In Vitro and in Vivo Study of Poly(Lactic–co–Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes
Abstract
:1. Introduction
2. Materials and Methods
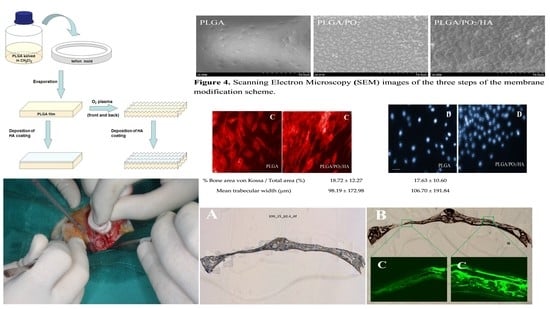
2.1. Preparation of the Membranes
2.2. Materials Characterizations
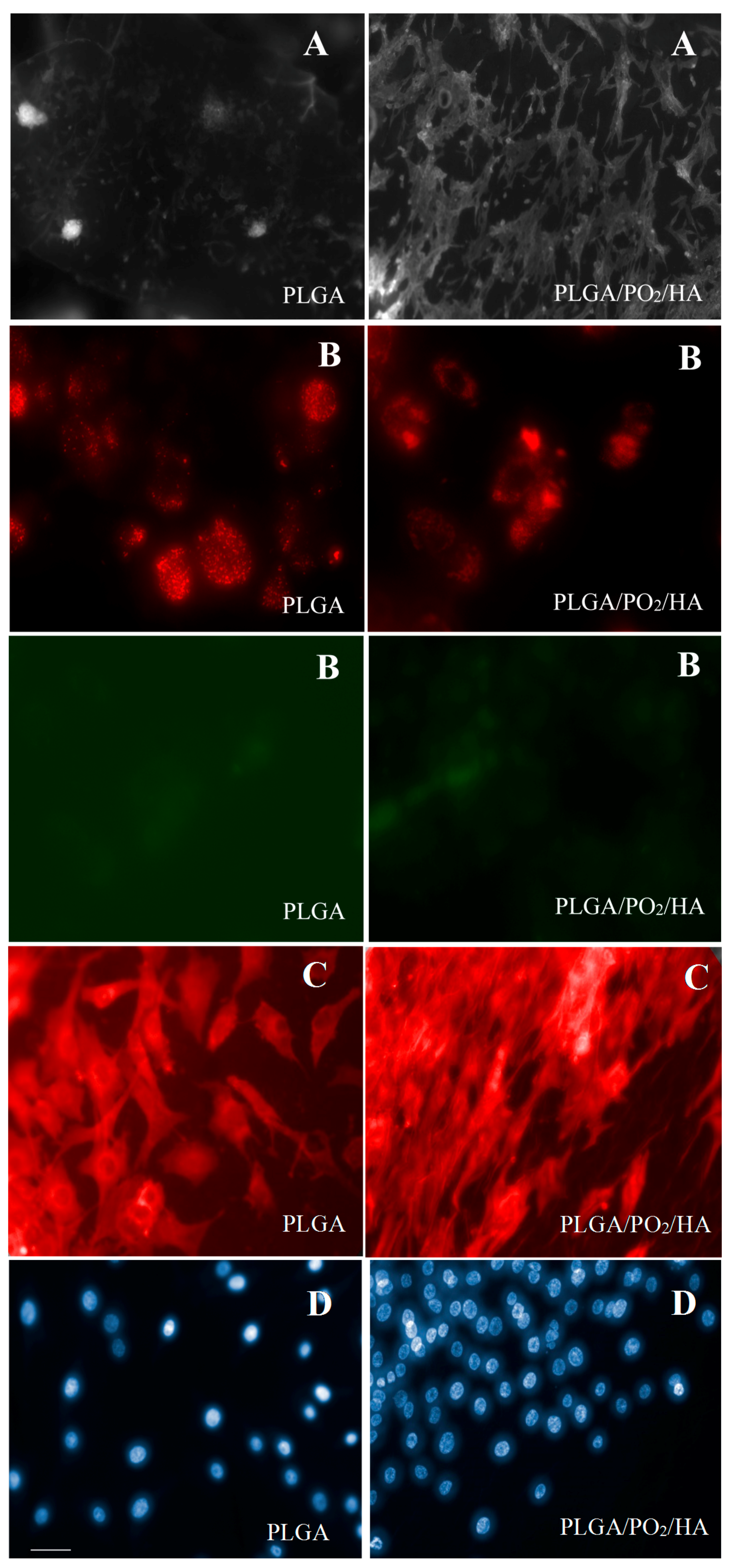
2.3. In Vitro Cell Cultures
2.4. Animal Experimentation Specimens
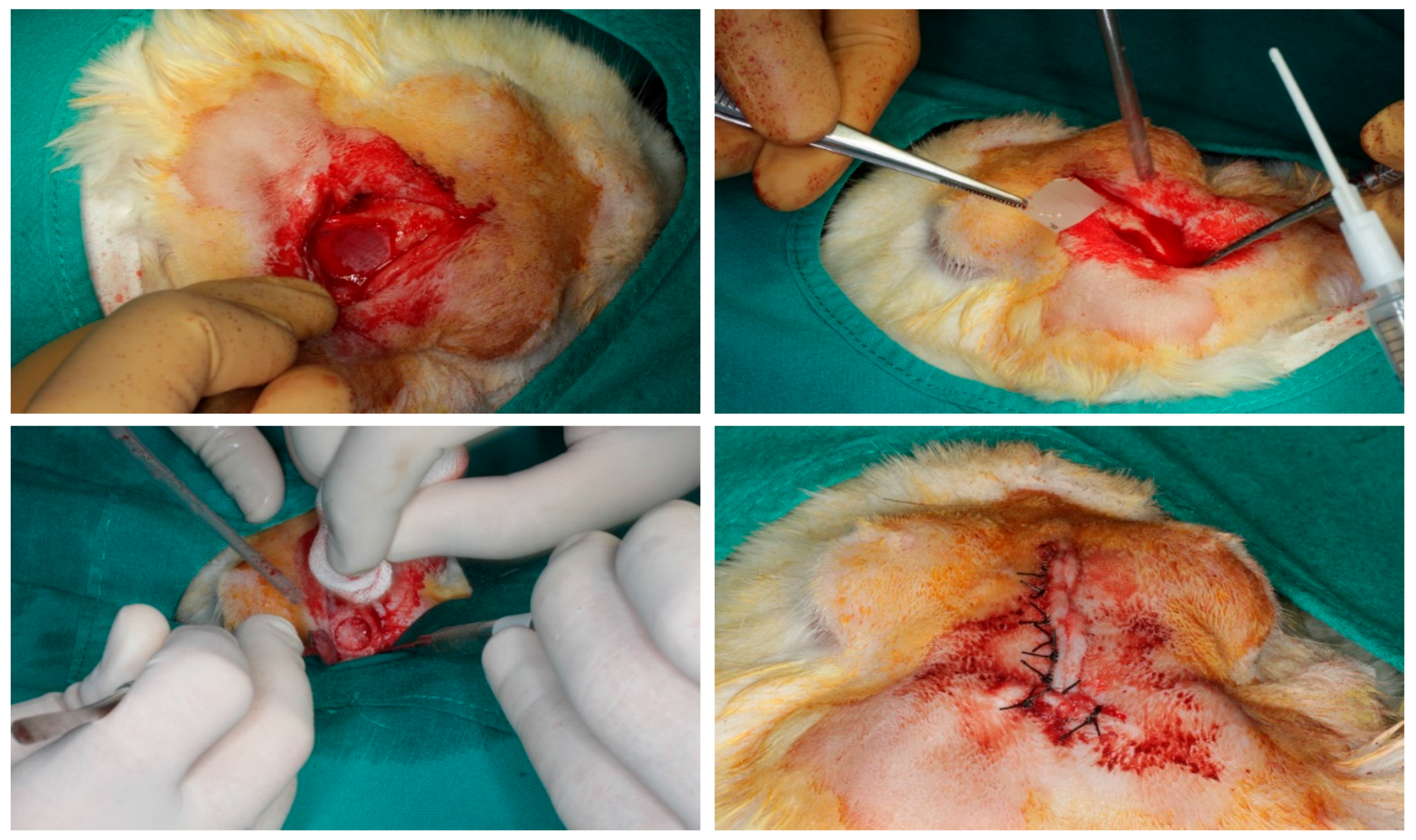
2.5. Surgical Procedure
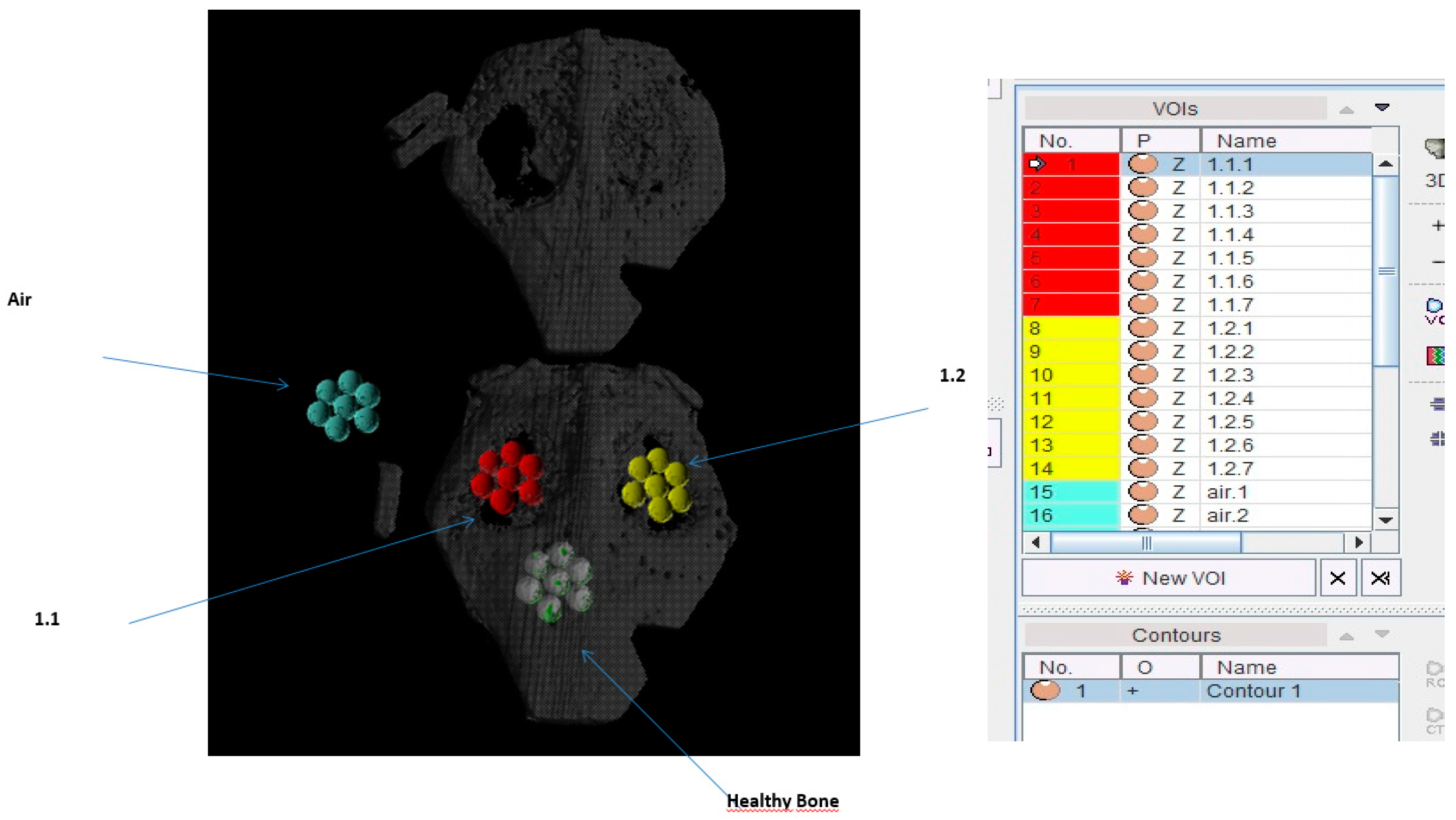
2.6. Comparison of Bone Density (BoneJ)
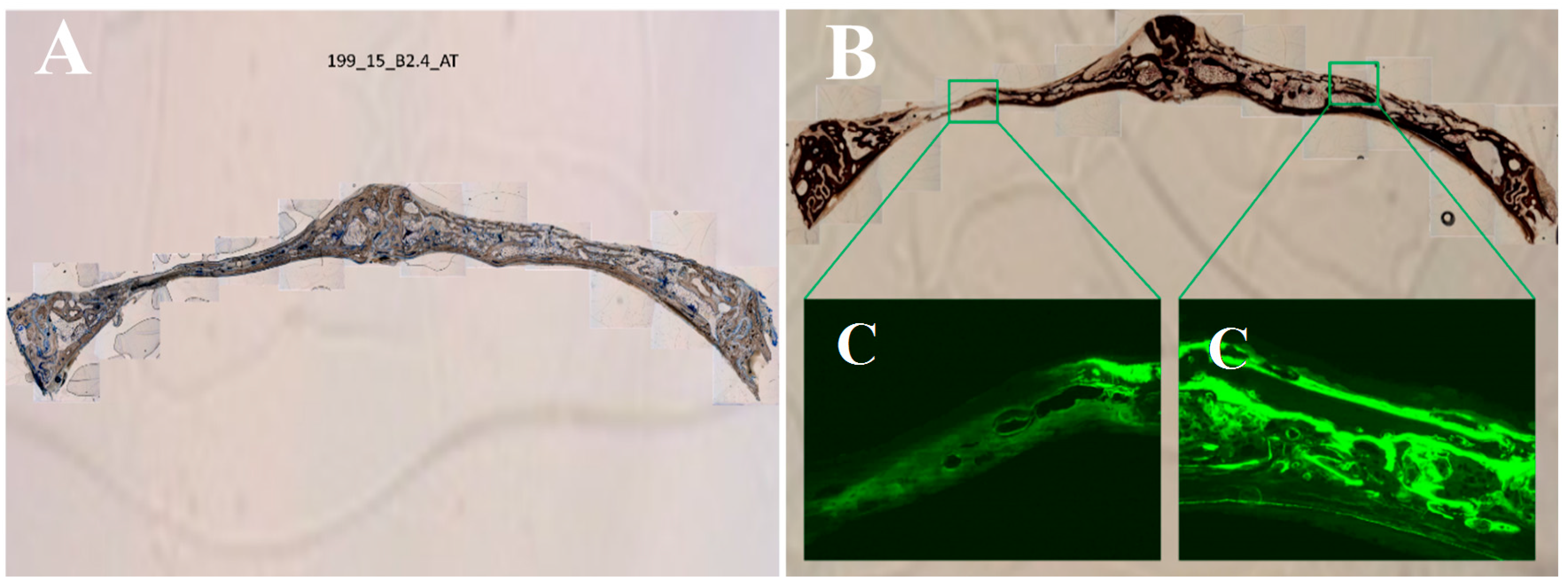
2.7. Histological Processing of Samples
2.8. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
- (1)
- We have verified the incorporation of nanometric layers of nanostructured HA films into PLGA membranes modified with PO2 are effective for the regeneration of bone defects when applied to skull defects in an animal model. We have verified the incorporation of nanometric layers of nanostructured HA films into PLGA membranes modified with PO2. These membranes showed good potential for the regeneration of bone defects when applied to skull defects in an animal model.
- (2)
- Compared to the untreated PLGA barriers, PLGA/PO2/HA membranes promote higher osteosynthetic activity, new bone formation and mineralisation levels that are comparable to those of the original bone tissue.
- (3)
- Further investigations of the new membranes in humans are required to develop new techniques that might improve the aesthetic and functional features of future restorations.
Acknowledgments
Author Contributions
Conflicts of Interest
References
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Batista-Cruzado, A.; López-Santos, C.; Rodríguez-González-Elipe, A.; Saffar, J.L.; Lynch, C.D.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Reliability of new poly (lactic–co–glycolic acid) membranes treated with oxygen plasma plus silicon dioxide layers for pre-prosthetic guided bone regeneration processes. Med. Oral Patol Oral Cir. Bucal 2017, 22, e242–e250. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista, A.; Barranco, A.; Cabezas-Talavero, J.; Lynch, C.D.; Barouk, B.; Llorens, A.; et al. In vivo comparative model of oxygen plasma and nanocomposite particles on PLGA membranes for guided bone regeneration processes to be applied in pre-prosthetic surgery: A pilot study. J. Dent. 2014, 42, 1446–1457. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Ma, P.X. Polymeric scaffolds for bone tissue engineering. Ann. Biomed. Eng. 2004, 32, 477–486. [Google Scholar] [CrossRef] [PubMed]
- Townsend-Nicholson, A.; Jayasinghe, S.N. Cell Electrospinning: A Unique Biotechnique for Encapsulating Living Organisms for Generating Active Biological Microthreads/Scaffolds. Biomacromolecules 2006, 7, 3364–3369. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.X.; He, Y.; Bi, L.; Qu, Z.H.; Zou, J.W.; Pan, Z.; Fan, J.J.; Chen, L.; Dong, X.; Liu, X.N.; et al. Enhancing the bioactivity of poly(lactic–co–glycolic acid) scaffold with a nano-hydroxyapatite coating for the treatment of segmental bone defect in a rabbit model. Int. J. Nanomed. 2013, 8, 1855–1865. [Google Scholar] [CrossRef] [PubMed]
- Castillo-Dalí, G.; Castillo-Oyagüe, R.; Terriza, A.; Saffar, J.L.; Batista-Cruzado, A.; Lynch, C.D.; Sloan, A.J.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D. Pre-prosthetic use of poly(lactic–co–glycolic acid) membranes treated with oxygen plasma and TiO2 nanocomposite particles for guided bone regeneration processes. J. Dent. 2016, 47, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Cavalcanti-Adam, E.A.; Volberg, T.; Micoulet, A.; Kessler, H.; Geiger, B.; Spatz, J.P. Cell spreading and focal adhesion dynamics are regulated by spacing of integrin ligands. Biophys. J. 2007, 92, 2964–2974. [Google Scholar] [CrossRef] [PubMed]
- Lim, J.Y.; Dreiss, A.D.; Zhou, Z.; Hansen, J.C.; Siedlecki, C.A.; Hengstebeck, R.W.; Cheng, J.; Winograd, N.; Donahue, H.J. The regulation of integrin-mediated osteoblast focal adhesion and focal adhesion kinase expression by nanoscale topography. Biomaterials 2007, 28, 1787–1797. [Google Scholar] [CrossRef] [PubMed]
- Riveline, D.; Zamir, E.; Balaban, N.Q.; Schwarz, U.S.; Ishizaki, T.; Narumiya, S.; Kam, Z.; Geiger, B.; Bershadsky, A.D. Focal contacts as mechanosensors: Externally applied local mechanical force induces growth of focal contacts by an mdia1-dependent and rock-independent mechanism. J. Cell Biol. 2001, 153, 1175–1186. [Google Scholar] [CrossRef] [PubMed]
- López-Santos, C.; Terriza, A.; Puértolas, J.; Yubero, F.; González-Elipe, A.R. Physiological Degradation Mechanisms of PLGA Membrane Films under Oxygen Plasma Treatment. J. Phys. Chem. C 2015, 119, 20446–20452. [Google Scholar] [CrossRef]
- Shen, H.; Hu, X.; Yang, F.; Bei, J.; Wang, S. Combining oxygen plasma treatment with anchorage of cationized gelatin for enhancing cell affinity of poly(lactide–co–glycolide). Biomaterials 2007, 28, 4219–4230. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.M.; Liu, X. Advancing biomaterials of human origin for tissue engineering. Prog. Polym. Sci. 2016, 53, 86–168. [Google Scholar] [CrossRef] [PubMed]
- Ngiam, M.; Liao, S.; Patil, A.J.; Cheng, Z.; Chan, CK.; Ramakrishna, S. The fabrication of nano-hydroxyapatite on PLGA and PLGA/collagen nanofibrous composite scaffolds and their effects in osteoblastic behaviour for bone tissue engineering. Bone 2009, 45, 4–16. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Wang, Z.; Dong, S.; Cai, Y.; Ni, Y.; Zhang, T.; Wang, L.; Zhou, Y. Designed Bilayer Poly(Lactic–co–Glycolic Acid)/nano-hydroxyapatite Membrane with Double Benefits of Barrier Function and Osteogenesis Promotion. Materials 2017, 10, 257. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.; Xu, Y.; Wang, Y.; Ito, Y.; Zhang, P.; Chen, X. Enhanced in Vitro Mineralization and in Vivo Osteogenesis of Composite Scaffolds through Controlled Surface Grafting of L–Lactic Acid Oligomer on Nanohydroxyapatite. Biomacromolecules 2016, 17, 818–829. [Google Scholar] [CrossRef] [PubMed]
- Witt, C.; Kissel, T. Morphological characterization of microspheres, films and implants prepared from poly(lactide–co–glycolide) and ABA triblock copolymers: Is the erosion controlled by degradation, swelling or diffusion? Eur. J. Pharm. Biopharm. 2001, 51, 171–181. [Google Scholar] [CrossRef]
- Nieh, T.G.; Jankowski, A.F.; Koike, J. Processing and characterization of hydroxyapatite coatings on titanium produced by magnetron sputtering. J. Mater. Res. 2001, 16, 3238–3245. [Google Scholar] [CrossRef]
- Wong-Lee, J.G.; Lovett, M. Rapid and sensitive PCR method for identification of Mycoplasma species in tissue culture. In Diagnostic Molecular Microbiology: Principles and Applications; Persing, D.H., Smith, T.F., Tenover, F.C., White, T.J., Eds.; American Society for Microbiology: Washington, DC, USA, 1993; pp. 257–260. [Google Scholar]
- Paz-Pumpido, F. Biocompatibilidad de los adhesivos dentinarios. Avances Odontoestomatol. 2005, 21, 339–345. [Google Scholar] [CrossRef]
- Di Toro, R.; Betti, V.; Spampinato, S. Biocompatibility and integrin-mediated adhesion of human osteoblasts to poly(dl–lactide–co–glycolide) copolymers. Eur. J. Pharm. Sci. 2004, 21, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Zhang, Z.; Lv, W.; Zhang, M.; Yang, S.; Yin, L.; Hong, J.; Han, D.; Chen, C.; Swarts, S.; et al. Interleukin 11 protects bone marrow mitochondria from radiation damage. Adv. Exp. Med. Biol. 2013, 789, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Waggoner, A.; DeBiasio, R.; Conrad, P.; Bright, G.R.; Ernst, L.; Ryan, K.; Nederlof, M.; Taylor, D. Multiple spectral parameter imaging. Methods Cell Biol. 1989, 30, 449–478. [Google Scholar] [PubMed]
- Kubista, M.; Aakerman, B.; Norden, B. Characterization of interaction between DNA and 4′,6-diamidino-2-phenylindole by optical spectroscopy. Biochemistry 1987, 26, 4545–4553. [Google Scholar] [CrossRef] [PubMed]
- Bornstein, M.M.; Reichart, P.A.; Buser, D.; Bosshardt, D.D. Tissue response and wound healing after placement of two types of bioengineered grafts containing vital cells in submucosal maxillary pouches: An experimental pilot study in rabbits. Int. J. Oral Maxillofac. Implants 2011, 26, 768–775. [Google Scholar] [PubMed]
- Landis, E.N.; Keane, D.T. X-ray microtomography. Mater. Charact. 2010, 61, 1305–1316. [Google Scholar] [CrossRef]
- Doube, M.; Kłosowski, M.M.; Arganda-Carreras, I.; Cordeliéres, F.; Dougherty, R.P.; Jackson, J.; Schmid, B.; Hutchinson, J.R.; Shefelbine, S.J. BoneJ: Free and extensible bone image analysis in ImageJ. Bone 2010, 47, 1076–1079. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Píriz, R.; Fernández, A.; Goyos-Ball, L.; Rivera, S.; Díaz, L.A.; Fernández-Domínguez, M.; Prado, C.; Moya, J.S.; Torrecillas, R. Performance of a New Al2O3/Ce–TZP Ceramic Nanocomposite Dental Implant: A Pilot Study in Dogs. Materials 2017, 10, 614. [Google Scholar] [CrossRef] [PubMed]
- Donath, K.; Breuner, G. A method for the study of undecalcified bones and teeth with attached soft tissues. The säge-schliff (sawing and grinding) technique. J. Oral Pathol. 1982, 11, 318–326. [Google Scholar] [CrossRef] [PubMed]
- Hannigan, A.; Lynch, C.D. Statistical methodology in oral and dental research: Pitfalls and recommendations. J. Dent. 2013, 41, 385–392. [Google Scholar] [CrossRef] [PubMed]
- González-Padilla, D.; García-Perla, A.; Gutiérrez-Pérez, J.L.; Torres-Lagares, D.; Castillo-Dalí, G.; Salido-Peracaula, M.; Vilches-Troya, J.; Vilches-Perez, J.I.; Terriza-Fernandez, A.; Barranco-Quero, A.; et al. Membrana Reabsorbible Para Regeneración Ósea 2012. Spanish Patent P-0201232018, 24 December 2012. [Google Scholar]
- Terriza, A.; Vilches-Pérez, J.; González-Caballero, J.L.; de la Orden, E.; Yubero, F.; Barranco, A.; Gonzalez-Elipe, A.R.; Vilches, J.; Salido, M. Osteoblasts interaction with PLGA membranes functionalized with titanium film nanolayer by PECVD. In vitro assessment of surface influence on cell adhesion during initial cell to material interaction. Materials 2014, 7, 1687–1708. [Google Scholar] [CrossRef] [PubMed]
- Terriza, A.; Vilches-Pérez, J.I.; de la Orden, E.; Yubero, F.; Gonzalez-Caballero, J.L.; González-Elipe, A.R.; Vilches, J.; Salido, M. Osteoconductive potential of barrier nanoSiO2 PLGA membranes functionalized by plasma enhanced chemical vapour deposition. Biomed. Res. Int. 2014, 2014, 253590. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.; Qu, X.; Lu, J.; Zhu, C.; Wan, L.; Yang, J.; Bei, J.; Wang, S. Characterization of surface property of poly(lactide–co–glycolide) after oxygen plasma treatment. Biomaterials 2004, 25, 4777–4783. [Google Scholar] [CrossRef] [PubMed]
- Socol, G.; Macovei, A.M.; Miroiu, F.; Stefan, N.; Duta, L.; Dorcioman, G.; Mihailescu, I.N.; Petrescu, S.M.; Stan, G.E.; Marcov, D.A.; et al. Hydroxyapatite thin films synthesized by pulsed laser deposition and magnetron sputtering on PMMA substrates for medical applications. Mater. Sci. Eng. B 2010, 169, 159–168. [Google Scholar] [CrossRef]
- Yamaguchi, T.; Tanaka, Y.; Ide-Ektessabi, A. Fabrication of hydroxyapatite thin films for biomedical applications using RF magnetron sputtering. Nucl. Instrum. Methods Phys. Res. Sect. B 2006, 249, 723–725. [Google Scholar] [CrossRef]
- Gumbiner, B.M. Cell adhesion: The molecular basis of tissue architecture and morphogenesis. Cell 1996, 84, 345–357. [Google Scholar] [CrossRef]
- Barthes, J.; Özçelik, H.; Hindié, M.; Ndreu-Halili, A.; Hasan, A.; Vrana, N.E. Cell microenvironment engineering and monitoring for tissue engineering and regenerative medicine: The recent advances. Biomed. Res. Int. 2014, 2014, 921905. [Google Scholar] [CrossRef] [PubMed]
- Gentile, P.; Chiono, V.; Carmagnola, I.; Hatton, P.V. An overview of poly(lactic–co–glycolic) acid (PLGA)-based biomaterials for bone tissue engineering. Int. J. Mol. Sci. 2014, 15, 3640–3659. [Google Scholar] [CrossRef] [PubMed]
- Kasuga, T.; Hosoi, Y.; Nogami, M.; Niinomi, M. Apatite formation on calcium phosphate invert glasses in simulated body fluid. J. Am. Ceram. Soc. 2001, 84, 450–452. [Google Scholar] [CrossRef]
- Wong, K.L.; Wong, C.T.; Liu, W.C.; Pan, H.B.; Fong, M.K.; Lam, W.M.; Cheung, W.L.; Tang, W.M.; Chiu, K.Y.; Luk, K.D.; et al. Mechanical properties and in vitro response of strontium-containing hydroxyapatite/polyetheretherketone composites. Biomaterials 2009, 30, 3810–3817. [Google Scholar] [CrossRef] [PubMed]
- Canillas, M.; Pena, P.; de Aza, A.H.; Rodríguez, M.A. Calcium phosphates for biomedical applications. Boletín SECV 2017, 56, 91–112. [Google Scholar] [CrossRef]
- Bose, S.; Tarafder, S. Calcium phosphate ceramic systems in growth factor and drug delivery for bone tissue engineering: A review. Acta Biomater. 2012, 8, 1401–1421. [Google Scholar] [CrossRef] [PubMed]
- Habraken, W.; Habibovic, P.; Epple, M.; Bohner, M. Calcium phosphates in biomedical applications: Materials for the future? Mater. Today 2016, 19, 69–87. [Google Scholar] [CrossRef]
- Baino, F.; Novajra, G.; Vitale-Brovarone, C. Bioceramics and scaffolds: A winning combination for tissue engineering. Front. Bioeng. Biotechnol. 2015, 3, 202. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Kerns, D.G. Mechanisms of guided bone regeneration: A review. Open Dent. J. 2014, 8, 56–65. [Google Scholar] [CrossRef] [PubMed]
- Palmer, L.C.; Newcomb, C.J.; Kaltz, S.R.; Spoerke, E.D.; Stupp, S.I. Biomimetic systems for hydroxyapatite mineralization inspired by bone and enamel. Chem. Rev. 2008, 108, 4754–4783. [Google Scholar] [CrossRef] [PubMed]
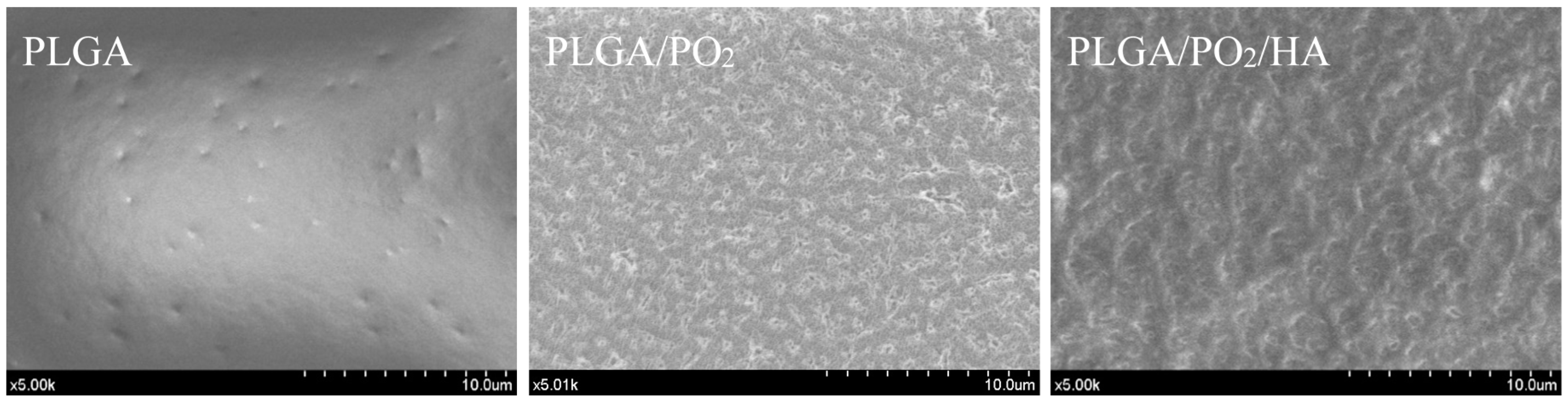
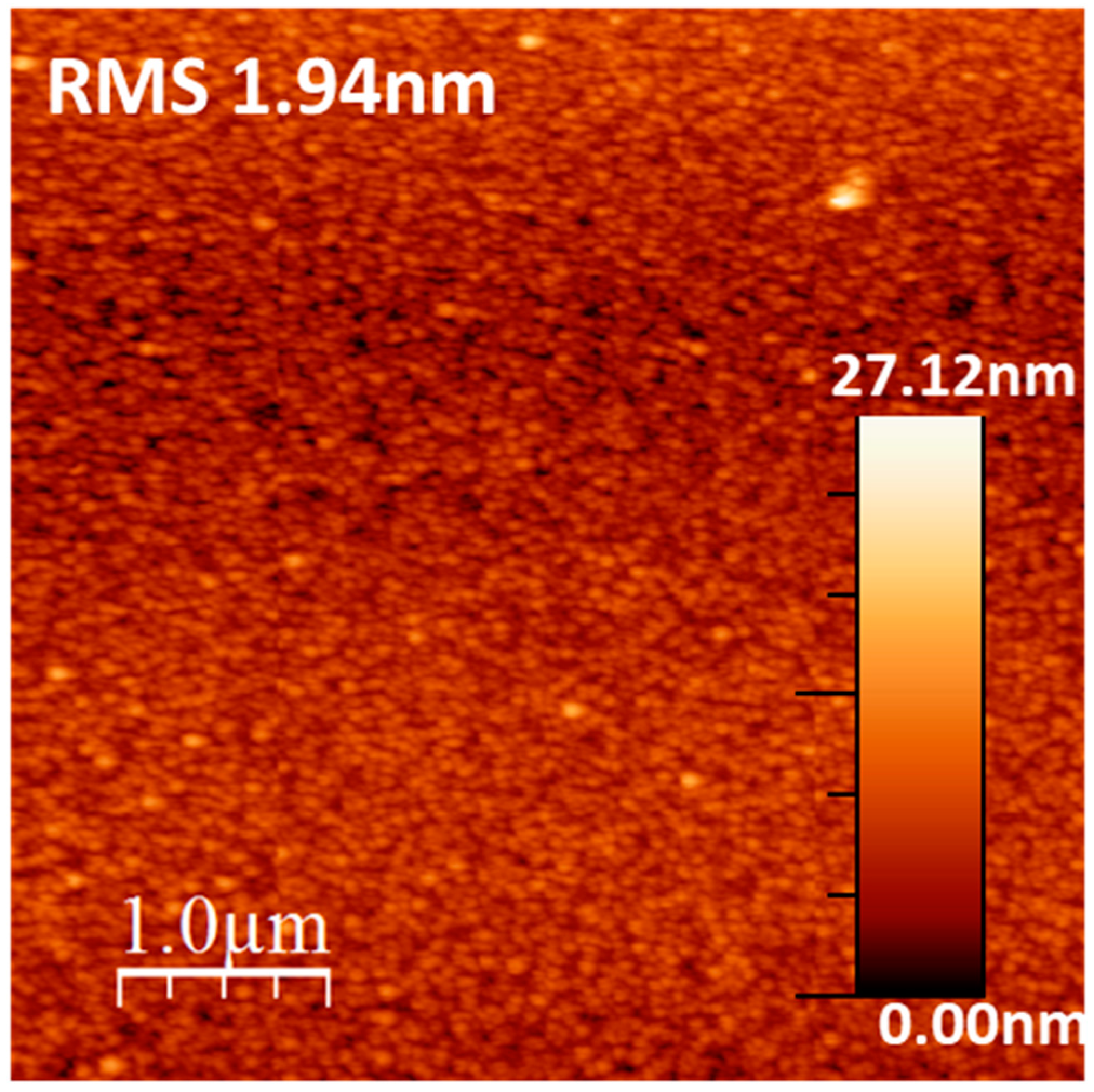
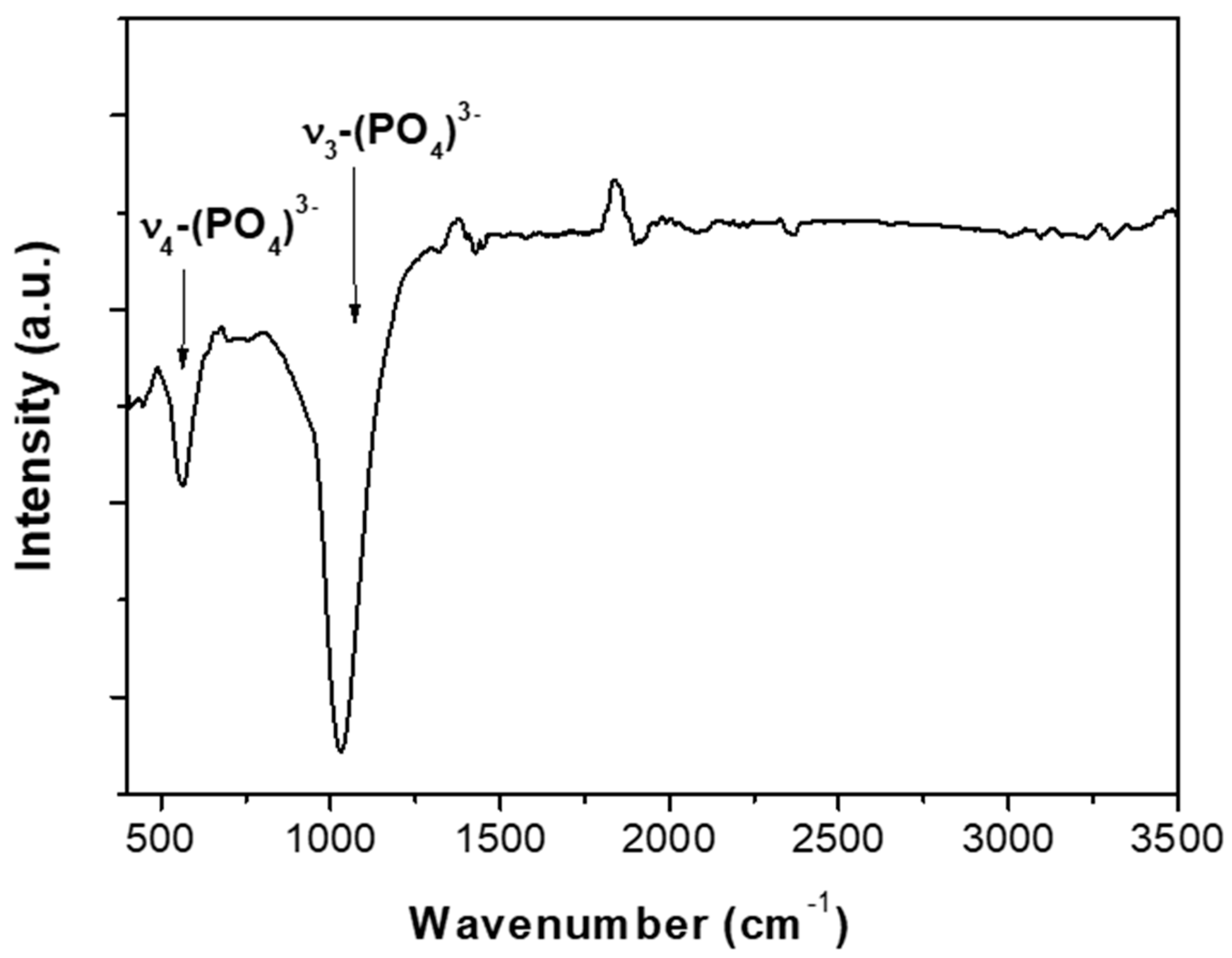
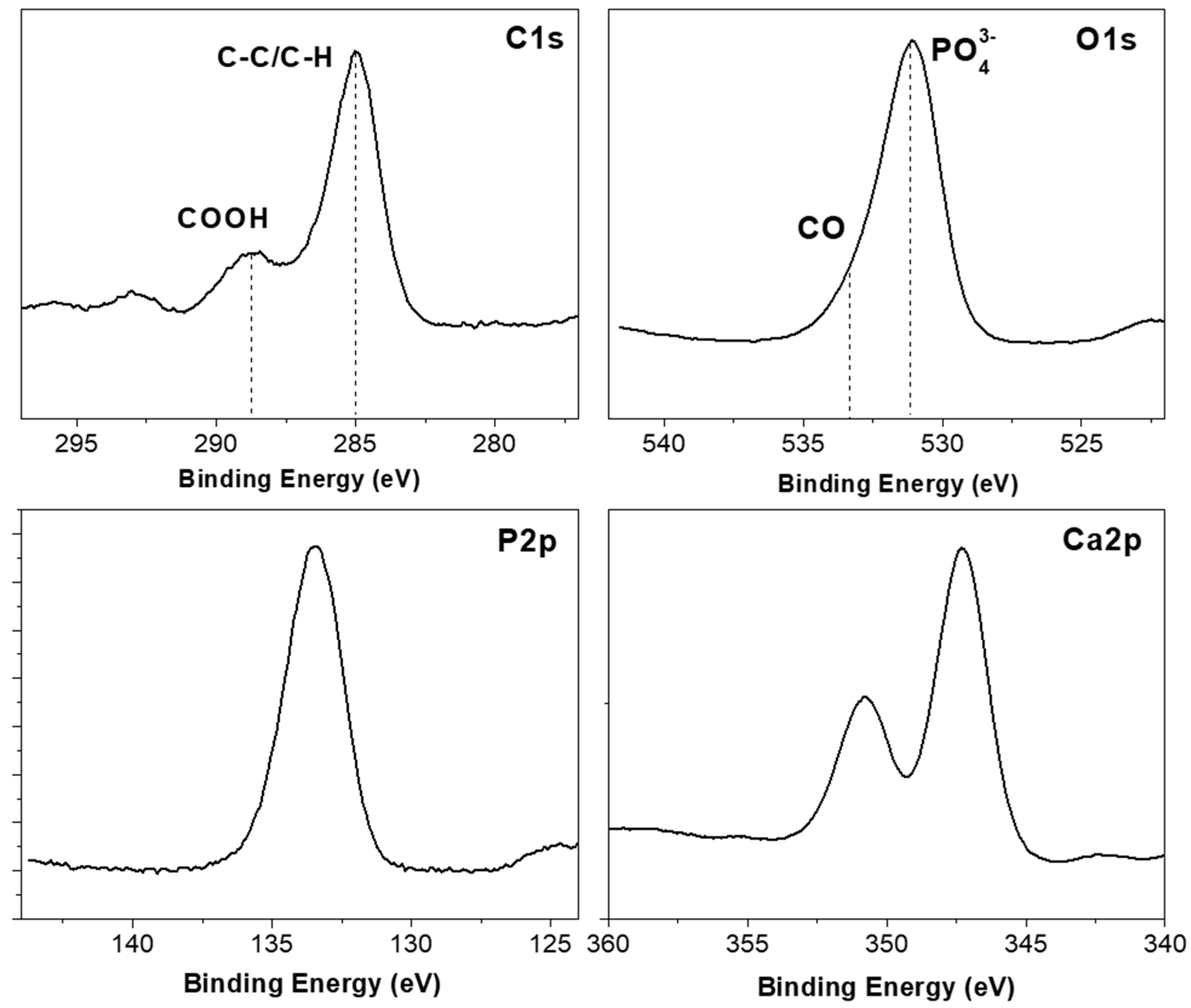
| Atomic | C1s | O1s | Ca2p | P2p |
|---|---|---|---|---|
| Composition (%) | 23.6 | 44.8 | 18.1 | 13.5 |
| PLGA (Control) | PLGA/PO2/HA (Experimental) | Statistical Significance | |
|---|---|---|---|
| Cell area (μm2) | 288 ± 124 | 379 ± 110 | |
| DAPI (nuclei area) (μm2) | 2.82 ± 2.0 | 2.55 ± 1.9 | |
| Probe JC-1 (red/green ratio) | 1.69 ± 0.41 | 2.57 ± 0.09 | p = 0.06 |
| Cells Viability (%) | 62.4 ± 6.2 | 78.2 ± 3.4 | p < 0.05 |
| Total cells (cells) | 2.1 × 105 ± 0.3 | 4.6 × 105 ± 0.4 | p < 0.05 |
| PLGA (Control) | PLGA/PO2/HA (Experimental) | Statistical Significance | |
|---|---|---|---|
| Bone density (HU) | 969.51 ± 145.7 | 1036.71 ± 241.3 | |
| Bone density (%) | 0.59 ± 0.08 | 0.63 ± 0.14 | |
| Bone Surface (pixels 2) | 5079.09 ± 1779.49 | 11,049.51 ± 4304.57 | * |
| Mean trabecular thickness (pixels) | 5.74 ± 1.24 | 6.29 ± 1.52 | |
| Max. trabecular thickness (pixels) | 9.13 ± 1.60 | 11.30 ± 1.75 | * |
| Bone volume (pixels 2) | 25,016.20 ± 9922.46 | 45,526.20 ± 15,275.48 | * |
| Total volume (pixels 2) | 8,120,601.20 ± 16,432.30 | 8,090,300.45 ± 17,742.30 | * |
| Bone volume/Total volume | 0.003 ± 0.001 | 0.005 ± 0.001 | * |
| Euler characteristic | −34.05 ± 17.49 | −74.55 ± 36.65 | * |
| Maximum branch length (pixels) | 38.89 ± 7.55 | 35.47 ± 12.38 | |
| Connectivity (mm−3) | 35.25 ± 17.45 | 75.75 ± 36.63 | * |
| Number of branches (branches) | 152.45 ± 83.62 | 265.70 ± 109.02 | * |
| Number of junctions (junctions) | 77.45 ± 43.02 | 139.70 ± 58.92 | * |
| Number of end-point voxels (voxels) | 41.20 ± 23.63 | 51.70 ± 17.78 | 0.08 |
| Number of junctions voxels (voxels) | 180.45 ± 97.38 | 323.70 ± 132.25 | * |
| Number of slab voxels (voxels) | 761.95 ± 434.86 | 1269.95 ± 467.48 | * |
| Average branch length (pixels) | 8.87 ± 1.67 | 8.64 ± 1.62 | |
| Number of triple points (points) | 97.20 ± 40.15 | 53.450 ± 30.40 | * |
| Number of quadruple points (points) | 17.45 ± 10.96 | 31.95 ± 14.74 | * |
| PLGA (Control) | PLGA/PO2/HA (Experimental) | Statistical Significance | |
|---|---|---|---|
| Bone height (μm) | 1718 ± 775 | 1729 ± 700 | |
| Trabecular area (μm2) | 129,558.45 ± 619,632.90 | 160,339.46 ± 654,020.23 | |
| Trabecular perimeter (μm) | 1282.57 ± 2525.41 | 1491.46 ± 2747.67 | |
| Number of trabeculae (trabeculae) | 27.75 ± 18.81 | 33.50 ± 33.40 | |
| Fluorescence (μm2) | 12.43 ± 6.61 | 14.11 ± 4.82 | |
| Median trabecular area (μm2) | 129,139.75 ± 618,508.04 | 160,339.47 ± 654,020.24 | |
| Total trabecular area (μm2) | 235,428.77 ± 638,030.19 | 186,611.42 ± 398,159.18 | |
| Osteoid area (μm2) | 4369.21 ± 8129.87 | 5974.17 ± 10,159.57 | |
| % Osteoid area/ Total area (%) | 0.0501 ± 0.0675 | 0.0854 ± 0.1172 | * |
| % Bone area von Kossa / Total area (%) | 18.72 ± 12.27 | 17.63 ± 10.60 | |
| Mean trabecular width (μm) | 98.19 ± 172.98 | 106.70 ± 191.84 | |
| % Bone area von Kossa / Total area (%) | 18.72 ± 12.27 | 17.63 ± 10.60 | |
| Mean trabecular width (μm) | 98.19 ± 172.98 | 106.70 ± 191.84 |
© 2017 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Torres-Lagares, D.; Castellanos-Cosano, L.; Serrera-Figallo, M.Á.; García-García, F.J.; López-Santos, C.; Barranco, A.; Rodríguez-Gonzalez Elipe, A.; Rivera-Jiménez, C.; Gutiérrez-Pérez, J.-L. In Vitro and in Vivo Study of Poly(Lactic–co–Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes. Polymers 2017, 9, 410. https://doi.org/10.3390/polym9090410
Torres-Lagares D, Castellanos-Cosano L, Serrera-Figallo MÁ, García-García FJ, López-Santos C, Barranco A, Rodríguez-Gonzalez Elipe A, Rivera-Jiménez C, Gutiérrez-Pérez J-L. In Vitro and in Vivo Study of Poly(Lactic–co–Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes. Polymers. 2017; 9(9):410. https://doi.org/10.3390/polym9090410
Chicago/Turabian StyleTorres-Lagares, Daniel, Lizett Castellanos-Cosano, María Ángeles Serrera-Figallo, Francisco J. García-García, Carmen López-Santos, Angel Barranco, Agustín Rodríguez-Gonzalez Elipe, Cristóbal Rivera-Jiménez, and José-Luis Gutiérrez-Pérez. 2017. "In Vitro and in Vivo Study of Poly(Lactic–co–Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes" Polymers 9, no. 9: 410. https://doi.org/10.3390/polym9090410
APA StyleTorres-Lagares, D., Castellanos-Cosano, L., Serrera-Figallo, M. Á., García-García, F. J., López-Santos, C., Barranco, A., Rodríguez-Gonzalez Elipe, A., Rivera-Jiménez, C., & Gutiérrez-Pérez, J.-L. (2017). In Vitro and in Vivo Study of Poly(Lactic–co–Glycolic) (PLGA) Membranes Treated with Oxygen Plasma and Coated with Nanostructured Hydroxyapatite Ultrathin Films for Guided Bone Regeneration Processes. Polymers, 9(9), 410. https://doi.org/10.3390/polym9090410