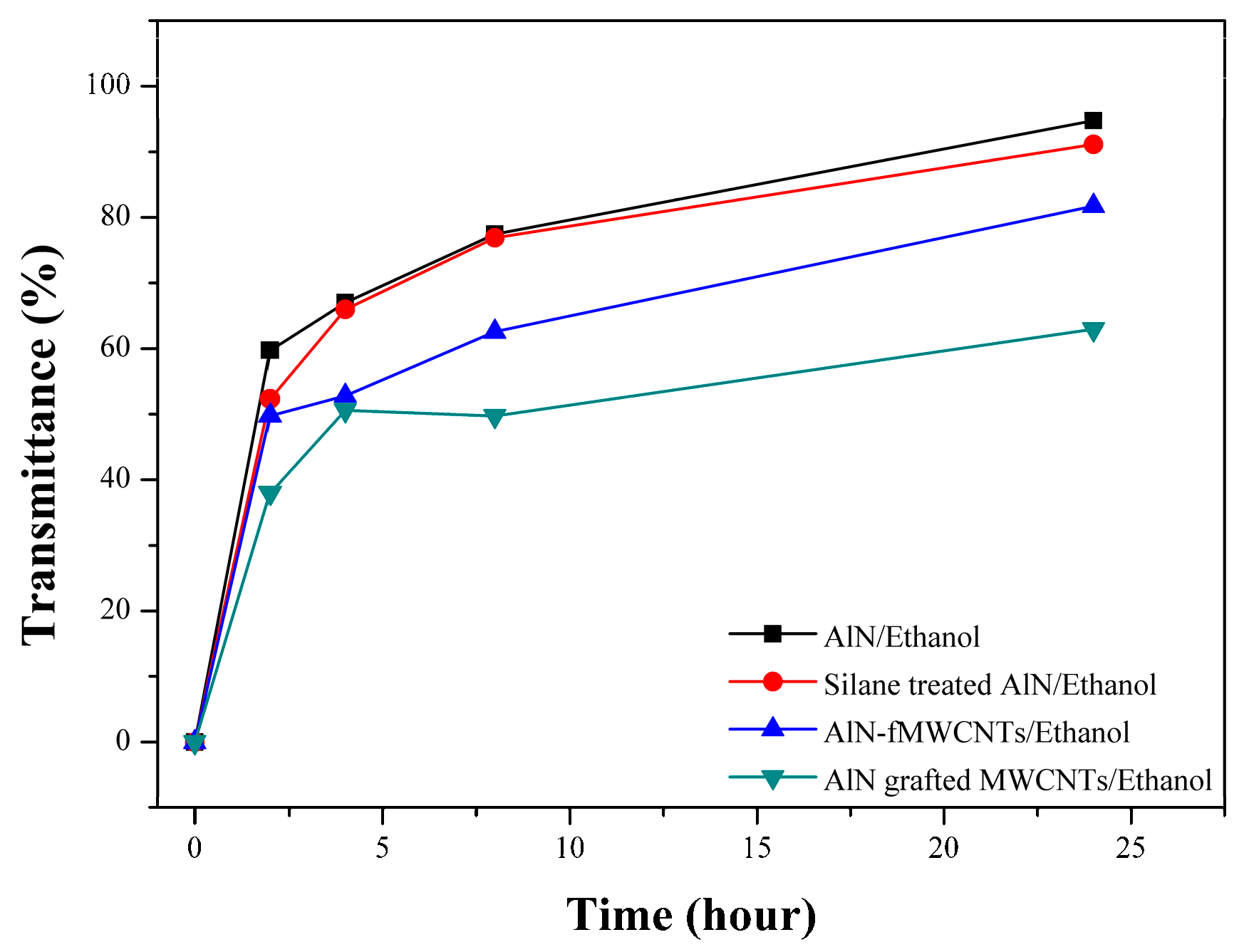
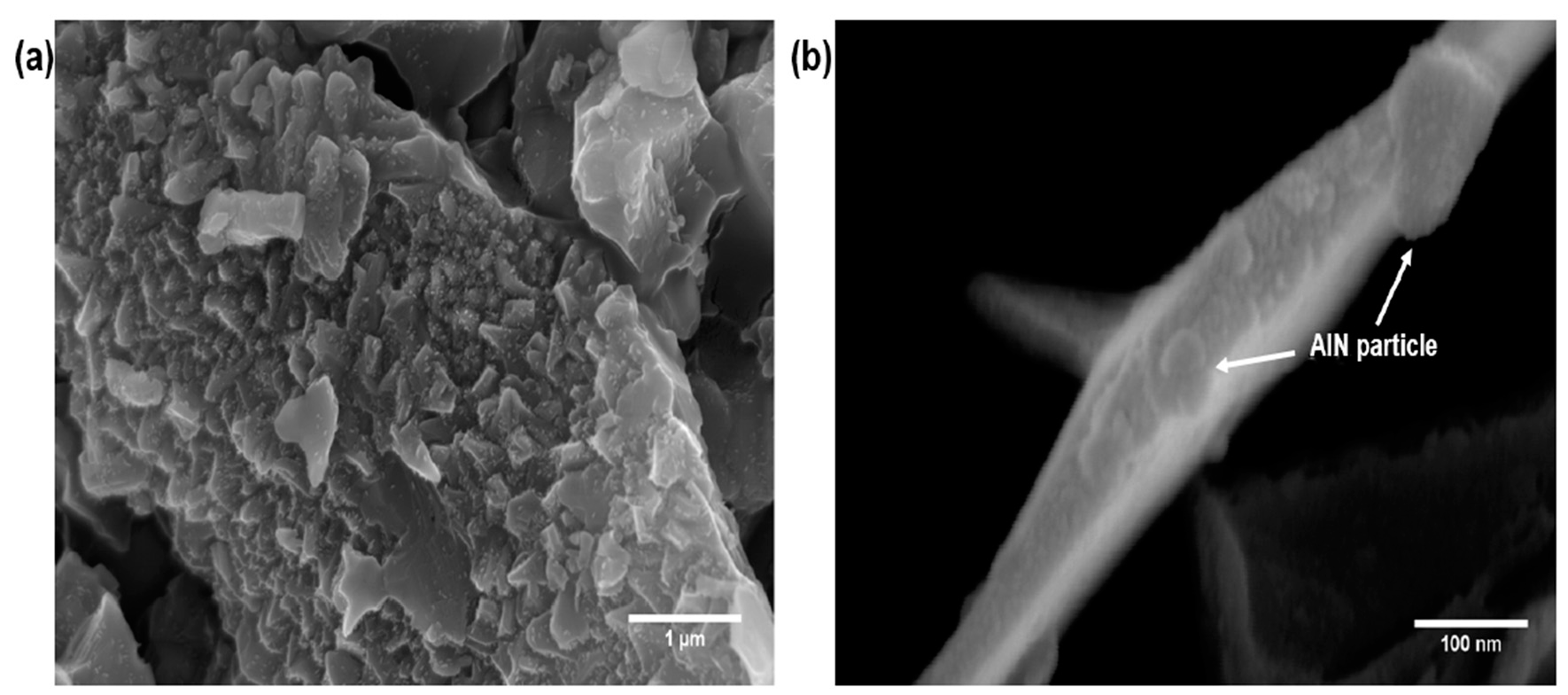
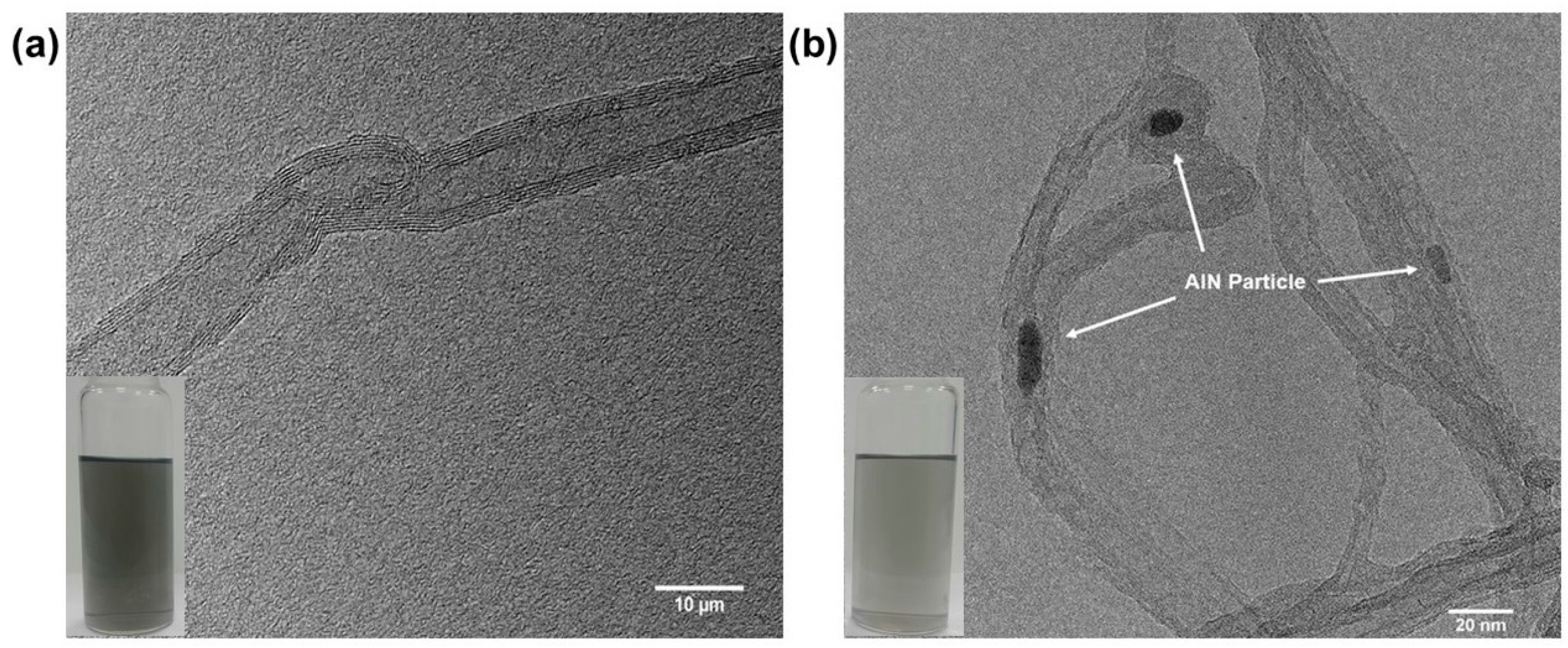
3.2. Characterization of the Grafting Reaction of AlN Particles on the MWCNTs
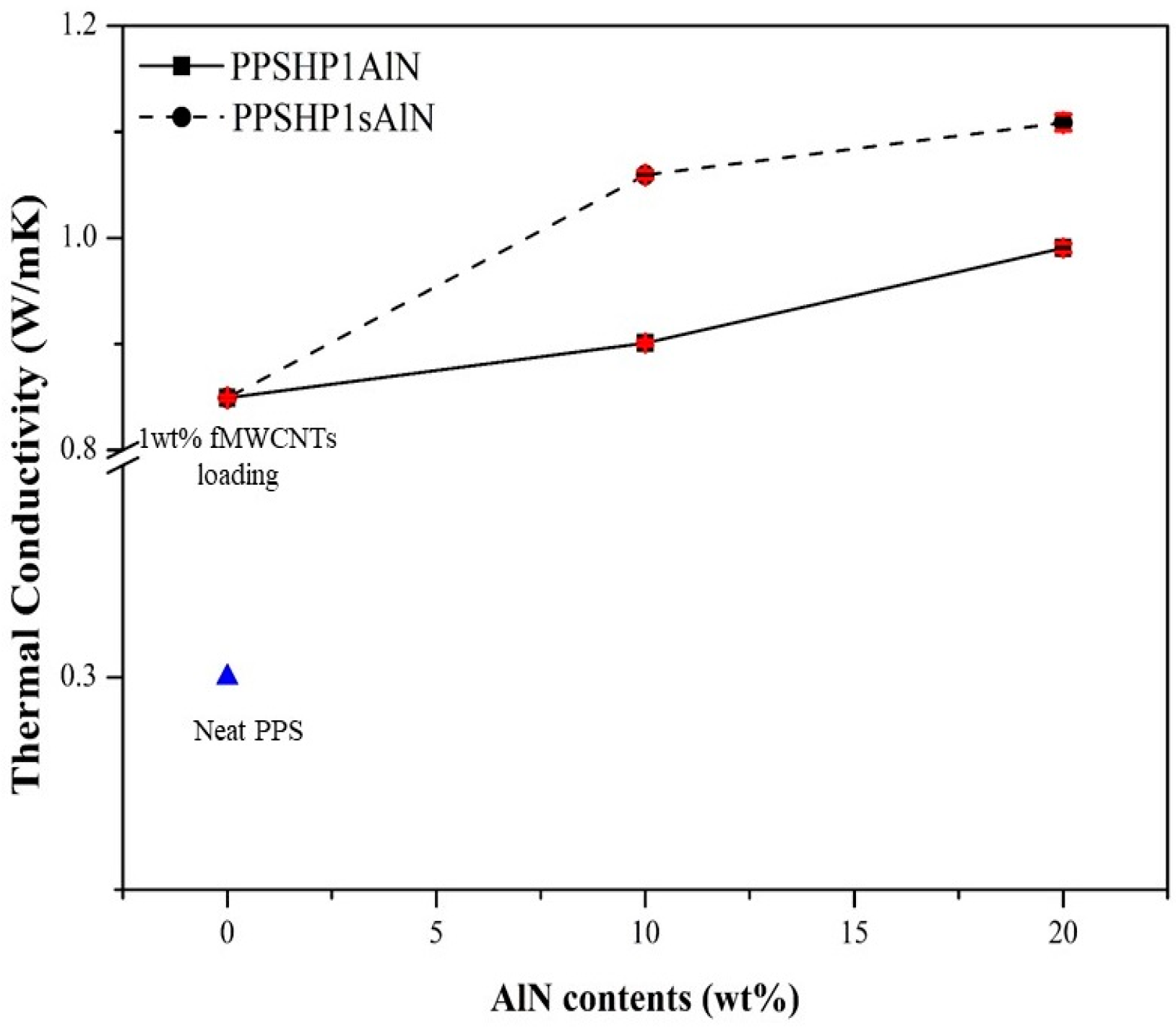
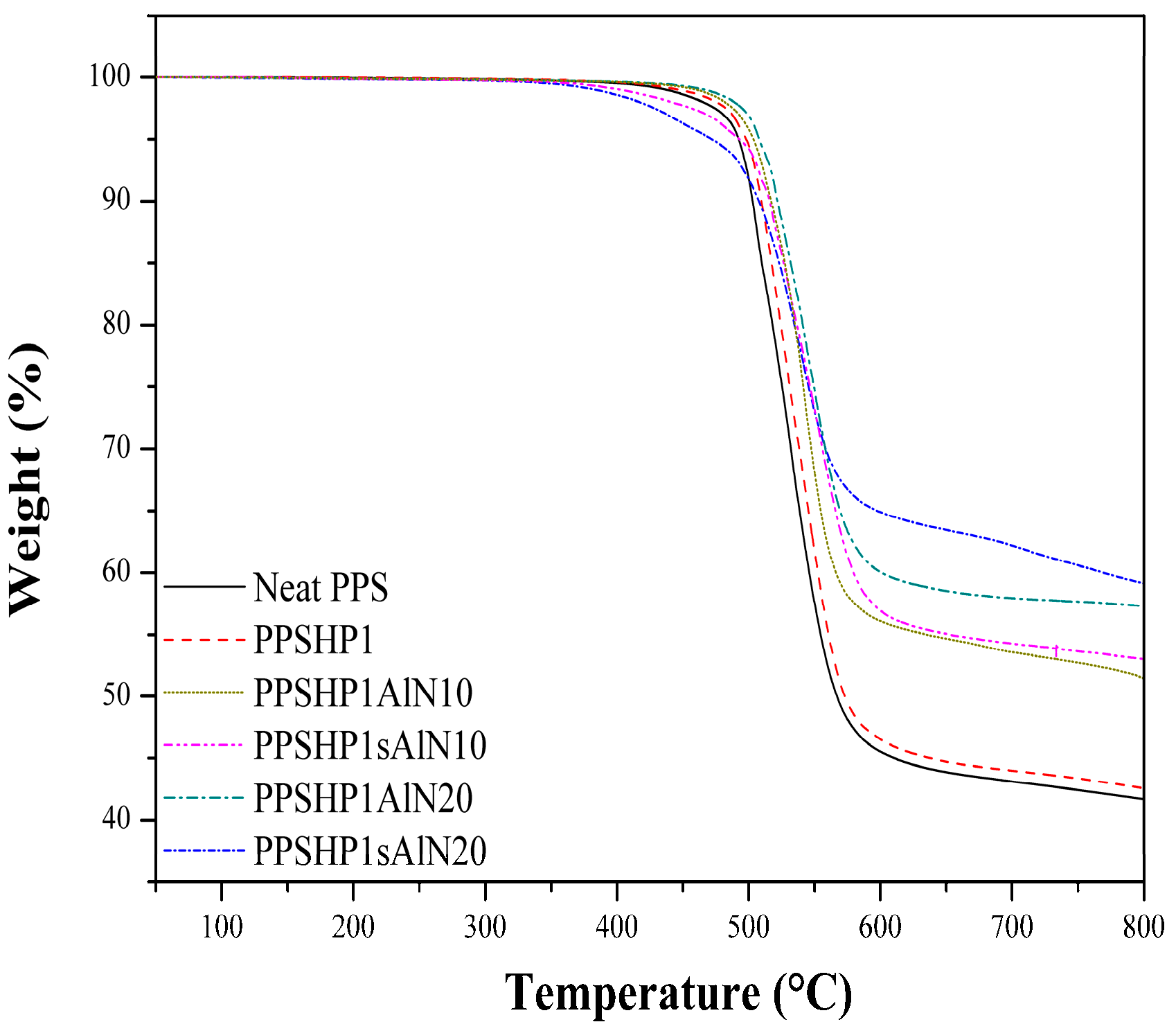
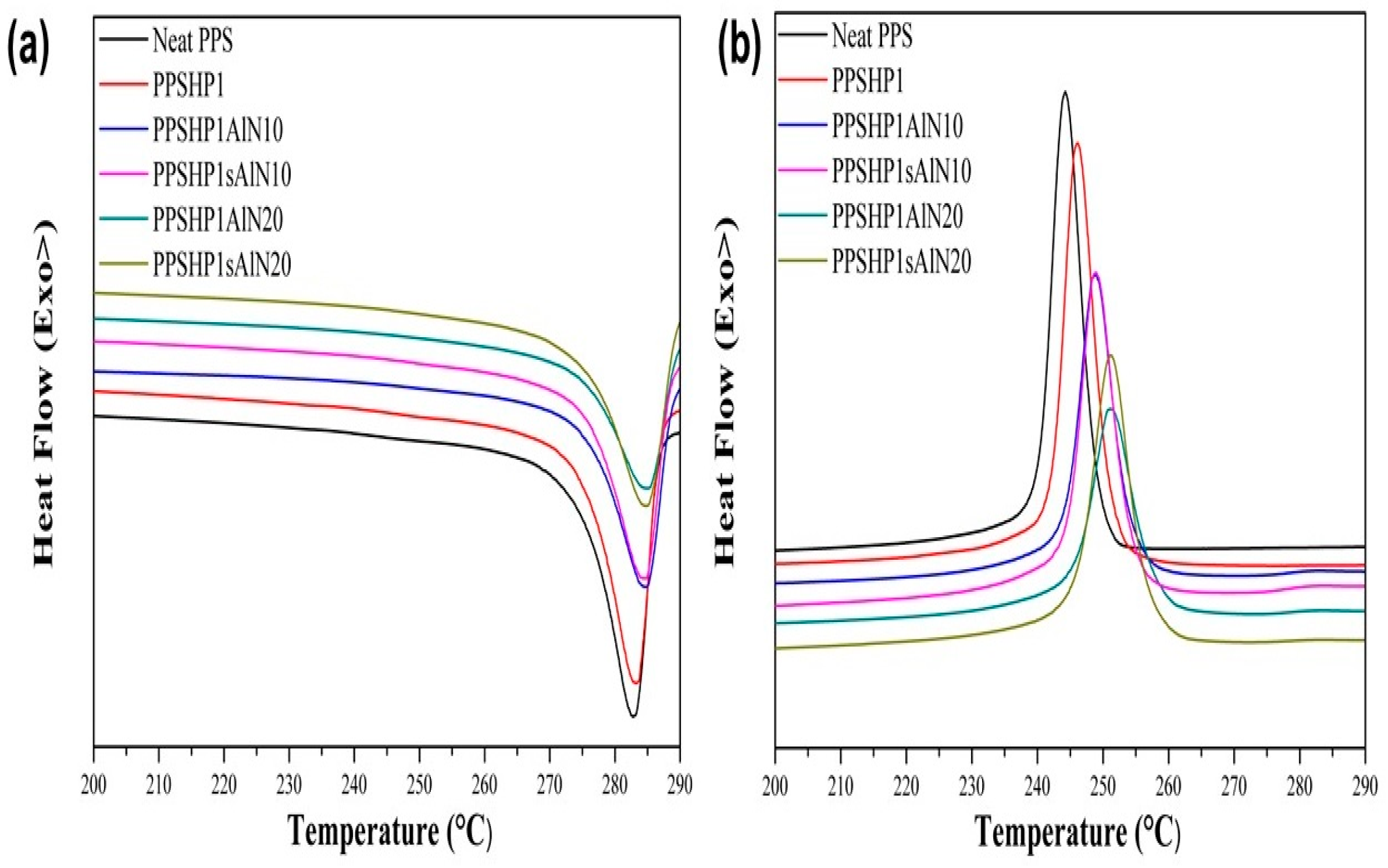
3.3. Thermal Properties of the PPS/MWCNTs/AlN Composites
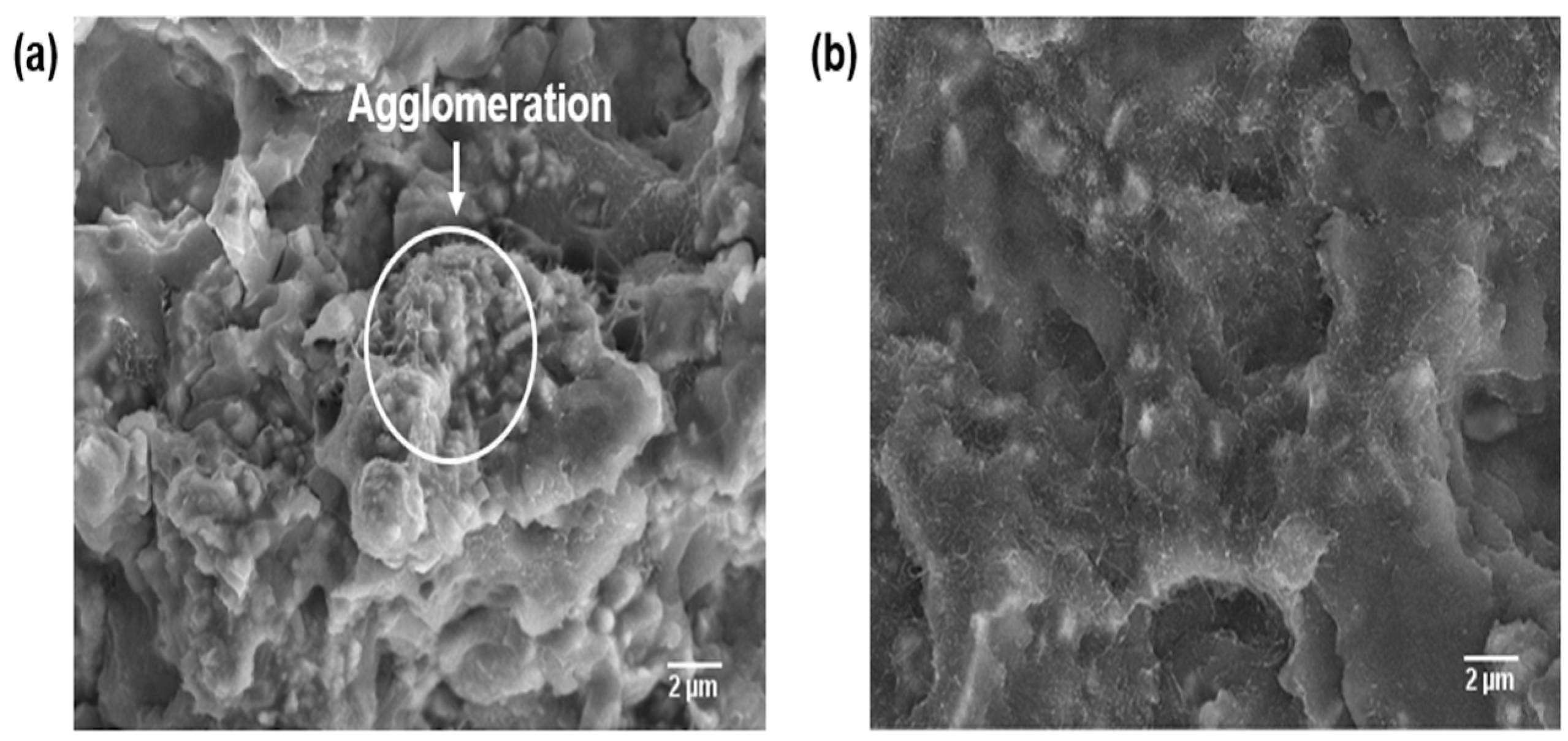
3.4. Morphological Differences of the PPS/MWCNTs/AlN Composites
4. Conclusions
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Rahate, A.S.; Nemade, K.R.; Waghuley, S.A. Polyphenylene sulfide (PPS): State of the art and applications. Rev. Chem. Eng. 2013, 29, 471–489. [Google Scholar] [CrossRef]
- Chen, Z.; Li, T.; Yang, Y.; Liu, X.; Lv, R. Mechanical and tribological properties of PA/PPS blends. Wear 2004, 257, 696–707. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56–58. [Google Scholar] [CrossRef]
- Moniruzzaman, M.; Winey, K.I. Polymer Nanocomposites Containing Carbon Nanotubes. Macromolecules 2006, 39, 5194–5205. [Google Scholar] [CrossRef]
- McEuen, P.L.; Bockrath, M.; Cobden, D.H.; Yoon, T.G.; Louie, S.G. Disorder, pseudospins, and backscattering in carbon nanotubes. Phys. Rev. Lett. 1999, 83, 5098–5101. [Google Scholar] [CrossRef]
- Uchida, T.; Kumar, S. Single wall carbon nanotube dispersion and exfoliation in polymers. J. Appl. Polym. Sci. 2005, 98, 985–989. [Google Scholar] [CrossRef]
- Sumita, M.; Sakata, K.; Asai, S.; Miyasaka, K.; Nakagawa, H. Dispersion of fillers and the electrical conductivity of polymer blends filled with carbon black. Polym. Bull. 1991, 25, 265–271. [Google Scholar] [CrossRef]
- Yoon, Y.S.; Oh, M.H.; Kim, A.Y.; Kim, N. The Development of Thermal Conductive Polymer Composties for Heat Sink. J. Chem. Chem. Eng. 2012, 6, 515–519. [Google Scholar] [CrossRef]
- Kostagiannakopoulou, C.; Fiamegkou, E.; Sotiriadis, G.; Kostopoulos, V. Thermal Conductivity of Carbon Nanoreinforced Epoxy Composites. J. Nanomater. 2016, 2016, 1847325. [Google Scholar] [CrossRef]
- Ciecierska, E.; Boczkowska, A.; Kurzydlowski, K.J.; Rosca, I.D.; Hoa, S.V. The effect of carbon nanotubes on epoxy matrix nanocomposites. J. Therm. Anal. Calorim. 2013, 111, 1019–1024. [Google Scholar] [CrossRef]
- Deng, S.; Lin, Z.; Xu, B.; Lin, H.; Du, C. Effects of Carbon Fillers on Crystallization Properties and Thermal Conductivity of Poly(phenylene sulfide). Polym. Plast. Technol. Eng. 2015, 54, 1017–1024. [Google Scholar] [CrossRef]
- Kim, S.; Kim, T.; Kim, Y.; Choi, H.; Lim, H.; Yang, S.; Park, C. Surface modification for the effective dispersion of carbon nanotubes in solvents and polymer. Carbon 2012, 50, 3–33. [Google Scholar] [CrossRef]
- Goyal, R.K.; Jadhav, P.; Tiwari, A.N. Preparation and Properties of New Polyphenylene Sulfide/AlN Composites for Electronic Packaging. J. Electron. Mater. 2011, 40, 1377–1383. [Google Scholar] [CrossRef]
- Mi, Y.; Liang, G.; Gu, A.; Zhao, F.; Yuan, L. Thermally Conductive Aluminum Nitride-Multiwalled Carbon Nanotube/Cyanate Ester Composites with High Flame Retardance and Low Dielectric Loss. Ind. Eng. Chem. Res. 2013, 52, 3342–3353. [Google Scholar] [CrossRef]
- Pak, S.; Kim, H.; Kim, S.; Youn, J. Synergistic improvement of thermal conductivity of thermoplastic composites with mixed boron nitride and multi-walled carbon nanotube fillers. Carbon 2012, 50, 4830–4838. [Google Scholar] [CrossRef]
- Teng, C.; Ma, C.M.; Chiou, K.; Lee, T. Synergic effect of thermal conductive properties of epoxy composties containing functionalized multi-walled carbon nanotubes and aluminum nitride. Compos. B Eng. 2012, 43, 265–271. [Google Scholar] [CrossRef]
- Balázsi, C.; Fényi, B.; Hegman, N.; Kövér, Z.; Wéber, F.; Vértesy, Z.; Kónya, Z.; Kiricsi, I.; Biró, L.P.; Arató, P. Development of CNT/Si3N4 composites with improved mechanical and electrical properties. Compos. B Eng. 2006, 37, 418–424. [Google Scholar] [CrossRef]
- Im, H.; Kim, J. Effect of homogeneous Al(OH)3 covered MWCNT addition on the thermal conductivity of Al2O3/epoxy-terminated poly(dimethylsiloxane) composites. J. Mater. Sci. 2012, 47, 6025–6033. [Google Scholar] [CrossRef]
- Sim, L.C.; Ramanan, S.R.; Ismail, H.; Seetharamu, K.; Goh, T. Thermal characterization of Al2O3 and ZnO reinforced silicone rubber as thermal pads for heat dissipation purposes. Thermochim. Acta 2005, 430, 155–165. [Google Scholar] [CrossRef]
- Rath, B.N.; Ghanwat, S.J.; Danani, C.; Kulkarni, R.V.; Alur, V.D.; Sathiyamoorthy, D.; Anantharaman, S. Thermal Conductivity of Composites of Beryllia and Lithium Titanate. J. Mater. Eng. Perform. 2013, 22, 3455–3460. [Google Scholar] [CrossRef]
- Naito, K.; Yang, J.; Xu, Y.; Kagawa, Y. Enhancing the thermal conductivity of polyacrylontrile- and pitch-based carbon fibers by grafting carbon nanotubes on them. Carbon 2010, 48, 1849–1857. [Google Scholar] [CrossRef]
- Zhao, J.; Du, F.; Zhou, X.; Chui, W.; Wang, X.; Zhou, H.; Xie, X.; Mai, Y. Thermal conductive and electrical properties of polyurethane/hyperbranched poly(urea-urethane)-grafted multi-walled carbon nanotube composites. Compos. B Eng. 2011, 42, 2111–2116. [Google Scholar] [CrossRef]
- Chung, D.D.L. Materials for thermal conduction. Appl. Therm. Eng. 2001, 21, 1593–1605. [Google Scholar] [CrossRef]
- Droval, G.; Feller, J.F.; Salagnac, P.; Glouannec, P. Thermal conductivity enhancement of electrically insulating syndiotactic poly(styrene) matrix for diphasic conductive polymer composites. Polym. Adv. Technol. 2006, 17, 732–745. [Google Scholar] [CrossRef]
- Thostenson, E.T.; Ren, Z.; Chou, T.W. Advances in the science and technology of carbon nanotubes and their composites: A review. Compos. Sci. Technol. 2001, 61, 1899–1912. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Roh, H.; Kang, H.; Park, S.; Oh, S.; Lee, J.; Park, J. Covalent functionalization of multi-walled carbon nanotubes surface via chemical treatment. J. Nanosci. Nanotechnol. 2017, 17, 2463–2470. [Google Scholar] [CrossRef]
- Zhou, Y.; Hu, J.; Chen, X.; Yu, F.; He, J. Thermoplastic Polypropylene/Aluminum Nitride Nanocomposites with Enhanced Thermal Conductivity and Low Dielectric Loss. IEEE Trans. Dielectr. Electr. Insul. 2016, 23, 2768–2776. [Google Scholar] [CrossRef]
- Hagio, T.; Takase, A.; Umebayashi, S. X-ray photoelectron spectroscopic studies of β-sialons. J. Mater. Sci. Lett. 1992, 11, 878–880. [Google Scholar] [CrossRef]
- Castanho, S.M.; Moreno, R.; Fierro, J.L.G. Influence of process conditions on the surface oxidation of silicon nitride green compacts. J. Mater. Sci. 1997, 32, 157–162. [Google Scholar] [CrossRef]
- Xu, Y.; Chung, D.D.L.; Mroz, C. Thermally conducting aluminum nitride polymer-matrix composites. Compos. A Appl. Sci. Manuf. 2001, 32, 1749–1757. [Google Scholar] [CrossRef]
- Zhou, W.; Qi, S.; Li, H.; Shao, S. Study on insulating thermal conductive BN/HDPE composites. Thermochim. Acta 2007, 452, 36–42. [Google Scholar] [CrossRef]
- Dashora, P.; Gupta, G. On the temperature dependence of the thermal conductivity of linear amorphous polymers. Polymer 1996, 37, 231–234. [Google Scholar] [CrossRef]
- Lee, G.; Park, M.; Kim, J.; Lee, J.; Yoon, H. Enhanced thermal conductivity of polymer composites filled with hybrid filler. Compos. A Appl. Sci. Manuf. 2006, 37, 727–734. [Google Scholar] [CrossRef]
- Kim, M.; Lee, J.; Roh, H.; Kim, D.; Byeon, J.; Jee, M.; Park, J. Effects of covalent functionalization of the MWCNTs on thermal properties and non-isothermal crystallization behaviors of the PPS composites. Polymers 2017. accepted. [Google Scholar]
- Choi, S.; Im, H.; Kim, J. The thermal conductivity of embedded nano-aluminum nitride-doped multi-walled carbon nanotubes in epoxy composites containing micro-aluminum nitride particles. Nanotechnology 2012, 23, 65303. [Google Scholar] [CrossRef] [PubMed]
- Gu, J.; Du, J.; Dang, J.; Geng, W.; Hu, S.; Zhang, Q. Thermal conductivities, mechanical and thermal properties of graphite nanoplatelets/polyphenylene sulfide composites. RSC Adv. 2014, 4, 22101–22105. [Google Scholar] [CrossRef]
- Zhou, G.; Xu, X.; Wang, S.; He, X.; He, W.; Su, X.; Wong, C.P. Surface grafting of epoxy polymer on CB to improve its dispersion to be filler of resistive ink for PCB. Results Phys. 2017, 7, 1870–1877. [Google Scholar] [CrossRef]
- Hay, J.N.; Luck, D.A. The conformation of crystalline poly(phenylene sulphide). Polymer 2001, 42, 8297–8301. [Google Scholar] [CrossRef]
- Jana, R.N.; Im, C.; Bhunia, H. Effect of multiwalled Carbon Nanotubes on Crystallization Behavior of Poly(ε-caprolactone)diol. J. Thermoplast. Compos. 2009, 22, 531–546. [Google Scholar] [CrossRef]
- Reyes-de Vaaben, S.; Aguilar, A.; Avalos, F.; Ramos-de Valle, L.F. Carbon Nanoparticles as Effective Nucleating Agents for Polypropylene. J. Therm. Anal. Calorim. 2008, 93, 947–952. [Google Scholar] [CrossRef]
- Guo, B.; Lin, Q.; Zhao, X.; Zhou, X. Crystallization of polyphenylene sulfide reinforced with aluminum nitride composite: Effects on thermal and mechanical properties of the composites. Iran. Polym. J. 2015, 24, 965–975. [Google Scholar] [CrossRef]
| Sample code | fMWCNTs (wt %) | AlN (wt %) | Silane treated AlN (wt %) |
|---|---|---|---|
| PPSHP1 | 1 | - | - |
| PPSHP1AlN10 | 10 | - | |
| PPSHP1AlN20 | 20 | ||
| PPSHP1sAlN10 | - | 10 | |
| PPSHP1sAlN20 | 20 |
| Sample | Elemental composition (at %) | ||||
|---|---|---|---|---|---|
| Al | N | C | O | Si | |
| AlN | 30.8 | 15.2 | 28.5 | 25.5 | - |
| Silane treated AlN | 20.7 | 9.2 | 35.2 | 30.3 | 4.6 |
| Sample | Temperature at weight loss (°C) | Heat-resistance index (°C) | |
|---|---|---|---|
| 5 wt % | 30 wt % | ||
| Neat PPS | 492.36 | 531.97 | 252.90 |
| PPSHP1 | 498.12 | 538.23 | 255.87 |
| PPSHP1AlN10 | 503.53 | 547.58 | 259.68 |
| PPSHP1AlN20 | 507.97 | 558.12 | 263.65 |
| PPSHP1sAlN10 | 492.84 | 555.99 | 260.06 |
| PPSHP1sAlN20 | 472.01 | 558.40 | 256.68 |
| Sample | (°C) | (°C) | (J/g) | (°C) | (J/g) | (%) | Ref |
|---|---|---|---|---|---|---|---|
| Neat PPS | 89.09 | 244.28 | 45.97 | 282.84 | 37.06 | 48.44 | [34] |
| PPSHP1 | 94.72 | 246.18 | 45.22 | 283.31 | 40.81 | 53.89 | |
| PPSHP1AlN10 | 100.75 | 248.77 | 37.69 | 284.65 | 34.21 | 50.25 | - |
| PPSHP1AlN20 | 99.71 | 251.13 | 32.40 | 285.04 | 30.69 | 50.78 | |
| PPSHP1sAlN10 | 102.40 | 248.97 | 39.16 | 284.52 | 35.56 | 52.23 | |
| PPSHP1sAlN20 | 95.29 | 251.08 | 39.39 | 284.79 | 37.03 | 61.27 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, M.; Park, S.; Park, J. Effect of the Grafting Reaction of Aluminum Nitride on the Multi-Walled Carbon Nanotubes on the Thermal Properties of the Poly(phenylene sulfide) Composites. Polymers 2017, 9, 452. https://doi.org/10.3390/polym9090452
Kim M, Park S, Park J. Effect of the Grafting Reaction of Aluminum Nitride on the Multi-Walled Carbon Nanotubes on the Thermal Properties of the Poly(phenylene sulfide) Composites. Polymers. 2017; 9(9):452. https://doi.org/10.3390/polym9090452
Chicago/Turabian StyleKim, Myounguk, Sunmin Park, and Jongshin Park. 2017. "Effect of the Grafting Reaction of Aluminum Nitride on the Multi-Walled Carbon Nanotubes on the Thermal Properties of the Poly(phenylene sulfide) Composites" Polymers 9, no. 9: 452. https://doi.org/10.3390/polym9090452
APA StyleKim, M., Park, S., & Park, J. (2017). Effect of the Grafting Reaction of Aluminum Nitride on the Multi-Walled Carbon Nanotubes on the Thermal Properties of the Poly(phenylene sulfide) Composites. Polymers, 9(9), 452. https://doi.org/10.3390/polym9090452





